Abstract
OBJECTIVE
To examine the evolution of the dysregulated glucagon responses to mixed-meal tolerance tests (MMTTs) in youth with recent-onset type 1 diabetes (T1D).
RESEARCH DESIGN AND METHODS
MMTTs were performed in 25 youth (9–18 years of age) with 1.5–12 months disease duration (year 1); 22 subjects were restudied 1 year later (year 2). Twenty nondiabetic (ND) control children were also studied.
RESULTS
In T1D children, MMTT-stimulated increases in glucagon were significantly greater than that in ND children (median increments: year 1, 21 pg/mL [16–30]; year 2, 25 pg/mL [16–30]; ND, 9 pg/mL [5–16]; P = 0.001 and P < 0.001, respectively).
CONCLUSIONS
In comparison with ND control children, exaggerated plasma glucagon responses to mixed-meal feedings are observed in youth with T1D within the first 2 years of diagnosis. Further studies to determine whether suppression of these abnormal responses may help to improve glycemic control are warranted.
Introduction
Many patients with type 1 diabetes (T1D) are vulnerable to severe hypoglycemic events because they fail to stimulate glucagon responses to falling blood glucose levels (1,2). Conversely, α-cell response to amino acid stimulation is exaggerated in T1D, which may contribute to postprandial hyperglycemia (3,4). However, the evolution of this aspect of α-cell dysfunction beyond the 1st year of T1D in children and adolescents has not been determined or compared with that in nondiabetic (ND) children. This study was undertaken to examine this question and to compare differences in the magnitude of glucagon responses to a standard mixed-meal feeding in T1D and ND children.
Research Design and Methods
Subjects
Twenty-five youth with T1D were enrolled at the five centers in the Diabetes Research in Children Network (DirecNet), as part of a study examining the loss of glucagon responses to hypoglycemia (5). They were between 9 and 18 years of age (13.4 ± 2.7 years) and had a duration of T1D between 1.5 and 12 months. Subjects were recruited into four disease duration bins: 5 subjects with duration of diabetes between 6 and 13 weeks, 7 between 14 and 26 weeks, 7 between 27 and 39 weeks, and 6 between 40 and 52 weeks; 22 of the 25 subjects (13.2 ± 2.7 years of age) were restudied ∼12 months later. Twenty healthy, age- and sex-matched ND children (13.5 ± 3.1 years of age) were also studied. Supplementary Table 1 summarizes the demographic data of the ND children to the T1D subjects on study entry.
Each center received approval of the study from their institutional review board. In subjects <18 years of age, informed written consent was obtained from the parents/guardians and written assent from the subjects. Subjects ≥18 years of age provided their own written consent.
Procedures
Mixed-meal tolerance tests (MMTTs) were performed after an overnight fast, with subjects drinking Boost High Protein (24% protein, 55% carbohydrate, and 21% fat) at a dose of 6 mL/kg (maximum dose 360 mL), as previously described (5). Blood was obtained for measurement of plasma glucose, C-peptide, and glucagon levels at −10, 0, 15, 30, 60, 90, 120, 150, 180, 210, and 240 min.
Laboratory Methods
C-peptide concentrations were measured at Northwest Lipid Metabolism and Diabetes Research Laboratories (Seattle, WA) using Tosoh AIA 1800. The lower limit of detection was 0.05 ng/mL (0.0167 nmol/L), and the median coefficient of variation was 6.5%. Plasma glucagon concentrations were measured at the University of Minnesota (Minneapolis, MN) by a radioimmunoassay (Linco Research, St. Charles, MO) with the primary antibody from guinea pig and the secondary antibody from goat. The lower limit of detection was 20 pg/mL (6 pmol/L).
Analytical Methods
Standard deviation of baseline glucagon levels (based on the coefficient of variation of split duplicate plasma glucagon measurements) was 4 pg/mL. A clinically relevant increase in plasma glucagon levels during the MMTT was defined as a rise in plasma glucagon concentrations ≥12 pg/mL or greater than three times the standard deviations above baseline concentrations, as previously described (5). Data are presented as median (25–75%). Wilcoxon rank sum tests were performed to compare the plasma glucagon and C-peptide responses in diabetic and ND subjects. Paired Student t tests or signed rank tests were used to assess changes in responses in the T1D subjects from year 1 to year 2. Fisher exact test was used to assess the presence of a glucagon response between cohorts of subjects. Calculations were performed using SAS 9.3.
Results
Mean plasma glucose, C-peptide, and glucagon levels during the MMTT are shown in Supplementary Fig. 1. Fasting and peak stimulated plasma C-peptide levels during the 1st year (0.29 nmol/L [0.25–0.36] and 0.87 nmol/L [0.57–1.12], respectively) were lower than corresponding values in ND subjects (0.43 nmol/L [0.37–0.57] and 1.92 nmol/L [1.70–2.43]; P < 0.001 for both). Nevertheless, only one T1D subject had a peak stimulated C-peptide value <0.2 mmol/L during year 1. By year 2, baseline and peak stimulated C-peptide values fell to 0.10 nmol/L (0.04–0.18) and 0.28 nmol/L (0.19–0.54), and seven subjects (32%) had peak stimulated values <0.2 nmol/L.
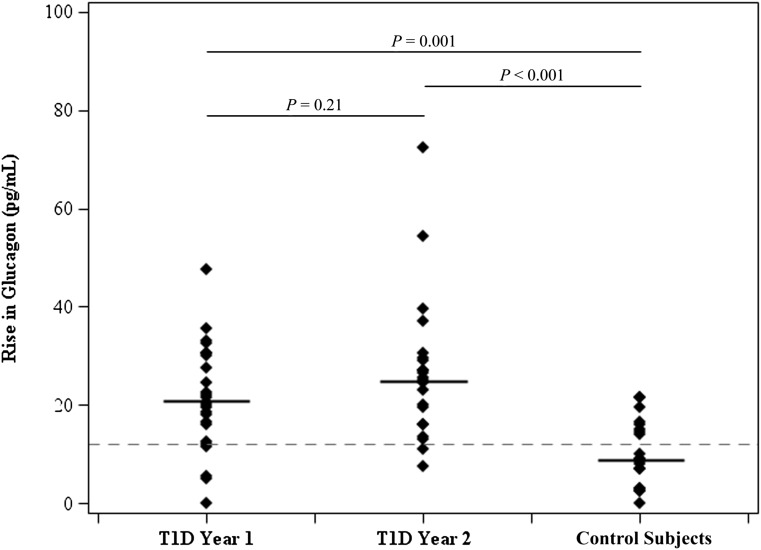
During years 1 and 2, fasting plasma glucagon levels in T1D subjects were similar to each other (44 pg/mL [34–53] and 46 pg/mL [41–54]) and to baseline concentrations in ND children (50 pg/mL [46–57]). As shown in Fig. 1, in years 1 and 2, the peak increments in plasma glucagon levels during the MMTT (21 pg/mL [16–30] and 25 pg/mL [16–30], respectively) were significantly greater than median increments in ND children (9 pg/mL [5–16]; P = 0.001 and P < 0.001, respectively). Moreover, all but two subjects with T1D in year 2 had clinically relevant increases in plasma glucagon (i.e., ≥12 pg/mL) versus only 40% of control subjects (P < 0.001).
Figure 1.
Glucagon response to MMTT.
A progressive rise in glucagon AUC was noted with increasing disease duration of T1D (P = 0.009) reaching values that were significantly greater than in ND control subjects (P < 0.03 and P < 0.002 at years 1 and 2, respectively).
Conclusions
More than 40 years ago, Unger et al. (6,7) suggested that diabetes was a bihormonal disease characterized by too little insulin and too much glucagon. Interest in this question faded during the early intensive treatment era when attention turned to impaired glucagon responses to hypoglycemia (8). The introduction of pharmacological agents that suppress plasma glucagon and difficulties in controlling postprandial hyperglycemia with external closed-loop systems that use the subcutaneous route of insulin delivery (9) have served to refocus attention on the role of α-cell dysregulation on postprandial hyperglycemia. Although concomitant use of insulin and glucagon during closed-loop control has been explored (10–12), fewer studies have investigated the benefits of glucagon suppression during closed-loop therapy (13).
The most important finding in this study was that exaggerated increases in plasma glucagon levels in response to mixed-meal feedings appear to be fully established during the 1st year of T1D, at a time when almost all of our subjects retained substantial residual C-peptide responses, corroborating the results of Brown et al. (4). Moreover, peak increments in plasma glucagon during the first 2 years of diabetes differed markedly from ND children, in whom plasma glucagon concentrations remained within error of the assay in many subjects. Additionally, the glucagon responses to mixed-meal feedings in our youngsters with T1D approximated the increase in plasma glucagon that we previously reported in ND young adults in response to hypoglycemia (median increment 38 pg/mL) (14). These observations provide a compelling rationale for further studies on the benefits of agents like pramlintide and GLP1 agonists that can suppress abnormal glucagon responses to feeding (15–18) in the treatment of youth with T1D.
Article Information
Acknowledgments. The authors thank all the subjects and their families for their participation.
Funding. This work was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants HD-41890-10, HD-41906-10, HD-41908-10, HD-41915, HD-41918, HD-56526, UL1-RR-024992, UL1-RR-024139, UL1-RR-024979, UL1-RR-025744, and UL1-RR-024150. J.S. received funding from the Pediatric Endocrine/Diabetes Physician Scientists, Grant K12-DK-094714-01.
Duality of Interest. Stanford University received funding from the Juvenile Diabetes Research Foundation, the Helmsley Foundation, Medtronic MiniMed, and Dexcom for industry-sponsored research. L.F. received payment for serving on the clinical advisory board of Tandem Diabetes Care. B.B. received research support for both principal investigator–initiated studies and industry-sponsored studies from Medtronic MiniMed; received payment for consultancy to Sanofi, BD, Roche, Glysense, Debiotech, Unomedical, Animas, and Bayer; received payment for development of educational presentations from Children with Diabetes; and received travel/accommodations/meeting expenses from the Helmsley Foundation for T1D Exchange. S.W. received payment for consultancy to Animas, Novo Nordisk, and BD; received payment for lectures including service on speaker bureaus for Eli Lilly and Company; and received payment from stock for InsuLine Medical. N.H.W. served on the data monitoring board and received payment from both Daiichi Sankyo and Novo Nordisk. A.M.A. received funding from the Harold Amos Medical Faculty Development Program, Grant KL2-RR-024994. C.K. received payment for consultancy to Medtronic. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.S. and W.V.T. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. E.T., L.F., B.B., S.W., N.H.W., A.M.A., C.K., K.J.R., P.C., and R.W.B. researched data, contributed to discussion, and reviewed and edited the manuscript. R.W.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Clinical Centers (Listed in alphabetical order. Personnel are listed as PI for principal investigator, I for co-investigator, and C for coordinator.) Stead Family Department of Pediatrics, University of Iowa Carver College of Medicine: Eva Tsalikian, MD (PI), Michael J. Tansey, MD (I), Julie Coffey, MSN (C), Joanne Cabbage (C), and Sara Salamati (C); Nemours Children’s Clinic: Nelly Mauras, MD (PI), Larry Fox, MD (I), Kim Englert, RN (C), Joe Permuy, ARNP (C), and Kaitlin Sikes (C); Division of Pediatric Endocrinology and Diabetes, Stanford University: Bruce Buckingham, MD (PI), Darrell M. Wilson, MD (I), Paula Clinton, RD, CDE (C), and Kimberly Caswell, APRN (C); Department of Pediatrics, Yale University School of Medicine: Stuart Weinzimer, MD (PI), William V. Tamborlane, MD (I), Jennifer Sherr, MD (I), Amy Steffen, BS (C), Kate Weyman, MSN (C), Melinda Zgorski, BSN (C), and Eileen Tichy, MMS (C); Washington University in St. Louis: Neil H. White, MD (PI), Ana Maria Arbelaez, MD (I), Lucy Levandoski, PA-C (C), and Angie Starnes, RN, BSN, CDE (C).
Coordinating Center Jaeb Center for Health Research: Roy W. Beck, MD, PhD, Katrina J. Ruedy, MSPH, Craig Kollman, PhD, Peiyao Cheng, MPH, and Beth Stevens.
National Institutes of Health Gilman D. Grave, MD, PhD, Karen K. Winer, MD, and Ellen Leschek, MD.
Data and Safety Monitoring Board Mark Sperling, MD, Dorothy M. Becker, MBBCh, Patricia Cleary, MS, Carla Greenbaum, MD, and Antoinette Moran, MD.
University of Minnesota Central Laboratory Michael W. Steffes, MD, PhD, Jean M. Bucksa, CLS, Maren L. Nowicki, CLS, and Vicky Makky, CLS.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-2612/-/DC1.
A complete list of the members of the Diabetes Research in Children Network can be found in the appendix.
References
- 1.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 1973;182:171–173 [DOI] [PubMed] [Google Scholar]
- 2.Bolli G, de Feo P, Compagnucci P, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 1983;32:134–141 [DOI] [PubMed] [Google Scholar]
- 3.Gerich JE, Charles MA, Grodsky GM. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest 1974;54:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care 2008;31:1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherr J, Xing D, Ruedy KJ, et al. Diabetes in Children Network Lack of association between residual insulin production and glucagon response to hypoglycemia in youth with short duration of type 1 diabetes. Diabetes Care 2013;36:1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger RH, Eisentraut AM, McCALL MS, Keller S, Lanz HC, Madison LL. Glucagon antibodies and their use for immunoassay for glucagon. Proc Soc Exp Biol Med 1959;102:621–623 [DOI] [PubMed] [Google Scholar]
- 7.Unger RH. Glucagon and the insulin: glucagon ratio in diabetes and other catabolic illnesses. Diabetes 1971;20:834–838 [DOI] [PubMed] [Google Scholar]
- 8.Cryer PE, Binder C, Bolli GB, et al. Hypoglycemia in IDDM. Diabetes 1989;38:1193–1199 [DOI] [PubMed] [Google Scholar]
- 9.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 10.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med 2010;2:27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care 2012;35:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinzimer SA, Sherr JL, Cengiz E, et al. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care 2012;35:1994–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbelaez AM, Xing D, Cryer PE, et al. for the Diabetes Research in Children Network (DirecNet) Study Group Blunted glucagon but not epinephrine responses to hypoglycemia occurs in youth with less than 1 yr duration of type 1 diabetes mellitus. Pediatr Diabetes. 28 August 2013 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levetan C, Want LL, Weyer C, et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care 2003;26:1–8 [DOI] [PubMed] [Google Scholar]
- 16.Neumiller JJ. Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors. J Am Pharm Assoc (2003) 2009;49(Suppl. 1):S16–S29 [DOI] [PubMed]
- 17.Raman VS, Mason KJ, Rodriguez LM, et al. The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care 2010;33:1294–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar G, Alattar M, Brown RJ, Quon MJ, Harlan DM, Rother KI. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care 2014;37:666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]