Summary
An anion exchange method was developed to separate selenium and arsenic for potential utility in a 72Se/72As generator. The separation of the daughter 72As from the 72Se parent is based on the relative acid-base behavior of the two oxo-anions in their highest oxidation states. At pH 1.5, selenate is retained on strongly basic anion exchange resin as HSeO4− and SeO42−, while neutral arsenic acid, H3AsO4, is eluted.
Keywords: As-72, Se-72, radionuclide generator, ion exchange
1. Introduction
Positron emission tomography (PET) has become a routine nuclear medicine imaging technique with the approval of 2-deoxy-(18F)-fluoroglucose (18F-FDG). PET imaging allows for better resolution nuclear medicine images than single photon emission computed tomography (SPECT) and allows for quantitation. Several positron emitting radionuclides are currently available but most are short-lived (e.g., t1/2 = 110 min for 18F, t1/2 = 20 min for 11C, t1/2 = 12.7 h for 64Cu) [1]. Longer-lived positron emitting radionuclides would allow the imaging of biological processes that require longer localization times such as radiolabeled antibodies. Zirconium-89 is being developed as a longer-lived, metallic positron emitter (t1/2 = 3.27 d), and enhanced chelate development is currently underway [2]. Iodine-124 is a long-lived, non-metallic, positron emitter (t1/2 = 4.176 d) being developed for molecular imaging applications, but minimizing radioiodine impurities and maximizing 124I production yield during either proton or deuteron irradiation remains a trade-off [3–6]. An alternative non-metallic, positron emitting radionuclide is 72As (t1/2 = 26.0 h, 88%, β+, 2.49 MeV endpoint energy, 12% EC), which is available from the decay of 72Se (t1/2 = 8.4 d, EC, 46 keV γ (58% abundant)) and makes this parent-daughter pair suitable for development of a 72Se/72As generator [7]. Compared to most PET radionuclides, the longer half-life of 72As allows for attachment to antibodies and proteins for quantitative imaging of biochemical and physiological processes and receptor mapping [8–12]. The chemistry of arsenic is diverse (oxidation states of 0, +3 and +5) allowing for the development of potentially valuable PET radiopharmaceuticals. Direct labeling of the monoclonal antibody Bavituximab with 72As for tumor-specific PET imaging has been reported [13].
Selenium-72, the parent of 72As, can be produced from charged particle reactions on Ge, Br, or As targets [14]. The most common production routes involve α or 3He irradiation of 70Ge, 72Ge, or natGe, generally as the oxide (e.g., 70Ge(α,2n)72Se or 70Ge(3He,n)72Se), or a proton induced nuclear reaction on stable arsenic (75As(p,5n)72Se) [15–18]. Cross-section data suggest that these are the best production methods [15,19,20]. Alternatively natural NaBr targets irradiated with higher energy protons (72–92 MeV) generate Se radioisotopes (72Se, 73Se, 75Se) with fewer by-products than higher energy reactions [7,21,22]. The higher beam currents available (average of 101.4 μA) at the Los Alamos National Laboratory Isotope Production Facility (LANL-IPF) make this a viable route although radioselenium impurities are generated [22]. Any of these routes will produce 72Se for generator development, with the 72Se generally separated from its target as Se(0) [15,17] or Se(IV) [16,18,20].
Development of a column generator for the separation of 72As from 72Se, similar to the Mo-99 alumina column generator for 99mTc (e.g., Ultra-TechneKow®) or the Sr-82 stannic oxide column for 82Rb (e.g., Cardiogen-82®) would be advantageous. Selenium-72/Arsenic-72 (72Se/72As) radionuclide generators have been proposed in the literature [14], however complex separation/isolation methods are required. Separation of the daughter from the parent can occur via repeated distillation of “grown-in” 72AsCl3 from carrier added 72Se stock solutions [16,21]; electroplating of 72Se as Cu2Se on Cu backings [23], solvent extraction [22] or solid phase extraction of 72Se [17,24]. However, with the exception of the solid phase extraction method, the reported methods require laborious and/or significant handling. The solid phase approach as reported in the literature is limited to very small dimensions (500 μL cartridge volume, few μCi of 72Se activity) involving reagents such as HF, requires Se in its elemental state under an inert atmosphere to prevent oxidation, and scale up to clinically useful 10 mCi batches may not be feasible [17,22,24].
Extraction chromatographic methods, including batch distribution ratio methods and column chromatography, have provided simple, robust methods for the separation of parent/daughter radionuclide pairs [25–27] including the development of a higher activity 137Cs/137mBa generator system for industrial and environmental applications [27]. Using several radioisotopes of arsenic and selenium, an anion exchange method was developed for the separation of As from Se with each element in its highest oxidation state, namely as selenate (Se(VI)O42−) and arsenic acid (H3As(V)O4). A low activity generator was prepared and evaluated.
2. Experimental
Caution! Selenium-75 (75Se), 72Se, 72,73,74,76As, and 22Na are radioactive and all work involving these radionuclides were carried out in approved laboratories following the appropriate radiation safety procedures. Selenium-75 (t1/2 = 119.78 d) used as a Se tracer in column and batch studies was produced at the University of Missouri Research Reactor (MURR) via the 74Se (n, γ) 75Se reaction of an encapsulated 74Se (as SeO2; 98.85%) target, which was dissolved in 1 M HNO3. Arsenic-76 (t1/2 = 1.0942 d) used as an As tracer in batch studies was produced at the MURR via the 75As (n, γ)76As reaction of an encapsulated 75As (as natAs2O3) target, which was dissolved in 1 M NaHCO3. A mixture of radioactive selenium (72,75Se), arsenic (72,73,74As), and sodium (22Na) in 0.1 M HCl and ~3 M NaCl (from processing) [22], produced by the irradiation of natural NaBr targets with 72–92 MeV protons at the Los Alamos National Laboratory – Isotope Production Facility (LANL-IPF), was evaluated for producing a 72Se/72As generator. On receipt, the sample contained 19.34 μCi of 22Na, 2.8 μCi of 72As (with 72Se in equilibrium), 62.51 μCi of 73As, 56.26 μCi of 74As, and 35.24 μCi of 75Se as determined by gamma spectroscopy (HPGe). Table 1 lists the radionuclides produced at LANL during irradiation of the NaBr target, their half-lives in days, the initial activities measured at LANL, and the activities measured at the University of Missouri after processing and receipt.
Table 1.
Activities of sample from LANL.
| Nuclide | Literature28 t1/2 (days) | Measured Initial Activity at LANL on 07/24/201122 | Measured Activity at MURR on 08/23/2011 |
|---|---|---|---|
| 22Na | 950.5 | Not determined | 19.34 μCi/mL |
| 71As | 2.72 | 0.19 ± 0.01 Ci | Decayed |
| 72As | 1.08 | Not determined | 2.8 μCi/mL |
| 73As | 80.3 | a Not determined | 62.51 μCi/mL |
| 74As | 17.77 | 0.046 ±0.001 Ci | 56.26 μCi/mL |
| 72Se | 8.4 | 0.03 ± 0.01 Ci | b Not determined |
| 75Se | 119.78 | 0.050 ± 0.002 Ci | 35.24 μCi/mL |
| 76Br | 0.68 | 8.1 ± 0.7 Ci | c Not present |
| 77Br | 2.38 | 4.2 ± 0.6 Ci | c Not present |
| 82Br | 1.47 | 0.27 ± 0.06 Ci | c Not present |
Accurate measurement of the low energy (56 keV) was impossible with the high activities present.
Accurate measurement of the low activity, low energy (46 keV) gamma was not possible.
Br isotopes had been removed prior to our receipt.
Table 2 lists the various radionuclides, their nuclear properties, and their specific activities as used in these studies.
Table 2.
Evaluated sodium, arsenic, and selenium isotopes and their associated gammas [28].
| Isotope | Half-Life | Gamma Emissions (Abundances) | Specific Activity |
|---|---|---|---|
| 22Na | 2.6027 a | 511 (180%), 1274.5 keV (99.9%) | 1.65 MBq/mg (4.5 × 10−5 Ci/mg)* |
| 72As | 26.0 h | 511 (176%), 834.0 keV (81.0%) | nca (6.18 GBq/mg; 1670 Ci/mg) |
| 73As | 80.30 d | 53.4 keV (10.3%) | nca (825 MBq/mg; 22.3 Ci/mg) |
| 74As | 17.77 d | 511 (58%), 595.8 keV (59.0%) | nca (3.67 GBq/mg; 99.3 Ci/mg) |
| 76As | 26.3 h | 559.1 keV (45.0%) | 5.74 MBq/mg (0.155 mCi/mg) at EOB* |
| 72Se | 8.4 d | 45.89 keV (57.2%) | nca (7.99 GBq/mg; 216 Ci/mg) |
| 75Se | 119.78 d | 121 (17%), 136.0 keV (58.3%) 264 (59%), 279 (25%), 400 (11%) |
nca in LANL sample 9.36 MBq/mg (0.253 Ci/mg)* |
nca = no carrier added; specific activities calculated.
Specific activities determined from target mass irradiated.
2.1 Materials
Reagents and solvents were purchased from Alfa Aesar (Ward Hill, MA), Fisher Scientific (Pittsburg, PA), Mallinckrodt (St. Louis, MO), and Sigma-Aldrich (St. Louis, MO) and used as received. Dowex anion exchange resins were obtained from Bio-Rad Corporation (Hercules, CA) and Sigma-Aldrich (St. Louis, MO). Alumina basic form (Sigma Aldrich), alumina neutral form (Sigma-Aldrich), alumina acidic form (Fisher Scientific), silica gel 60 (Brinkman, Des Plaines, IL), magnesium oxide (Mallinkrodt), Florisil (Fisher Scientific), and zirconium oxide (Alfa Aesar) were evaluated. All water used was purified on-site (deionized water from a Millipore system to >18MΩcm).
2.2 Instrumental analysis
Ion pairing HPLC analysis was performed using a Perkin-Elmer Model 410 LC pump with controller and Turbochrome software equipped with a Rainin Dynamax UV-1 variable UV-visible detector. An analytical reversed phase C18 column (Alltech Hypersil, 120 Å, 0.46 cm × 25 cm × 5 μm) with an isocratic mobile phase of 20:80 acetonitrile: phosphate buffer (60 mM, pH 7.2, 20 mM tetrabutylammonium hydroxide as the ion pair agent) at a flow rate of 1 mL/min and UV detection at 210 nm was utilized for HPLC. Retention times for selenite, arsenate and selenate were 3.1, 3.4 and 3.9 minutes, respectively.
Radiochemical assays for samples containing solely 75Se were determined by gamma counting using a Harshaw integral line NaI(Tl) solid scintillation well detector with a Canberra HV power supply and Ortec preamp.
Radiochemical assays for 22Na, 72, 73,74As, and 75Se were performed by γ-ray spectroscopy using a Canberra Model GC2018S HPGe detector system. The detector diameter was 60.5 mm, detector length was 30.5 mm, and the distance from the window was 5 mm. The detector’s specified FWHM at 1.33 MeV was 1.8 keV. Spectral analyses were performed by a Canberra Model 9600 multichannel analyzer.4
2.3 Determination of optimum chromatographic media
For the development of a 72Se/72As generator, various support media were evaluated to elute arsenate while retaining selenate. Silica gel, acidic alumina, neutral alumina, basic alumina, Florisil, zirconium oxide, magnesium oxide, Dowex 1-X8 and Dowex 1-X2 were tested for their ability to retain selenate while allowing arsenate to elute under neutral to slightly acidic conditions (Table 3). Columns were separately loaded with non-radioactive sodium selenate and sodium arsenate dibasic heptahydrate dissolved in water, and were then evaluated for ion retention by elution with DI H2O and either dilute HCl (<1 M) or dilute HNO3 (<1 M). The collected elution fractions were evaluated for selenate and arsenate using ion pairing reversed phase HPLC analysis described above.
Table 3.
Column and eluent conditions evaluated.
| Matrix (volume) | Eluent | Arsenate | Selenate |
|---|---|---|---|
| Silica (1.5 mL) | Water or 0.12 M HCl | Elutes rapidly | Elutes rapidly |
| Acidic Alumina (1.5 mL) | Water, neutral saline, 0.12 M or 0.24 M HCl | Did not elute | Did not elute |
| Neutral Alumina (1.5 mL) | Water or normal saline | Did not elute | Elutes |
| Basic Alumina (1.5 mL) | Water, normal saline, 0.12 M or 0.24 M HCl | Did not elute | Elutes |
| Florisil (1.5 mL) | Water, normal saline, 0.012 M or 0.12 M HCl | Elutes | Elutes |
| Zirconium Oxide* (0.5 mL) | 0.001 M, 0.01 M or 0.1 M HNO3 | Elutes | Elutes |
| Magnesium Oxide* (0.5 mL) | 0.012 M HCl | Elutes | Elutes |
| Dowex 1-X2 or X8 (1.5 mL) | Water or normal saline | Did not elute | Did not elute |
| 0.012 M or 0.12 M HCl | Elutes | Did not elute | |
| 1.2 M HCl | Elutes | Begins to elute very slowly |
Fine powders; smaller volumes for reasonable flow rates.
2.4 Optimization of elution parameters
To optimize the elution parameters on Dowex anion exchange resin, sorption studies were performed via the batch method for selenite, selenate, and arsenate. Prior to use, samples were verified to contain purely selenate, selenite, or arsenate by comparison to standards using HPLC. All experiments were performed at room temperature (20°C). The removal of selenium and arsenic from varying pH (−1 to 7) solutions was measured by mixing 50 mg of AG 1-X8 resin with 1.5 mL of a 75Se- or 76As-spiked solution. The selenium tracer was 117 kBq (3.16 μCi)/sample and the arsenic tracer was 146 kBq (3.95 μCi)/sample. The liquid-solid system was mixed by vortexing for 2 minutes and immediately centrifuged for 2 minutes at 7500 rpm. Two 500-microliter aliquots of the supernatant (As) were transferred into clean counting vials. Additionally, a 500-microliter aliquot of the original solution (A0) was transferred to a clean counting vial to determine the original activity. The distribution ratio, Kd, is calculated as follows:
A0 is the original selenium or arsenic activity of the aqueous solution. As is the selenium or arsenic activity remaining in solution following contact with the resin. (A0 − As) is the amount of activity adsorbed by the resin. The volume (V) of the aqueous solution is measured in milliliters and the mass (m) is measured in grams resulting in the distribution ratio having units of mL/g. A larger distribution ratio correlates to a more effective removal of selenate, selenite, or arsenate from the aqueous solution.
2.5 Oxidation of Se(IV) to Se(VI)
An oxidation method was developed to treat the samples to ensure that selenium was present as Se(VI). The optimum method was as follows: samples of 75Se were treated using 1 mL of 30% H2O2 and refluxed for 1 hour. Each sample was quantitatively loaded onto a 1 mL bed volume of Dowex AG1-X8 (100–200 mesh) column and washed with DI H2O (4 × 1 mL), 0.03 N HNO3 (4 × 1 mL), and 0.6 N HNO3 (4 × 1 mL) with all fractions collected and counted using the NaI(Tl) well detector. Selenous acid (H2SeO3) eluted with 0.3 N HNO3 and selenic acid (H2SeO4) eluted with 0.6 N HNO3, allowing determination of Se oxidation state present.
The LANL-IPF mixture (222 kBq; 6 μCi/sample) was evaluated by this technique to determine the Se oxidation state. Treated and untreated control samples were eluted from Dowex columns in the manner described above and the fractions were counted on the HPGe detector, and evaluated for 22Na, 73As, and 75Se content (Table 1).
2.6 Application of method to Se/As generator column
A large, preparative column (2.2 cm i.d. × 12.5 cm) packed with a 48 mL bed volume of Dowex 1-X2 (50–100 mesh) resin was conditioned with 3 M HCl followed by DI H2O (~10 bed volumes). Approximately 11.1 MBq (300 μCi) of the LANL-IPF sample was neutralized, oxidized and diluted (total volume = 300 mL), and then added to this pre-concentrating column followed by DI H2O washes (2 × 50 mL). Arsenate was eluted from the column with 0.03 N HNO3 (6 × 50 mL) followed by DI H2O (2 × 50 mL). Selenate was then eluted from the column with 0.6 N HNO3 (6 × 50 mL) followed by DI H2O (2 × 50 mL). The 2nd and 3rd fractions of the 0.6 N HNO3 elution (containing the majority of the selenate) were combined, the volume slowly reduced to 0.1 mL by evaporation in a hot water bath under air flow, and then neutralized (1 M NaHCO3), oxidized (30% H2O2), and diluted with DI H2O to 32 mL. This sample was loaded onto a generator Dowex 1-X2 column (50–100 mesh; 4 mL wet resin volume; 0.7 cm i.d. × 10 cm; conditioned as above), washed with DI H2O (10 mL), and then eluted after 1, 3, and 8 days with 0.03 N HNO3 (5 × 5 mL) and DI H2O (2 × 10 mL). The fractions were counted on the HPGe to analyze for 72,73,74As and 75Se content.
2.7 Effect of radiation on Dowex resin
With the anticipated, large radiation dose to the resin expected from a 72Se/72As generator, the Dowex 1-X8 resin was exposed to 2880 Gy (288 MRad) over 2 weeks using a 60Co source. The elution profile of two columns (0.6 cm i.d. × 4.2 cm; 1.2 mL wet volume), one containing the exposed resin and one the control unexposed resin, were prepared as described above to evaluate any difference in breakthrough of 75Se. The same activity of 75Se (1850 Bq (50 nCi); 0.24 ng; 0.206 Ci/mg) was applied to each column, with 75Se used as radiotracer for 72Se for easier counting.
3. Results and discussion
Several methods have been reported to separate 72As from 72Se, none are straight-forward and most require extractions or harsh chemical treatments (e.g., HF) to generate 72As in the +3 oxidation state, which is readily oxidized at the tracer level to the +5 oxidation state. To this end, an anion exchange method has been developed that takes advantage of the difference in acid strength between selenic acid (H2SeO4) and arsenic acid (H3AsO4) with the 72As in its +5 oxidation state.
3.1 Determination of optimum chromatographic media
Several chromatographic materials were evaluated for their ability to retain selenate and elute arsenate, with Dowex anion exchange resins (1X-8 and 1X-2) determined to be the best suited (Table 2). At pH = 1.5 the arsenate ion is nearly fully protonated and is eluted from the anion exchange resin while the selenate ion retains a negative charge and is not readily displaced. None of the hydrous metal oxides evaluated gave a suitable separation as they retained arsenate and eluted selenate or both species were similarly retained.
3.2 Optimization of elution parameters
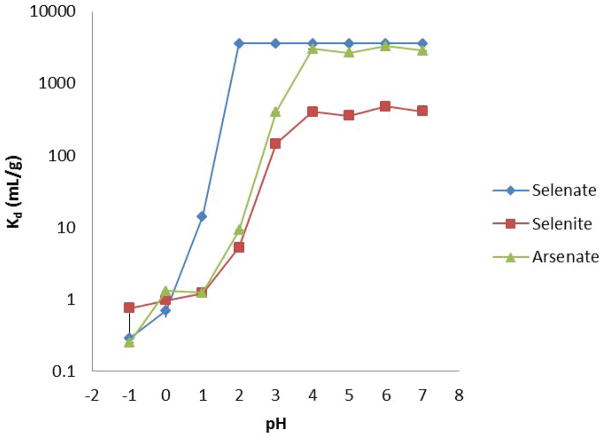
To determine the best pH for selenate retention and arsenate elution, equilibrium distribution ratios were acquired as a function of pH. The retention of selenate, selenite, and arsenate was impeded by the high ionic strength of the pH −1 and 0 solutions. Since selenic acid is a strong acid, selenate was readily retained by the resin and quickly reached its maximum Kd. Maximum Kd is a function of the minimal detectable activity of the detector and the activity of the spike; the maximum Kd in this experiment is 3570 mL/g. Since selenous and arsenic acids are weaker acids, selenite (pKa1 = 2.62) and arsenate (pKa1 = 2.19) are weakly retained until reaching a solution pH where the anionic species is the primary form. The several orders of magnitude difference in the retention of selenite and selenate highlights the necessity of an oxidation step to prevent loss of selenium from the column. Additionally, the results indicate eluting at pH 2 allows the parent, selenate, to be retained on the column while the arsenate daughter is removed (Figure 1).
Figure 1.
Distribution coefficient for selenate, selenite, and arsenate as a function of pH (n = 6; the error bars are included).
3.3 Oxidation of Se(IV) to Se(VI)
The first pKa value of selenous acid (H2SeO3) is close to that of arsenic acid (H3AsO4) (2.62 and 2.19, respectively), which would make their separation very difficult as shown in Figure 1. Thus, a method was developed to ensure that all of the selenium was present as selenic acid to afford a separation from the weak arsenic acid. An ion pairing reversed phase HPLC method was used to determine the speciation of selenate, selenite and arsenate present and to optimize the conditions for the oxidation of Se(IV) to Se(VI). Refluxing 30% H2O2 was found to completely convert selenite to selenate. Variations in pH had no effect on the conversion or elution.
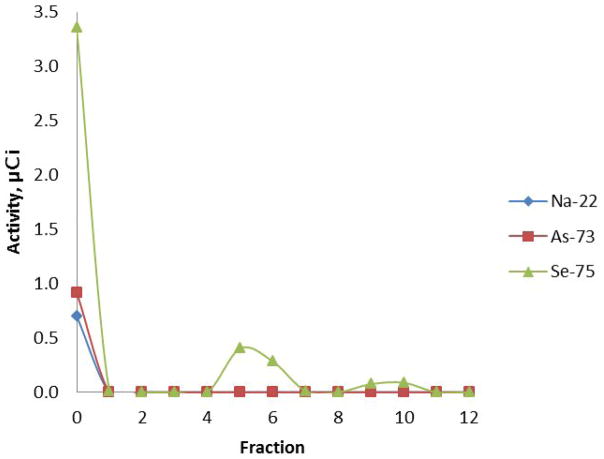
The LANL-IPF sample, which contained radioisotopes of Se, As and Na, and had been oxidized with hydrogen peroxide/HCl to remove bromide isotopes as Br2 prior to shipping to the University of Missouri Research Reactor (MURR), required additional treatment with refluxing 30% H2O2 to convert all of the selenium to selenate. Either the original oxidation was insufficient to convert the selenium to Se(VI) or over time the selenium originally converted was reduced to a range of lower oxidation states (Figure 2). The sample contained principally 22Na, 73As, 74As and 75Se as observed by HPGe gamma spectroscopy with 75Se in the greatest abundance. At low levels of 72Se activity the 72Se gamma signal is not readily quantitated from the high Compton background at 45 keV. Chromatographic separation of the non-oxidized sample showed selenium in at least three different forms: an uncharged or cationic species eluting with water, selenite eluting with 0.03 N HNO3 and selenate eluting with 0.6 N HNO3. The arsenic present eluted with water as an uncharged or cationic species and the sodium also eluted with water.
Figure 2.
Un-oxidized aliquot of the LANL-IPF sample evaluated on Dowex 1X-8 (100–200 mesh). Eluent of fractions: 0 = sample addition; 1–4 = water; 5–8 = 0.03 M HNO3 (pH 1.5); 9–12 = 0.6 M HNO3 (pH 0.2). Selenium is present in three different chemical forms: Se(0) in fraction 0; selenite in fractions 5–6; selenate in fractions 9–11.
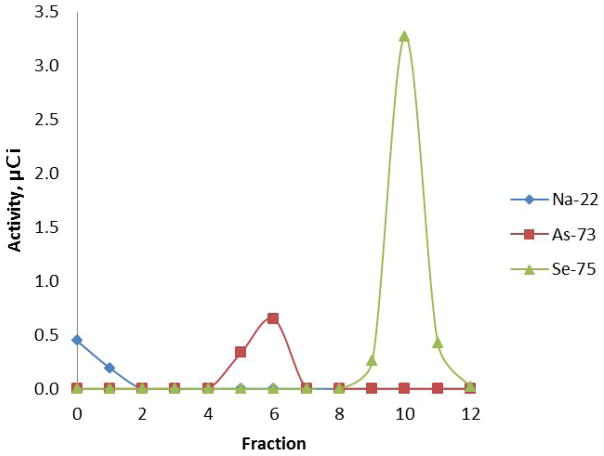
Treatment with refluxing 30% H2O2 oxidized the arsenic to arsenate, and the selenium to selenate. No lower oxidation state As or Se species were observed (Figure 3).
Figure 3.
Oxidation of LANL-IPF sample with 30% H2O2 evaluated on Dowex 1X-8 (100–200 mesh). Eluent of fractions: 0 = sample addition; 1–4 = water; 5–8 = 0.03 M HNO3 (pH 1.5); 9–12 = 0.6 M HNO3 (pH 0.2). Arsenate elutes in fractions 5 and 6, while selenate elutes in fractions 9–11.
3.4 Application of method to Se/As generator column
To examine the practical application of the separation method to a generator preparation, a portion of the neutralized, oxidized, diluted LANL-IPF sample was pre-concentrated to reduce the volume and remove Na and As radioisotopes produced during irradiation with 72–92 MeV protons. A portion of the neutralized, oxidized, diluted LANL-IPF sample was directly added to a preparative column; the eluent resulting from the addition yielded 481 kBq (13 μCi) of 22Na and trace amounts of 74As. No selenium was detected during the addition. Elution with 0.03 N HNO3 removed approximately 24 μCi of 74As and 41 μCi of 73As and a much smaller amount of 72As. Again no Se was detected. Elution with 0.6 N HNO3 yielded over 1.61 MBq (43.5 μCi) of 75Se and trace quantities of 72,73,74As. From the concentrated fractions, more than 97 % of the 75Se was transferred to the 4 mL (bed volume) generator column. No radioisotopes were detected in the resulting eluent from addition of the sample to the generator column. Results from elution with 0.03 N HNO3 are illustrated in Figure 4. After the first elution, which removed the residual arsenic isotopes, 72As was the only arsenic radioisotope eluted. The activity of the LANL sample was measured by HPGe to contain 35.2 μCi/mL of 75Se and 2.8 μCi/mL of 72As (and also 72Se in equilibrium) on 08/23/2010. These activities were decay corrected to the expected activities during the generator column experiment, 26.4 μCi/mL of 75Se and 0.045 μCi of 72As (72Se). Note the significantly higher activity for 75Se over 72Se at this time. The final elution of the generator column yielded 50 nCi of 72As and 1.5 nCi of 75Se. The expected 72Se breakthrough was determined from the 75Se:72Se ratio present in the sample at this time. Only 0.0026 nCi of 72Se would have co-eluted with the 72As, which represented 0.005% of the 72Se activity on the column.
Figure 4.
Elution profile of generator column. Each elution included 0.03 M HNO3(4 × 5 mL) followed by a water rinse (2 × 5 mL).
3.5 Effect of radiation on Dowex resin
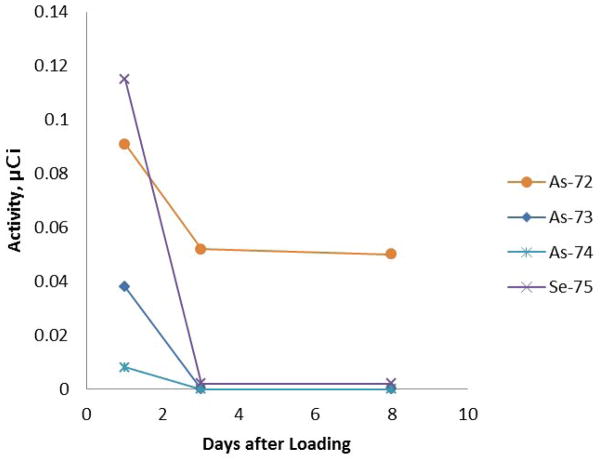
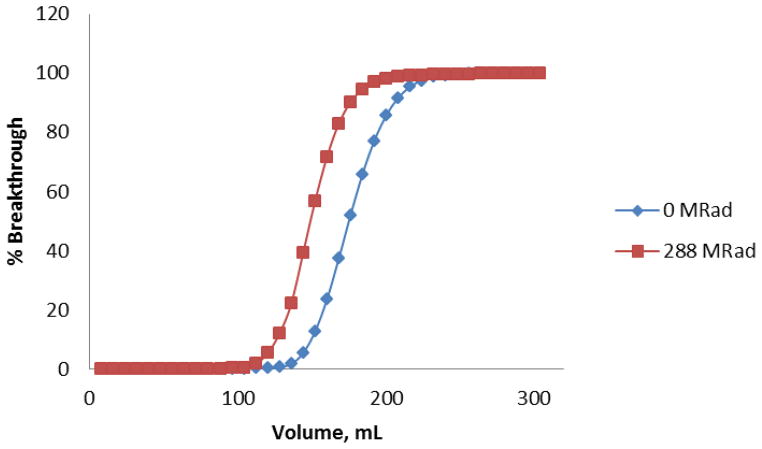
Additionally, a test was performed to assess possible changes in selenium breakthrough upon exposure of the Dowex anion exchange resin to gamma radiation. After irradiation with 2880 Gy (288 MRads) cumulative dose over two weeks using a 60Co source, the resin was darker in color and did not wet as well as the untreated resin. The column prepared from the irradiated resin appeared to have a slightly higher flow rate than the column prepared from the original resin (visually observed; not measured). Breakthrough of Se(VI) occurred on day 16 (elution 12) for the 60Co irradiated resin, and on day 23 (elution 16) for the untreated resin. The column capacity to retain Se(VI) was only marginally affected by the strong extended gamma radiation conditions. The complete breakthrough curves are shown in Figure 5.
Figure 5.
Se(VI) breakthrough comparison of irradiated and non-irradiated Dowex 1×8 resins. The columns were eluted with 0.03 M HNO3 (4 × 1 mL) followed with a water rinse (4 × 1 mL).
4. Conclusions
The development of new drugs utilizing longer-lived radionuclides will allow for the continued growth and increased application of PET imaging for quantitation, especially radioimmunoimaging. Arsenic-72, a long-lived, non-metallic positron emitter, is best produced in high specific activity from the decay of its 72Se parent. A 72Se/72As generator system was developed and will greatly increase the availability and application of 72As. Dowex 1-X2 and 1-X8 resins retained selenate while allowing no-carrier-added 72As arsenate to be eluted.
Distribution coefficient studies showed selenite has over an order of magnitude weaker retention on Dowex 1-X8 than selenate, which would lead to undesired 72Se breakthrough and product loss. A simple oxidation method was developed to ensure selenium is completely in the selenate form.
A 72Se/72As generator, prepared from an irradiated NaBr target, demonstrated the production and elution of 72As from the decay of 72Se. It was further demonstrated that Dowex 1-X8 held up relatively well to radiation damage, which should allow for elutions to be performed over a longer time period without 72Se breakthrough due to radiolysis.
Future work will include preparation and evaluation of a 72Se/72As generator containing higher activities similar to those used in a clinical setting. This would allow determination of radiation resistance of the polystyrene-divinylbenzene copolymer backbone of Dowex AG 1-X8 or 1-X2 resins or whether other resin backbones are needed. A 72Se/72As generator would make 72As available as a tracer for medical, toxicological and environmental studies.
Highlights.
Ion exchange separation for 72Se and 72As developed.
Oxidation method to Se(VI) for column loading developed.
H3AsO4 eluted at pH 1.5 while SeO42−/HSeO4− retained.
Kd values reported for As(V), Se(IV) and Se(VI).
Resin gamma irradiation has minimal effects on Se breakthrough.
Acknowledgments
The authors acknowledge support from the Department of Energy, Office of Basic Energy Sciences, Isotope Research program under Grant No. DE-SC0003851, and trainee support from the National Science Foundation under IGERT award DGE-0965983 (M. D. Gott) and NIBIB Training Grant NIBIB 5 T32 EB004822 (A. J. DeGraffenreid). The 72Se production was funded by the United States Department of Energy, Office of Science via an award from The Isotope Development and Production for Research and Applications subprogram in the Office of Nuclear Physics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qaim SM. Radiochim Acta. 2012;100:635. doi: 10.1524/ract.2012.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. Nucl Med Biol. 2013;40:3. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler L, Gagnon K, McQuarrie S, Wuest F. Molecules. 2010;15:2686–2718. doi: 10.3390/molecules15042686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pentlow KS, Graham MC, Lambrecht RM, Daghighian F, Bacharach SL, Bendriem B, Finn RD, Jordan K, Kaaigian H, Karp JS, Robeson WR, Larson SM. J Nucl Med. 1996;37:1557–1562. [PubMed] [Google Scholar]
- 5.Belov VV, Bonab AA, Fischman AJ, Heartlein M, Calias P, Papisov MI. Molecular Pharmaceutics. 2011;8:736–747. doi: 10.1021/mp100358f. [DOI] [PubMed] [Google Scholar]
- 6.Stafford JH, Hao G, Best AM, Sun X, Thorpe PE. PLOS One. 2013;8(12):1–10. doi: 10.1371/journal.pone.0084864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips DR, Hamilton VT, Nix DA, Taylor WA, Jamriska DJ, Staroski RC, Lopez RA, Emran AM. New Trends in Radiopharmaceutical Synthesis. Quality Assurance and Regulatory Control. 1991:173. [Google Scholar]
- 8.Le Loirec C, Champion C. Nucl Instrum Meth Phys Res A. 2007;582:665. [Google Scholar]
- 9.Jennewein M, Hermanne A, Mason RP, Thorpe PE, Rösch F. Nucl Instrum Meth Phys Res A. 2006;569:512. [Google Scholar]
- 10.Jahn M, Radchenko V, Filosofov D, Hauser H, Eisenhut M, Rösch F, Jennewein M. Radiochim Acta. 2010;98:807. [Google Scholar]
- 11.Nayak TK, Brechbiel MW. Bioconjugate Chem. 2009;20:825. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer RB, Born JL. 6,106,804. United States Patent.
- 13.Jennewein M, Lewis A, Zhao D, Tsyganov E, Slavine N, He J, Watkins L, Antich PP, Hermanne A, Rösch FM, Mason RP, Thorpe PE. Clin Cancer Res. 2008;14:1377. doi: 10.1158/1078-0432.CCR-07-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard B, Nortier FM, Birnbaum ER, John KD, Phillips DR, Fassbender ME. Current Radiopharmaceuticals. 2012;5:264. doi: 10.2174/1874471011205030264. (and references therein; a current review) [DOI] [PubMed] [Google Scholar]
- 15.Al-Kouraishi SH, Boswell GGJ. Int J Appl Radiat Isot. 1978;29:607. [Google Scholar]
- 16.Jennewein M, Schmidt A, Novgorodov AF, Qaim SM, Roesch F. Radiochim Acta. 2004;92:245. [Google Scholar]
- 17.Jennewein M, Qaim SM, Kulkarni PV, Mason RP, Hermanne A, Rosch F. Radiochim Acta. 2005;93:579. [Google Scholar]
- 18.Mudrova B, Kopecky P, Svoboda K. Int J Appl Radiat Isot. 1973;24:610. doi: 10.1016/0020-708x(73)90132-4. [DOI] [PubMed] [Google Scholar]
- 19.Guillaume M, Lambrecht RM, Wolf AP. Int J Appl Radiat Isot. 1978;29:411. doi: 10.1016/0020-708x(78)90104-7. [DOI] [PubMed] [Google Scholar]
- 20.Qaim SM, Mushtaq A, Uhl M. Phys Rev C. 1988;38:645. doi: 10.1103/physrevc.38.645. [DOI] [PubMed] [Google Scholar]
- 21.Phillips DR. 5405589 United States Patent.
- 22.Ballard B, Wycoff D, Birnbaum ER, John KD, Lenz JW, Jurisson SS, Cutler CS, Nortier FM, Taylor WA, Fassbender ME. Appl Radiat Isot. 2012;70:595. doi: 10.1016/j.apradiso.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Phillips DR, Moody DC, Taylor WA, Segura NJ, Pate BD. Appl Radiat Isot. 1987;38(7):521. [Google Scholar]
- 24.Chajduk E, Katarzyna D, Polkowska-Motrenko H, Bilewicz A. Appl Radiat Isot. 2012;70:819. doi: 10.1016/j.apradiso.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Dutta S, Mohapatra PK, Raut DR, Manchandra VK. J Chromatogr A. 2011;1218:6483. doi: 10.1016/j.chroma.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Kim SY, Ito T, Nakazawa K, Funaki Y, Tada T, Hitomi K, Ishii K. J Chromatogr A. 2012;1263:28. doi: 10.1016/j.chroma.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarty R, Ram R, Pillai KT, Pamale Y, Kamat RV, Dash A. J Chromatogr A. 2012;1220:82. doi: 10.1016/j.chroma.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 28.National Nuclear Data Center. http://www.nndc.bnl.gov.