Abstract
The availability of insulin analogs has offered insulin replacement strategies that are proposed to more closely mimic normal human physiology. Specifically, there are a considerable number of reports demonstrating that prandial insulin analogs (lispro, aspart, glulisine) have pharmacokinetic and pharmacodynamic profiles closer to normal, with resulting faster onset and offset of insulin effect when compared with regular human insulin. In addition, basal insulin analogs (glargine, detemir) have been reported to offer longer duration of action, less variability, more predictability, less hypoglycemia (especially nocturnal), and a favorable effect on weight. However, an argument against use of analog insulins as compared with use of regular or NPH insulin is one that states that the effectiveness and risk of hypoglycemia are the only two valid clinical outcomes that should be used to compare the analog and human insulins. Thus, there remains a debate in some circles that analog insulins are no more effective than human insulins, yet at a much higher financial cost. To provide an in-depth understanding of both sides of the argument, we provide a discussion of this topic as part of this two-part point-counterpoint narrative. In the counterpoint narrative presented here, Dr. Davidson provides his argument and defends his opinion that outside of a few exceptions, analog insulins provide no clinical benefit compared with human insulins but cost much more. In the preceding point narrative, Dr. Grunberger provides a defense of analog insulins and their value in clinical management and suggests that when evaluating the “cost” of therapy, a much more global assessment is needed.
—William T. Cefalu
Editor in Chief, Diabetes Care
Introduction
In 2011, global insulin sales cost $16.7 billion, of which $8.3 billion was spent in the U.S. (1). Some of this high cost, of course, is due the increasing number of people with diabetes. However, the unit cost of insulin is increasing at a rate far outstripping the rate of inflation, which was 17.5% over the 7 years from 2005 to 2011 (2). For instance, the increases over that period of time in the price per vial of Humulin R (Eli Lilly, Indianapolis, IN) and Novolin N (Novo Nordisk, Plainsboro, NJ) were 114%, 134% for lispro insulin, 116% for glargine insulin, and 117% for aspart insulin FlexPens (2). The wholesale prices for insulin in the past 2 years from 2011 to 2013 have increased on average by 43% (3).
In 2011, the cost per unit of insulin was twice as much for an analog compared with a generic preparation (2). In 2013, the wholesale cost of a vial of rapid-acting insulin was 81% more expensive than regular insulin, a vial of analog basal insulin (glargine, detemir) was 126% more expensive than NPH insulin, and a vial of analog premixed insulin 92% was more expensive than premixed NPH/regular insulin (3).
Given the high and increasing costs of insulin, it behooves us to examine whether analog preparations are worth their extra costs. What should be the outcomes used to compare analog insulins with human insulins? Pharmacokinetic and pharmacodynamic data are generated in acute studies and do not speak to clinical outcomes. For instance, both show that the rapid-acting analogs have significantly different pharmacokinetic and pharmacodynamic dynamics than human insulin. Based on these results, it is widely believed that regular insulin should be injected 20–30 min before a meal to yield lower postprandial glucose concentrations than if injected just prior to eating. Yet a recent study by Müller et al. (4) showed that both preprandial and postprandial glucose values measured at home were the same whether regular insulin was injected 20 min before or just prior to meals. This may not be too surprising given that the absorption and action of regular insulin varies over 20% in the same individual from day to day (5,6). There is little evidence that variability of glucose levels per se affects clinical outcomes (7,8). On the other hand, there is good evidence that lower overall glycemia, as reflected in A1C levels, leads to less microvascular diabetes complications (9–11). Therefore, changes in A1C levels and hypoglycemia should be the basis upon which analog and human insulins are compared.
The outcomes in 60 randomized control trials comparing analog and human insulins are summarized in Table 1. As there is little difference among the three rapid-acting insulins (lispro, aspart, glulisine) and the two basal insulins (glargine, detemir) regarding these outcomes, the results in each class of analog insulins are combined. Studies in type 1 and type 2 diabetic patients are presented separately. It is important to realize that a negative value for ∆A1C means a better response for the analog insulins. Also, note that regarding the ratios in Table 1, the numerator is the number of studies in which the outcome for the analog insulins was statistically significant compared with human insulins with the arrow signifying the direction of the difference and the denominator is the number of studies in which the outcome was measured. Regarding efficacy, across evaluations of all comparisons, only 15 of 64 (23%) showed a significant increase in the lowering of A1C levels with analog insulins compared with human insulins. The weighted mean difference between the change in A1C levels between analog and human insulins across all comparisons ranged from –0.01 to −0.23%, with an average difference of −0.09%—hardly of clinical importance in my opinion.
Table 1.
A1C and hypoglycemia outcomes in 64 comparisons between analog and human insulins in randomized control trials
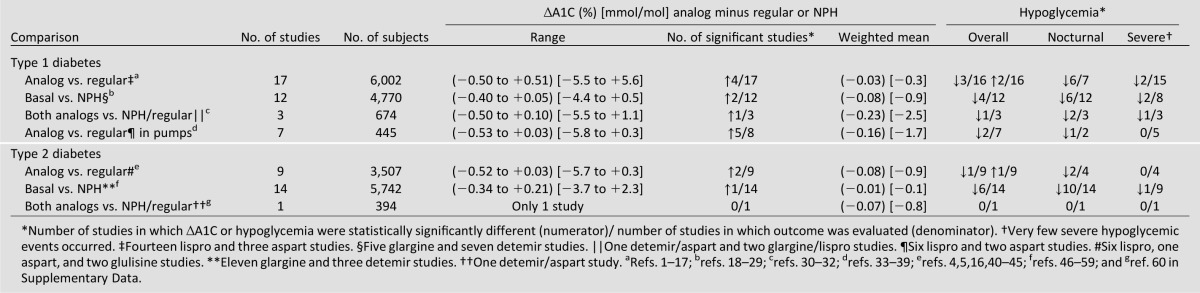
Regarding hypoglycemia, overall hypoglycemia was evaluated in 62 comparisons. In 17 of them, hypoglycemia was significantly less with analog insulins, while in 3 it was significantly increased. The most striking difference occurred in nocturnal hypoglycemia; in the 43 comparisons in which it was evaluated, it was significantly decreased in 27. In the 45 comparisons in which severe hypoglycemia was evaluated, it was significantly decreased by analog insulins in only 6. Thus, hypoglycemia occurred less often in patients receiving analog insulins, especially overnight. However, in none of the 60 studies was a bedtime snack recommended. In our practice, we insist that patients taking insulin eat a small bedtime snack and very few experience nocturnal hypoglycemia. As the vast majority of people with type 2 diabetes are overweight or obese, we instruct them to switch some calories from their largest meal to their bedtime snack.
Patients taking detemir insulin gained significantly less weight than those taking either NPH or glargine insulin in a number of studies (12–17). However, the differences were only 0.4–1.3 kg, which are not clinically significant.
Some would argue that treatment satisfaction and cost-effectiveness should play a role in the decision to consider using analog insulins. At least six studies have evaluated treatment satisfaction. Treatment satisfaction was assessed in three studies utilizing the Diabetes Treatment Satisfaction Questionnaire (18), which is an eight-item questionnaire with six questions evaluating treatment satisfaction and two questions related to perceived hyperglycemia and hypoglycemia. The maximum score for all eight questions is 48. One found no difference between glargine and NPH insulin (19). In a comparison between lispro and regular insulin in pump patients evaluating all eight questions, lispro insulin users scored 35.2 ± 4.2 versus 32.4 ± 5.9 for regular insulin users, which apparently was statistically significant (P < 0.001) by the nonparametric Friedman rank sum test (20). In a comparison between glargine and NPH insulins in which the two questions related to the perception of hyperglycemia and hypoglycemia were not reported, treatment satisfaction rose from 12.6 at baseline to 16.6 in the analog group versus 12.5 to 16.0 in those using NPH, which apparently also was statistically significant (P < 0.02) by pairwise ANCOVA (21). In a comparison between lispro and regular insulin, it was simply stated that there were no differences in the domains of energy/fatigue, health distress, or treatment flexibility but significant improvement was seen in the treatment satisfaction domain (no data were provided) (22). Two studies comparing lispro (14) or aspart (23) to regular insulin found the analogs provided significantly more flexibility than the human insulin, but this might be expected given that patients were instructed to take regular insulin 30 min before eating versus immediately before eating for the analogs. This difference in timing may not be considered important in the future given the results of Müller et al. (4), which showed that home glucose levels were the same whether regular insulin was injected 20 min before or just prior to a meal. These data on treatment satisfaction between analog and human insulins do not present a strong argument favoring the analogs.
There are reports based on modeling studies that analog insulins are more cost-effective than human insulin (24,25). Cost-effectiveness was defined as an incremental value of less than $50,000 per quality-adjusted life-year. The time horizon for the models ranged from 10 to 60 years and some for the lifetime of the patient. The various analyses use three major inputs: changes in A1C levels (affecting future diabetes complications that are the major drivers of costs for diabetes), costs for hypoglycemia (emergency room/hospital), and fear of hypoglycemia. The latter is considered important because with less analog-induced hypoglycemia, there would presumably be better adherence to insulin therapy; in the general population, better adherence to medications is associated with less medical care costs. It is difficult, of course, to project costs and complications for 10–60 years because of potential changes in the natural history of diabetes as well as noninsulin changes in treatments affecting diabetes complications. The data in Table 1 do not support assumptions that there are meaningful clinical changes in A1C levels between analog and human insulins. Although there are statistically significant reported differences in hypoglycemia between analogs and human insulin, these mainly occur overnight and bedtime snacks minimize this difference. Fear of hypoglycemia and its effect on adherence to insulin therapy is based on assumptions that are difficult to quantitate. In my view, these data on cost-effectiveness are not a strong argument for preferring the more expensive analog insulins.
In conclusion, regular insulin is just as effective as the rapid-acting insulin analogs (lispro, aspart, glulisine). Similarly, NPH insulin is just as effective as the basal insulin analogs (glargine, detemir). Without a bedtime snack, overnight hypoglycemia may be more common with regular insulin taken before supper and NPH insulin taken at bedtime than their corresponding analogs. In general, analog insulins are twice as expensive as human insulins. For those who wish to use a much less expensive, evidence-based approach to insulin therapy, analog insulins are not preferred with two important exceptions. Type 1 diabetic patients require basal analog insulin because they produce no endogenous insulin and need 24-h coverage. In the unusual occurrence of overnight hypoglycemia in type 2 diabetic patients who ingest a bedtime snack, it may be helpful to prescribe an analog rapid-acting insulin before supper (and presumably before the other meals to simplify the regimen) if the hypoglycemia occurs early overnight, and a basal analog insulin if it occurs toward morning. With these two exceptions, analog insulins provide no clinical benefit compared with human insulins, but cost much more.
Article Information
Acknowledgments. The author is grateful to Sharon Ng, Venice Family Clinic, for obtaining the average wholesale prices of the insulin preparations.
Funding. M.B.D. received partial salary support from National Institutes of Health (NIH) National Institute on Minority Health and Health Disparities grant US54-MD-007598 (Axis grant, formerly U54-RR-026138) and NIH National Center for Advancing Translational Sciences grant UL1-TR-000124.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2915/-/DC1.
See accompanying article, p. 1767.
References
- 1.Lowenstein LS, Ran N, Shivers JP, Yarchoan M, Close KL. Opportunities and challaenges for biosimilars: what’s on the horizon in the global insulin market? Clin Diabetes 2012;30:138–150 [Google Scholar]
- 2.Drugstore.com www.drugstore.com Accessed 28 April 2012
- 3.UpToDate, Inc. www.uptodate.com Accessed 5 February 2014
- 4.Müller N, Frank T, Kloos C, Lehmann T, Wolf G, Müller UA. Randomized crossover study to examine the necessity of an injection-to-meal interval in patients with type 2 diabetes and human insulin. Diabetes Care 2013;36:1865–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziel FH, Davidson MB, Harris MD, Rosenberg CS. The variability in the action of unmodified insulin is more dependent on changes in tissue sensitivity than on insulin absorption. Diabet Med 1988;5:662–666 [DOI] [PubMed] [Google Scholar]
- 6.Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther 2002;4:673–682 [DOI] [PubMed] [Google Scholar]
- 7.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev 2010;31:171–182 [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Kilpatrick ES. Glycemic variability: both sides of the story. Diabetes Care 2013;36(Suppl. 2):S272–S275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995;44:968–983 [PubMed] [Google Scholar]
- 10.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117 [DOI] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1999;354:602]. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 12.McAdam-Marx C, Bouchard J, Aagren M, Nelson R, Brixner D. Analysis of glycaemic control and weight change in patients initiated with human or analog insulin in an US ambulatory care setting. Diabetes Obes Metab 2010;12:54–64 [DOI] [PubMed] [Google Scholar]
- 13.van Golen LW, IJzerman RG, Huisman MC, et al. Cerebral blood flow and glucose metabolism in appetite-related brain regions in type 1 diabetic patients after treatment with insulin detemir and NPH insulin: a randomized controlled crossover trial. Diabetes Care 2013;36:4050–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holleman F, Schmitt H, Rottiers R, Rees A, Symanowski S, Anderson JH, The Benelux-UK Insulin Lispro Study Group Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. Diabetes Care 1997;20:1827–1832 [DOI] [PubMed] [Google Scholar]
- 15.Vague P, Selam J-L, Skeie S, et al. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care 2003;26:590–596 [DOI] [PubMed] [Google Scholar]
- 16.Home P, Bartley P, Russell-Jones D, et al. ; Study to Evaluate the Administration of Detemir Insulin Efficacy, Safety and Suitability (Steadiness) Study Group. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes. Diabetes Care 2004;27:1081–1087 [DOI] [PubMed] [Google Scholar]
- 17.Standl E, Lang H, Roberts A. The 12-month efficacy and safety of insulin detemir and NPH insulin in basal-bolus therapy for the treatment of type 1 diabetes. Diabetes Technol Ther 2004;6:579–588 [DOI] [PubMed] [Google Scholar]
- 18.Bradley C. Diabetes treatment satisfaction questionnaire (DTSQ). In Handbook of Psychology and Diabetes. Bradley C, Ed. U.K., Gordon & Breach, 1994, p. 111–132 [Google Scholar]
- 19.Hsia SH. Insulin glargine compared to NPH among insulin-naïve, U.S. inner city, ethnic minority type 2 diabetic patients. Diabetes Res Clin Pract 2011;91:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renner R, Pfutzner A, Trautmann M, Harzer O, Sauter K, Landgraf R; German Humalog-CSII Study Group. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Diabetes Care 1999;22:784–788 [DOI] [PubMed] [Google Scholar]
- 21.Eliaschewitz FG, Calvo C, Valbuena H, et al. HOE 901/4013 LA Study Group Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch Med Res 2006;37:495–501 [DOI] [PubMed] [Google Scholar]
- 22.Pfustner A, Kustner E, Forst T, et al. Intensive insulin therapy with insulin lispro in patients with type 1 diabetes reduces the frequency of hypoglycemic episodes. Exp Clin Endocrinol Diabetes 1996;104:25–30 [DOI] [PubMed] [Google Scholar]
- 23.Gy T, Marre M, Astorga R, Dedov I, Jacobsen J, Lindholm A; Insulin Aspart Study Group. Glycaemic control in type 1 diabetic patients using optimized insulin aspart or human insulin in a randomized multinational study. Diabetes Res Clin Pract 2001;54:105–114 [DOI] [PubMed] [Google Scholar]
- 24.Brixner DI, McAdam-Marx C. Cost-effectiveness of insulin analogs. Am J Manag Care 2008;14:766–775 [PubMed] [Google Scholar]
- 25.Cameron CG, Bennett HA. Cost-effectiveness of insulin analogues for diabetes mellitus. CMAJ 2009;180:400–407 [DOI] [PMC free article] [PubMed] [Google Scholar]


