Abstract
Purpose
The prevalence of macrolide-resistant Mycoplasma pneumoniae (MRMP) has increased worldwide. The aim of this study was to estimate the proportion of MRMP in a tertiary hospital in Korea, and to find potential laboratory markers that could be used to predict the efficacy of macrolides in children with MRMP pneumonia.
Methods
A total of 95 patients with M. pneumoniae pneumonia were enrolled in this study. Detection of MRMP was based on the results of specific point mutations in domain V of the 23S rRNA gene. The medical records of these patients were reviewed retrospectively and the clinical course and laboratory data were compared.
Results
The proportion of patients with MRMP was 51.6% and all MRMP isolates had the A2063G point mutation. The MRMP group had longer hospital stay and febrile period after initiation of macrolides. The levels of serum C-reactive protein (CRP) and interleukin-18 in nasopharyngeal aspirate were significantly higher in patients who did not respond to macrolide treatment. CRP was the only significant factor in predicting the efficacy of macrolides in patients with MRMP pneumonia. The area under the curve for CRP was 0.69 in receiver operating characteristic curve analysis, indicating reasonable discriminative power, and the optimal cutoff value was 40.7 mg/L.
Conclusion
The proportion of patients with MRMP was high, suggesting that the prevalence of MRMP is rising rapidly in Korea. Serum CRP could be a useful marker for predicting the efficacy of macrolides and helping clinicians make better clinical decisions in children with MRMP pneumonia.
Keywords: Mycoplasma pneumoniae, Antibiotic resistance, Child, C-reactive protein, Predictive value of tests
Introduction
Mycoplasma pneumoniae is a common causative organism of community-acquired pneumonia in children and young adults. M. pneumoniae infections occur both endemically and in cyclic epidemics every 3 to 7 years, and account for 10%-40% of community acquired pneumonia cases in school-aged children1). It can also cause a wide array of extrapulmonary manifestations.
Because of its lack of a cell wall, M. pneumonia is intrinsically resistant to β-lactams and other antibiotics that target the cell wall. Therefore, macrolides and related antibiotics such as tetracyclines and fluoroquinolones are used in clinical practice for the treatment of M. pneumonia infections. However, only macrolides are usually considered as the drug of choice for children with these infections because of potential age-related side effects of the alternatives.
Macrolide resistant M. pneumonia (MRMP) has been emerging worldwide since 2000. Although the prevalence of MRMP varies from country to country, it has generally increased2). The clinical relevance of the increased prevalence of MRMP has not been definitely established; however, the clinical course of MRMP pneumonia appears to be prolonged3,4), and recent case reports revealed that MRMP can cause severe complications5,6).
Although there are few alternative antibiotics available for children with MRMP infections, minocycline or doxycycline could be considered in the management of serious MRMP infections, as recently reported by Okada et al.7). In addition, systemic corticosteroids with antimicrobial therapy are often proposed for the treatment of macrolide non-responsive M. pneumoniae infections8). However, these drugs should be used with caution, and only in macrolide nonresponsive patients, until the effects and safety issues are resolved in pediatric patients. Therefore, prediction of the efficacy of macrolides in patients with MRMP infection is important to clinical practice.
In Korea, M. pneumoniae epidemics have been observed in three- to four-year cycles from the mid-1980s. The clinical features of the 2011 epidemic seemed different from previous epidemics. However, few comprehensive studies of MRMP pneumonia in Korea have been reported. The aim of this study was to estimate the proportion of MRMP in a tertiary hospital in Korea and to find potential laboratory markers that could be used to predict the efficacy of macrolides in patients with MRMP pneumonia.
Materials and methods
1. Subjects
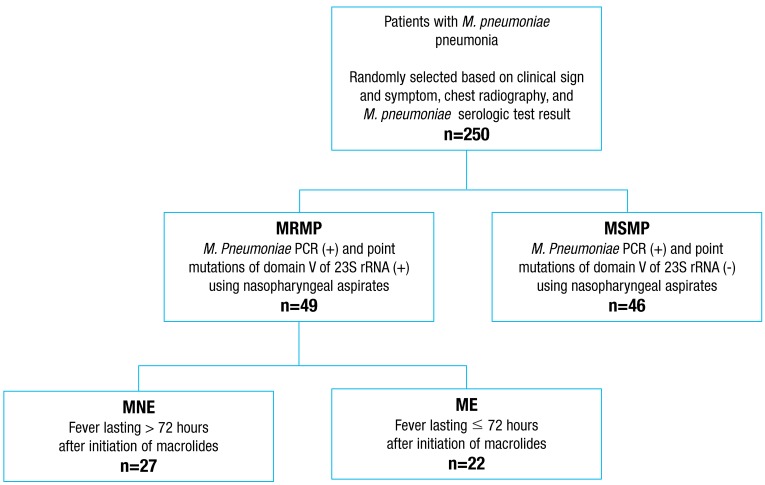
A retrospective cross sectional study was carried out among patients with M. pneumoniae pneumonia admitted to the Department of Pediatrics, Korea University Guro Hospital between August and December 2011 (during a wide-spread outbreak of M. pneumoniae). The diagnosis of M. pneumoniae pneumonia was based on clinical symptoms, signs of lower respiratory tract infection, chest radiography, and serologic test for M. pneumoniae (≥1:160). Two hundred fifty patients were selected randomly among those diagnosed with M. pneumoniae pneumonia for this study (Fig. 1). Based on the results of M. pneumoniae polymerase chain reaction (PCR) and specific point mutations of domain V of 23S rRNA using nasopharyngeal aspirates, children with M. pneumoniae pneumonia were divided into macrolide-resistant (MRMP) and macrolide-susceptible (MSMP) groups. To find potential markers for the prediction of the efficacy of macrolides in patients with MRMP pneumonia, the MRMP group was divided into macrolide effective (ME) and macrolide noneffective (MNE) subgroups based on defervescence within 72 hours after initiation of macrolides. Defervescence was defined as a body temperature below 38℃ for at least 24 hours without the use of antipyretics. Patients with preexisting underlying diseases such as congenital heart disease, bronchopulmonary dysplasia, immunodeficiency, or malignancy and coinfected with viruses with the potential of causing lower respiratory tract infection were excluded. The Institutional Review Board of Korea University Guro Hospital approved this study on anonymized retrospective samples and medical records and waived the need for informed consent.
Fig. 1.
Study population. MRMP, macrolide-resistant Mycoplasma pneumoniae ; MSMP, macrolide-susceptible Mycoplasma pneumoniae ; MNE, macrolide-noneffective; ME, macrolide-effective; PCR, polymerase chain reaction.
2. Specimens and clinical data
All blood samples and nasopharyngeal aspirates were obtained at the time of admission. Nasopharyngeal aspirates were stored at -80℃ until they were used for PCR assay and enzyme-linked immunosorbent assay. The medical records of patients with positive M. pneumoniae PCR were reviewed retrospectively and demographic data, physical findings, radiographic findings, and laboratory data on admission were collected. Clinical course, including treatment prior to admission, febrile days before and after macrolide administration, length of hospital stay, and related complications, was also analyzed.
3. Identification of MRMP
M. pneumoniae DNA was detected by conventional PCR targeting a conserved part of the P1 cytadhesin gene9). The point mutations at sites 2063 and 2064 in domain V of 23S rRNA genes conferring resistance to macrolides were searched using a direct sequencing method in specimens with a positive PCR result, as previously reported10).
4. IL-18 measurements
Levels of interleukin (IL) 18 in nasopharyngeal aspirate were measured using commercial enzyme-linked immunosorbent assay kits (MBL international, Woburn, MA, USA) according to the manufacturer's instructions. The sensitivity of the assay was 12.5 pg/mL.
5. Statistical analysis
All data are expressed as mean and standard deviation, unless otherwise indicated. Continuous variables were compared with the Student t test or the Mann-Whitney test. Differences in categorical variables were assessed with chi-square test. Multivariate analysis was performed using a stepwise logistic regression model. Receiver operating characteristic (ROC) curve analysis was used to analyze the discriminative power of the laboratory markers for prediction of the efficacy of macrolides in MRMP pneumonia. SigmaPlot software (Systat Software Inc., San Jose, CA, USA) was used for statistical analysis and P values<0.05 were considered statistically significant.
Results
1. Proportion of MRMP
A total of 95 patients with positive M. pneumoniae PCR were enrolled in this study. Among these 95 M. pneumoniae isolates, 49 (51.6%) showed macrolide resistance. All MRMP isolates had the A2063G point mutation in domain V of 23S rRNA gene. The point mutations at site 2064 in domain V of 23S rRNA genes were not observed.
2. Clinical course of MRMP and MSMP pneumonia
The clinical course and laboratory findings of patients with MRMP or MSMP pneumonia are summarized in Table 1. Demographic data were not significantly different between the two groups. The total number of febrile days, febrile days before initiation of macrolides, laboratory findings and chest radiologic findings on admission were also not significantly different. However, compared with the MSMP group, the MRMP group had longer hospital stays (8.4±5.6 vs. 6.1±2.4, P=0.007), longer febrile periods after initiation of macrolides (4.8±6.0 vs. 1.9±2.0, P=0.002), and had a higher proportion of patients who had a fever lasting more than 72 hours after initiation of macrolides (55.1 vs. 15.2, P<0.001).
Table 1.
Clinical course, laboratory, and radiologic findings in children with MRMP and MSMP pneumonia
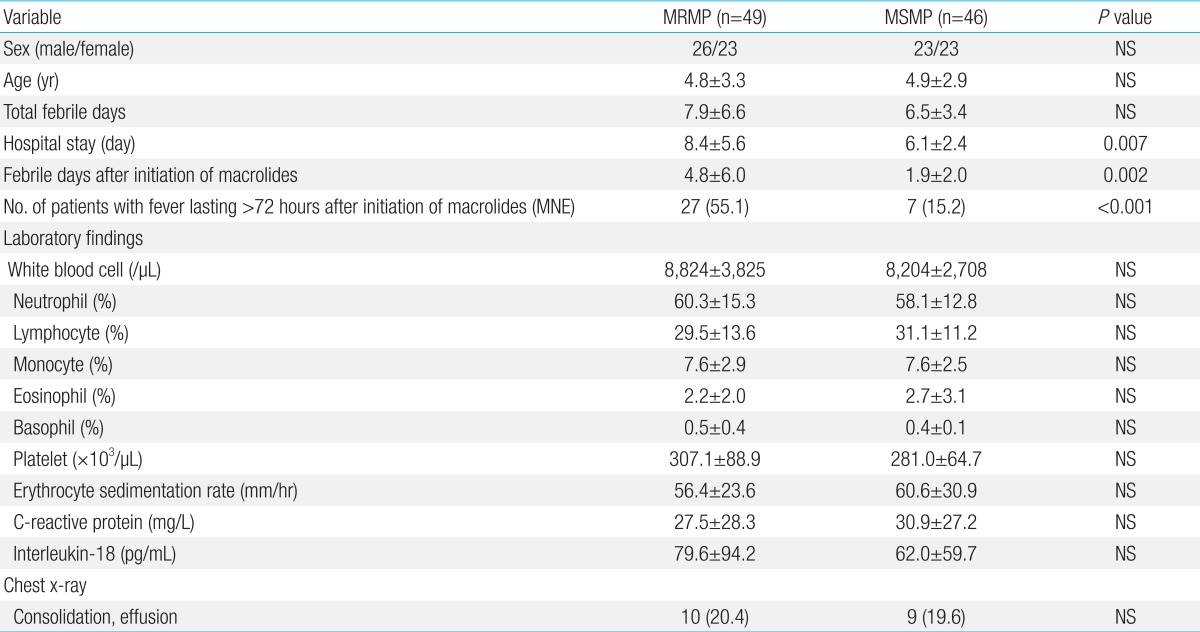
Values are presented as mean±standard deviation or number (%).
MRMP, macrolide-resistant Mycoplasma pneumoniae ; MSMP, macrolide-susceptible Mycoplasma pneumoniae ; MNE, macrolide-noneffective; NS, not significant.
3. Comparison based on the clinical efficacy of macrolides in patients with MRMP pneumonia
The demographic and laboratory findings at the time of admission in the MNE and ME subgroups are summarized in Table 2. Compared with the ME subgroup, the MNE subgroup showed significantly higher levels of serum CRP (45.3±36.9 vs. 16.1±14.7, P=0.018) and IL-18 in nasopharyngeal aspirate (107.8±117.1 vs. 49.5±49.7, P=0.042). No other laboratory or radiologic findings were significantly different between the two subgroups.
Table 2.
Comparison of demographic, laboratory, and radiologic findings based on the efficacy of macrolides in children with MRMP pneumonia
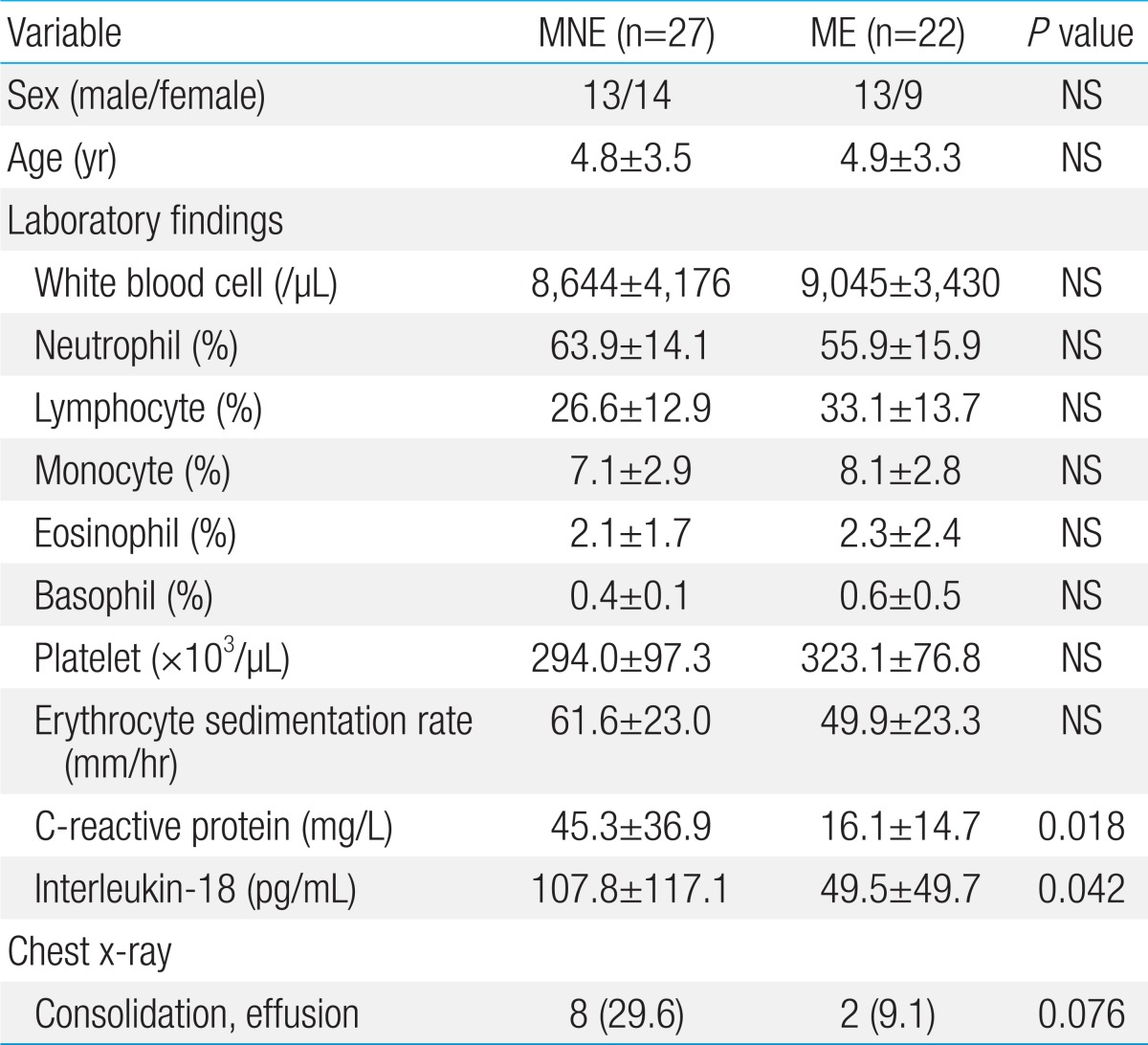
Values are presented as mean±standard deviation or number (%).
MRMP, macrolide-resistant Mycoplasma pneumoniae ; MNE, macrolide-noneffective; ME, macrolide-effective; NS, not significant.
4. Logistic regression and ROC curve analysis
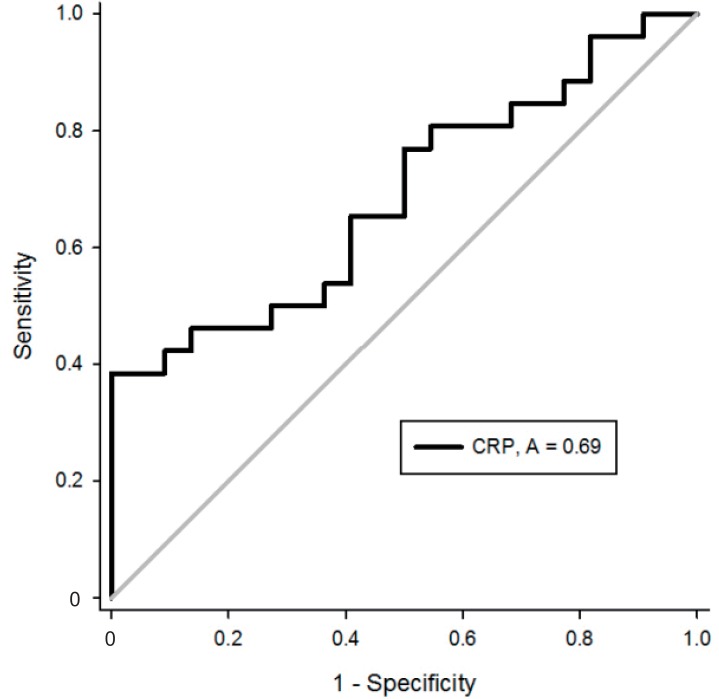
In stepwise logistic regression analysis, CRP was the single significant factor predicting the efficacy of macrolides in patients with MRMP pneumonia (odds ratio, 1.04; 95% confidence interval [CI], 1.01-1.08; P=0.022). The area under the curve (AUC) of CRP was 0.69 (95% CI, 0.54-0.84) in ROC curve analysis (Fig. 2). The optimal cutoff value of CRP was 40.7 mg/L, with a sensitivity of 38.5% and a specificity of 100% (positive predictive value, 100%; negative predictive value, 60.9%).
Fig. 2.
Receiver operating characteristic curve of C-reactive protein (CRP) in prediction of the efficacy of macrolides in children with macrolide-resistant Mycoplasma pneumoniae pneumonia.
Discussion
Okazaki et al.11) first revealed the nucleotide mutations associated with resistance to macrolides in Japanese pediatric patients in 2001. Since then, the emergence of MRMP has been reported worldwide. Antibiotic resistance is mainly due to point mutations at nucleotide positions A2063, A2064, or C2617 of domain V of 23S rRNA, or the ribosomal target of macrolides. Mutations at positions A2063 or A2064 confer a high level of resistance to macrolides, whereas mutations at positions A2067 and C2617 are related to a lower level of resistance12). A2063G is the most common mutation, followed by A2064G, but C2617A and mutations in the ribosomal proteins L4 and L22 have been reported rarely.
The prevalence of MRMP has been reported to be as high as 92% in China and is over 40% in Japan and 23% in Taiwan, although it has been reported as less than 10% in Europe2,13,14,15,16). Regardless of the prevalence, resistance has been consistently observed to be more frequent in children than adults. Although the highest prevalence of MRMP has been observed in East Asia, it is rising worldwide. In Japan, a gradual increase in MRMP from 2002 to 2008 was found, and more than 80% of the isolates were resistant to macrolides during a recent outbreak4,7). In the United States (US), resistance has been reported in only a few cases, but a recent study found the prevalence of resistant strains rising to 8.3% in the central US17). In Italy and Israel, over 20% of M. pneumoniae isolates were resistant strains in 2010, which exceeded the general prevalence in Europe18,19). The reason for the rapid rise in the prevalence of resistant strains may be related to the widespread empirical use of macrolides in children with suspected M. pneumoniae pneumonia18,20). The prevalence of MRMP in Korea has been found to be 27%, with mutation of the L4 ribosomal protein as the most common mutation21). However, in this study, the proportion of MRMP was 51.6% and all had the A2063G point mutation.
Previous reports about the clinical course of MRMP pneumonia have suggested that resistant strains do not increase the severity of the disease. However, signs and symptoms last relatively longer and the efficacy of macrolides is low in patients with resistant strains10). The results of this study also showed similar clinical features for MRMP. In comparison with the MSMP group, both hospital stay and febrile period after initiation of macrolides were prolonged in the MRMP group. The proportion of patients who had a fever lasting more than 72 hours after initiation of macrolides was also higher in the MRMP group. In addition, the proportion of patients who had lobar consolidation and/or effusion on chest radiography was also higher in the MRMP group than in the MSMP group, although the difference did not reach statistical significance due to the small number of patients. Furthermore, recent severe cases of life-threatening pneumonia or Stevens-Johnson syndrome have been reported in patients with resistant strains5,6).
Real-time PCR methods for the rapid detection of mutations in 23S rRNA directly from respiratory specimens are now available and allow treatment to be adjusted rapidly if a resistant genotype is detected22). Unfortunately, the problem is that there are few alternative antibiotics available for children with MRMP infection. Okada et al.7) recently reported rapid effectiveness of minocycline and doxycycline against MRMP infections in a 2011 outbreak among Japanese children. Of 176 patients with MRMP pneumonia, 125 were treated with minocycline or doxycycline, which were more effective than fluoroquinolones in achieving clinical improvement within 24 hours and in decreasing M. pneumoniae DNA copy numbers after 3 days of treatment.
Another alternative therapeutic strategy is the administration of systemic corticosteroids in addition to antimicrobials. The host inflammatory response to M. pneumoniae plays a central role in the pathogenesis of the clinical disease. In particular, significant production of IL-8 and IL-18, which induce interferon-γ production and promote type 1 cytokine response, has been suggested to be associated with the severity of M. pneumoniae pneumonia23,24,25). In this study, the level of IL-18 in nasopharyngeal aspirates was not different between the MRMP and MSMP groups, but was significantly higher in the MNE subgroup than in the ME subgroup. These results suggest that the host hyperimmune response is associated with the low efficacy of macrolides. Therefore, early administration of immune-modulators such as corticosteroids may be helpful in alleviating host hyperimmune response in macrolide nonresponsive patients. Some experimental studies have reported beneficial effects of corticosteroids on M. pneumoniae infections26,27), and Lee et al.8) reported that the use of corticosteroids for antibiotic nonresponsive M. pneumoniae pneumonia patients was effective in improving clinical and radiographic findings. However, further prospective controlled clinical studies for alternative antibiotics and corticosteroids in children with MRMP infection are needed.
To prevent the emergence of new resistant strains and to minimize the risk of well-known adverse effects, the limited use of these drugs in selective cases is appropriate. Therefore, the next issue is how to predict the efficacy of macrolides in children with MRMP infection. For this purpose, we performed stepwise logistic regression and ROC curve analysis. In stepwise logistic regression analysis, CRP was the single significant factor predicting the efficacy of macrolides in patients with MRMP pneumonia, and the AUC for CRP was 0.69 in ROC curve analysis, indicating fair discriminative power. Although IL-18 in nasopharyngeal aspirate also showed a similar discriminative power and there was no significant difference in the AUC comparison between IL-18 and CRP, IL-18 in nasopharyngeal aspirate is not routinely measured in most hospital laboratories; thus, CRP should be a more useful laboratory marker to predict the efficacy of macrolides in children with MRMP pneumonia in clinical practice.
Finally, a number of important limitations of this study should be considered, including its retrospective design. First, all children with M. pneumoniae pneumonia were not included. Patients were enrolled based on the results of mycoplasma specific PCR, and thus patients who had no available nasopharyngeal aspirate were excluded. Second, mutations in the ribosomal proteins L4 were not searched in this study because resistance to macrolides in East Asia has been mainly due to 23S rRNA mutation, and thus the proportion of MRMP may be underestimated. Third, the enrolled patients were from a single tertiary center, and therefore our results cannot be extrapolated to the general pediatric population in Korea. Lastly, we could not measure other important cytokines associated with M. pneumoniae infection because of sample limitations. To our knowledge, this study is the first to examine the predictive value of laboratory markers in the response to macrolides in pediatric MRMP pneumonia patients. Further prospective and large-scale studies are needed to resolve these limitations and to validate our findings.
In conclusion, the proportion of MRMP in children with M. pneumoniae pneumonia was over 50%, which suggests the possibility that the prevalence of MRMP is rising rapidly in Korea. Signs and symptoms are relatively prolonged and the efficacy of macrolides is low in patients with resistant strains. Early prediction of the efficacy of macrolides in children with MRMP pneumonia is crucial for the proper management of these patients. The results of this study suggests that CRP could be a useful marker to predict the efficacy of macrolides, which could help clinicians make better clinical decisions and lead to improved outcomes in children with MRMP pneumonia.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lee KY. Pediatric respiratory infections by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther. 2008;6:509–521. doi: 10.1586/14787210.6.4.509. [DOI] [PubMed] [Google Scholar]
- 2.Morozumi M, Takahashi T, Ubukata K. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J Infect Chemother. 2010;16:78–86. doi: 10.1007/s10156-009-0021-4. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki S, Yamazaki T, Narita M, Okazaki N, Suzuki I, Andoh T, et al. Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother. 2006;50:709–712. doi: 10.1128/AAC.50.2.709-712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, et al. Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother. 2008;52:348–350. doi: 10.1128/AAC.00779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson TP, Boppana S, Theos A, Clements LS, Xiao L, Waites K. Stevens-Johnson syndrome in a boy with macrolide-resistant Mycoplasma pneumoniae pneumonia. Pediatrics. 2011;127:e1605–e1609. doi: 10.1542/peds.2010-2624. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh YC, Tsao KC, Huang CG, Tong S, Winchell JM, Huang YC, et al. Life-threatening pneumonia caused by macrolide-resistant Mycoplasma pneumoniae. Pediatr Infect Dis J. 2012;31:208–209. doi: 10.1097/INF.0b013e318234597c. [DOI] [PubMed] [Google Scholar]
- 7.Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, et al. Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55:1642–1649. doi: 10.1093/cid/cis784. [DOI] [PubMed] [Google Scholar]
- 8.Lee KY, Lee HS, Hong JH, Lee MH, Lee JS, Burgner D, et al. Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2006;41:263–268. doi: 10.1002/ppul.20374. [DOI] [PubMed] [Google Scholar]
- 9.Ieven M, Ursi D, Van Bever H, Quint W, Niesters HG, Goossens H. Detection of Mycoplasma pneumoniae by two polymerase chain reactions and role of M. pneumoniae in acute respiratory tract infections in pediatric patients. J Infect Dis. 1996;173:1445–1452. doi: 10.1093/infdis/173.6.1445. [DOI] [PubMed] [Google Scholar]
- 10.Kawai Y, Miyashita N, Yamaguchi T, Saitoh A, Kondoh E, Fujimoto H, et al. Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology. 2012;17:354–362. doi: 10.1111/j.1440-1843.2011.02102.x. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki N, Narita M, Yamada S, Izumikawa K, Umetsu M, Kenri T, et al. Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol Immunol. 2001;45:617–620. doi: 10.1111/j.1348-0421.2001.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 12.Cardinale F, Chironna M, Dumke R, Binetti A, Daleno C, Sallustio A, et al. Macrolide-resistant Mycoplasma pneumoniae in paediatric pneumonia. Eur Respir J. 2011;37:1522–1524. doi: 10.1183/09031936.00172510. [DOI] [PubMed] [Google Scholar]
- 13.Xin D, Mi Z, Han X, Qin L, Li J, Wei T, et al. Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob Agents Chemother. 2009;53:2158–2159. doi: 10.1128/AAC.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu PS, Chang LY, Lin HC, Chi H, Hsieh YC, Huang YC, et al. Epidemiology and clinical manifestations of children with macrolideresistant Mycoplasma pneumoniae pneumonia in Taiwan. Pediatr Pulmonol. 2013;48:904–911. doi: 10.1002/ppul.22706. [DOI] [PubMed] [Google Scholar]
- 15.Pereyre S, Charron A, Renaudin H, Bebear C, Bebear CM. First report of macrolide-resistant strains and description of a novel nucleotide sequence variation in the P1 adhesin gene in Mycoplasma pneumoniae clinical strains isolated in France over 12 years. J Clin Microbiol. 2007;45:3534–3539. doi: 10.1128/JCM.01345-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumke R, von Baum H, Luck PC, Jacobs E. Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect. 2010;16:613–616. doi: 10.1111/j.1469-0691.2009.02968.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, Buller R, Bledsoe S, Storch GA. Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr Infect Dis J. 2012;31:409–411. doi: 10.1097/INF.0b013e318247f3e0. [DOI] [PubMed] [Google Scholar]
- 18.Chironna M, Sallustio A, Esposito S, Perulli M, Chinellato I, Di Bari C, et al. Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J Antimicrob Chemother. 2011;66:734–737. doi: 10.1093/jac/dkr003. [DOI] [PubMed] [Google Scholar]
- 19.Averbuch D, Hidalgo-Grass C, Moses AE, Engelhard D, Nir-Paz R. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg Infect Dis. 2011;17:1079–1082. doi: 10.3201/eid1706.101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo SJ, Kim HB, Choi SH, Lee SO, Kim SH, Hong SB, et al. Differences in the frequency of 23S rRNA gene mutations in Mycoplasma pneumoniae between children and adults with community-acquired pneumonia: clinical impact of mutations conferring macrolide resistance. Antimicrob Agents Chemother. 2012;56:6393–6396. doi: 10.1128/AAC.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh CE, Choi EH, Lee HJ. Detection of genetic mutations associated with macrolide resistance of Mycoplasma pneumoniae. Korean J Pediatr. 2010;53:178–183. [Google Scholar]
- 22.Bebear C, Pereyre S, Peuchant O. Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol. 2011;6:423–431. doi: 10.2217/fmb.11.18. [DOI] [PubMed] [Google Scholar]
- 23.Narita M, Tanaka H, Abe S, Yamada S, Kubota M, Togashi T. Close association between pulmonary disease manifestation in Mycoplasma pneumoniae infection and enhanced local production of interleukin-18 in the lung, independent of gamma interferon. Clin Diagn Lab Immunol. 2000;7:909–914. doi: 10.1128/cdli.7.6.909-914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narita M, Tanaka H. Cytokines involved in the severe manifestations of pulmonary diseases caused by Mycoplasma pneumoniae. Pediatr Pulmonol. 2007;42:397. doi: 10.1002/ppul.20445. [DOI] [PubMed] [Google Scholar]
- 25.Oishi T, Narita M, Matsui K, Shirai T, Matsuo M, Negishi J, et al. Clinical implications of interleukin-18 levels in pediatric patients with Mycoplasma pneumoniae pneumonia. J Infect Chemother. 2011;17:803–806. doi: 10.1007/s10156-011-0265-7. [DOI] [PubMed] [Google Scholar]
- 26.Tagliabue C, Salvatore CM, Techasaensiri C, Mejias A, Torres JP, Katz K, et al. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J Infect Dis. 2008;198:1180–1188. doi: 10.1086/591915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirao S, Wada H, Nakagaki K, Saraya T, Kurai D, Mikura S, et al. Inflammation provoked by Mycoplasma pneumoniae extract: implications for combination treatment with clarithromycin and dexamethasone. FEMS Immunol Med Microbiol. 2011;62:182–189. doi: 10.1111/j.1574-695X.2011.00799.x. [DOI] [PubMed] [Google Scholar]