Abstract
The hippocampus is said to be involved in “navigation” and “memory” as if these were distinct functions. In this issue of Neuron, Singer et al. (2013) provide evidence that the hippocampus retrieves spatial sequences in support of memory, strengthening a convergence between the two perspectives on hippocampal function.
In 1989, Richard Morris criticized the notion that place cells—hippocampal principal neurons that fire when a rat occupies a particular location in the environment—had anything to do with memory (Morris, 1989). He emphasized that the existing data showed place cells that tell us only where the animal is at the present time and offer no information about where it might go based on memories of what is found at distant locations. This and many other disconnects have long characterized a separation between “navigation” and “memory” literatures of hippocampal function. However, in the current issue of Neuron, observations by Singer et al. (2013) seem to address Morris’s concern, providing compelling evidence that hippocampal neural ensembles retrieve memories of alternative paths, composed as different sequences of place cell activations, which could lead the animal to a desired goal.
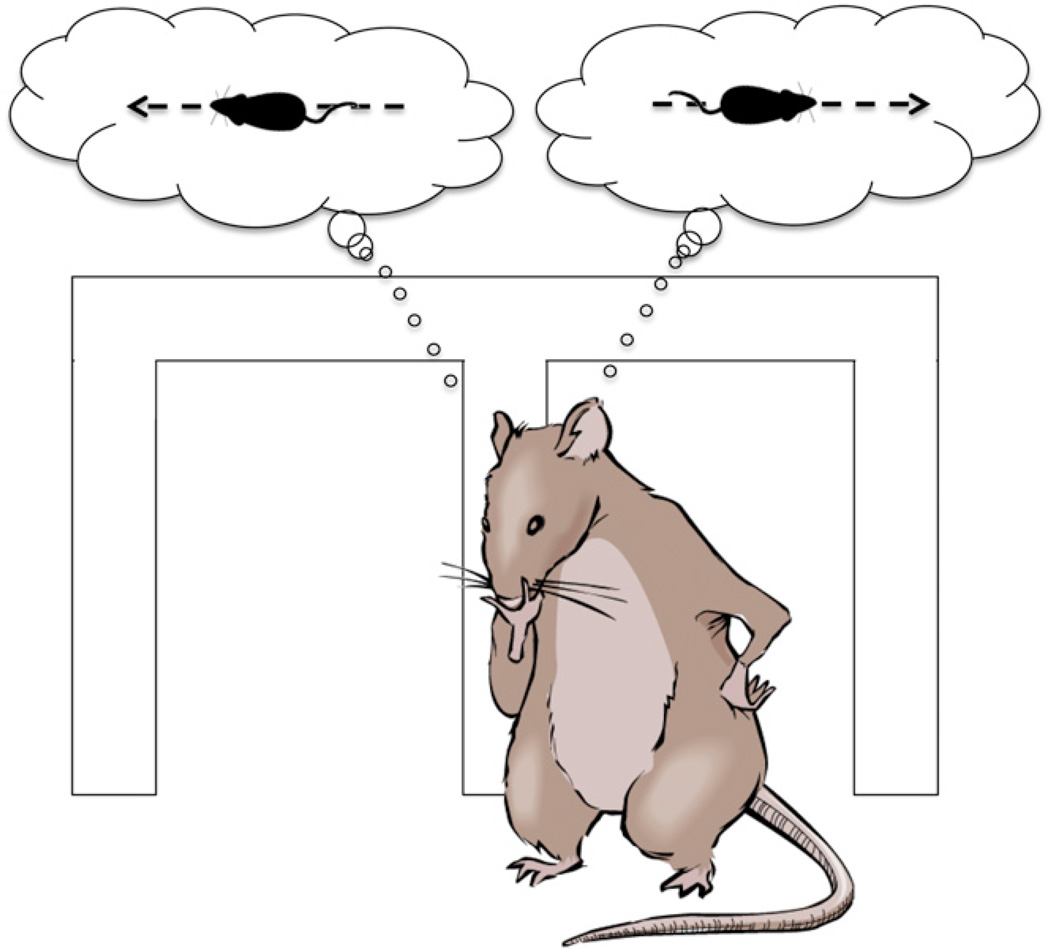
Singer et al. (2013) recorded from CA1 and CA3 principal cells in rats performing a spatial alternation task in a “W-shaped” maze (Figure 1). They examined neuronal activity during local field potential events known as sharp-wave ripples (SWRs), in which several earlier reports have shown a speeded “replay” of neuronal firing sequences that had occurred in earlier experiences. Specifically, their analyses focused on SWRs when the rat was relatively still while outbound on the center arm, heading toward the critical choice between the left or right arm as having the next reward. During these SWR events, they identified replays as coactivations of place cell activity that typically occurred during actual runs toward the left or right goals. There were three main findings. First, more replays occurred preceding subsequent correct choices than incorrect choices and, in the latter, the likelihood of replay was at chance level. Second, there were usually multiple replays at these times, corresponding to both the correct and incorrect choice paths. Third, replays were common early in learning but no longer appeared when rats had mastered the task. Thus, associated with the course of learning, the hippocampus replays alternative paths just before a critical choice between those paths is made, and the occurrence of replay increases the accuracy of the subsequent choice.
Figure 1. Retrieving Memories to Make Decisions.
To receive reward at the ends of maze arms, the rat had to run back and forth through the maze, from left to center to right, back to center, then left, and so on. The critical choice point was as the rat left the center arm and had to remember whether it had most recently come from the left or right arm and choose the alternate direction. The main finding in this study is that, as animals approached the critical choice point, the amount of hippocampal replay of both left and right routes predicted accurate choices.
These findings build on many earlier observations about hippocampal replay, including, in particular, that hippocampal neural ensembles replay both recent paths and paths not recently taken (Gupta et al., 2010). Also, the occurrence of replays is greater after novel experiences and correlates with memory performance (Dupret et al., 2010). And replays of alternative paths have also been observed when rats investigate possible choices during vicarious trial and error at a critical decision point (Johnson and Redish, 2007). Here the trial-by-trial prediction of accuracy by the proportion of replays of alternative paths suggests that hippocampal replay reflects the retrieval of multiple relevant memories that can be evaluated to guide the correct subsequent choice, and this is of particular value early in learning (Figure 1).
The findings on hippocampal replay and its association with memory are paralleled by several observations on trajectory- dependent activity of place cells (reviewed in Shapiro et al., 2006). In these studies, rats traverse overlapping routes through a maze and a typical observation is distinct place cell firing sequences for each route, including different firing patterns when the rat is traversing the overlapping part of different routes. Similar to the findings of Singer et al. (2013) on replays, trajectory-dependent activity of place cells is also strongly linked to memory performance, as its occurrence both prior to a memory delay and, during memory retrieval, predicts subsequent trial-by-trial memory accuracy (Robitsek et al., 2013). The combined evidence on replay and trajectory-dependent firing strongly suggests that the activity of place cells in spatial memory tasks reflects the encoding and retrieval of sequences of places traversed that compose the memories of routes taken.
From the broader perspective on the role of the hippocampus, these findings point to a reconciliation of the largely separate literatures that have long suggested mostly separate roles for the hippocampus in spatial navigation (Moser et al., 2008) and memory (Squire, 2009), and this distinction is getting ever more fuzzy. Consistent with a convergence of these literatures, Buzsáki and Moser (2013) proposed that the neuronal mechanisms of the hippocampus underlying spatial navigation and memory are the same and suggested that common circuitry and computational algorithms could support both functions. They did begin with the caveat that hippocampus-supported navigation phylogenetically precedes the role in memory processing, but the evidence equally well supports the alternative conclusion that fundamental mechanisms of hippocampal memory processing support navigation as an example of memory processing. Of particular relevance to the findings on hippocampal replay and trajectory-specific firing sequences, Buzsáki and Moser (2013) emphasized that a fundamental contribution of the hippocampus to navigation is to encode and retrieve the temporal organization of locations traversed in paths taken, and they likened this function to temporal organization as a defining feature of episodic memory. Indeed, the replay of hippocampal spatial firing sequences provides the best current evidence for “mental time travel” in a case for evolutionary continuity of episodic memory in animals and humans (Corballis, 2013).
Notably, the role of hippocampal ensembles in the temporal organization of memories extends to nonspatial as well as spatial events that compose episodic memories (reviewed in Eichenbaum, 2013). The hippocampus is critical to memory for temporal organization independent of space and the same neurons that are place cells when rats traverse maze paths fire sequentially when rats run in one location and when rats bridge gaps between remembered events independent of behavior and location. These findings are examples of a growing set of studies that show a prominent role of the hippocampus in memory for temporal order in animals and humans and providing a broad range of evidence for sequential activation of hippocampus neurons during memory retrieval of serial events in rats, monkeys, and humans. In particular, the existence of hippocampal “time cells” that encode moments in temporally extended memories, much as place cells encode locations in spatially extended environments, suggests that time, not place, is the fundamental dimension of hippocampal representation that is common to navigation and memory. Furthermore, recent evidence revealed temporal organization in hippocampal ensembles that exists prior to experiences, to which learning attaches specific memories (Dragoi and Tonegawa, 2011). This observation of “preplay,” which anticipates subsequent replay, suggests that temporal organization is primary and may provide the scaffolding onto which spatial and nonspatial memories are hung. The convergence of literatures on retrieval-associated replay in spatial memory and temporal organization in a broad variety of situations offers considerable promise for a comprehensive understanding of the role of the hippocampus in memory.
ACKNOWLEDGMENTS
H. Eichenbaum is supported by NIMH MH094263, MH095297, MH51570, MH52090, ONR N00014-10-1-0936.
REFERENCES
- Buzsáki G, Moser EI. Nat. Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis MC. Trends Cogn. Sci. 2013;17:5–6. doi: 10.1016/j.tics.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. Nat. Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Trends Cogn. Sci. 2013;17:81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Redish AD. J. Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM. Does the hippocampus play a disproportionate role in spatial memory? In: Squire LR, Mishkin M, Shimamura A, editors. FESN Study Group on Learning and Memory. Geneva: Elsevier; 1989. pp. 39–45. [Google Scholar]
- Moser EI, Kropff E, Moser MB. Annu. Rev. Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, White JA, Eichenbaum H. Behavioural Brain Research. 2013 doi: 10.1016/j.bbr.2012.12.034. Published online January 4, 2013. http://dx.doi.org/10.1016/j.bbr.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Kennedy PJ, Ferbinteanu J. Curr. Opin. Neurobiol. 2006;16:701–709. doi: 10.1016/j.conb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Singer AC, Carr MF, Karlsson MP, Frank LM. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. J. Neurosci. 2009;29:12711–12716. doi: 10.1523/JNEUROSCI.3575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]