Abstract
Marine Synechococcus cyanobacteria constitute a monophyletic group that displays a wide latitudinal distribution, ranging from the equator to the polar fronts. Whether these organisms are all physiologically adapted to stand a large temperature gradient or stenotherms with narrow growth temperature ranges has so far remained unexplored. We submitted a panel of six strains, isolated along a gradient of latitude in the North Atlantic Ocean, to long- and short-term variations of temperature. Upon a downward shift of temperature, the strains showed strikingly distinct resistance, seemingly related to their latitude of isolation, with tropical strains collapsing while northern strains were capable of growing. This behaviour was associated to differential photosynthetic performances. In the tropical strains, the rapid photosystem II inactivation and the decrease of the antioxydant β-carotene relative to chl a suggested a strong induction of oxidative stress. These different responses were related to the thermal preferenda of the strains. The northern strains could grow at 10 °C while the other strains preferred higher temperatures. In addition, we pointed out a correspondence between strain isolation temperature and phylogeny. In particular, clades I and IV laboratory strains were all collected in the coldest waters of the distribution area of marine Synechococus. We, however, show that clade I Synechococcus exhibit different levels of adaptation, which apparently reflect their location on the latitudinal temperature gradient. This study reveals the existence of lineages of marine Synechococcus physiologically specialised in different thermal niches, therefore suggesting the existence of temperature ecotypes within the marine Synechococcus radiation.
Keywords: adaptation, ecotype, marine cyanobacteria, Synechococcus, temperature
Introduction
Marine picocyanobacteria belonging to the Prochlorococcus and Synechococcus genera are major contributors to carbon biomass and global oceanic primary production and may contribute up to half of the fixed carbon in some oceanic regions (Li, 1994; Liu et al., 1997; Buitenhuis et al., 2012). Although phylogenetically closely related, these two cyanobacteria exhibit distinct traits of ecology, physiology and evolution (Partensky et al., 1999; Partensky and Garczarek, 2010). Prochlorococcus thrives in warm waters of the latitudinal 45°N–40°S band, whereas Synechococcus cells prefer coastal and mesotrophic open ocean waters, with a much wider latitudinal distribution ranging from the equator to the polar fronts (Not et al., 2005; Zwirglmaier et al., 2008; Huang et al., 2011). In addition, Synechococcus has no obvious depth preference, the highest cell densities being often observed in the upper mixed layer, whereas Prochlorococcus shows strong depth partitioning, with two main ecotypes that are both physiologically and genetically distinct: a high light (HL)-adapted ecotype, occupying the upper part of the euphotic zone and a low light(LL)-adapted ecotype, dominating the bottom of the euphotic layer (Moore et al., 1998). The HL ecotype has been further subdivided into HLI and HLII, which exhibit distinct latitudinal distributions, HLII dominating between 28°S and 33°N and HLI above 32°S or 38°N, a difference seemingly linked to the different temperature growth optima of their cultured representatives (Johnson et al., 2006; Zinser et al., 2007).
Using the 16S ribosomal RNA and Internal Transcribed Spacer genetic markers, respectively, 10 and 15 different clades (Rocap et al., 2002; Fuller et al., 2003) have been delineated within the main group of the marine Synechococcus radiation (cluster 5.1; sensu Herdman et al., 2001). However, a recent study by Mazard et al. (2012a) has highlighted a greater genetic microdiversity than previously thought in this subcluster. Indeed, using the cytochrome b6 gene petB, they could define >30 different subclades of marine Synechococcus. As a result of this large number of lineages, the environmental factors that have directed the diversification of marine Synechococcus appear much more complicated to understand than in the case of Prochlorococcus. Through a vast phylogeographic study, Zwirglmaier et al. (2007, 2008) have, however, shown that, out of the dozen of marine Synechococcus 16S ribosomal RNA clades, only four (I–IV) predominate in the oceans. Clades I and IV generally co-occur at latitudes above 30°N/S and at depth, whereas clade II seems to prevail in warm, coastal or shelf areas (Zwirglmaier et al., 2007, 2008; Huang et al., 2011; Mella-Flores et al., 2011; Ahlgren and Rocap, 2012). The latitudinal distribution of clade III appears to be broader, but with an apparent predominance in oligotrophic, offshore waters (Fuller et al., 2005; Zwirglmaier et al., 2008; Mella-Flores et al., 2011; Post et al., 2011). The other clades are usually detected at low concentrations and their distribution patterns are less clearly defined (Zwirglmaier et al., 2008; Huang et al., 2011).
To understand whether some Synechococcus lineages are adapted to specific ecological niches, both phylogeography and comparative physiology studies are necessary. These two approaches indeed allow pointing out possible correspondences between cell performances, phylogeny and ecological niches. So far, most of the comparative studies of marine Synechococcus physiology have dealt with adaptation and acclimation capacities to light (Six et al., 2004, 2007b) and nutrients variations (for example, Liu et al., 2012; Mazard et al., 2012b). These works suggest that marine Synechococcus strains can grow over large ranges of irradiance, explaining that cells of this organism are detected from surface down to 150 m (Kana and Glibert, 1987; Olson et al., 1990; Moore et al., 1995; Six et al., 2004, 2007b). Light quality also influences marine Synechococcus distribution (Olson et al., 1990; Lantoine and Neveux, 1997; Wood et al., 1998; Sherry and Wood, 2001). The pigmentation of the light-harvesting complex, the phycobilisome (PBS), is indeed highly variable among strains (Six et al., 2007b, 2007c) and optimised to collect the most abundant wavelengths in a given light niche. These different pigment types, however, most often are not restricted to specific Synechococcus lineages (Palenik 2001; Six et al., 2007c), and therefore do not constitute ecotypes sensu stricto. Similarly, although nutrient availability contribute to some of the variability of the Synechococcus community structure (Zwirglmaier et al., 2008; Mazard et al., 2012a), a clear delineation of nutrient ecotypes (equivalent to Prochlorococcus light ecotypes) within the Synechococcus radiation remains globally elusive (Palenik et al., 2006; Scanlan et al., 2009; Stuart et al., 2009). A notable exception concerns the response to phosphorus limitation (including cell size change and the ability to accumulate polyphosphate) that appears somewhat consistent with Synechococcus phylogeny and their inferred ecology of the different clades (Mazard et al., 2012a). However, a large part of the variation observed in the Synechococcus community structure and the hierarchy of environmental factors shaping ecotype genomes remains largely unexplained.
Adaptation to temperature variations among marine Synechococcus has been so far poorly explored. This environmental factor can significantly constrain growth, as the activity of most enzymes and biomembranes directly depends on it, thus impacting major metabolic processes. Among them, photosynthesis is known to be particularly affected by temperature variations, notably because of the resulting changes in thylakoidal fluidity that eventually lead to photosystem (PS) II inactivation (Murata and Los, 1997; Takahashi and Murata, 2008). Like for Prochlorococcus (Johnson et al., 2006; Zinser et al., 2007), temperature might have thus played an important role in the differentiation of the Synechococcus lineages, possibly influencing significantly their genome shaping. However, given the scarcity of physiological studies of the response of open ocean Synechococcus isolates to temperature (Moore et al., 1995; Fu et al., 2007), it is difficult to assert whether the large latitudinal distribution of these picocyanobacteria is rather the result of broad acclimation capacities to temperature, or of adaptation processes underlying the existence of different ‘thermotypes' that would display distinct temperature optima for growth.
To explore these questions, we describe in this paper the short- and long-term responses to temperature of a panel of six Synechococcus strains, isolated at various latitudes, in mesotrophic waters of the North Atlantic Ocean. We aimed at pointing out different resistance and acclimation capacities among the strains, with a focus on their capability to tune light utilisation in response to temperature variations. We furthermore highlight ecophylogenetic features of marine Synechococcus and discuss the importance of temperature in the diversification of this radiation.
Materials and methods
Growth conditions and experimental design
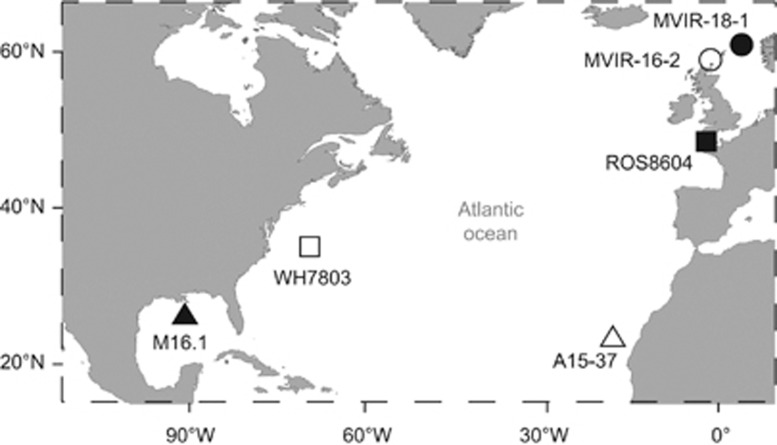
In order to minimise the differences regarding other physicochemical parameters than temperature among isolation sites, we selected six marine phycoerythrobilin-rich (pigment type 3a, Six et al., 2007c; Humily et al., in press) Synechococcus strains that were isolated in the North Atlantic Ocean, at similar distance from both the West or East coast, but at very different latitudes, from the polar circles to intertropical zone (Figure 1). Clonal Synechococcus spp. strains A15-37, M16.1, WH7803, ROS8604, MVIR-16-2 and MVIR-18-1 were retrieved from the Roscoff culture collection (Table 1; http://www.sb-roscoff.fr/Phyto/RCC/; Vaulot et al., 2004) and grown in polystyrene flasks (Nalgene, Rochester, NY, USA; or Sarsted, Nümbrecht, Germany) in PCR-S11 culture medium (Rippka et al., 2000) supplemented with 1 mM sodium nitrate. The seawater was reconstituted using Red Sea Salt (Houston, TX, USA) and distilled water. Continuous white light was provided by fluorescent tubes (Sylvania Daylight F18W/54-765 T8) at 80 μmol photons m−2 s−1 irradiance. Cultures of the six strains were long-term acclimated to a range of temperatures, from 10 °C to 35 °C, within temperature-controlled chambers (Liebherr-Hausgeräte, Lienz, Austria) in order to measure growth rates as a function of temperature.
Figure 1.
Location of isolation sites of the six marine Synechococcus spp. used in this study.
Table 1. Information regarding the Synechococcus strains used in this study.
| Strain name | A15-37 | M16.1 | WH7803 | ROS8604 | MVIR-16-2 | MVIR-18-1 |
|---|---|---|---|---|---|---|
| RCC # | 2526 | 791 | 752 | 2380 | 1594 | 2385 |
| Pigment type | 3a | 3a | 3a | 3a | 3a | 3a |
| Isolation site | Offshore Mauritania | Gulf of Mexico | Sargasso Sea | English Channel | Southern Norwegian Sea | Southern Norwegian Sea |
| Isolation latitude | 23° 33′ N | 27° 42′ N | 33° 45′ N | 48° 43′ N | 60° 19′ N | 61° 00′ N |
| Isolation longitude | 19° 59′ W | 91° 18′ W | 67° 30′ W | 3° 59′ W | 3° 29′ W | 1° 59′ E |
| Isolation date | 29/09/2004 | 09/02/2004 | 03/07/1978 | 24/11/1986 | 21/07/2007 | 23/07/2007 |
| Isolation Depth (m) | 10 | 275 | 25 | 1 | 10 | 25 |
| Isolation temperature (°C) | 24.53 | 24.15 | 25.85 | 12.81 | 11.99 | 13.98 |
The pigment type nomenclature is described in Six et al., 2007c and Humily et al. (in press).
For cold stress experiments, early exponentially growing cultures (1.5 l) of the six Synechococcus strains grown at 22±1 °C were transferred to 13±1 °C under 80 μmol photons m−2 s−1 of continuous white light. This temperature amplitude was chosen to be large enough to observe physiological readjustments in the cold resistant strains, while not causing too abrupt culture collapsing of the sensitive ones. After the transfer, about 5 h were necessary for the cultures to reach 13 °C. The cultures were daily sampled for cell counting, fluorescence measurements and pigment analyses as described below. These experiments were repeated at least four times and compared with control cultures, that is, grown routinely at 22 °C. Following the cold period of 6 days, cultures were shifted back to 22 °C, in order to estimate their recovery capacities.
Flow cytometry
For cell density measurements, aliquots of cultures were preserved within 0.25% glutaraldehyde grade II (Sigma Aldrich, St Louis, MO, USA) and stored at −80 °C until analysis (Marie et al., 1999). Cell concentrations were determined using a flow cytometer (FACSCanto II, Becton Dickinson, San Jose, CA, USA) for which laser emission was set at 488 nm and using distilled water as sheath fluid. For steady-state acclimated cultures, growth rates (μ, in day−1) were computed as the slope of a Ln(Nt) vs time plot, where Nt is the cell concentration at time t. Orange and red fluorescence levels (emission at 585±21 nm and 660±10 nm, respectively) were normalised with standard 0.95 μm YG beads (Polysciences Warrington, PA, USA) after analysing list mode files with the custom-designed freeware CYTOWIN (Vaulot, 1989) or the FCS Express 4 Flow Research Edition software (De Novo Software, Los Angeles, CA, USA).
In vivo fluorescence measurements
PSII quantum yield (FV/FM) and non-photochemical quenching associated to state transition (NPQST) were measured upon excitation at 520 nm using a Pulse Amplitude Modulation fluorometer (Phyto-PAM, Walz, Effeltrich, Germany) equipped with a temperature-controlled cuvette holder and connected to a chart recorder (Vernier, LabPro, Beaverton, OR, USA). After 5-min acclimation to dark, the maximal fluorescence levels were measured in the dark (FMd) and under bright red light (655 nm, 2000 μmol photons m−2 s−1; FM) in the presence of 100 μM of the PSII blocker 3-(3,4-dichlorophenyl)-1,1-dimethylurea, by triggering saturating light pulses (655 nm; 5000 μmol photons m−2 s−1). The PSII quantum yield was calculated as:
FV/FM=(FM–F0)/FM
where F0 is the basal fluorescence level and FV is the variable fluorescence (Campbell et al., 1998; Six et al., 2007a, 2009). The NPQST factor was calculated as:
NPQST=(FM–FMd)/FM
In addition, fluorescence emission spectra were recorded at experimental temperature (22 °C or 13 °C) upon excitation at 530 nm, with a LS-50B spectrofluorometer (Perkin-Elmer, Waltham, MA, USA), as described by Six et al. (2004, 2007b). In order to study the phycobiliprotein coupling in the PBS during the cold period, the phycoerythrin (PE) to phycocyanin (PC) fluorescence emission ratio was calculated by dividing values measured at the PE maximum (565–575 nm) to the PC maximum (645–655 nm).
D1 protein and β-chain PE immunoblotting
Cell pellets were resuspended in extraction buffer (140 mM Tris base, 105 mM Tris-HCl, 0.5 mM ethylenediaminetetraacetic acid, 2% lithium dodecyl sulphate, 10% glycerol and 0.1 mg ml−1 PefaBloc protease inhibitor; Roche, Basel, Switzerland) and lysed applying freezing–thawing cycles and sonication. After centrifugation, total protein concentration was determined using a Lowry protein assay kit and bovine serum albumin as protein standard (Bio-Rad, Hercules, CA, USA). Samples were then denaturated by heating for 2 min at 80 °C in the presence of 50 mM dithiotreitol and an amount of 4 (PsbA protein) or 8 (β-chain PE) μg total protein was loaded on a 4–12% acrylamide gradient precast NuPAGE Bis-Tris mini-gel (Invitrogen, Carlsbad, CA, USA). Gels were electrophoresed and the proteins were transferred onto a polyvinylidene fluoride membrane, then immediately immersed into Tris Buffer Saline-Tween (TBS-T) buffer, pH 7.6 (0.1% Tween 20, 350 mM sodium chloride, 20 mM Trizma base) containing 2% (w:v) blocking agent (Amersham Biosciences, Piscataway, NJ, USA) for 45 min. Aliquots of rabbit primary antibodies against β-subunit PE proteins (directed against the β-subunit PE of Prochlorococcus sp. SS120; courtesy of Wolfgang R Hess, University of Freiburg, Germany; Six et al., 2007b; Hess et al., 1999) or PsbA (D1 protein; Agrisera) were diluted at 1:50 000 in TBS-T in the presence of 0.5% blocking agent and membranes were soaked into this solution for 1 h with agitation. After extensive washing of the membrane with TBS-T buffer, anti-rabbit secondary antibodies (Bio-Rad) were applied with the same procedure as for primary antibodies. Membranes were developed by chemoluminescence using the ECL Advance reagent kit (Amersham Biosciences) and visualised with a LAS4000 imager equipped with a CCD camera (GE Healthcare, Waukesha, WI, USA).
Pigment analyses
After extraction in 100% methanol, pigment extracts were supplemented with 10% water in order to avoid peak distortion. Hydrophobic pigments were then measured by high pressure liquid chromatography using an HPLC 1100 Series System (Hewlett-Packard, St Palo Alto, CA, USA), equipped with a Kromasil C8 column (150 × 4.6 mm, 3.5 μm particles size) according to a procedure published elsewhere (Six et al., 2005).
Phylogenetic analyses
petB sequences of Synechococcus strains obtained from the Roscoff Culture Collection (RCC, http://www.sb-roscoff.fr/Phyto/RCC/) were amplified as previously described (Mazard et al., 2012a). Briefly, 550 bp petB fragments from MVIR-16-1 (RCC2570), MVIR-11-1 (RCC1695), MVIR-7-1 (RCC1648), MVIR-18-1 (RCC2385), MVIR-16-2 (RCC3010), MVIR-10-1 (RCC1688), MVIR-1-1 (RCC1708) and A15-44 (RCC2527) were amplified directly from cultures at an annealing temperature of 55 °C. Sequences were deposited in the GenBank nucleotide sequence database under the accession numbers mentioned in Supplementary Table S2. A multiple alignment of 74 sequences from cultured isolates was generated with MAFFT v6.818 using the G-INS-I option (Katoh and Toh, 2008) and trimAL 1.4 to remove poorly aligned regions and gaps (Capella-Gutiérrez et al., 2009). Phylogenetic reconstructions were performed on 559 aligned amino acids using three different methods: maximum likelihood, Bayesian inference and neighbour joining. Neighbour-joining analyses were performed using Phylip 3.69 (Felsenstein, 1989) as previously described (Mella-Flores et al., 2011). The phylogenetic inference by maximum likelihood was performed with the Message Passing Interface version of PhyML v3.0 (Guindon and Gascuel, 2003) using the TrN+I+G, a submodel of the general time-reversible, with invariant sites and gamma distribution. This model was selected using JModeltest 2.1.3 according the Akaike Information Criterion (Darriba et al., 2012). Bayesian inference was conducted using MrBayes 3.1.2 after partitioning according to the position in the codon (Huelsenbeck and Ronquist, 2001). Four Markov Chain Monte Carlo simulations were run for five millions generations that were sampled every 100 generations, the first 12 500 trees being discarded. The topology of the tree was obtained after ML analyses and the robustness of inferred topologies was supported by 1000 nonparametric bootstrap samplings for ML and neighbour joining.
Field temperature data collection
Average sea surface temperature at the strain isolation sites (resolution of 5° squares) and at the month and year of collection were determined using the satellite data available from the National Oceanic and Atmospheric Administration (NOAA, http://iridl.ldeo.columbia.edu/SOURCES/.NOAA/.NCEP/.EMC/.CMB/.GLOBAL/.Reyn_ SmithOIv2/.monthly/.sst/). Regarding strains isolated before 1981 (Supplementary Table S2) for which satellite data are not available, we used the seasonal average temperature at the isolation site (grid of 5° squares) over 10 years (2002–2012). For the strain for which isolation date is not available, Synechococcus sp. SYN20, we used the annual average surface seawater temperature over the 10 years. Seawater thermal amplitude at the isolation sites were estimated through the difference between summer and winter average temperature over the 10 years.
Results
Photophysiological differences among the Synechococcus strains
When grown in identical conditions at 22 °C, the six strains exhibited PSII quantum yields close to 0.6, indicating efficient photosynthetic activity (Table 2). The parameters related to the light-harvesting system showed nevertheless significant differences. The emission spectra indeed displayed a number of strain specificities, including the wavelengths of the maxima, the relative width of the emission bands and the PE to PC ratio (hereafter PE:PC) that ranged from 2.0 to 3.5 (Table 2 and Supplementary Figure S1). Furthermore, the amplitude of the state transition process, as estimated by the NPQST parameter (see Materials and methods section), varied significantly among strains from 0.2 to 0.5. Synechococcus sp. M16.1 showed weak state transitions with a fairly high fluorescence PE:PC, whereas Synechococcus spp. WH7803 and MVIR-18-1 displayed strong state transitions and a low PE:PC. The most striking differences among the six strains lied in their pigment cell contents. The tropical Synechococcus spp. A15-37 and M16.1 displayed the lowest membrane pigment (Chl a, β-carotene and zeaxanthin) contents while it was significantly higher in the mid-latitude strains WH7803 and ROS8604 (Table 2). Similar trends were observed for flow cytometric fluorescence signals with Synechococcus sp. A15-37 emitting weak orange fluorescence in contrast to WH7803 and ROS8604 cells, which were brightly fluorescent.
Table 2. Photophysiological features of the six marine Synechococcus strains, acclimated at 22 °C under 80 μmol photons m−2 s−1.
| Strain | A15-37 | M16.1 | WH7803 | ROS8604 | MVIR-16-2 | MVIR-18-1 |
|---|---|---|---|---|---|---|
| Orange fluorescence | 0.05±0.01 | 0.18±0.01 | 0.80±0.06 | 1.10±0.22 | 0.45±0.04 | 0.21±0.07 |
| PE:PC | 2.70±0.10 | 3.50±0.70 | 2.00±0.20 | 2.70±0.20 | 2.40±0.10 | 2.00±0.30 |
| Fv:FM | 0.64±0.03 | 0.57±0.07 | 0.61±0.07 | 0.53±0.03 | 0.57±0.01 | 0.60±0.02 |
| NPQST | 0.48±0.04 | 0.20±0.07 | 0.47±0.09 | 0.36±0.01 | 0.43±0.02 | 0.50±0.01 |
| Chl a (fg cell−1) | 2.21±0.81 | 2.62±0.81 | 17.5±1.41 | 9.52±1.61 | 2.75±0.39 | 5.57±0.26 |
| Zeaxanthin (fg cell−1) | 1.47±0.54 | 2.06±0.59 | 5.20±0.62 | 6.54±1.71 | 2.05±0.25 | 3.05±0.07 |
| β-Carotene (fg cell−1) | 0.08±0.01 | 0.15±0.05 | 1.16±0.16 | 0.88±0.09 | 0.22±0.02 | 0.40±0.02 |
| Zeaxanthin: Chl a (g/g) | 0.67±0.07 | 0.80±0.06 | 0.30±0.03 | 0.69±0.08 | 0.75±0.03 | 0.53±0.04 |
| β-Carotene: Chl a (g/g) | 0.059±0.028 | 0.085±0.010 | 0.070±0.001 | 0.094±0.018 | 0.081±0.004 | 0.071±0.02 |
Abbreviations: PC, phycocyanin; PE, phycoerythrin.
Variations of cell density and fluorescence upon cold stress
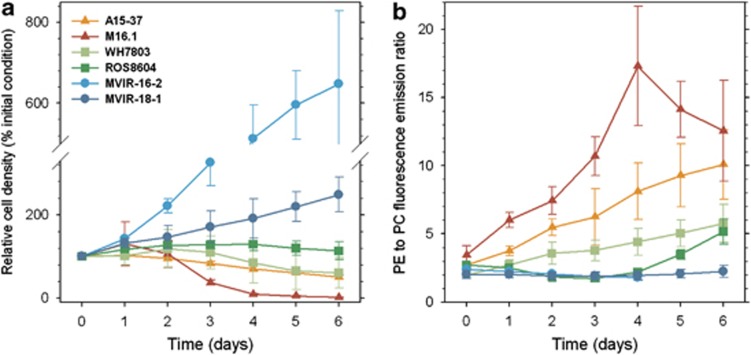
In order to highlight possible differences in temperature stress response, cultures fully acclimated to 22 °C were shifted to 13 °C. The time course variations of cell abundances differed significantly between the six strains (Figure 2a). For the tropical strain Synechococcus sp. M16.1, the cell density slightly increased after 1 day, then markedly dropped down to 2% of the initial cell density after 6 days of cold stress. In the cultures of strains A15-37 and WH7803, the cell density decreased by half after 6 days. For Synechococcus sp. ROS8604, cell density increased during the first 3 days, reaching 140% of the initial cell density and then started to decrease. For these four strains, cell density kept decreasing if the cold stress was prolonged over 6 days (data not shown). By contrast, the two high-latitude strains, Synechococcus spp. MVIR-16-2 and MVIR-18-1, exhibited significant growth (0.40±0.04 day−1 and 0.16±0.03 day−1, respectively), which was comparable to acclimated cultures at 13 °C. The former strain reached stationary phase after 4–5 days at 13 °C. As classically observed in stationary phase cultures, drastic physiological changes occurred in the culture, including a drop of the FM fluorescence level and an increase of the PE fluorescence yield (see below). As this downregulation of photosynthesis is under no circumstances related to a temperature effect and could lead to misinterpretation, data subsequent to the fourth day are not shown in the following results for Synechococcus sp. MVIR-16-2.
Figure 2.
Variations of relative cell density (cell ml−1) expressed as % of initial cell density at the start of the experiment (a) and PE to PC fluorescence emission ratio (b) in the tropical (triangles), mid-latitude (squares) and high-latitude (circles) marine Synechococcus strains during 6 days of cold stress (n⩾4).
Red fluorescence per cell, a proxy for chl a cell content, was measured by flow cytometry (Supplementary Figure S2A). This parameter decreased down to about 30% of the initial value in the strains isolated at low latitude. In Synechococcus sp. ROS8604, the red fluorescence per cell kept fairly stable until the third day and then dropped sharply. In the northern strains, this parameter slightly decreased and stabilised to a level corresponding at about 80% of the initial values. After the temperature shift, all strains reduced their orange fluorescence by about 50% (Supplementary Figure S2B).
Phycobiliprotein coupling upon cold stress
Fluorescence emission spectra were recorded for each strain at each time point, and used to calculate the PE to PC fluorescence emission ratio (Figure 2b). This parameter rapidly increased for the low-latitude strains Synechococcus spp. A15-37 and M16.1, most likely due to an increase of leaking energy between PE and PC, therefore indicating a lower coupling of the PBS rod phycobiliproteins (Six et al., 2007b). This process was particularly notable for the tropical Synechococcus sp. M16.1, which exhibited a 5.5-fold increase after 4 days of cold stress. The PE:PC decrease during the last days of the cold period indicates PE degradation, which was also suggested by the bleaching of the cultures at this stage of the experiment. The PE:PC increase was moderate and immediate for strain WH7803, and delayed for ROS8604, starting at the fourth day. The high-latitude strains did not show any variation of the PE to PC ratio. Very similar trends were observed among the six strains for the PE to PBS terminal acceptor ratio (data not shown; Supplementary Figure S1).
State transition amplitude upon cold stress
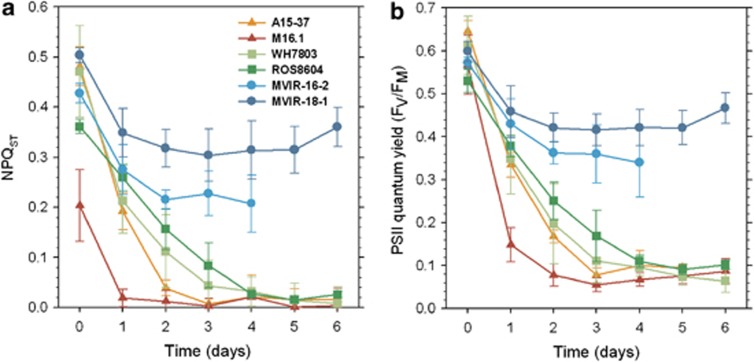
We used the non-photochemical quenching of fluorescence associated to state transitions, NPQST, as an index of the amplitude of this process, which balances the distribution of light energy between the two PS. This mechanism was markedly affected by low temperature in all Synechococcus strains considered, except for the high-latitude strains MVIR-16-2 and MVIR-18-1, which showed a lesser decrease of this parameter (45% and 20% of initial condition, respectively; Figure 3a). State transitions were totally inhibited after 1–2 days of cold stress in the low-latitude strains. In Synechococcus spp. WH7803 and ROS8604, NPQST decreased progressively to reach a minimal level after 4 days of cold stress (Figure 3a).
Figure 3.
Variations of state transition amplitude as measured by non-photochemical quenching of fluorescence associated to state transition (NPQST; (a)) and photosystem II quantum yield (FV/FM; (b)) in the tropical (triangles), mid-latitude (squares) and high-latitude (circles) marine Synechococcus strains during 6 days of cold stress (n⩾4).
The recovery capacities of temperate and tropical strains were estimated by placing the cultures back to the initial temperature (22 °C, Supplementary Table S1). The tropical strain A15-37 could never recover from the cold stress and its western counterpart, M16.1, needed >13 days to recover its photosynthetic parameters, and thereafter growth. Synechococcus spp. WH7803 and ROS8604 recovered more than twice faster than M16.1.
PSII quantum yield upon cold stress
Upon a 9 °C downward temperature shift, we observed a gradient of photosynthetic responses among the six Synechococcus strains (Figure 3b). The PSII quantum yield (FV/FM) of the strains isolated at low and medium latitude was markedly affected by low temperature. Synechococcus spp. A15-37 and M16.1 reached minimal yield values after 2–3 days of cold stress, with very low fluorescence signal after a couple of days. The fluorescence signal of the intermediate latitude strains reached minimum values more slowly, after 4–5 days. By contrast, strains isolated at high latitude, MVIR-16-2 and MVIR-18-1, suffered only a moderate decrease of the PSII quantum yield, reaching a steady state of ca 0.4 after 2 days. Similarly reduced FV/FM (and NPQST) values were observed for both strains once acclimated for several weeks to 13 °C (data not shown), suggesting that these cultures had already reached an acclimated level for these parameters after 4–5 days at 13 °C.
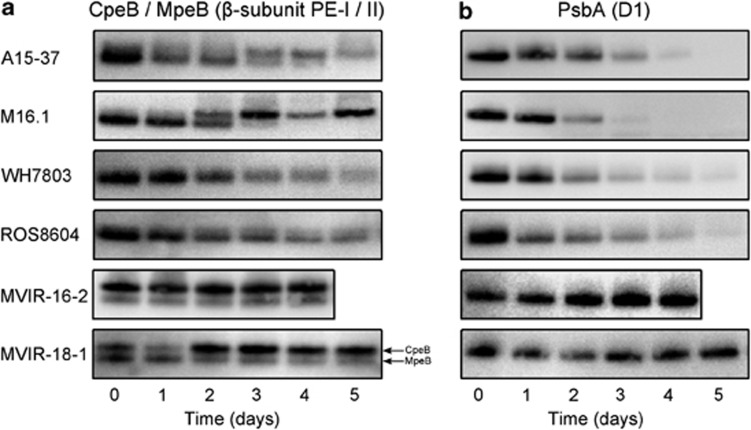
Time course relative variations of β-subunit PEs and D1 protein
Immunoreactions on whole-cell proteins using antibodies directed against PE β-subunits and D1 allowed analysing the variations of the relative quantity of these proteins during the cold stress experiment (Figure 4). Both antibodies gave clean immunoblots with no parasite bands. The antibody raised against PE β-subunits cross-reacted with both CpeB (PEI) and MpeB (PEII), as previously tested on purified PE (Six et al., 2007a, 2007b, 2007c). In all marine Synechococcus genomes, CpeB (∼19.1 kDa) is systematically heavier than MpeB (∼18.5 kDA). Therefore, the immunoblot profiles often showed two bands whose intensity reflected the quantity of CpeB or MpeB proteins relative to total proteins (Figure 4a). The intensity of the bands cannot be compared across strains or between subunits because the affinity of the antibody differs depending on amino-acid sequences.
Figure 4.
Relative variations of the β-subunit of PEs I and II (a) and of the D1 protein (b) estimated by immunoreactions in tropical, mid-latitude and high-latitude marine Synechococcus strains during 5 days of cold stress.
In the tropical strains Synechococcus A15-37 and M16.1, cold stress primarily induced MpeB degradation, as indicated by the disappearance of the lower band. CpeB variations were more variable but there was no such strong degradation. In Synechococcus spp. WH7803 and ROS8604, the two PE bands were less well separated but a general decreasing trend was clearly observed. In the northern strains, no PE degradation was observed.
We also investigated the relative quantity variations of the D1 protein (∼32 kDa), encoded by the psbA gene family, during the cold period. The cellular pool of this reaction centre II protein decreased rapidly in tropical strains, the signal being hardly detectable after day 3. The D1 band was progressively reduced in mid-latitudes strains Synechococcus WH7803 and ROS8604 until quasi disappearance at day 5. In the high-latitude strains, MVIR-16-2 and MVIR-18-1, the D1 relative quantity remains fairly constant along the stress experiment.
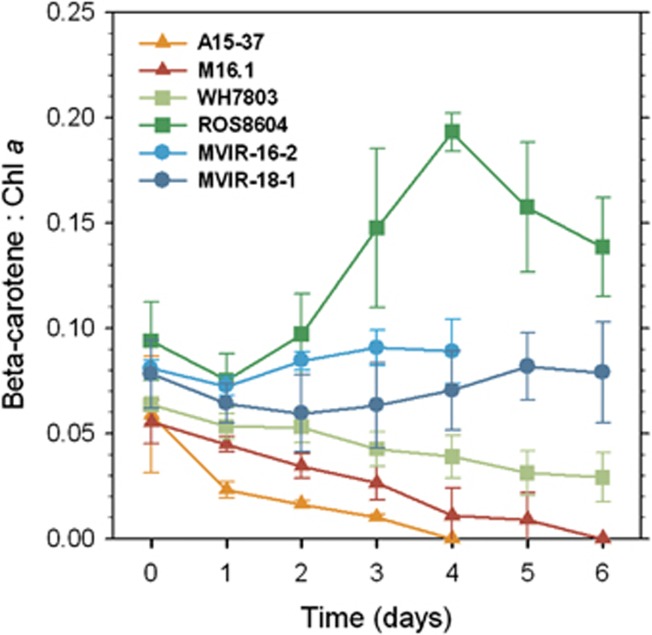
Time course variations of pigment cell content upon cold stress
High-performance liquid chromatography pigment analyses revealed the occurrence of three major pigments identified as chl a, β-carotene and zeaxanthin, as classically observed in marine Synechococcus (see for example, Kana et al., 1988; Six et al., 2004), along with minor xanthophylls such as β-cryptoxanthin. The low-latitude strains showed a pronounced decrease of the β-carotene to chl a ratio, which reached zero at about the fifth day of stress (Figure 5). The mid-latitude strains exhibited different pigment responses to thermal stress. Synechococcus sp. WH7803 underwent a decrease of about 50% of its initial β-carotene to chl a ratio, whereas low temperature induced a notable increase of this ratio in Synechococcus sp. ROS8604 by a factor two at the fourth day. The large increase of this ratio while the chl a fluorescence per cell (reflecting chl a cell content) remains unchanged indicates a synthesis of β-carotene. In contrast, the two northern strains did not show any clear trends in the variations of the β-carotene to chl a ratio, which remained fairly constant.
Figure 5.
Variations of the β-carotene to chl a ratio (g/g) in the tropical (triangles), mid-latitude (squares) and high-latitude (circles) marine Synechococcus strains during 6 days of cold stress (n⩾4).
We also measured the variations of the zeaxanthin to chl a ratio (data not shown). This ratio moderately increased in northern strains due to a decrease in chl a cell content (see whole-cell chl a fluorescence; Supplementary Figure S2A), whereas no obvious variation was observed in the three lower latitude strains, indicating a similar degradation rate of the two pigments.
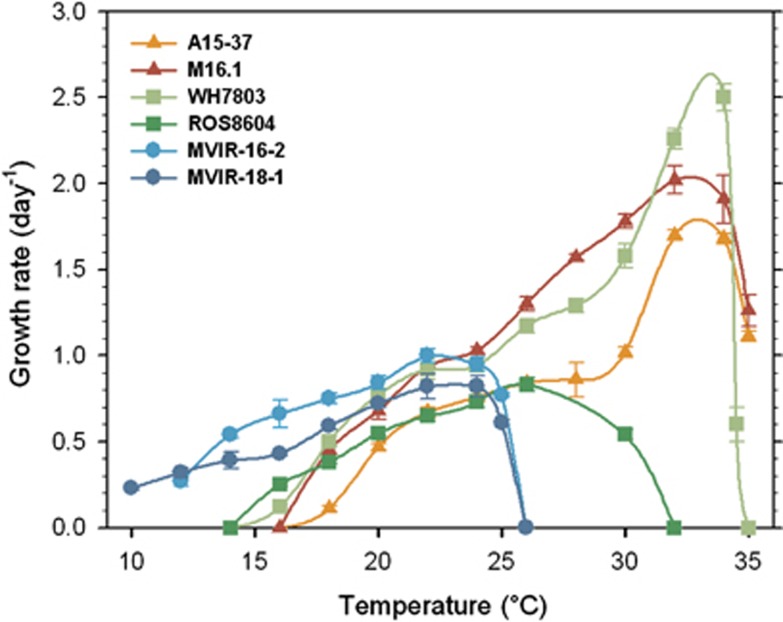
Growth rates vs temperature
In order to define their temperature optima for growth and compare their thermal growth range in our culture conditions, strains were acclimated to a wide range of temperatures (Figure 6). The strains showed quite different acclimation capabilities to temperature. We were able to acclimate Synechococcus spp. A15-37 and M16.1, both isolated at latitude lower than 30 °N, at temperatures ranging from 18 °C to 35 °C, with a maximum growth rate of about 32 °C. These strains exhibited different maximal growth rates, with Synechococcus sp. A15-37 growing more slowly than M16.1 (1.70±0.03 day−1 and 2.02±0.08 day−1 at 32 °C, respectively). Despite a similar pattern to M16.1 with a high maximal growth rate value of 2.50±0.08 day−1 at 34 °C, Synechococcus sp. WH7803 could, however, grow at lower temperatures than the two tropical strains. For the three above-mentioned strains, growth rate sharply decreased at 35 °C. We managed to acclimate the strain ROS8604 within a thermal range from 18 °C to 30 °C, with an optimal temperature at 26 °C, corresponding to a growth rate value of 0.83±0.02 day−1. At last, strains MVIR-16-2 and MVIR-18-1, isolated near the polar circle, showed thermal niches shifted toward lower temperatures, MVIR-18-1 being able to cope with temperature down to 10 °C (and probably less) and 12 °C for MVIR-16-2, but none of these two strains were able to acclimate at temperature higher than 25 °C. Furthermore, similar optimal temperatures were observed for the two northern strains, with Synechococcus sp. MVIR-16-2 and MVIR-18-1 growing optimally at about 22 °C (1.00±0.04 day−1 and 0.82±0.07 day−1, respectively).
Figure 6.
Growth rate as a function of temperature in the tropical (triangles), mid-latitude (squares) and high-latitude (circles) marine Synechococcus strains. Error bars are s.d. from the mean based on four replicates (n⩾4).
Phyloecology of marine Synechococcus isolates
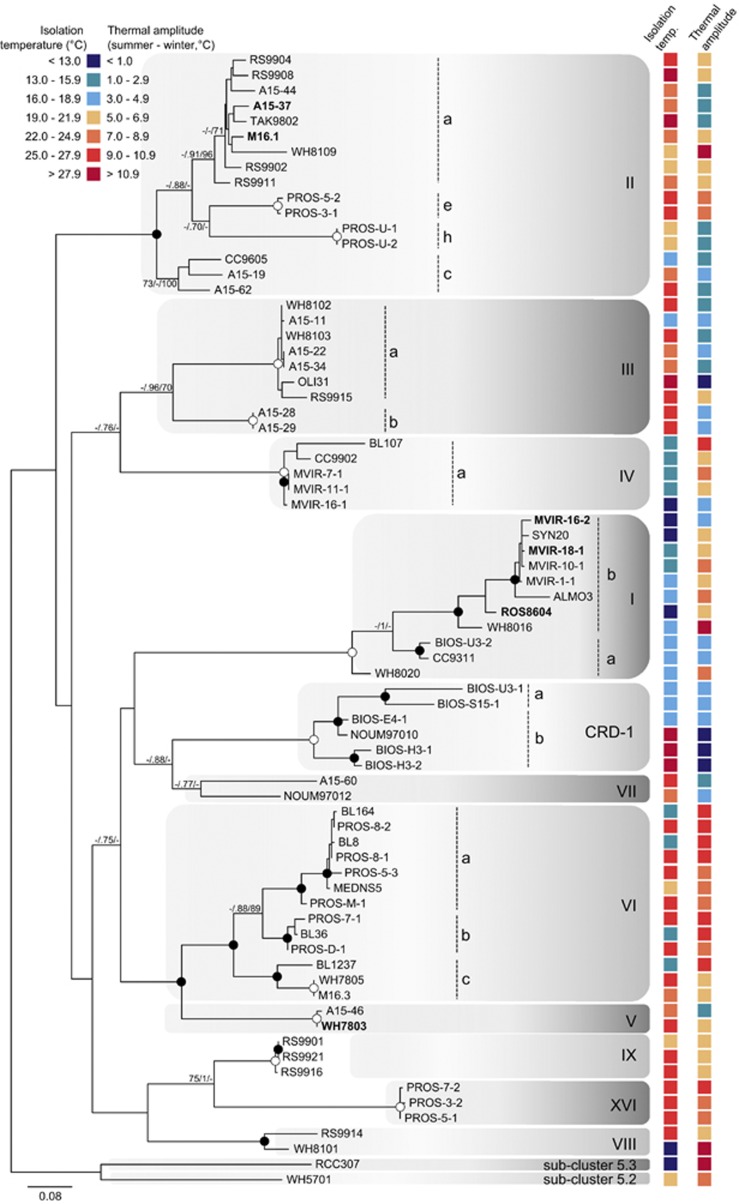
In order to unveil potential relationships between Synechococcus phylogeny and the temperature of strain isolation site, a phylogenetic analysis was conducted on the petB sequence of 74 cultured strains isolated from a wide range of latitudes, across different oceans and seas (Figure 7, Supplementary Table S2). This marker was chosen because it provides a better phylogenetic resolution of Synechococcus strains (at the subclade level) than more typical markers, such as 16S ribosomal RNA or Internal Transcribed Spacer (Mazard et al., 2012a). Our data set includes a number of strains from the Roscoff Culture Collection, whose phylogenetic position was so far unknown, in particular strains isolated during the MICROVIR cruise (July 2007, North Sea; Supplementary Table S2). The data set mainly included representatives of subcluster 5.1 and the phylogenetic tree was rooted with one representative of each of the two other subclusters (5.2 and 5.3; sensu Dufresne et al., 2008). As in a previous study using the petB gene (Mazard et al., 2012a), members of subcluster 5.1 were divided into 11 clades and a number of these clades were further subdivided into 14 subclades (Figure 7). It is worth noting that we found a slight difference with regard to Mazard et al. study concerning subclade Ib, which does not constitute a monophyletic group in our analysis. This disparity is probably imputable to the differences in the phylogenetic methods used between the two studies. This tree allowed us to assign the tropical Synechococcus spp. A15-37 and M16.1 to subclade IIa, Synechococcus spp. ROS8604, MVIR-16-2 and MVIR-18-1 to subclade Ia, whereas Synechococcus sp. WH7803 is one of the rare representatives of clade V available in culture.
Figure 7.
Maximum likelihood (ML) analysis of the petB gene (based on 559 aligned amino acids) retrieved from 74 marine cultured isolates of Synechococcus. For each strain, sea surface temperatures at the isolation site are indicated according to the colour scale. Numbers at nodes correspond to bootstrap values from ML, posterior probability of Bayesian inference (BI; ranging between 0 and 1) and bootstraps for neighbour-joining (NJ) method. Bootstraps, represented as a percentage, were obtained through 1000 repetitions for each method, and only values higher than 70% are shown on the phylogenetic tree. Filled circles correspond to nodes supported by values higher than 70/0.8/80 for ML/BI/NJ methods, respectively. Empty circles correspond to nodes fully supported by the three methods. The six Synechococcus strains used in this study are in bold. Synechococcus sp. WH5701, affiliated to subcluster 5.2, was used as an outgroup. The nomenclature described by Mazard et al. (2012a) was retained for the clade numbers.
In parallel to the phylogenetic analyses, the average seawater temperatures at the isolation sites were retrieved for the 74 strains. Comparison between the phylogeny and the average isolation temperatures underlined that clades I and IV strains were isolated in significantly colder waters (15.03±2.53 °C and 15.05±2.13 °C, respectively) than the isolates from clades II and III (23.93±3.03 °C and 24.86±2.84 °C, respectively), as shown by one-way analysis of variance (F=36.26, P-value<0.01).
Discussion
In this study, we compare the physiology of six marine Synechococcus strains isolated at different latitudes (from 23°N to 61°N; Table 1; Figure 1) in near coastal areas (that is, mesotrophic waters) of the North Atlantic Ocean. All of them belong to pigment type 3a (Six et al., 2007c; Humily et al., in press), but in spite of this similar PBS composition, the six strains exhibited large differences in pigment cell content (chl a, carotenoids and PE; Table 2), with the mid-latitude strains being much more pigmented than their northern and tropical counterparts. These sharp differences are probably associated with different thylakoidal surfaces per cell and/or different cell biovolumes among strains. Moreover, although fluorescence excitation spectra were quite comparable, fluorescence emission spectra showed differences in wavelength maxima, maxima ratios and apparent width of the PE emission band (Supplementary Figure S1), revealing the occurrence of distinct levels of PBP coupling. These six strains, which display very similar fluorescence phycourobilin to phycoerythrobilin ratio (ca 0.4), could thus be differentiated by other photosynthetic features (Table 2). These observations highlight the functional microdiversity that exists within the marine Synechococcus pigment types defined in previous studies (Six et al., 2007c; Humily et al., in press).
Marine Synechococcus show differential resistance to cold stress
Cold stress experiments revealed large differences in thermal flexibility among the six marine Synechococcus strains used in this study. Tropical strains were unable to stand the temperature drop challenge and temperate strains maintained a nearly constant cell density (Figure 2a). By contrast, the northern strains kept dividing, at similar growth rates than cells fully acclimated to 13 °C (Figure 2a). In these photosynthetic organisms, being able to sustain efficient light absorption capacities and utilisation is crucial for growth. In order to better understand the differences among the strains, we first followed the response of their photosynthetic antenna, the PBS, during the cold period (Figures 2b and 4a). In contrast to the northern strains, for which PBS fluorescence spectra and PE cell content remained fairly constant, the strong increase in PE:PC fluorescence ratio observed in Synechococcus spp. A15-37, M16.1, WH7803 and ROS8604 indicates an impairment of the phycobiliprotein coupling, which likely led to the physical disconnection of PE molecules from the rest of the PBS structure (Six et al., 2007b). PBS impairment was especially severe for Synechococcus sp. M16.1, for which fast specific degradation of the distal PBS hexamers (PEII) was evidenced by MpeB immunoblotting (Figure 4a). Our results are comparable to previous studies led in other cyanobacteria that pointed out serious PBS perturbations in response to ultraviolet (Lao and Glazer, 1996; Rajagopal and Murthy, 1996; Rinalducci et al., 2006; Six et al., 2007b), HL (Tamary et al., 2012) and temperature stress (Li et al., 2001; Stoitchkova et al., 2007), and thus suggests that PBS uncoupling and dismantling is a usual consequence of environmental stress in cyanobacteria. It is, however, worth noting that the highly fluorescent-free PE may also constitute a way of energy dissipation through fluorescence during a stress period. In any case, the cold-induced destruction of the antenna considerably decreased cell absorption capacities and must have had a strong impact on the growth capacities of the temperate and tropical strains studied here.
Cyanobacteria balance excitation energy notably through PBS state transitions, which regulate the energy transfer from PBS to PSI and PSII in response to environmental factors or metabolism demands (Joshua and Mullineaux, 2004; Mullineaux and Emlyn-Jones, 2004). The drastic decrease observed in state transitions amplitude during cold shock for the tropical and mid-latitude strains generally arose before the PBS uncoupling in these strains, indicating that the decline in state transition during the first few days was not directly related to PBS dismantling (Figures 2b and 3a). These early perturbations of the state transition process might originate in the cold-induced changes in the fluidity of the thylakoidal membranes, as low temperature is well known to induce membrane stiffening (Wada and Murata, 1990; Murata and Los, 1997; Mikami and Murata, 2003). Later during the cold period, PBS dismantling likely contributed to the complete inhibition of state transitions. The high-latitude Synechococcus strains. MVIR-16-2 and MVIR-18-1 showed much better capacities to regulate state transitions, possibly through active regulation of thylakoid fluidity (Murata and Wada, 1995; Murata and Los, 1997).
The PSII quantum yield (FV/FM) is a good proxy for the efficiency with which the reaction centre II converts the energy funnelled by the PBS into electron flux. The most likely explanation for the major non-reversible decrease of the PSII quantum yield during cold stress in tropical and temperate strains is the photoinactivation of the reaction centre II (for review, see for example, Campbell and Tyystjärvi, 2012). This phenomenon is unavoidably induced by light in any oxygenic photosynthetic organism but is further accelerated by environmental stresses that disturb the balance between light absorption and utilisation, such as temperature variations (Takahashi and Murata, 2008). Cold stress is indeed thought to decrease the repair rate of the D1 protein, the key protein of the PSII reaction centre, thus inducing the concomitant drop in D1 cell content and PSII quantum yield reported in the sensitive strains (Figures 3b and 4b). The observation that high-latitude strains did not suffer from PSII inactivation during the cold period raise questions about the physiological processes that allow such tolerance, and calls for further research.
Cold stress, protection and acclimation
To counteract the photoinhibition induced by reduced metabolic rates at low temperature (Burns et al., 2005), reversible physiological adjustments are induced by photosynthetic organisms, referred as photoacclimation. Acclimation of the photosynthetic apparatus to low temperature usually shows strong similarities to HL acclimation processes (Oquist, 1983; Maxwell et al., 1994; Huner et al., 1998), as it is also based on an imbalance between light energy absorption and its downstream utilisation. We indeed observed in the northern strains hints of HL-like acclimation (see for example, Six et al., 2004, 2005), such as a reduction in pigment fluorescence per cell (Supplementary Figure S2) and chl a (data not shown), likely associated with the reduction of the thylakoïdal surface (Kana and Glibert, 1987).
The PSII quantum yield of the northern strains decreased until a level close to that measured in cultures fully acclimated to 13 °C (data not shown), whereas the D1 cell content kept stable (Figure 4b). This suggests the induction of non-photochemical quenching associated with light energy dissipation, characterised by a reduction of the amount of energy processed by the reaction centre (for review, see Campbell et al., 1998; Kirilovsky and Kerfeld, 2012, 2013). The results thus suggest that, in contrast to the tropical and mid-latitude strains, the northern ones managed to adjust their light utilisation capacities to the cold-induced slowing down of their metabolism, an ability which likely limits the formation of reactive oxygen species (Asada, 1994; Latifi et al., 2009). In cyanobacteria, the best known non-photochemical quenching mechanism associated with light energy dissipation involves the so-called orange carotenoid protein, encoded by the ocp gene, which seemingly acts as a photoreceptor and intercepts light energy at the PBS core, before it reaches reaction centre II (Wilson et al., 2006; Kirilovsky and Kerfeld, 2012). The effects of cold stress on the activity of this protein, whose gene is present in most marine Synechococcus strains (Scanlan et al., 2009), remain to be studied.
A number of β-carotene molecules bound to the reaction centre II act as quenchers of singlet oxygen and under light, are regenerated through a repair cycle (Telfer 2005; Ishikita et al., 2007; Mella-Flores et al., 2012). This carotenoid is essential for the protection of reaction centres (Cazzaniga et al., 2012) and thus may be used as an indicator of oxidative stress intensity. The β-carotene measurements revealed that tropical strains suffered from strong oxidative stress during the cold period (Figure 5). The sharp degradation of β-carotene, probably due to an impairment of the turnover of this antioxidant, observed for Synechococcus spp. A15-37, M16.1 and to a lesser extent WH7803, implies that the PSII of these strains were subjected to direct oxidative damages (Blot et al., 2011). Furthermore, oxidative stress is also thought to prevent the repair of the PSII key protein, D1 (Nishiyama et al., 2006), thus greatly enhancing PSII inactivation. Interestingly, Synechococcus sp. ROS8604 responded differently, as it transiently increased its β-carotene cell content until the fourth day of stress, probably by boosting the β-carotene cycle. This rapid synthesis likely allowed a better protection against oxidative stress and may partially explain the moderate growth of this strain at the beginning of the cold period (Figure 2a). However, cultures of Synechococcus sp. ROS8604 eventually did not manage to cope with oxidative stress, as seen by the rising of PBS uncoupling (Figure 2b) and the cell density decrease (Figure 2a), starting from the fourth day of stress. Variability in carotenoid response to temperature has also been observed among cyanobacterial strains isolated from high-latitude freshwaters (Tang et al., 1997).
Differences in cold resistance capacities were also noticeable when considering the recovery capacities (Supplementary Table S1). Synechococcus sp. M16.1 needed much longer time to recover and the cold period turned out to be lethal for Synechococcus sp. A15-37. The WH7803 and ROS8604 strains were not able to maintain growth and photosynthetic activity during the cold period, but they both recovered quite quickly when replaced to initial temperature, indicating significant thermal flexibility. These strains thus appear quite well adapted to temperate environments, in which temperature fluctuates depending on the seasons.
Marine Synechococcus strains have different thermal preferenda
The different physiological responses of the six marine Synechococcus strains to cold stress are associated with distinct long-term acclimation capacities to different temperatures. Our study thus demonstrates for the first time that marine Synechococcus strains exhibit different thermal preferenda and, therefore, strongly suggests the existence of strains adapted to different local temperature ranges. These preferences appear to be related to the latitude of isolation of each strain, which is directly linked to the latitudinal gradient of seawater temperature. The temperature vs growth curve of the WH8103 strain, isolated in the Sargasso Sea at 28.5°N, exhibits a maximum at 28 °C (Moore et al., 1995) and thus supports this conclusion. Our observations support recent surveys which stated that optimum temperature for growth of marine phytoplankton strains is related to the latitude and temperature of their isolation site (Thomas et al., 2012; Boyd et al., 2013), and support field observations that the abundances of the Synechococcus of the 5.1 cluster fall to very low values in the polar oceans, of about hundred cells per ml (Gradinger and Lenz, 1995; Vincent 2000; Not et al., 2004; Cottrell and Kirchman, 2009; Vincent and Quesada, 2012).
The two northern Synechococcus strains we studied cannot be considered as true psychrophilic organisms but rather as psychrotolerant ones (Tang et al., 1997). Indeed, although strains such as Synechococcus sp. MVIR-18-1 (one of the culture isolates which, to our knowledge, shows the northernmost isolation latitude) can probably survive at temperature below 10 °C, true psychrophilic phytoplankton exhibit optimal temperature lower than 15 °C and can generally not grow over 20 °C (Vincent, 2000; Lovejoy et al., 2007). Although we cannot exclude the existence of true psychrophilic strains within the Synechococcus 5.1 group, as it might be the case for natural Synechococcus subcluster 5.2 populations (Cottrell and Kirchman, 2009; Huang et al., 2011), it appears that northern 5.1 strains might have only moderately decreased their optimal temperature during the evolution and acquired the capacity to stand low temperatures.
Our results suggest that the capacity to grow at low temperature may only be possible to the detriment of the ability to develop high growth rates, as observed for the low-latitude strains A15-37, M16.1 and WH7803 (Figure 6). This apparent trade-off between growth performances and the capacity to grow over a given temperature range, which has also been observed for Prochlorococcus picocyanobacteria (Johnson et al., 2006), might constitute a general adaptative trait of marine picocyanobacteria to different thermal niches. In addition, it is worth noting that Synechococcus strains display shapes of the growth vs temperature curves similar to Prochlorococcus ecotypes (Moore et al., 1995; Johnson et al., 2006). The typical shoulder in the first part of the curve (Figure 6) may reflect the triggering of a thermophysiological mechanism that allows efficient acclimation to higher temperature, a mechanism that was possibly lost by the northern strains.
Marine Synechococcus display temperature specialised clades
The combination of phylogenetic analyses of a large number of Synechococcus culture isolates (Figure 7; Supplementary Table S2) with their isolation temperatures provides an interesting complement to the field studies of Zwirglmaier et al. (2007, 2008). This approach allowed us to highlight differences in the thermal niches of some of the Synechococcus clades. Laboratory strains belonging to clades I and IV were isolated in colder waters than those from clades II and III (Figure 7). This result is coherent with the latitudinal distribution of the Synechococcus clades, as field studies reported that clades I and IV are generally confined to latitudes higher than 30° N/S. The two other dominant clades II and III seem to prevail in the waters of the warm temperate and tropical/sub-tropical regions of the ocean (Zwirglmaier et al., 2007, 2008; Huang et al., 2011). It should be noted that strains CC9605 (clade III) and A15-11 (clade II), for which temperature isolation was below 19 °C, were collected in upwelling areas (California current and Mauritania coast, respectively) where water temperature is usually colder and more variable. Strains from clade VI seem to be easily brought to culture, as shown by the relatively high number of isolates available in culture collections (data not shown), but are not very abundant in the field (Huang et al., 2011). The wide spectrum of temperature at which these strains have been isolated (Figure 7) suggests that clade VI might gather strains with high physiological flexibility regarding temperature.
The temperature adaptation capacities of the six strains considered in this study are in line with their phylogenetic position. The tropical strains A15-37 and M16.1, which showed high optimal temperature for growth and could not grow below ca 18 °C, belong to clade II. Similar observations were made for the clade III strain Synechococcus sp. WH8103 (Moore et al., 1995). Among the strains we studied, Synechococcus spp. ROS8604, MVIR-16-2 and MVIR-18-1 belong to clade I and appear to be the best adapted to cold waters. It is, however, worth noting that the three strains show differential responses to cold stress (Figures 2, 3, 4, 5) and different temperature preferenda (Figure 6), with a good correspondence to their isolation latitude. Thus, clade I Synechococcus strains, living at the northernmost limit of distribution (near the polar circle), show better capacities to cope with low temperature than those isolated from cold temperate waters. This highlights the existence of significant functional microdiversity regarding adaptative capacities to temperature within marine Synechococcus clade I.
Conclusion
This study demonstrates for the first time that marine Synechococcus strains exhibit considerably different physiological capacities to cope with temperature variations and display different thermal preferenda. These abilities might rely on the capacities to maintain the photosynthetic apparatus in a state that limits the induction of oxidative stress. Furthermore, our study suggests a correlation between the ability to cope with temperature variations and specific clades of the marine Synechococcus radiation. Our results suggest that clade I and probably clade IV Synechococcus have a physiology preferentially adapted to cold thermal niches. These observations, which are quite in line with phylogeographic field studies (Zwirglmaier et al., 2007, 2008), suggest the existence of low temperature ecotypes, that is, cold thermotypes, among marine Synechococcus and underline an important role of temperature in the diversification of marine Synechococcus at least at high latitudes. The deep understanding of the differentiation of cold Synechococcus thermotypes at high latitudes will, however, not be easy, given the functional microdiversity occurring within Synechococcus clade I, a diversity which likely reflects adaptation the continuous latitudinal gradient of temperature. Although clade II (and III; Moore et al., 1995) cells seem more adapted to warm waters, more work is necessary to evaluate the importance of temperature relative to other environmental factors in the Synechococus diversification at lower latitudes.
The absence of comparative data on the thermophysiology of marine Synechococcus has so far made it impossible to build predictive models of picocyanobacteria distribution taking in account the thermal flexibility of these organisms (Flombaum et al., 2013). As our results suggest that the composition of the Synechococcus natural communities may experience major changes in the context of possible climatic changes due to the occurrence of temperature ecotypes, future modelling efforts should incorporate growth rate data in order to better predict the global response of marine Synechococcus to environmental changes.
Acknowledgments
This work was supported by the French program ANR PELICAN (PCS-09-GENM-200), the EMBRC France (INFRA-2010-2.2.5) and the European Union programs MicroB3 and MaCuMBA (grant agreements 287589 and 311975, respectively). We certify that there is no conflict of interest with any financial organisation regarding the material discussed in the manuscript. Justine Pittera was supported by the French Ministry of Higher Education and Research. We warmly thank Frédéric Partensky for improving the manuscript as well as Morgane Ratin for her help with the cultures and the gene sequencing. We are also grateful to the Roscoff Culture Collection for maintaining the Synechococcus strains used in this study and in particular to Florence Legall who isolated some of them.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Ahlgren NA, Rocap G. Diversity and distribution of marine Synechococcus: multiple gene phylogenies for consensus classification and development of qPCR assays for sensitive measurement of clades in the ocean. Front Microbiol. 2012;3:213–213. doi: 10.3389/fmicb.2012.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K.1994Production and action of active oxygen species in photosynthetic tissuesIn: Foyer CH, Mullineaux PM, (eds)Causes of Photooxidative Stress and Amelioration of Defence Systems in Plants 77–104.
- Blot N, Mella-Flores D, Six C, Le Corguille G, Boutte C, Peyrat A, et al. Light history influences the response of the marine cyanobacterium Synechococcus sp. WH7803 to oxidative stress. Plant Physiol. 2011;156:1934–1954. doi: 10.1104/pp.111.174714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd PW, Rynearson TA, Armstrong EA, Fu F, Hayashi K, Hu Z, et al. Marine phytoplankton temperature versus growth responses from polar to tropical waters – Outcome of a scientific community-wide study. PLoS One. 2013;8:e63091. doi: 10.1371/journal.pone.0063091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitenhuis ET, Li WKW, Vaulot D, Lomas MW, Landry M, Partensky F, et al. Picophytoplankton biomass distribution in the global ocean. Earth System Science Data. 2012;4:37–46. [Google Scholar]
- Burns RA, MacDonald CD, McGinn PJ, Campbell DA. Inorganic carbon repletion disrupts photosynthetic acclimation to low temperature in the cyanobacterium Synechococcus elongatus. J Phycol. 2005;41:322–334. [Google Scholar]
- Campbell D, Hurry V, Clarke AK, Gustafsson P, Oquist G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev. 1998;62:667–683. doi: 10.1128/mmbr.62.3.667-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DA, Tyystjärvi E. Parameterization of photosystem II photoinactivation and repair. Biochim Biophys Acta Bioenerg. 2012;1817:258–265. doi: 10.1016/j.bbabio.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Capella-Gutiérrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzaniga S, Li Z, Niyogi KK, Bassi R, Dall'Osto L. The Arabidopsis szl1 mutant reveals a critical role of beta-carotene in photosystem I photoprotection. Plant Physiol. 2012;159:1745–1758. doi: 10.1104/pp.112.201137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell MT, Kirchman DL. Photoheterotrophic microbes in the Arctic ocean in summer and winter. Appl Environ Microbiol. 2009;75:4958–4966. doi: 10.1128/AEM.00117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne A, Ostrowski M, Scanlan DJ, Garczarek L, Mazard S, Palenik BP, et al. Unraveling the genomic mosaic of a ubiquitous genus of marine cyanobacteria. Genome Biol. 2008;9:R90. doi: 10.1186/gb-2008-9-5-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP - Phylogeny Inference Package (Version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- Flombaum P, Gallegos JL, Gordillo RA, Rincón J, Zabala LL, Jiao N, et al. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proc Natl Acad Sci USA. 2013;110:9824–9829. doi: 10.1073/pnas.1307701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu F-X, Warner ME, Zhang Y, Feng Y, Hutchins DA. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus. J Phycol. 2007;43:485–496. [Google Scholar]
- Fuller NJ, Marie D, Partensky F, Vaulot D, Post AF, Scanlan DJ. Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl Environ Microbiol. 2003;69:2430–2443. doi: 10.1128/AEM.69.5.2430-2443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller NJ, West NJ, Marie D, Yallop M, Rivlin T, Post AF, et al. Dynamics of community structure and phosphate status of picocyanobacterial populations in the Gulf of Aqaba, Red Sea. Limnol Oceanogr. 2005;50:363–375. [Google Scholar]
- Gradinger R, Lenz J. Seasonal occurrence of picocyanobacteria in the Greenland Sea and central Arctic Ocean. Polar Biol. 1995;15:447–452. [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Herdman M, Castenholz RW, Waterbury JB, Rippka R.2001Form-genus XIII. SynechococcusIn: Boone DR, Castenholz RW, (eds)Bergey's Manual of Systematic Bacteriology2nd edn.Springer-Verlag: New York; 508–512. [Google Scholar]
- Hess WR, Steglich C, Lichtlé C, Partensky F. Phycoerythrins of the oxyphotobacterium Prochlorococcus marinus are associated to the thylakoid membrane and are encoded by a single large gene cluster. Plant Mol Biol. 1999;40:507–521. doi: 10.1023/a:1006252013008. [DOI] [PubMed] [Google Scholar]
- Huang S, Wilhelm SW, Harvey HR, Taylor K, Jiao N, Chen F. Novel lineages of Prochlorococcus and Synechococcus in the global oceans. ISME J. 2011;6:285–297. doi: 10.1038/ismej.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Humily F, Partensky F, Six C, Farrant G, Ratin M, Marie D, et al. (submitted). A gene island with dual evolutionary origin is involved in chromatic acclimation in marine Synechococcus PLoS One(in press). [DOI] [PMC free article] [PubMed]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- Ishikita H, Loll B, Biesiadka J, Kern J, Irrgang K-D, Zouni A, et al. Function of two beta-carotenes near the D-1 and D-2 proteins in photosystem II dimers. Biochim Biophys Acta Bioenerg. 2007;1767:79–87. doi: 10.1016/j.bbabio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Johnson ZI, Zinser ER, Coe A, McNulty NP, Woodward EM, Chisholm SW. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science. 2006;311:1737–1740. doi: 10.1126/science.1118052. [DOI] [PubMed] [Google Scholar]
- Joshua S, Mullineaux CW. Phycobilisome diffusion is required for light-state transitions in cyanobacterial. Plant Physiol. 2004;135:2112–2119. doi: 10.1104/pp.104.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana TM, Glibert PM. Effect of irradiances up to 2000 μE m−2 s−1 on marine Synechococcus WH7803—I. Growth, pigmentation, and cell composition. Deep Sea Res A Oceanograph Res Papers. 1987;34:479–495. [Google Scholar]
- Kana TM, Glibert PM, Goericke R, Welschmeyer NA. Zeaxanthin and beta-carotene in Synechococcus WH7803 respond differently to irradiance. Limnol Oceanogr. 1988;33:1623–1627. [Google Scholar]
- Katoh K, Toh H. Improved accuracy of multiple ncRNA alignment by incorporating structural information into a MAFFT-based framework. BMC Bioinfo. 2008;9:212. doi: 10.1186/1471-2105-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilovsky D, Kerfeld CA. The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim Biophys Acta Bioenerg. 2012;1817:158–166. doi: 10.1016/j.bbabio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Kirilovsky D, Kerfeld CA. The orange carotenoid protein: a blue-green light photoactive protein. Photochem Photobiol Sci. 2013;12:1135–1143. doi: 10.1039/c3pp25406b. [DOI] [PubMed] [Google Scholar]
- Lantoine F, Neveux J. Spatial and seasonal variations in abundance and spectral characteristics of phycoerythrins in the tropical northeastern Atlantic Ocean. Deep Sea Res, Part I. 1997;44:223–246. [Google Scholar]
- Lao K, Glazer AN. Ultraviolet-B photodestruction of a light-harvesting complex. Proc Natl Acad Sci USA. 1996;93:5258–5263. doi: 10.1073/pnas.93.11.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifi A, Ruiz M, Zhang C-C. Oxidative stress in cyanobacteria. FEMS Microbiol Rev. 2009;33:258–278. doi: 10.1111/j.1574-6976.2008.00134.x. [DOI] [PubMed] [Google Scholar]
- Li WKW. Primary production of prochlorophytes, cyanobacteria, and eucaryotic ultraphytoplankton: measurements from flow cytometric sorting. Limnol Oceanogr. 1994;39:169–175. [Google Scholar]
- Li Y, Zhang JP, Xie J, Zhao JQ, Jiang LJ. Temperature-induced decoupling of phycobilisomes from reaction centers. Biochim Biophys Acta Bioenerg. 2001;1504:229–234. doi: 10.1016/s0005-2728(00)00250-4. [DOI] [PubMed] [Google Scholar]
- Liu H, Nolla H, Campbell L. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat Microb Ecol. 1997;12:39–47. [Google Scholar]
- Liu S, Juneau P, Qiu B. Effects of iron on the growth and minimal fluorescence yield of three marine Synechococcus strains (Cyanophyceae) Phycol Res. 2012;60:61–69. [Google Scholar]
- Lovejoy C, Vincent WF, Bonilla S, Roy S, Martineau M-J, Terrado R, et al. Distribution, phylogeny, and growth of cold-adapted Picoprasinophytes in Artic sea. J Phycol. 2007;43:78–89. [Google Scholar]
- Marie D, Brussaard C, Partensky F, Vaulot D.1999Flow cytometric analysis of phytoplankton, bacteria and virusesIn: Sons JW, (ed)Current Protocols in Cytometry. International Society for Analytical Cytology John Wiley & Sons Inc.: New York, USA; 11.11.11–11.11.15. [Google Scholar]
- Maxwell DP, Falk S, Trick CG, Huner NPA. Growth at low-temperature mimics high light acclimation in Chlorella-vulgaris. Plant Physiol. 1994;105:535–543. doi: 10.1104/pp.105.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazard S, Ostrowski M, Partensky F, Scanlan DJ. Multi-locus sequence analysis, taxonomic resolution and biogeography of marine Synechococcus. Environ Microbiol. 2012;14:372–386. doi: 10.1111/j.1462-2920.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- Mazard S, Wilson WH, Scanlan DJ. Dissecting the physiological response to phosphorus stress in marine Synechococcus isolates. J Phycol. 2012;48:94–105. doi: 10.1111/j.1529-8817.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- Mella-Flores D, Mazard S, Humily F, Partensky F, Mahe F, Bariat L, et al. Is the distribution of Prochlorococcus and Synechococcus ecotypes affected by global warming. Biogeosciences. 2011;8:2785–2804. [Google Scholar]
- Mella-Flores D, Six C, Ratin M, Partensky F, Boutte C, Le Corguillé G, et al. Prochlorococcus and Synechococcus have evolved different adaptive mechanisms to cope with light and UV stress. Front Microbiol. 2012;3:285. doi: 10.3389/fmicb.2012.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami K, Murata N. Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog Lipid Res. 2003;42:527–543. doi: 10.1016/s0163-7827(03)00036-5. [DOI] [PubMed] [Google Scholar]
- Moore LR, Goericke R, Chisholm SW. Comparative physiology of Synechococcus and Prochlorococcus: influence of light and temperature on growth, pigments, fluorescence and absorptive properties. Mar Ecol Progr Ser. 1995;116:259–275. [Google Scholar]
- Moore LR, Rocap G, Chisholm SW. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- Mullineaux CW, Emlyn-Jones D. State transitions: an example of acclimation to low-light stress. J Exp Bot. 2004;56:389–393. doi: 10.1093/jxb/eri064. [DOI] [PubMed] [Google Scholar]
- Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N, Los DA. Membrane fluidity and temperature perception. Plant Physiol. 1997;115:875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Not F, Latasa M, Marie D, Cariou T, Vaulot D, Simon N. A single species, Micromonas pusilla (Prasinophyceae), dominates the eukaryotic picoplankton in the western English channel. Appl Environ Microbiol. 2004;70:4064–4072. doi: 10.1128/AEM.70.7.4064-4072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Not F, Massana R, Latasa M, Marie D, Colson C, Eikrem W, et al. Late summer community composition and abundance of photosynthetic picoeukaryotes in Norwegian and Barents Seas. Limnol Oceanogr. 2005;50:1677–1686. [Google Scholar]
- Olson RJ, Chisholm SW, Zettler ER, Armbrust EV. Pigments, size, and distribution of Synechococcus in the North Atlantic and Pacific Oceans. Limnol Oceanogr. 1990;35:45–58. [Google Scholar]
- Oquist G. Effetcs of low-temperature on photosynthesis. Plant Cell Environ. 1983;6:281–300. [Google Scholar]
- Palenik B. Chromatic adaptation in marine Synechococcus strains. Appl Environ Microbiol. 2001;67:991–994. doi: 10.1128/AEM.67.2.991-994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenik B, Ren Q, Dupont CL, Myers GS, Heidelberg JF, Badger JH, et al. Genome sequence of Synechococcus CC9311: insights into adaptation to a coastal environment. Proc Natl Acad Sci USA. 2006;103:13555–13559. doi: 10.1073/pnas.0602963103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partensky F, Blanchot J, Vaulot D. Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: a review. Marine Cyanobacteria Bulletin de l'Institut Oceanographique: Monaco. 1999. pp. 457–475.
- Partensky F, Garczarek L. Prochlorococcus: advantages and limits of minimalism. Annu Rev Mar Sci. 2010;2:305–331. doi: 10.1146/annurev-marine-120308-081034. [DOI] [PubMed] [Google Scholar]
- Post AF, Penno S, Zandbank K, Paytan A, Huse SM, Welch DM. Long term seasonal dynamics of Synechococcus population structure in the gulf of Aqaba, northern Red Sea. Front Microbiol. 2011;2:131–131. doi: 10.3389/fmicb.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Murthy SDS. Short term effect of ultraviolet-B radiation on photosystem 2 photochemistry in the cyanobacterium Synechococcus 6301. Biol Plant. 1996;38:129–132. [Google Scholar]
- Rinalducci S, Hideg É, Vass I, Zolla L. Effect of moderate UV-B irradiation on Synechocystis PCC 6803 biliproteins. Biochem Biophys Res Commun. 2006;341:1105–1112. doi: 10.1016/j.bbrc.2006.01.070. [DOI] [PubMed] [Google Scholar]
- Rippka R, Coursin T, Hess W, Lichtlé C, Scanlan DJ, Palinska KA, et al. Prochlorococcus marinus Chisholm et al., 1992 subsp. pastoris subsp. nov. strain PCC 9511, the first axenic chlorophyll a2/b2-containing cyanobacterium (Oxyphotobacteria) Int J Syst Evol Microbiol. 2000;50:1833–1847. doi: 10.1099/00207713-50-5-1833. [DOI] [PubMed] [Google Scholar]
- Rocap G, Distel DL, Waterbury JB, Chisholm SW. Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl Environ Microbiol. 2002;68:1180–1191. doi: 10.1128/AEM.68.3.1180-1191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan DJ, Ostrowski M, Mazard S, Dufresne A, Garczarek L, Hess WR, et al. Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev. 2009;73:249–299. doi: 10.1128/MMBR.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry ND, Wood MA.2001Phycoerythrin-containing picocyanobacteria in the Arabian Sea in February 1995: diel patterns, spatial variability, and growth rates Deep Sea Res Part II481263–1283. [Google Scholar]
- Six C, Thomas JC, Brahamsha B, Lemoine Y, Partensky F. Photophysiology of the marine cyanobacterium Synechococcus sp. WH8102, a new model organism. Aquat Microb Ecol. 2004;35:17–29. [Google Scholar]
- Six C, Worden AZ, Rodriguez F, Moreau H, Partensky F. New insights into the nature and phylogeny of Prasinophyte antenna proteins: Ostreococcus tauri, a case study. Mol Biol and Evol. 2005;22:2217–2230. doi: 10.1093/molbev/msi220. [DOI] [PubMed] [Google Scholar]
- Six C, Finkel ZV, Irwin AJ, Campbell DA. Light variability illuminates niche-partitioning among marine picocyanobacteria. PLoS One. 2007;2:e1341. doi: 10.1371/journal.pone.0001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six C, Joubin L, Partensky F, Holtzendorff J, Garczarek L. UV-induced phycobilisome dismantling in the marine picocyanobacterium Synechococcus sp. WH8102. Photosynth Res. 2007;92:75–86. doi: 10.1007/s11120-007-9170-4. [DOI] [PubMed] [Google Scholar]
- Six C, Thomas JC, Garczarek L, Ostrowski M, Dufresne A, Blot N, et al. Diversity and evolution of phycobilisomes in marine Synechococcus spp.—a comparative genomics study. Genome Biol. 2007;8:R259. doi: 10.1186/gb-2007-8-12-r259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six C, Sherrard R, Lionard M, Roy S, Campbell DA. Photosystem II and pigment dynamics among ecotypes of the green alga Ostreococcus. Plant Physiol. 2009;151:379–390. doi: 10.1104/pp.109.140566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoitchkova K, Zsiros O, Javorfi T, Pali T, Andreeva A, Gombos Z, et al. Heat- and light-induced reorganizations in the phycobilisome antenna of Synechocystis sp PCC 6803. Thermo-optic effect. Biochim Biophys Acta, Bioenerg. 2007;1767:750–756. doi: 10.1016/j.bbabio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Stuart RK, Dupont CL, Johnson DA, Paulsen IT, Palenik B. Coastal strains of marine Synechococcus species exhibit increased tolerance to copper shock and a distinctive transcriptional response relative to those of open-ocean strains. Appl Environ Microbiol. 2009;75:5047–5057. doi: 10.1128/AEM.00271-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition. Trends Plant Sci. 2008;13:178–182. doi: 10.1016/j.tplants.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Tamary E, Kiss V, Nevo R, Adam Z, Bernat G, Rexroth S, et al. Structural and functional alterations of cyanobacterial phycobilisomes induced by high-light stress. Biochim Biophys Acta Bioenerg. 2012;1817:319–327. doi: 10.1016/j.bbabio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Tang PY, Tremblay R, Vincent W. Cyanobacterial dominance of polar freshwater ecosystems: are high-latitude mat-formers adapted to low temperature. J Phycol. 1997;33:181. [Google Scholar]
- Telfer A. Too much light? How beta-carotene protects the photosystem II reaction centre. Photochem Photobiol Sci. 2005;4:950–956. doi: 10.1039/b507888c. [DOI] [PubMed] [Google Scholar]
- Thomas MK, Kremer CT, Klausmeier CA, Litchman E. A global pattern of thermal adaptation in marine phytoplankton. Science. 2012;338:1085–1088. doi: 10.1126/science.1224836. [DOI] [PubMed] [Google Scholar]
- Vaulot D. CYTOPC: processing software for flow cytometric data. Signal Noise. 1989;2:8. [Google Scholar]
- Vaulot D, Le Gall F, Marie D, Guillou L, Partensky F. The Roscoff Culture Collection (RCC): a collection dedicated to marine picoplankton. Nova Hedwigia. 2004;79:49–70. [Google Scholar]
- Vincent W.2000Cyanobacterial dominance in the polar regionsIn: Whitton BA, Potts M, (eds)The Ecology of Cyanobacteria Kluwer Dordrecht:321–340. [Google Scholar]
- Vincent W, Quesada A. Ecology of Cyanobacteria II: Their Diversity in Space and Time. Springer: New York; 2012. Cyanobacteria in high latitude lakes, rivers and seas Whitton BA, eds; pp. 371–385. [Google Scholar]
- Wada H, Murata N. Temperature-induced changes in the fatty-acid composition of the cyanobacterium, Synechocystis PCC6803. Plant Physiol. 1990;92:1062–1069. doi: 10.1104/pp.92.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Ajlani G, Verbavatz JM, Vass I, Kerfeld CA, Kirilovsky D. A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria. Plant Cell. 2006;18:992–1007. doi: 10.1105/tpc.105.040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AM, Phinney DA, Yentsch CS. Water column transparency and the distribution of spectrally distinct forms of phycoerythrin- containing organisms. Mar Ecol Prog Ser. 1998;162:25–31. [Google Scholar]
- Zinser ER, Johnson ZI, Allison C, Karaca E, Veneziano D, Chisholm SW. Influence of light and temperature on Prochlorococcus ecotype distributions in the Atlantic Ocean. Limnol Oceanogr. 2007;52:2205–2220. [Google Scholar]
- Zwirglmaier K, Heywood JL, Chamberlain K, Woodward EM, Zubkov MV, Scanlan DJ. Basin-scale distribution patterns of picocyanobacterial lineages in the Atlantic Ocean. Environ Microbiol. 2007;9:1278–1290. doi: 10.1111/j.1462-2920.2007.01246.x. [DOI] [PubMed] [Google Scholar]
- Zwirglmaier K, Jardillier L, Ostrowski M, Mazard S, Garczarek L, Vaulot D, et al. Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ Microbiol. 2008;10:147–161. doi: 10.1111/j.1462-2920.2007.01440.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.