Abstract
OBJECTIVE
The aim of this study was to investigate the relationship between diabetes and different phenotypes of peripheral vascular disease (lower extremity peripheral artery disease [PAD], carotid artery stenosis [CAS], and abdominal aortic aneurysm [AAA]).
RESEARCH DESIGN AND METHODS
Prevalence of vascular disease was evaluated in 3,696,778 participants of the Life Line Screening survey between 2003 and 2008. PAD was defined as ankle-brachial pressure index <0.90 or prior revascularization, CAS as ≥50% stenosis or prior revascularization, and AAA as infrarenal aortic diameter ≥3 cm or prior repair. Odds ratios (ORs) and 95% CIs were assessed using logistic regression modeling.
RESULTS
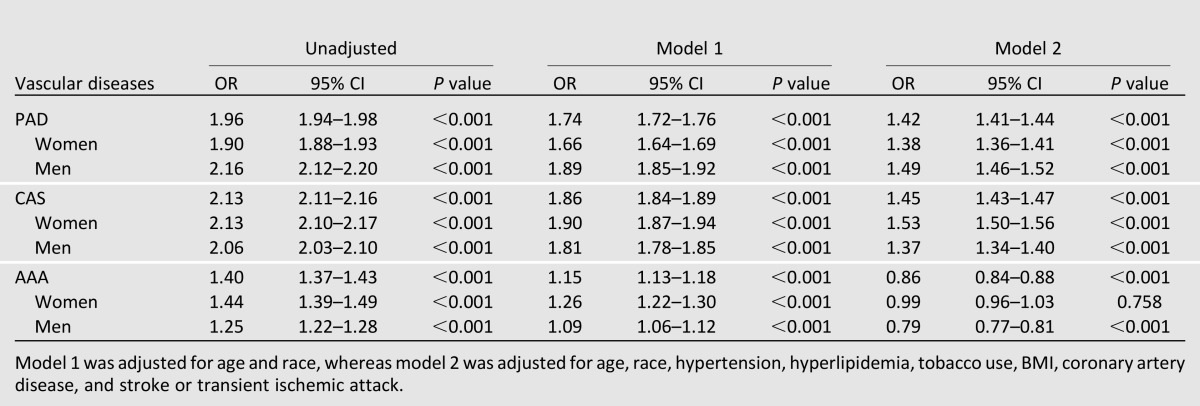
Diabetes mellitus was present in 10.8% of participants (n = 399,884). Prevalence of PAD, CAS, and AAA was significantly higher (P < 0.0001) in participants with compared with those without diabetes. After multivariate adjustment for baseline demographics and clinical risk factors, a significant interaction existed between diabetes and vascular disease phenotype (P < 0.0001). Diabetes was associated with increased odds of PAD (OR 1.42 [95% CI 1.41–1.4]; P < 0.0001) and CAS (1.45 [1.43–1.47]; P < 0.0001) but decreased odds of AAA (0.86 [0.84–0.88]; P < 0.0001). The strength of association increased with increasing severity of disease in each vascular phenotype, and this association persisted in the population with asymptomatic vascular disease.
CONCLUSIONS
In a large population-based study, the association between diabetes and vascular disease differed according to vascular phenotype. Future studies exploring the mechanism for these vascular-specific differences are needed.
Introduction
Diabetes is a growing epidemic affecting more than 346 million people worldwide (1–3). The metabolic abnormalities associated with diabetes lead to altered platelet, endothelial cell, and smooth muscle cell function (4–7). Together, these changes promote systemic atherosclerosis and lead to the traditional micro- and macrovascular complications commonly observed in diabetes.
Morbidity and mortality in the population with diabetes are largely due to complications of atherosclerosis. Furthermore, vascular disease in one arterial bed increases the risk for concomitant vascular disease in other arterial territories (8,9). Vascular disease in the peripheral arteries presents a significant burden to health care expenditure and represents a major cause of myocardial infarction, stroke, and limb morbidity (10–12). In addition, both diabetes and disease in the peripheral vasculature are associated with increased long-term mortality (13–16). However, the peripheral neuropathy associated with diabetes masks symptoms of atherosclerosis, making the true prevalence of disease in the peripheral arteries difficult to ascertain without additional testing and presenting a challenge to early identification and prevention of disease progression (12,17).
Given the known clustering of traditional cardiovascular risk factors and coronary heart disease with diabetes, this study sought to investigate the independent association between diabetes and the presence and severity of vascular disease in the peripheral arteries. While several studies have evaluated the association between diabetes and lower extremity peripheral artery disease (PAD), few studies have explored carotid artery stenosis (CAS) or abdominal aortic aneurysms (AAA) in populations with and without diabetes, and no study to date has evaluated the association between diabetes and all three major vascular disease subtypes in a large unselected population. In this study, we aim to evaluate the association between diabetes and the presence and severity of PAD, CAS, and AAA in more than 3 million subjects.
Research Design and Methods
Study Population
A total of 3,696,778 primarily self-referred people participated in the Life Line Screening (LLS; Independence, Ohio) vascular survey at more than 20,000 screening sites, representing all 50 states and broad geographical and socioeconomic characteristics, between 2003 and 2008. As noted previously, the prevalence of different cardiovascular risk factors in this population database is similar to that of the general U.S. population (18). Using U.S. census data linked by the subject’s zip code, we categorized individuals based on income and education and found fairly good representation across different levels of socioeconomic status (19). A variety of costs was incurred by the individuals based on the package of tests purchased. All LLS sites use identical protocols and are subject to a quality control program (18). In accord with the Office for Human Research Protections, this study is exempt from review by an institutional review board (20).
Covariates
Participants completed a two-page questionnaire, providing their demographic information, height and weight, and medical and surgical history, including cardiovascular risk factors, physical activity, nutrition, and family history of cardiovascular disease. Obesity was identified as BMI ≥30 kg/m2, and tobacco use was defined as use of at least 100 cigarettes during their lifetime and currently smoking (current) or not currently smoking (former). Hypertension was defined as systolic blood pressure of ≥140 mmHg in either upper extremity or self-reported physician diagnosis or medication use. Hypercholesterolemia was defined as self-reported physician diagnosis or medication use. History of coronary artery disease was defined as having a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft. Diabetes was defined as self-reported physician diagnosis or medication use.
Vascular Disease Outcomes
Presence of PAD was assessed with the bilateral ankle-brachial pressure index (ABI), defined as systolic blood pressure in the left and right ankle (measured in the posterior tibial artery or dorsalis pedis artery if a posterior tibial artery Doppler signal was inaudible) divided by the highest of the two systolic blood pressures in the left or right arm (brachial artery). In the overall population, 5.6% (n = 207,818) did not have recorded ABI results. PAD was defined as either left or right ABI ≤0.9 or history of peripheral arterial intervention. PAD was defined as symptomatic if participants had a prior revascularization procedure or answered yes to, “Do you have aching or pain in the legs that is worse with walking or running” or “…relieved within a few minutes by rest.” Degree of PAD was categorized as normal (ABI 0.91–1.4), mild (ABI 0.81–0.90), moderate (ABI 0.61–0.80), severe (ABI ≤0.60), or unable to compress artery (ABI >1.4) (17).
Presence of CAS was assessed using duplex ultrasound, and in the population of interest, 2.9% (n = 107,318) did not have carotid ultrasound results recorded. CAS was defined as CAS ≥50% or history of carotid artery revascularization procedure. Symptomatic CAS was defined as participants with a prior stroke, transient ischemic attack, or operation on the carotid arteries. The degree of CAS was categorized as moderate for 50–69% stenosis or severe for ≥70% stenosis.
All participants had abdominal aortic ultrasound results recorded, and AAA was defined as presence of an infrarenal abdominal aortic diameter of ≥3 cm on ultrasound (the greater of the anteroposterior or transverse measurement was used) or prior surgical or special procedure to repair an AAA. If a participant had a history of AAA repair, they were considered symptomatic.
Statistical Analysis
Each individual was assigned a unique identifier, and the investigators had access only to de-identified data. The population of interest was categorized into those with versus those without diabetes. Baseline characteristics and the presence of PAD, CAS, and AAA between participants with and without diabetes were compared using the χ2 test for proportions and a two-sided independent sample t test for continuous variables. Odds ratios (ORs) and 95% CIs were assessed using a logistic regression model to examine the strength of association between diabetes and PAD, CAS, and AAA. In addition to unadjusted OR, model 1 adjusted for age, sex, and race, whereas model 2 adjusted for age, sex, race, BMI, hypertension, hyperlipidemia, tobacco use, coronary artery disease, and stroke or transient ischemic attack. In sex-specific analysis, the strength of association between diabetes and PAD, CAS, and AAA was evaluated using the logistic regression model in model 2. The Wald test was used for testing interaction between the presence of diabetes and sex for the presence of each of the vascular disease subtypes. All statistical analyses were performed with PASW (version 18.0; SPSS Inc., Chicago, IL), SAS (version 9.12; SAS Institute Inc., Cary, NC), and the R package (R Development Core Team; available from http://www.r-project.org/).
Results
Baseline Characteristics
Among 3,696,778 participants in this analysis, 399,884 (10.8%) had diabetes. Baseline characteristics of participants with and without diabetes are shown in Supplementary Table 1. Consistent with prior observations, participants with diabetes were older, more frequently male, less frequently white, and more likely to have other cardiovascular risk factors. These differences were consistent across the spectrum of vascular disease. Subjects with vascular disease had a higher prevalence of risk factors than subjects without vascular disease. Subjects with AAA were more likely to be men and had a higher prevalence of heart disease and stroke than subjects with PAD or CAS (Supplementary Table 1).
Prevalence of Vascular Disease
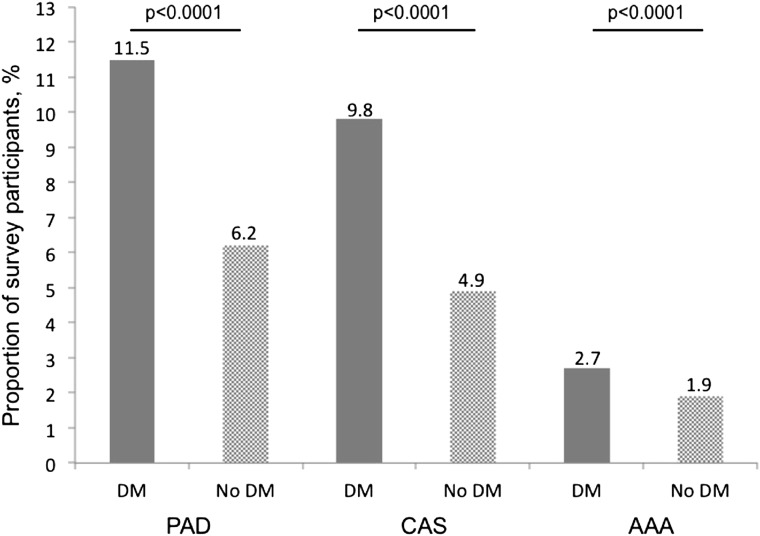
Among the 3,696,778 participants in the study, 422,501 participants had any vascular disease; 233,958 participants had PAD, 193,734 participants had CAS, and 73,443 participants had AAA. Participants with diabetes had a higher prevalence of any vascular disease (unadjusted OR 1.98 [95% CI 1.96–2.00]; P < 0.0001) (Fig. 1). Diabetes was associated with a higher odds of PAD (unadjusted OR 1.96 [95% CI 1.94–1.98]; P < 0.0001), CAS (unadjusted OR 2.13 [95% CI 2.11–2.16]; P < 0.0001), and AAA (unadjusted OR 1.40 [95% CI 1.37–1.43]; P < 0.0001) compared with those without diabetes (Table 1). Vascular disease in more than one peripheral vascular bed was also more common in participants with diabetes. Compared with participants without diabetes, participants with diabetes were more likely to have peripheral vascular disease in one territory (15.1% vs. 9.0%; P < 0.0001), two territories (2.9% vs. 1.2%; P < 0.0001), or three territories (0.7% vs. 0.4%; P < 0.0001) (Supplementary Fig. 1).
Figure 1.
Proportion of survey participants with lower extremity PAD, CAS, and AAA in the populations with and without diabetes (DM).
Table 1.
Odds of vascular disease by degree of severity in the presence of diabetes

After adjustment for age, sex, race, hypertension, hyperlipidemia, smoking status, BMI, coronary artery disease, and stroke or transient ischemic attack, diabetes was significantly associated with increased odds of any vascular disease (adjusted OR 1.41 [95% CI 1.39–1.42]; P < 0.0001). A significant interaction existed between diabetes and vascular disease phenotype (P < 0.0001); diabetes was significantly associated with increased odds of PAD (adjusted OR 1.42 [95% CI 1.41–1.44]; P < 0.0001) and CAS (adjusted OR 1.45 [95% CI 1.43–1.47]; P < 0.0001). In contrast, after multivariable adjustment, diabetes was significantly associated with lower odds of AAA (adjusted OR 0.86 [95% CI 0.84–0.88]; P < 0.001) (Table 1).
Vascular Disease Severity
The relationship between severity of vascular disease and diabetes across the spectrum of vascular phenotypes is shown in Table 1 and Fig. 2. Subjects with diabetes were more likely to have more severe PAD than subjects without diabetes. The association between diabetes and PAD became more pronounced with severity of disease (adjusted ORs: mild PAD, 1.37; moderate PAD, 1.77; severe PAD, 2.16; P for trend < 0.001). Although less striking, a similar observation was noted in CAS; the association between diabetes and CAS increased with severity of disease (adjusted OR 1.53 [95% CI 1.50–1.56]; P < 0.001 for moderate CAS and adjusted OR 1.60 [95% CI 1.56–1.63]; P < 0.001 for severe CAS). For the end point of AAA, diabetes was associated with a 20% and 25% lower odds of smaller and larger AAA, respectively (adjusted OR 0.80 [95% CI 0.77–0.83]; P < 0.001 for 3- to 5-cm-diameter aneurysm and adjusted OR 0.75 [95% CI 0.66–0.85]; P < 0.001 for >5-cm-diameter aneurysm).
Figure 2.
Proportion of survey participants with lower extremity PAD (A), CAS (B), and AAA (C) by disease severity in the population with and without diabetes (DM).
Supplementary Fig. 2 shows the prevalence of vascular disease stratified by symptoms in participants with and without diabetes. Participants with diabetes were more likely to have both asymptomatic disease and symptomatic disease. To minimize recall bias in our analysis, we excluded subjects with symptomatic disease. Results were not greatly altered, with increased odds of PAD (adjusted OR 1.54 [95% CI 1.50–1.57]; P < 0.0001) and CAS (adjusted OR 1.57 [95% CI 1.55–1.60]; P < 0.0001) and decreased odds of AAA (adjusted OR 0.80 [95% CI 0.77–0.83]; P < 0.0001).
Sex Differences
We further investigated the association between diabetes and different phenotypes of vascular disease in women and men (Table 2). A significant association between diabetes and PAD and CAS was present in women and men, respectively. The association between diabetes and AAA seemed to differ by sex. Diabetes was associated with a 21% decreased odds of AAA in men, but no significant association between diabetes and AAA was noted among women (adjusted OR 0.99 [95% CI 0.96–1.03]; P = 0.76).
Table 2.
Association between diabetes and vascular disease in women and men
Conclusions
In this large population-based analysis, all three major forms of vascular disease were significantly higher in people with diabetes compared with those without diabetes. However, after adjustment for baseline demographics and traditional risk factors, diabetes was significantly associated with an increased odds of PAD and CAS but lower odds of AAA. In support of this observation, the strength of association was more pronounced with increasing severity of disease in each of the three vascular phenotypes. Moreover, the positive association between diabetes and PAD and CAS, as well as the negative association between diabetes and AAA, remained robust in subjects with asymptomatic disease. Finally, the increased association between diabetes and PAD and CAS was similar in women and men; however, diabetes was associated with lower odds of AAA in men, which was not observed in women.
The results of this study are consistent with a recent analysis of more than 40,000 men without a history of cardiovascular disease at baseline who were followed for 25 years in the prospective Health Professionals Follow-up Study, which demonstrated an independent association between diabetes and PAD (21). Another study using National Health and Nutrition Examination Survey (NHANES) data demonstrated almost double the proportion of individuals with PAD in the population with diabetes compared with the overall population (22). In contrast to the current study, though, the ABI in the NHANES was measured using systolic blood pressure in only the right upper extremity, and the diagnosis of PAD did not include history of prior lower extremity revascularization (22). Furthermore, the authors noted an increase in the proportion of individuals with PAD in the elderly, non-Hispanic black, and Mexican American populations but did not perform adjusted analyses (22).
Our results demonstrating a significant positive association between diabetes and CAS are consistent with those reported in prior smaller cohorts. One study evaluating 1,058 patients referred for Doppler ultrasonography of the carotid arteries reported a significant association between diabetes and severe CAS (OR 2.77) in a multivariate logistic regression analysis that included age, sex, and ischemic heart disease (23). Another small prospective study evaluated 782 subjects (14% with diabetes) with high-resolution B-mode ultrasound and demonstrated a nonsignificant association between the presence of diabetes without cardiovascular disease and CAS (adjusted for age and sex: OR 2.16 [95% CI 0.96–4.86]; adjusted for age, sex, and cardiovascular risk factors: OR 2.19 [95% CI 0.92–5.20]) (24).
Unlike PAD and CAS, however, the odds of AAA in participants with diabetes significantly decrease after multivariate adjustment. Similar to our findings, a cross-sectional screening study of 73,451 veterans with no history of AAA demonstrated a negative association between the presence of diabetes and AAA in a multivariable model accounting for demographics and traditional cardiovascular risk factors (OR 0.68 [95% CI 0.60–0.77] for AAA 3.0–3.9 cm and OR 0.54 [95% CI 0.44–0.65] for AAA ≥4.0 cm, compared with <3.0 cm) (25). Consistent with these results, a report of 6,142 veterans screened for AAA demonstrated that the presence of diabetes was associated with a normal aortic size (26). Another international, prospective, observational outpatient registry of 68,236 patients ≥45 years of age with established coronary, cerebral, or peripheral arterial disease or at least three vascular disease risk factors also demonstrated an inverse relationship between diabetes and AAA in a multivariate analysis (OR 0.59 [95% CI 0.52–0.66]) (27). Although these studies demonstrate a lower OR then that presented in the current study, the populations studied are different. For example, the studies from the Veterans Affairs System consisted almost entirely of men, and we found sex differences in the association between diabetes and AAA, whereas the international registry represents a highly selective population with established vascular disease or at high risk for vascular disease.
The significant interaction between diabetes and vascular phenotype requires important exploration. Although AAA is considered an atherosclerotic disease process (similar to PAD and CAS), an experimental mouse model demonstrated elevated glucose concentrations to be associated with increased plasminogen activator inhibitor-1 concentrations (concomitant with increased aneurysmal aortic PAI-1 gene expression), reduced plasmin generation, and reduced abdominal aortic diameter, suggesting a role for the fibrinolytic pathway in AAA pathophysiology and a possible mechanism of inhibition of AAA disease in the presence of diabetes (28). A prospective cohort study of predominately men demonstrated the development of thicker aortic walls in diabetes, a reduction in the secretion of matrix metalloproteinase, and, thereby, reduced aortic wall stress and subsequent development of AAA (29). This is consistent with a recent report of 360 patients (96% men) with small AAA; the study demonstrated an inverse correlation between diabetes and aneurysm growth (30).
It is interesting that we note a neutral association between diabetes and AAA in women. The majority of studies investigating AAA have included mostly men. The current study is one of the few with a large number of women (>2 million). One report of 9,342 women did demonstrate a six-times lower prevalence of AAA in women than men but did not comment on the prevalence of AAA by diabetes status (31). In addition, one meta-analysis of more than 15,000 patients from 18 studies, the majority of which consisted of more than 80% men, demonstrated that female sex was not associated with aneurysm growth; however, it did not comment on the association between female sex and the prevalence of AAA (32). The underlying mechanism for the sex disparities noted in the prevalence of AAA, and particularly in subjects with diabetes, needs further elucidation.
Limitations
There are several limitations to this study, including those inherent to a retrospective observational study design. First, participants were provided access to the LLS measures for a fee; therefore the cohort may underrepresent people from lower socioeconomic backgrounds. Second, the diagnosis of diabetes was based on participant self-report and was not verified by medical records. Nonetheless, we found that the prevalence of diabetes was 10.8%, which is similar to the prevalence of diabetes noted in more representative populations (e.g., NHANES [9.7%] and 2013 American Heart Association heart disease and stroke statistics [11.8%]). Third, while ABI was measured during PAD screening in this survey, the toe brachial pressure index could be used to establish the diagnosis of PAD in people in whom PAD is clinically suspected, but ABI tests are unreliable because of noncompressible vessels, such as in people with long-standing diabetes or advanced age. However, this population comprises only 1.2% of the total cohort with available ABI data and is therefore unlikely to have a significant effect on the differences detected. In addition, in this study, ABI was measured using the posterior tibial artery; the dorsalis pedis artery was used only when the posterior tibial artery was inaudible. The highest of the dorsalis pedis or posterior tibial artery pressures at the ankle traditionally is used to form the calculation (17). However, a recent report compared this traditional method with an alternative method using the lower of the two ankle artery pressures, and both methods had similar diagnostic and predictive accuracy for all-cause and cardiovascular mortality, suggesting the utility of the alternate method in identifying a clinically meaningful population with PAD (33). Moreover, we expect any resulting misclassification to bias toward a null result. Fourth, severe PAD is defined in this study as ABI ≤0.6, whereas some definitions use ABI ≤0.4 to define severe PAD. We did evaluate the population with ABI <0.4 and demonstrated similar results (adjusted OR 2.23 [95% CI 2.07–2.41]; P < 0.001). Finally, while a small percentage (7.5%) of individuals did not have ABI calculated, did not receive carotid ultrasound screenings, or both, subjects excluded because of incomplete data were similar to the subjects included in the final analyses. Despite these limitations, this is the largest evaluation of the relationship between vascular disease in the peripheral arteries and diabetes, as well as the only study to examine all three major vascular subtypes in one cohort.
Conclusion
In a large contemporary survey, after adjustment of demographics and traditional risk factors, the presence of diabetes is associated with higher odds of PAD and CAS but lower odds of AAA. Future studies exploring this difference in vascular subtype and assessing the risks versus benefits of screening for PAD and CAS in asymptomatic people with diabetes are needed.
Article Information
Acknowledgments. Data analysis and statistical support was provided by New York University School of Medicine Cardiovascular Outcomes Group. The statistical analysis for this work used computing resources at the High Performance Computing Facility of the Center for Health Informatics and Bioinformatics at New York University’s Langone Medical Center.
Funding. B.S. was partially funded by National Institutes of Health/National Heart, Lung, and Blood Institute grants (T32-HL-098129 in 2012 and UL1-TR-000038 in 2013). J.S.B. was funded in part by a Doris Duke Clinical Scientist Award (2010055).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.S. developed the study concept and design, interpreted data analyses, and wrote the manuscript. C.B.R., J.C., A.Z.S., H.S.W., M.A.A., and T.S.R. designed the study and edited the final manuscript. Y.G. designed the study, analyzed data, and edited the final manuscript. J.S.B. developed the study concept and design, interpreted data analyses, and edited the final manuscript. J.S.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Some of these data were presented at the American College of Cardiology Scientific Sessions, San Francisco, CA, 9 March 2013, and at the Northwestern Cardiovascular Young Investigator Forum, Chicago, IL, 21 September 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2432/-/DC1.
References
- 1.World Health Organization. Diabetes. Fact sheet no. 312, reviewed October 2013. Available from http://www.who.int/mediacentre/factsheets/fs312/en/ Accessed 30 September 2013
- 2.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–1340 [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Coull AJ, Silver LE, et al. Oxford Vascular Study Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773–1783 [DOI] [PubMed] [Google Scholar]
- 4.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002;287:2570–2581 [DOI] [PubMed] [Google Scholar]
- 5.Shah B, Sha D, Xie D, Mohler ER, 3rd, Berger JS. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999–2004. Diabetes Care 2012;35:1074–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001;24:1476–1485 [DOI] [PubMed] [Google Scholar]
- 7.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1996;27:567–574 [DOI] [PubMed] [Google Scholar]
- 8.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006;114:688–699 [DOI] [PubMed] [Google Scholar]
- 9.Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. J Vasc Surg 2013;58:673–681, e1 [DOI] [PubMed] [Google Scholar]
- 10.European Stroke Organisation. Tendera M, Aboyans V, Bartelink ML, et al. ESC Committee for Practice Guidelines ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851–2906 [DOI] [PubMed] [Google Scholar]
- 11.Hirsch AT, Haskal ZJ, Hertzer NR, et al. American Association for Vascular Surgery. Society for Vascular Surgery. Society for Cardiovascular Angiography and Interventions. Society for Vascular Medicine and Biology. Society of Interventional Radiology. ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease. American Association of Cardiovascular and Pulmonary Rehabilitation. National Heart, Lung, and Blood Institute. Society for Vascular Nursing. TransAtlantic Inter-Society Consensus. Vascular Disease Foundation ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–e654 [DOI] [PubMed] [Google Scholar]
- 12.Wolf PA, Clagett GP, Easton JD, et al. Preventing ischemic stroke in patients with prior stroke and transient ischemic attack : a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke 1999;30:1991–1994 [DOI] [PubMed] [Google Scholar]
- 13.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med 1992;326:381–386 [DOI] [PubMed] [Google Scholar]
- 14.Bryan DS, Carson J, Hall H, et al. Natural history of carotid artery occlusion. Ann Vasc Surg 2013;27:186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosevski M, Peovska I. Clinical usefulness of assessment of ankle-brachial index and carotid stenosis in type 2 diabetic population—three-year cohort follow-up of mortality. Angiology 2013;64:64–68 [DOI] [PubMed] [Google Scholar]
- 16.Nevitt MP, Ballard DJ, Hallett JW., Jr Prognosis of abdominal aortic aneurysms. A population-based study. N Engl J Med 1989;321:1009–1014 [DOI] [PubMed] [Google Scholar]
- 17.Aboyans V, Criqui MH, Abraham P, et al. American Heart Association Council on Peripheral Vascular Disease. Council on Epidemiology and Prevention. Council on Clinical Cardiology. Council on Cardiovascular Nursing. Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–2909 [DOI] [PubMed] [Google Scholar]
- 18.Savji N, Rockman CB, Skolnick AH, et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 2013;61:1736–1743 [DOI] [PubMed] [Google Scholar]
- 19.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106 [DOI] [PubMed] [Google Scholar]
- 20.Office for Human Protections. Human subject regulations decision charts. Chart 1: is an activity research involving human subjects covered by 45 CFR part 46? [Internet], 24 Sept 2004. Washington, DC: US Department of Health and Human Services. Available from http://www.hhs.gov/ohrp/policy/checklists/decisioncharts.html#c1 Accessed 15 January 2014
- 21.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA 2012;308:1660–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregg EW, Sorlie P, Paulose-Ram R, et al. 1999–2000 National Health and Nutrition Examination Survey Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care 2004;27:1591–1597 [DOI] [PubMed] [Google Scholar]
- 23.Göksan B, Erkol G, Bozluolcay M, Ince B. Diabetes as a determinant of high-grade carotid artery stenosis: evaluation of 1,058 cases by Doppler sonography. J Stroke Cerebrovasc Dis 2001;10:252–256 [DOI] [PubMed] [Google Scholar]
- 24.Inchiostro S, Dalfollo M, Marzano A, et al. Prevalence of diabetes and/or ischaemic heart disease in classes of increasing carotid artery atherosclerosis: an ultrasonographic study. Diabet Med 2003;20:670–676 [DOI] [PubMed] [Google Scholar]
- 25.Lederle FA, Johnson GR, Wilson SE, et al. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Intern Med 1997;126:441–449 [DOI] [PubMed] [Google Scholar]
- 26.Chun KC, Teng KY, Chavez LA, et al. Risk factors associated with the diagnosis of abdominal aortic aneurysm in patients screened at a regional Veterans Affairs health care system. Ann Vasc Surg 2014;28:87–92 [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner I, Hirsch AT, Abola MT, et al. REACH Registry Investigators Cardiovascular risk profile and outcome of patients with abdominal aortic aneurysm in out-patients with atherothrombosis: data from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. J Vasc Surg 2008;48:808–814 [DOI] [PubMed] [Google Scholar]
- 28.Dua MM, Miyama N, Azuma J, et al. Hyperglycemia modulates plasminogen activator inhibitor-1 expression and aortic diameter in experimental aortic aneurysm disease. Surgery 2010;148:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golledge J, Karan M, Moran CS, et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J 2008;29:665–672 [DOI] [PubMed] [Google Scholar]
- 30.De Rango P, Cao P, Cieri E, et al. Comparison of Surveillance vs. Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators Group Effects of diabetes on small aortic aneurysms under surveillance according to a subgroup analysis from a randomized trial. J Vasc Surg 2012;56:1555–1563 [DOI] [PubMed] [Google Scholar]
- 31.Scott RAP, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002;89:283–285 [DOI] [PubMed] [Google Scholar]
- 32.Sweeting MJ, Thompson SG, Brown LC, Powell JT, RESCAN Collaborators Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012;99:655–665 [DOI] [PubMed] [Google Scholar]
- 33.Nead KT, Cooke JP, Olin JW, Leeper NJ. Alternative ankle-brachial index method identifies additional at-risk individuals. J Am Coll Cardiol 2013;62:553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]