Abstract
This report provides a summary of deliberations conducted under the charge for members of Module C Panel participating in the Naphthalene State-of-the-Science Symposium (NS3), Monterey, CA, October 9–12, 2006. The panel was charged with reviewing the current state of knowledge and uncertainty about naphthalene metabolism in relation to anatomy, physiology and cytotoxicity in tissues observed to have elevated tumor incidence in these rodent bioassays. Major conclusions reached concerning scientific claims of high confidence were that: (1) rat nasal tumor occurrence was greatly enhanced, if not enabled, by adjacent, histologically related focal cellular proliferation; (2) elevated incidence of mouse lung tumors occurred at a concentration (30 ppm) cytotoxic to the same lung region at which tumors occurred, but not at a lower and less cytotoxic concentration (tumorigenesis NOAEL = 10 ppm); (3) naphthalene cytotoxicity requires metabolic activation (unmetabolized naphthalene is not a proximate cause of observed toxicity or tumors); (4) there are clear regional and species differences in naphthalene bioactivation; and (5) target tissue anatomy and physiology is sufficiently well understood for rodents, non-human primates and humans to parameterize species-specific physiologically based pharmacokinetic (PBPK) models for nasal and lung effects. Critical areas of uncertainty requiring resolution to enable improved human cancer risk assessment were considered to be that: (1) cytotoxic naphthalene metabolites, their modes of cytotoxic action, and detailed low-dose dose–response need to be clarified, including in primate and human tissues, and neonatal tissues; (2) mouse, rat, and monkey inhalation studies are needed to better define in vivo naphthalene uptake and metabolism in the upper respiratory tract; (3) in vivo validation studies are needed for a PBPK model for monkeys exposed to naphthalene by inhalation, coupled to cytotoxicity studies referred to above; and (4) in vivo studies are needed to validate a human PBPK model for naphthalene. To address these uncertainties, the Panel proposed specific research studies that should be feasible to complete relatively promptly. Concerning residual uncertainty far less easy to resolve, the Panel concluded that environmental, non-cytotoxic exposure levels of naphthalene do not induce tumors at rates that can be predicted meaningfully by simple linear extrapolation from those observed in rodents chronically exposed to far greater, cytotoxic naphthalene concentrations.
Keywords: Naphthalene; Cancer; Carcinogenicity; Tumorigenesis; Mode-of-action; Mechanism of action; Cytotoxicity; Olfactory epithelial neuroblastoma; Respiratory epithelial adenoma; Inflammation; Metabolism; Cytochrome P450; 1,2-Naphthoquinone; 1,4-Naphthoquinone; 1,2-Epoxide; Glutathione depletion; Species differences; Age-dependent differences; Gender differences; PBPK modeling
1. Introduction
The current state of knowledge and uncertainty about naphthalene metabolism in relation to anatomy, physiology and cytotoxicity in tissues observed to have elevated tumor incidence in rodent bioassays was the focus of the review for Panel C of the Naphthalene State of the Science Symposium (NS3) (Belzer et al., 2008). As discussed by North et al. (2008), these bioassays found increased incidences of nasal respiratory epithelial adenomas and of rare nasal olfactory epithelial neuroblastomas in female rats, and of nasal respiratory epithelial adenomas in male rats exposed to naphthalene vapor concentrations of 10, 30, or 60 ppm for 2 years (NTP, 2000; Abdo et al., 2001; Long et al., 2003). There were increased incidences of alveolar/bronchiolar adenomas or carcinoma in female (but not male) B6C3F1 mice exposed to 30 (but not to 10) ppm naphthalene for 2 years (NTP, 1992; Abdo et al., 1992). A previous study found increased tumor multiplicity in tumor-bearing A/J strain mice exposed to 10 or 30 ppm for 6 months (Adkins et al., 1986). The few case reports of cancer in naphthalene-exposed humans involve four laryngeal cancer cases among smokers in workers at an East German naphthalene purification plant (Kup, 1978; Wolf, 1976,1978), and a series of 11 colorectal carcinoma patients 18 to 30 years old admitted to a Nigerian hospital, “half” of whom “gave a definitive history of ingesting” the “naphthalene compound” kafura used as part of “local indigenous treatment for ‘piles’ or any anorectal problem” (Ajao et al., 1988). These case reports have been viewed as insufficient to evaluate human carcinogenicity of naphthalene (EPA, 1998,2004,2002).
The proposed U.S. Environmental Protection Agency (EPA) reclassification of potential human carcinogenicity of naphthalene from “possible” to “likely” and accompanying linear no-threshold risk extrapolations (EPA, 2004), follows a classification of naphthalene by the International Agency for Cancer Research (IARC, 2002) as a 2B carcinogen (possibly carcinogenic to humans), based on the same bioassay data. The EPA proposal was based primarily on the proposition that combined data from life-time bioassays of cancer induced in B6C3F1 mice and F344/N rats exposed chronically to naphthalene by inhalation (NTP, 1992,2000) are plausibly consistent with an exclusively or predominantly genotoxic mode of action. The EPA (1998) had previously classified naphthalene as a possible human carcinogen (Group C, inadequate human and limited animal data), concluding that its human carcinogenic potential could not be determined from available suggestive rodent tumor data, and that “it appears unlikely that naphthalene represents a genotoxic carcinogen.” The proposed EPA (2004) reclassification of naphthalene as a likely human carcinogen with a plausibly genotoxic mode of action thus hinged pivotally on interpretation of recent NTP (2000) bioassay data of naphthalene in rats, further supported by evidence that key naphthalene metabolites, such as 1,2- and/or 1,4-naphthoquinone, showed apparent genotoxic and/or mutagenic activity in a relatively small subset of prokaryotic and eukaryotic in vitro studies (Arfsten et al., 1994; Flowers-Geary et al., 1996; NTP, 1992; Wilson et al., 1996; Yu et al., 2002; Schreiner, 2003; and Brusick, 2008). No consideration, however, was given by the EPA to dose–response relationships observed in genotoxicity assays interpreted as “positive.” Specifically, the EPA (2004) considered neither whether cytotoxic oxidative damage and attendant DNA fragmentation may have occurred in “positive” genotoxicity assays observed for naphthalene or its metabolites, nor whether these endpoints occurred with similar and substantially non-linear low-dose dose–response relationships consistent with a cytotoxic mechanism of apparent “genotoxic” action.
Current bioassay data clearly establish that chronic inhalation of naphthalene can induce respiratory tract tumors. Classification of naphthalene “carcinogenicity,” per se, begs the critical scientific question of whether at very low environmental concentrations this compound presents a cancer risk either to humans or to rodents. Major conclusions reached by Panel C on factual issues quintessential for assessing cancer risk from low-level environmental naphthalene exposures are summarized below. These conclusions address the Panel’s charge to summarize conclusions it reached concerning: (1) scientific claims considered to be of high confidence, (2) key uncertainties that could be addressed by cost-effective research feasible to complete relatively promptly and how corresponding research results should be interpreted, and (3) the Panel’s best scientific judgments concerning those quintessential uncertainties not resolvable by prompt, cost-effective research.
2. Scientific claims with high confidence
2.1. NTP rat nasal tumor occurrence was greatly enhanced, if not enabled, by adjacent, histologically related focal cellular proliferation
Histopathology findings obtained from the NTP (2000) bioassay strongly suggest that nasal tumor formation in rats chronically exposed to naphthalene by inhalation was associated with, if not enabled solely, by chronic tissue damage and associated regenerative and focal hyperplasia. The extent of chronic nasal cytotoxicity and hyperplasia detected in nearly 100% of all exposed animals (regardless of dose group) was described by Long et al. (2003) as follows (bold added):
Neuroblastomas occurred amid a complex spectrum of non-neoplastic lesions of the olfactory epithelium. The principal non-neoplastic proliferative lesion was atypical hyperplasia, which… consisted of proliferating nests of dysplastic olfactory epithelial cells… and/or multifocal nodular proliferations of basal cells extending into the submucosa… The hyperplastic cells were deeply basophilic and, in many areas, continuous with the neoplasms. Such continuity was most clearly observed in association with small neuroblastomas. Atrophy of olfactory epithelium was characterized by… loss of epithelial cells… there was also loss of olfactory neurons. The most severe lesions had complete loss of sustentacular cells and neurons, leaving only basal epithelial cells.
Respiratory epithelial adenomas also occurred amid a spectrum of non-neoplastic lesions of the respiratory epithelium and the submucosal glandular epithelium.…In a few animals, focal proliferation of hyperplastic cuboidal respiratory epithelium resembled early adenoma formation.
…Although the incidence and severity of these non-neoplastic lesions frequently increase in an exposure-dependent manner, they commonly occur with no evidence of nasal carcinogenicity, indicating that factors other than the extent of tissue injury from chronic nasal toxicity contribute to nasal carcinogenesis… Atypical hyperplasia of the olfactory basal cells occurred at very high frequencies in all male and female groups exposed to naphthalene. This was considered an unusual proliferative lesion, because it had not been reported in previous NTP inhalation studies. Morphologically, these cells were similar to, and frequently formed a continuum with, those of the neuroblastomas. This appearance suggests that the atypical hyperplasia may represent a precursor for nasal olfactory carcinogenesis. In addition, a few animals had localized proliferative changes of the respiratory epithelium that were morphologically similar to respiratory epithelial adenomas.
This description of cytotoxicity and hyperplasia in direct association with both tumor types (nasal neuroblastomas and nasal respiratory epithelial adenomas) is similar to descriptions of multistep neoplastic transformation from focal hyperplastic tissue to nodular hyperplasia to adenomas or carcinomas observed in chemically induced or promoted carcinogenesis in the rodent liver and gastrointestinal tract (Farber, 1984; Farber and Cameron, 1984; Pitot et al., 2000). While initiation with a genotoxic agent is typically used in these experimental rodent carcinogenesis systems in order to generate observable tumor rates, subsequent promotion involving either enhanced cell proliferation, or just oxidative stress associated with additional genotoxic or cytotoxic exposure(s) (Sanchez-Perez et al., 2005), can be required to elevate these rates to observable levels. Naphthalene was clearly cytotoxic to epithelial and neural cells in nasal tissue of exposed NTP (2000) bioassay rats. The cytotoxic damage and regenerative hyperplasia strongly support the notion that these effects likely amplified the incidence of tumor occurrence in that study, through clonal expansion of premalignant cell populations that then became available for subsequent malignant transformation. A partly genotoxic mode of action cannot be ruled out, as may be indicated by absence of nasal tumors in mice chronically exposed to 10 or 30 ppm naphthalene, despite evidence of nasal irritation, nasal respiratory epithelium hyperplasia, and nasal olfactory-epithelium meta-plasia in these mice (NTP, 1992; Abdo et al., 1992). However, in contrast to the spectrum of non-neoplastic nasal lesions including atypical, strongly basophilic, multi-focal and/or “unusual” types—some involving moderate to severe atrophy—detected in naphthalene-exposed rats (Long et al., 2003) discussed above, average severity scores reported for non-neoplastic nasal lesions that occurred in similarly exposed male and female mice all fell within a “minimal” to “mild” range, below scores ≥3 associated with “moderate” or “severe” toxicity (Abdo et al., 1992). The combined rodent bioassay evidence therefore indicates a likely predominant cytotoxic mode of action contributing to the nasal tumors observed in naphthalene-exposed rats. Consequently, cancer risk associated with a plausible genotoxic mode-of-action component cannot meaningfully be either estimated at bioassay doses, or extrapolated to low environmental doses, from the NTP (2000) rat nasal tumor data.
2.2. Elevated incidence of mouse lung tumors occurred at a concentration (30 ppm) cytotoxic to the same lung region at which tumors occurred, but not at a lower and less cytotoxic concentration (tumorigenesis NOAEL = 10 ppm)
The conclusion regarding mode-of-action for nasal tumors in naphthalene-exposed rats is also appropriate for alveolar/bronchiolar adenomas (and one carcinoma) in female B6C3F1 mice, which tumors were observed after chronic exposure to 30 ppm but not 10 ppm naphthalene (NTP, 1992,2002; Abdo et al., 1992). The respective chronic naphthalene exposures produced dose-dependent cytotoxicity in the same (distal-bronchial/alveolar) lung region in mice, but not in rats (West et al., 2001). Non-ciliated Clara cells exhibited relatively high susceptibility to naphthalene-induced cytotoxicity. These cells also demonstrate the greatest capacity to metabolize naphthalene (see below).
2.3. Naphthalene cytotoxicity requires metabolic activation; unmetabolized naphthalene is not a proximate cause of observed toxicity or tumors
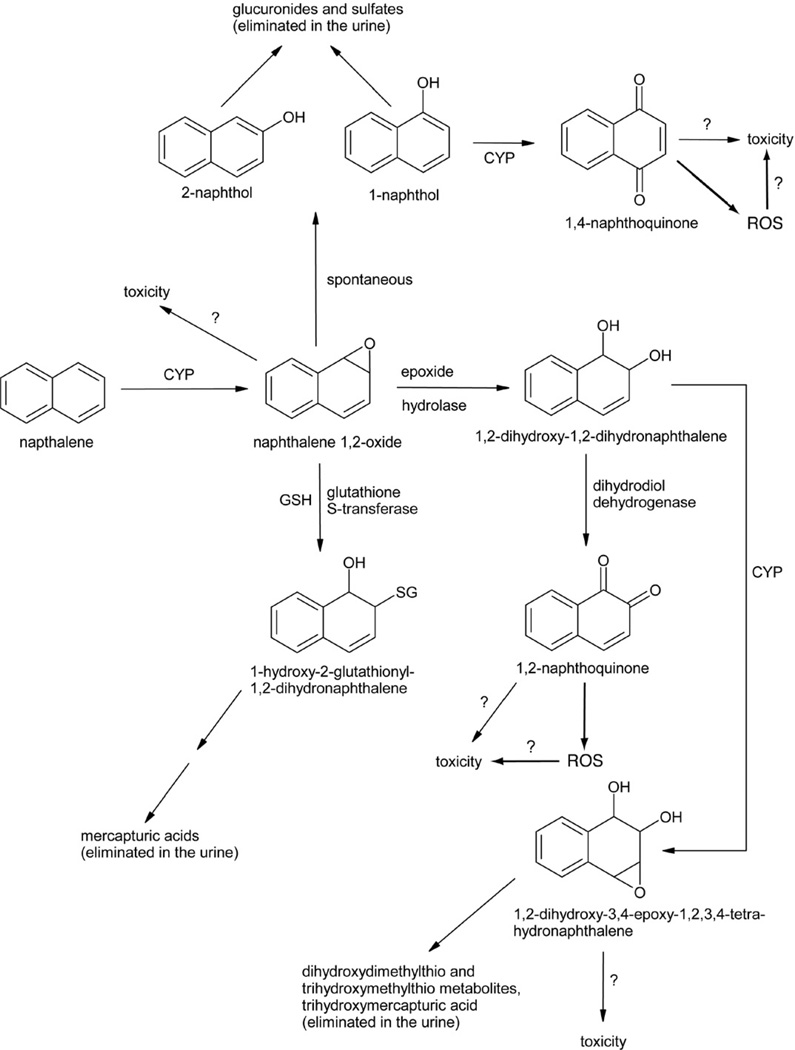
Fairly detailed pathways of naphthalene metabolism have been identified (reviewed in Buckpitt and Franklin, 1989; Buckpitt et al., 2002; Waidyanatha et al., 2002; EPA, 2004; ATSDR, 2005). Briefly, naphthalene is metabolically activated by one or more forms of cytochrome P450 (CYP) to a chiral 1,2-epoxide, which can react directly to form covalent adducts with cellular nucleophiles such as DNA and protein, or undergo subsequent transformation to other reactive metabolites or to detoxified intermediates that are excreted, primarily in urine (Fig. 1). Glutathione-S-transferase (GST) appears to play a key detoxification role in ameliorating the cytotoxicity associated with the naphthalene 1,2-epoxide derivative, as glutathione depletion before naphthalene exposure enhances acute naphthalene-induced injury in mouse-lung Clara cells, cells that are likely targeted by naphthalene metabolites during the initiation process of mouse lung tumorigenesis (Warren et al., 1982; West et al., 2000; Plopper et al., 2001; Phimister et al., 2004). In isolated murine Clara cells, decreased cell viability was non-detectable at naphthalene concentrations ≤0.1 mM but was substantial (≥63%) at naphthalene concentrations ≥0.5 mM, and the latter cytotoxic effects were blocked by preincubation with the CYP inhibitor, piperonyl butoxide (Chichester et al., 1993). In those isolated cells, incubation with 0.5 mM 1,2-dihydrodioxy-1,2-dihydronaphthalene (henceforth referred to as dihydrodiol), 1-naphthol, or 1,2-naphthoquinone decreased cell viability approximately as effectively as 0.5 mM naphthalene, whereas incubation with 0.5 M naphthalene oxide or 1,4-naphthoquinone significantly decreased viability more than the same concentration of parent compound; moreover, naphthalene-oxide-induced cytotoxicity was not blocked with piperonyl butoxide (Chichester et al., 1993). In isolated mouse-lung Clara cells exposed to naphthalene, 1,2-naphthoquinone was one of two detected types of covalent, naphthalene-related protein adduct (Zheng et al., 1997). Incubation of isolated murine hepatocytes with the (1S,2R)-naphthalene-oxide enantiomer—which is converted much more slowly to the dihydrodiol than the (1R,2S)-epoxide—resulted in nearly complete loss of cell viability, whereas incubation with the (1R,2S) enantiomer with a shorter half life had almost no effect on cell viability (Buonarati et al., 1989).
Fig. 1.
Scheme for naphthalene metabolism and formation of multiple reactive metabolites that may be involved in naphthalene toxicity. Adapted from Buckpitt et al., 2002; Waidyanatha et al., 2002; ATSDR, 2005; CYP, cytochrome P450 enzymes(s) including (but perhaps not limited to) CYP2F; SG, glutathionyl moiety; GSH, reduced glutathione; ROS, reactive oxygen species.
Naphthalene did not activate transcription mediated by aryl hydrocarbon receptor (AHR) in an in vitro luciferase reporter assay, and while knockout mice homozygous for deficiency in AHR, in CYP1A1 or in CYP1A2 dosed with naphthalene—regardless of pretreatment with the CYP2F inhibitor 5-phenyl-1-pentyne—all exhibited olfactory toxicity, CYP1A1- and CYP1A2-null mice pretreated with that CYP2F inhibitor exhibited no naphthalene olfactory toxicity (Genter et al., 2006). These studies demonstrated that CYP1A and CYP1A2 genes, which are inducible by AHR in the mouse respiratory tract, do not function to influence naphthalene-induced toxicity, and confirm previous observations (Phimister et al., 2004) that CYP2F2 bioactivates naphthalene in that target tissue to one or more reactive metabolites that induce cytotoxicity after depleting glutathione.
Pooled human liver microsomes (pHLMs) were found to metabolize naphthalene to trans-1,2-dihydro-1,2-naphthalenediol (dihydrodiol), 1-naphthol, and 2-naphthol, with corresponding kinetics characterized by Km values of 23, 40, and 116 µM and Vmax values of 2860, 268, and 22 pmol/mg protein/min, respectively (Cho et al., 2006). CYP isoform screening in this study identified CYP1A2 as the most efficient isoform for producing dihydrodiol and 1-naphthol, and CYP3A4 as the most effective for 2-naphthol production. Whereas further pHLM metabolism of 2-naphthol was found to produce 2,6- and 1,7-dihydroxynaphthalene, dihydrodiol and 1-naphthol were not efficiently metabolized by pHLMs (Cho et al., 2006). CYP1A2 and 2D6*1 were identified as the most active isoforms for producing 1,4-naphthoquinone, and CYP3A4 and CYP2A6 the most active at metabolizing dihydrodiol, though at rates less than those at which 1-naphthol was observed to be metabolized (Cho et al., 2006).
Necrosis of bronchial epithelial (Clara) cells in mice (O’Brien et al., 1985, 1989; Tong et al., 1981) and necrosis of olfactory epithelial cells in mice, rats and hamsters (Plopper et al., 1992) following intraperitoneal injection of naphthalene strongly indicate that metabolic activation in target tissues plays a dominant, and possibly exclusive, role in site-specific naphthalene cytotoxicity. There is no evidence that unmetabolized naphthalene is cytotoxic, or that unmetabolized naphthalene is genotoxic at non-cytotoxic concentrations. No tumors were observed in tissues where naphthalene was not cytotoxic.
2.4. There are clear regional and species differences in naphthalene bioactivation
Naphthalene bioactivation varies considerably among species and among different anatomical regions of the respiratory tract (Buckpitt and Bahnson, 1986; Buckpitt et al., 1992,1995,2002; Thornton-Manning and Dahl, 1997; Baldwin et al., 2004,2005; Boland et al., 2004). Relative to microsomal preparations made from mouse, those derived from hamster, rat, and rhesus macaque lung tissue were observed to metabolize naphthalene to dihydrodiol and glutathione conjugates at relative rates of 37%, 12%, and 1%, respectively. The enantiomeric form of the metabolites also vary among organs. For example, the mean (±1SD) of lung-to-liver ratios for values of the ratio between 1R,2S- and 1S,2R-epoxide enantiomers formed by organ-specific mouse microsomal preparations was measured to be 7.9 ± 2.0 at substrate concentrations ranging from 0.06 to 1 mM naphthalene, which contrasts with a lung-to-liver ratio of 1.3 ± 0.3 observed for the total rate of metabolic activation over this range of substrate concentrations (calculated from Table 1 of Buckpitt et al., 1992). Studies investigating naphthalene bioactivation in lung to dihydrodiol and glutathione conjugates demonstrated that the distal bronchiole region possesses the greatest activity in mice, rats and hamsters (Buckpitt et al., 1995). The total metabolic activity in this region was about 10-fold greater in mice than in rats. In contrast, GST levels varied only about 2-fold across species or lung tissue region (Buckpitt et al., 1995).
Table 1.
Summary of proposed studies of uptake and metabolism in rodents and monkeys
| Species | Endpointa | Number (n) of animals per time point |
Proposed sacrifice times |
Commentsa |
|---|---|---|---|---|
| Rodents (B6C3F1 mice, F344/Nrats) | URT uptake | 8 to 10 per group | 1 h | 2 or 3 inspiratory flow rates, in both normal and metabolically (CYP450-) inhibited animals |
| Initial body burden | 4 | 0 h | ||
| Tissue distribution and clearance | 3 | 0.5, 1, 4, 8, 24, 48, and 72 h | ||
| Excretion | ≥5 | 7 days | Adjust n as needed to provide sufficient sample for use by collaborating scientists biomarkers | |
| Non-human primates | Initial body burden | 3 | 0 h | |
| URT uptake | 3 | 0 h | 2 or 3 inspiratory flow rates in each animal | |
| Tissue distribution and clearance | 3 | 1, 8, 24, 48 and 72 h | Modify as needed based on rodent study results | |
| Excretion | 5 | 7 days | Make samples available to co-investigators of parallel studies |
Each study should use ≥ 4 concentrations spanning those in each species that show toxicity and those corresponding to the no e.ect level for respiratory tract lesions, including ≥ 2 intermediate concentrations allowing quantitative characterizations of cytotoxic dose–response.
In the nose, naphthalene bioactivation using microsomal preparations made from the olfactory region of the mouse, hamster, rat nasal mucosa was 2- to 3-fold greater than from septum or lateral-wall regions (Buckpitt et al., 1992). The 1R,2S-epoxide was the predominant enantiomer formed in all three species (Buckpitt et al., 1992).
Rates of formation of naphthalene 1R,2S-oxide in mouse, rat, and hamster airway explant incubations correlate well with immunolocalization of CYP2F2, but not with CYP2B4 that also is found in pulmonary Clara cells; and CYP2B4 inhibition does not block naphthalene metabolism by mouse lung microsomal enzymes (Buckpitt et al., 1995; Roberts et al., 1993). A subsequent immunolocalization study (complemented by peptide mass fingerprinting, and RT-PCR analysis of CYP2F mRNA expression) failed to detect CYP2F in rhesus macaque tissue of any kind studied other than nasal ethmoturbinates, where levels were 10- and 20-fold lower than in corresponding rat and mouse tissue, respectively (Baldwin et al., 2004). However, human liver microsomes convert naphthalene to its dihydrodiol intermediate at faster rates than mouse and rat liver microsomes (Kitteringham et al., 1996).
The human enzyme that is orthologous to the mouse CYP2F2 enzyme is CYP2F1. The CYP2F1 mRNA has been identified in human respiratory tissues by a number of different laboratories (see Raunio et al., 1999; Ding and Kaminsky, 2003). The CYP2F1 enzyme was expressed in lymphoblastoid cells and shown to metabolize naphthalene to its epoxide, albeit at very low rates (Lanza et al., 1999). This enzyme is highly unstable (Baldwin et al., 2005), but it has been over-expressed in a human bronchial epithelial cell line (Nichols et al., 2003), and used to evaluate the mechanisms of cytotoxicity of 3-methylindole, a prototypical pneumotoxicant, and the bioactivation of benzene (Sheets et al., 2004). This cell line could be used for cytotoxicity and mutagenesis studies with naphthalene.
Because naphthalene is not considered capable of inducing cytotoxicity (or genotoxicity) without metabolic activation, and because there are clear regional and species differences in naphthalene bioactivation, estimation of potential human cancer risk associated with naphthalene exposure—regardless of assumed mode of action—cannot be done meaningfully based on rodent bioassay results characterized simply in terms of bioassay-administered naphthalene doses or concentrations. Administered naphthalene doses or concentrations must first be converted to an estimated biologically effective dose at the target tissue. Estimation of an average dose in the entire target organ (e.g., rate of metabolism of naphthalene in the lung per gram of lung tissue) would be less satisfactory than an estimate for the region in which toxicity is observed (e.g., the nasal olfactory region or the terminal bronchiolar region of the lung). A number of different surrogates for the biologically effective dose could be considered, depending on the richness of the data available on the metabolism of naphthalene and its metabolites in the target tissues of both the experimental animal and the human. At a minimum, administered dose or concentration should be replaced with peak or average daily rate of metabolism in the target tissue (but not total metabolism in the body, which would be dominated by the liver). If sufficient data are available, a preferred surrogate closer to the ultimate toxic form could be used, such as mean or peak intracellular concentration of (total, or only 1R,2S enantiomeric) naphthalene-oxide, or of 1,2-naphthoquinone in the target tissue. Physiologically based pharmacokinetic (PBPK) models developed for naphthalene (e.g., Sweeney et al., 1996; Quick and Shuler, 1999; Ghanem and Shuler, 2000; Willems et al., 2001) demonstrate a reasonable approach to estimate such reasonably plausible measures of surrogate biologically effective dose as a function of bioassay-administered dose. However, the current models are only capable of estimating doses for the total lung. Additional elaboration of these models would be required to extend dosimetry to the nose as well as to support regional dosimetry within the nose and lung.
2.5. Target tissue anatomy and physiology is sufficiently well understood for rodents, non-human primates and humans to parameterize species-specific PBPK models for nasal and lung effects
Based on animal carcinogenicity and cytotoxicity data, the target tissue of primary concern for potential human cancer risk posed by environmental naphthalene exposure is respiratory tract (including nasal) epithelium. Current understanding of the anatomy and physiology of nasal/respiratory tissues in rodents, primates and humans is adequate to extend the PBPK models developed for naphthalene nasal and lung effects (e.g., Sweeney et al., 1996; Quick and Shuler, 1999; Ghanem and Shuler, 2000; Willems et al., 2001) to include a more detailed description of target tissues. An existing model for a similar compound, styrene (Sarangapani et al., 2002), would help to inform this effort. Further improvements would result from additional data, particularly on (i) blood:air and tissue:air partition coefficients for naphthalene; (ii) the concentration- and airflow-dependence of naphthalene uptake in the upper respiratory tract (URT) in rodents and primates; (iii) region-specific metabolism of naphthalene in rodent, primate and human tissues; and (iv) relative toxicities of naphthalene and its metabolites in different regions of the nose and lungs of rodents and non-human primates. The human bronchial epithelial cell line that over-expresses CYP2F1 could be used to evaluate metabolism and cellular effects in a relevant human cell line.
3. Uncertainties quintessential for human cancer risk assessment, and feasible experiments that could be done to reduce or eliminate these uncertainties
3.1. Cytotoxic naphthalene metabolites, their modes of cytotoxic action, and detailed low-dose dose–response need to be clarified, including in rodent, primate and human tissues, and in neonatal tissues
The possibility that low-dose linear risk extrapolation of tumor risk from data gathered at high, cytotoxic doses may introduce profound conservative bias hinges on the assumption that cytotoxicity and regenerative hyperplasia have a quasi-threshold type of dose–response. In vitro experiments can quantify and characterize the low-dose dose–response for cell killing in relevant target tissues and species, allowing meaningful inter-species and dose extrapolation for this critical endpoint.
A series of studies over 1 to 2 years could apply short-term in vitro assays to quantify naphthalene- or metabolite-induced reduction in target-cell viability in B6C3F1 mouse, F344/N rat, primate and human explants of regionally defined respiratory and nasal olfactory epithelia. Selection of particular regions and target cell types for focused investigation should be informed by results of experiments proposed in this issue by North et al. (2008) concerning species differences in acute and subchronic toxicity to inhaled naphthalene. Alternative measures of putative biologically effective dose (BED) should be characterized, such as protein binding, DNA adducts, abasic DNA sites, GST levels, and oxidative stress, measured using F-2 isoprostanes in exposed tissue and/or in culture media or by other means. The F2-isoprostanes are viewed as the most reliable, sensitive, and specific biomarkers of oxidative stress (Montuschi et al., 2004; Morrow, 2005). Parallel experiments done using CYP-isozyme-specific enzyme inhibitors (see, e.g., Hynes et al., 1999; Born et al., 2002), by suppressing enzymatic activity through iRNA, or by using explant tissue derived from gene-knockout strains, could provide straightforward tests of alternative metabolic pathway assumptions. The tissue-specific activity or concentration determined to correlate best with reduced cell viability will define the best available corresponding BED metric for that observed cytotoxicity. The quantitative dose–response relation between naphthalene concentration and the BED metric(s) identified will provide key information for corresponding species-specific PBPK models (discussed further in Sections 3.3 and 3.4 below), and are likely to generate testable hypotheses concerning the existence of a non-genotoxic mode of action for naphthalene-induced tumorigenesis. A non-genotoxic mode of action would be supported by evidence that induced cell killing has a sigmoidal, substantially non-linear dose–response relationship with either administered naphthalene concentration or corresponding BED, coupled with evidence that any naphthalene-induced genotoxicity is undetectable at non-cytotoxic naphthalene concentrations.
It will be important to include neonatal tissues among test explants to be examined, in order to investigate potential age-dependent differences in susceptibility to naphthalene-mediated cytotoxicity or developmental toxicity. If feasible, to more reliably characterize potential on developmental variation in rate-limiting metabolic activities governing naphthalene activation and deactivation pathways, remaining questions concerning the ontogeny of CYP2F enzymes should also be investigated (Choudhary et al., 2003,2005). Additional studies may be required to characterize other enzymes associated with the production and clearance of the key metabolites of naphthalene, including naphthalene-oxide (both enantiomers) and 1,2-naphthoquinone, as indicated by the evidence regarding the most appropriate measure of BED. These additional enzyme studies should be performed in rodent, primate, and human tissues.
3.2. Mouse, rat, and monkey inhalation studies are needed to better define in vivo naphthalene uptake and metabolism in the upper respiratory tract (URT)
Better understanding of comparative URT uptake and in situ metabolism of naphthalene is required to interpret observed species differences in URT cytotoxicity. A study requiring approximately 1 year to complete could apply acute (and, as feasible, repeated acute-dose) in vivo assays to quantify and compare URT uptake and metabolism of naphthalene administered by inhalation, as outlined in Table 1. Parallel studies with and without CYP inhibition will allow confirmation of hypotheses generated using more detailed in vitro explant studies. These in vivo studies should be done using different naphthalene concentrations expected (based on in vitro studies and in vivo inhalation studies) to induce a range of cytotoxic severity, again focusing on concentrations expected to shed the greatest light on low-dose dose–response relations (but also including 10 and 30 ppm naphthalene as bioassay-related reference points). Tissue- and region-specific CYP enzymes and their relative activities should be identified and quantified in these studies. This study should be coupled to the PBPK-model validation study proposed below. Selection of specific concentrations for focused investigation should be informed by results of experiments proposed by North et al. (2008) concerning species differences in acute and subchronic toxicity to inhaled naphthalene.
3.3. In vivo validation studies are needed for a PBPK model for monkeys exposed to naphthalene by inhalation, coupled to cytotoxicity studies referred to above
A monkey PBPK model needs to be validated in order to link data gathered from naphthalene cytotoxicity and DNA-damage studies in naphthalene-exposed monkeys and rodents to improved understanding of cancer bioassay results, and thereby to improve biologically based human risk prediction. A study requiring approximately 1 year to complete could be performed to collect blood and tissue data on naphthalene, naphthalene-oxide, urinary adducts, and related measures from naphthalene-exposed monkeys. In these studies, monkeys in metabolism cages would be exposed to 14C-radiolabeled naphthalene for one or repeated 6-h periods. Tissues would be obtained for analysis upon serial sacrifice. Tissue- and region-specific P450 enzymes and their relative activities should be identified and quantified in these studies. Such data are needed to obtain reliable parameter estimates for a PBPK model of naphthalene in monkeys, similar to models cited above already developed for rodents. Such a study should include determinations of blood:air and tissue:air partition coefficients for naphthalene for monkey, rat and mouse, as well as the human blood:air partition coefficient for naphthalene.
3.4. In vivo studies are needed to validate a human PBPK model for naphthalene
A human PBPK model needs to be validated in order to link data gathered from proposed studies of naphthalene cytotoxicity, and of naphthalene DNA-damage (Brusick, 2008), in naphthalene-exposed monkeys and rodents to improved understanding of cancer bioassay results, and thereby to improved biologically based human risk prediction. Validation in this context would focus on estimation of key parameters that are feasible to measure in a 1-year time-frame in a series of short-term experiments using human volunteers under an IRB- and USEPA-HSRB-approved study protocol. These experiments would involve controlled administration of approximately 1 part per trillion of 14C-radiolabeled naphthalene in air (i.e., a concentration that is a fraction of ambient levels of naphthalene typically found in indoor air, as discussed in Griego et al., 2008 by inhalation for 6 h. Naphthalene and metabolites in blood and urine at these exposure levels can be analyzed readily by accelerator mass spectrometry (AMS) (see, e.g., Bogen et al., 1998; Dingley et al., 1998; Williams et al., 2002; Cupid et al., 2004). Measured samples should include at least hourly blood samples. Interindividual variation in metabolic capacity can be assessed in vivo by experiments involving a larger number of (e.g., ≥40) subjects exposed for just one hour. To assess possible saturation-related non-linearity in naphthalene metabolism or adduct formation, results from the acute 6-h study done at very low concentration should be compared to results obtained using the same administered concentration of 14C-radiolabeled naphthalene diluted in a substantially larger concentration of unlabeled naphthalene (e.g., 0.1 or 1 ppm in air from a mothball that is partly unwrapped to expose a precisely defined uncovered surface area).
4. Best scientific judgment about quintessential uncertainty not resolvable by prompt cost-effective research
4.1. Could naphthalene induce tumors at environmental, non-cytotoxic exposure levels at rates predictable from currently available data?
The panel’s unanimous opinion is that, based on currently available data, it is extremely improbable that environmental, non-cytotoxic exposure levels of naphthalene induce tumors at rates that can be predicted meaningfully by simple linear extrapolation from those observed in rodents chronically exposed to far greater, cytotoxic naphthalene concentrations. Results from studies proposed in Section 2 are required to confirm this hypothesis beyond a reasonable doubt.
Acknowledgments
This article was developed in part under EPA Assistance Agreement No. 83330401-0 awarded by the U.S. Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Additional grant support was provided by the Electric Power Research Institute, the American Petroleum Institute, the Naphthalene Council, Inc., the Association of Railroads, the American Coke and Coal Chemicals Institute, the Asphalt Institute, and the National Petrochemical Refiners Association. It has not been reviewed by any of these sponsors, and does not necessarily represent their views.
Finally, Regulatory Checkbook, the 501(c) (3) nonprofit organization that coordinated the project, also provided funding from unrestricted support and retained earnings. More details about project objectives, organization, structure, and charge can be found in Belzer et al., pages 1–5, in this issue.
Footnotes
Conflict of interest disclosure
The authors declare that they have no conflicts of interest. Each received an honorarium from Regulatory Checkbook, PO Box 319, Mt. Vernon, VA 22121, for service as set forward in Belzer et al. (2008).
References
- Abdo KM, Eustis SL, McDonald M, Jokinen MP, Adkins B, Jr, Haseman JK. Naphthalene: a respiratory tract toxicant and carcinogen for mice. Inhal. Toxicol. 1992;4:393–409. [Google Scholar]
- Abdo KM, Grumbein S, Chou BJ, Herbert R. F344 rats following 2 years of whole-body exposure to naphthalene vapors. Inhal. Toxicol. 2001;13:931–950. doi: 10.1080/089583701752378179. [DOI] [PubMed] [Google Scholar]
- Adkins KM, Van Stee EW, Simmons JE, Eustis SL. Oncogenic response of strain A/J mice to inhaled chemicals. Toxicol. Environ. Health. 1986;17:311–322. doi: 10.1080/15287398609530825. [DOI] [PubMed] [Google Scholar]
- Ajao OG, Adenuga MO, Ladipo JK. Colorectal carcinoma in patients under the age of 30 years: a review of 11 cases. J. R. Coll. Surg. Edinb. 1988;33:277–279. [PubMed] [Google Scholar]
- Arfsten DP, Davenport R, Schaeffer DJ. Reversion of bioluminescent bacteria (MutatoxTM) to their luminescent state upon exposure to organic compounds, munitions, and metal salts. Biomed. Environ. Sci. 1994;7:144–149. [PubMed] [Google Scholar]
- Agency for Toxicological Substance Disease Registry (ATSDR) Toxicological profile for naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. August 2005. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, ATSDR; 2005. [Google Scholar]
- Baldwin RM, Jewell WT, Fanucchi MV, Plopper CG, Buckpitt AR. Comparison of pulmonary/nasal CYP2F expression levels in rodents and Rhesus Macaque. J. Pharmacol. Exp. Ther. 2004;309:127–136. doi: 10.1124/jpet.103.062901. [DOI] [PubMed] [Google Scholar]
- Baldwin RM, Shultz MA, Buckpitt AR. Bioactivation of the pulmonary toxicants naphthalene and 1-nitronaphthalene by rat CYP2F4. J. Pharmacol. Exp. Ther. 2005;312:857–865. doi: 10.1124/jpet.104.075440. [DOI] [PubMed] [Google Scholar]
- Belzer RB, Bus JS, Cavalieri EL, Lewis SC, North DW, Pleus RC. Naphthalene State of the Science Symposium: objectives, organization, structure, and charge. Regul. Toxicol. Pharmacol. 2008;51(Suppl. 1):S1–S5. doi: 10.1016/j.yrtph.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Bogen KT, Keating GA, Meissner S, Vogel JS. Initial uptake kinetics in human skin exposed to dilute aqueous trichloroethylene in vitro. J. Expo. Anal. Environ. Epidemiol. 1998;8:253–271. [PubMed] [Google Scholar]
- Boland B, Lin CY, Morin D, Miller L, Plopper C, Buckpitt A. Site-specific metabolism of naphthalene and 1-nitronaphthalene in dissected airways of Rhesus Macaques. J. Pharmacol. Exp. Ther. 2004;310(2):546–554. doi: 10.1124/jpet.103.063669. [DOI] [PubMed] [Google Scholar]
- Born SL, Caudill D, Fliter KL, Purdon MP. Identification of the cytochromes P450 that catalyze coumarin 3,4-epoxidation and 3-hydroxylation. Drug Metab. Dispos. 2002;30(5):483–487. doi: 10.1124/dmd.30.5.483. [DOI] [PubMed] [Google Scholar]
- Brusick DJ, Small MJ, Cavalieri EL, Chakravarti D, Ding X, Longfellow DG, Nakamura J, Rogan EG, Swenberg JA. Possible genotoxic modes of action for naphthalene. Regul. Toxicol. Pharmacol. 2008;51(Suppl. 1):S43–S50. doi: 10.1016/j.yrtph.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Buckpitt AR, Bahnson LS. Naphthalene metabolism by human lung microsomal enzymes. Toxicology. 1986;41:331–341. doi: 10.1016/0300-483x(86)90186-1. [DOI] [PubMed] [Google Scholar]
- Buckpitt AR, Franklin RB. Relationship of naphthalene and 2-methylnaphthalene metabolism to pulmonary bronchiolar epithelial cell necrosis. Pharm. Ther. 1989;41:393–410. doi: 10.1016/0163-7258(89)90116-2. [DOI] [PubMed] [Google Scholar]
- Buckpitt A, Buonarati M, Avey LB, Chang AM, Morin D, Plopper CG. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. II. Comparison of stereo selectivity of naphthalene epoxidation in lung and nasal mucosa of mouse, hamster rat, and rhesus monkey. J. Pharmacol. Exp. Ther. 1992;261(1):364–372. [PubMed] [Google Scholar]
- Buckpitt A, Chang AM, Weir A, Van Winkle L, Duan X, Philpot R, Plopper C. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol. Pharmacol. 1995;47(1):74–81. [PubMed] [Google Scholar]
- Buckpitt A, Boland B, Isbell M, Shultz M, Baldwin R, Chan K, Karlsson A, Lin C, Taff A, West J, Fanucchi M, Van Winkle L, Plopper C. Naphthalene-induced respiratory tract toxicity: metabolic mechanisms of toxicity. Drug Metab. Rev. 2002;34(4):791–820. doi: 10.1081/dmr-120015694. [DOI] [PubMed] [Google Scholar]
- Buonarati M, Morin D, Plopper C, Buckpitt A. Glutathione depletion and cytotoxicity by naphthalene 1,2-oxide in isolated hepatocytes. Chem. Biol. Interact. 1989;71:147–165. doi: 10.1016/0009-2797(89)90031-8. [DOI] [PubMed] [Google Scholar]
- Chichester CH, Buckpitt AR, Chang A, Plopper CG. Metabolism and cytotoxicity of naphthalene and its metabolites in isolated murine Clara cells. Mol. Pharmacol. 1993;45:664–672. [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Schenkman JB, Sarfarazi M, Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch. Biochem. Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1-4) during development and in different adult tissues. Arch. Biochem. Biophys. 2005;436:50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Cho TM, Rose RL, Hodgson E. In vitro metabolism of naphthalene by human liver microsomal cytochrome P450 enzymes. Drug Metab. Dispos. 2006;34:176–183. doi: 10.1124/dmd.105.005785. [DOI] [PubMed] [Google Scholar]
- Cupid BC, Lightfoot TJ, Russell D, Gant SJ, Turner PC, Dingley KH, Curtis KD, Leveson SH, Turteltaub KW, Garner RC. The formation of AFB(1)-macromolecular adducts in rats and humans at dietary levels of exposure. Food Chem. Toxicol. 2004;42(4):559–569. doi: 10.1016/j.fct.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- Dingley KH, Freeman SPHT, Nelson DO, Garner RC, Turteltaub KW. Covalent binding of 2-Amino-3,8-dimethylimidazo[ 4,5-f]quinoxaline to albumin and hemoglobin at environmentally relevant doses: comparison of human subjects and F344 rats. Drug Metab. Dispos. 1998;26(8):825–828. [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA) Toxicological Review of Naphthalene (CAS No. 91-20-3) In Support of Summary Information on the Integrated Risk Information System (IRIS) Washington, DC: U.S. EPA Office of Research and Development; 1998. [Google Scholar]
- Environmental Protection Agency (EPA) Toxicological Review of Naphthalene (CAS No. 91-20-3) In Support of Summary Information on the Integrated Risk Information System (IRIS). NCEA-S-1707, June 2004, External review draft. Washington, DC: U.S. EPA Office of Research and Development; 2004. [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984;44:4217–4223. [PubMed] [Google Scholar]
- Farber E, Cameron R. The sequential analysis of cancer development. Adv. Cancer Res. 1984;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Flowers-Geary L, Bleczinski W, Harvey RG, Penning TM. Cytotoxicity and mutagenicity of polycyclic aromatic hydrocarbon o-quinones produced by dihydrodiol dehydrogenase. Chem. Biol. Interact. 1996;99:55–72. doi: 10.1016/0009-2797(95)03660-1. [DOI] [PubMed] [Google Scholar]
- Genter MB, Marlowe J, Kerzee JK, Dragin N, Puga A, Dalton TP, Nebert DW. Naphthalene toxicity in mice and aryl hydrocarbon receptor-mediated CYPs. Biochem. Biophys. Res. Commun. 2006;348:120–123. doi: 10.1016/j.bbrc.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Ghanem A, Shuler ML. Combining cell culture analogue reactor designs and PBPK models to probe mechanisms of naphthalene toxicity. Biotechnol. Prog. 2000;16(3):334–345. doi: 10.1021/bp9901522. [DOI] [PubMed] [Google Scholar]
- Griego FY, Bogen KT, Price PS, Weed DL. Exposure, epidemiology and human cancer incidence of naphthalene. Regul. Toxicol. Pharmacol. 2008;51(Suppl. 1):S22–S26. doi: 10.1016/j.yrtph.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Hynes DE, DeNicola DB, Carlson GP. Metabolism of styrene by mouse and rat isolated lung cells. Toxicol. Sci. 1999;51:195–201. doi: 10.1093/toxsci/51.2.195. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 82. Lyon, France: IARC; 2002. Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. [PMC free article] [PubMed] [Google Scholar]
- Kitteringham NR, Davis C, Howard N, Pirmohamed M, Park BK. Interindividual and interspecies variation in hepatic microsomal epoxide hydrolase activity: studies with cis-stilbene oxide, carbamazepine 10,11-epoxide and naphthalene. J. Pharmacol. Exp. Ther. 1996;278:1018–1027. [PubMed] [Google Scholar]
- Kup W. Work-related origin of cancer in the nose, mouth, throat, and larynx. Akad. Wiss. 1978;2:20–25. (original in German, cited in NTP 1992 and abstracted in Carcinogenesis Abstracts). [Google Scholar]
- Lanza DL, Code e, Crespi CL, Gonzalez FJ, Yost GS. Specific dehydrogenation of 3-methylindole and epoxidation of naphthalene by recombinant human CYP2F1 expressed in lymphoblastoid cells. Drug Metab. Dispos. 1999;27:798–803. [PubMed] [Google Scholar]
- Long PH, Herbert RA, Peckham JC, Grumbein SL, Shackelford CC, Abdo KM. Morphology of nasal lesions in F344/N rats following chronic inhalation exposure to naphthalene vapors. Toxicol. Pathol. 2003;31:655–664. doi: 10.1080/01926230390242016. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes PJ, Roberts LJI. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscl. Thromb. Vasc. Biol. 2005;25:279–286. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (NTP) Technical Report Series No 410. Research Triangle Park, NC: NTP; 1992. Toxicology and Carcinogenesis Studies of Naphthalene in B6C3F1 mice (Inhalation Studies) [PubMed] [Google Scholar]
- National Toxicology Program (NTP) Technical Report Series No 500. Research Triangle Park, NC: NTP; 2000. Toxicology and Carcinogenesis Studies of Naphthalene (CAS No. 91-20-3) in F344/N Rats (Inhalation Studies) [PubMed] [Google Scholar]
- National Toxicology Program (NTP) Report on Carcinogens Background Document for Naphthalene. Research Triangle Park, NC: NTP; 2002. http://ntp-server.niehs.nih.gov/newhomeroc/roc11/NaphthalenePub.pdf. [Google Scholar]
- Nichols WK, Mehta R, Skordos K, Mace K, Pfeifer AM, Carr BA, Minko T, Burchiel SW, Yost GS. 3-Methylindole-induced toxicity to human bronchial epithelial cell lines. Toxicol. Sci. 2003;71:229–236. doi: 10.1093/toxsci/71.2.229. [DOI] [PubMed] [Google Scholar]
- North DW, Abdo KM, Benson JM, Dahl AR, Morris JB, Renne R, Witschi H. A review of whole animal bioassays of the carcinogenic potential of naphthalene. Regul. Toxicol. Pharmacol. 2008;51(Suppl. 1):S6–S14. doi: 10.1016/j.yrtph.2007.09.022. [DOI] [PubMed] [Google Scholar]
- O’Brien KAF, Smith LL, Cohen GM. Differences in naphthalene-induced toxicity in the mouse and rat. Chem. Biol. Interact. 1985;55:109–122. doi: 10.1016/s0009-2797(85)80122-8. [DOI] [PubMed] [Google Scholar]
- O’Brien KA, Suverkropp C, Kanekal S, Plopper CG, Buckpitt AR. Tolerance to multiple doses of the pulmonary toxicant, naphthalene. Toxicol. Appl. Pharmacol. 1989;99(3):487–500. doi: 10.1016/0041-008x(89)90156-7. [DOI] [PubMed] [Google Scholar]
- Phimister AJ, Lee MG, Morin D, Buckpitt AR, Plopper CG. Glutathione depletion is a major determinant of inhaled respiratory toxicity and naphthalene metabolism. Toxicol. Sci. 2004;82:268–278. doi: 10.1093/toxsci/kfh258. [DOI] [PubMed] [Google Scholar]
- Pitot HC, Hikita H, Dragan Y, Sargent L, Haas M. The stages of gastrointestinal carcinogenesis – application of rodent models to human disease. Aliment. Pharmacol. Ther. 2000;14(Suppl. 1):153–160. doi: 10.1046/j.1365-2036.2000.014s1153.x. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Suverkropp C, Morin D, Nishio S, Buckpitt A. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract in mice, rats and hamsters after parenteral administration of naphthalene. J. Pharmacol. Exp. Ther. 1992;261(1):353–363. [PubMed] [Google Scholar]
- Plopper CG, Van Winkle LS, Fanucchi MV, Malburg SRC, Nishio SJ, Chang A, Buckpitt AR. Early events in naphthalene-induced acute clara cell toxicity: 11. Comparison of glutathione depletion and histopathology by airway location. Am. J. Respir. Cell Mol. Biol. 2001;24:272–281. doi: 10.1165/ajrcmb.24.3.4247. [DOI] [PubMed] [Google Scholar]
- Quick DJ, Shuler ML. Use of in vitro data for construction of a physiologically based pharmacokinetic model for naphthalene in rats and mice to probe species differences. Biotechnol. Prog. 1999;15(3):540–555. doi: 10.1021/bp990057t. [DOI] [PubMed] [Google Scholar]
- Raunio H, Hakkola J, Hukkanen J, Lassila A, Paivarinta K, Pelkonen O, Anttila S, Piipari R, Boobis A, Edwards RJ. Expression of xenobiotic-metabolizing CYPs in human pulmonary tissue. Exp. Toxicol. Pathol. 1999;51:412–417. doi: 10.1016/S0940-2993(99)80031-1. [DOI] [PubMed] [Google Scholar]
- Roberts E, Hopkins N, Alaolth W, Hollenberg P. Mechanism based investigation of cytochrome P450 2B1 by 2-ethylnaphthalene: an identification of an active site peptide. Chem. Res. Toxicol. 1993;6:470–479. doi: 10.1021/tx00034a013. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez Y, Carrasco-Legleu C, Garcia-Cuellar C, Perez-Carreon J, Hernandez-Garcia S, Salcido-Neyoy M, Aleman-Lazarini L, Villa-Trevino S. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Lett. 2005;217(1):25–32. doi: 10.1016/j.canlet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Sarangapani R, Teeguarden JG, Cruzan G, Clewell HJ, Andersen ME. Physiologically based pharmacokinetic modeling of styrene and styrene oxide respiratory-tract dosimetry in rodents and humans. Inhal. Toxicol. 2002;14(8):789–834. doi: 10.1080/08958370290084647. [DOI] [PubMed] [Google Scholar]
- Schreiner CA. Genetic toxicity of naphthalene: a review. J. Toxicol. Environ. Health B Crit. Rev. 2003;6(2):161–183. doi: 10.1080/10937400306472. [DOI] [PubMed] [Google Scholar]
- Sheets PL, Yost GS, Carlson GP. Benzene metabolism in human lung cell lines BEAS-2B and A549 and cells overexpressing CYP2F1. J. Biochem. Mol. Toxicol. 2004;18:92–99. doi: 10.1002/jbt.20010. [DOI] [PubMed] [Google Scholar]
- Sweeney LM, Shuler ML, Quick DJ, Babish JG. A preliminary physiologically based pharmacokinetic model for naphthalene and naphthalene oxide in mice and rats. Ann. Biomed. Eng. 1996;24:305–320. doi: 10.1007/BF02667357. [DOI] [PubMed] [Google Scholar]
- Thornton-Manning JR, Dahl AR. Metabolic capacity of nasal tissue interspecies comparisons of xenobiotic-metabolizing enzymes. Mutat. Res. 1997;380:43–59. doi: 10.1016/s0027-5107(97)00126-7. [DOI] [PubMed] [Google Scholar]
- Tong SS, Hirokata Y, Trush MA, Mimnaugh EG, Ginsburg E, Lowe MC, Gram TE. Clara cell damage and inhibition of pulmonary mixed-function oxidase activity by naphthalene. Biochem. Biophys. Res. Commun. 1981;100(3):944–950. doi: 10.1016/0006-291x(81)91914-8. [DOI] [PubMed] [Google Scholar]
- Waidyanatha S, Troester MA, Lindstrom AB, Rappaport SM. Measurement of hemoglobin and albumin adducts of naphthalene-1,2-oxide, 1,2-naphthoquinone and 1,4-naphthoquinone after administration of naphthalene to F344 rats. Chem. Biol. Interact. 2002;141:189–210. doi: 10.1016/s0009-2797(02)00048-0. [DOI] [PubMed] [Google Scholar]
- Warren DL, Brown DL, Buckpitt AR. Evidence for cytochrome P-450 mediated metabolism in the bronchiolar damage by naphthalene. Chem. Biol. Interact. 1982;40:287–303. doi: 10.1016/0009-2797(82)90152-1. [DOI] [PubMed] [Google Scholar]
- West JA, Buckpitt AR, Plopper CG. Elevated airway GSH resynthesis confers protection to Clara cells from naphthalene injury in mice made tolerant by repeated exposures. J. Pharmacol. Exp. Ther. 2000;294(2):516–523. [PubMed] [Google Scholar]
- West JA, Pakehham G, Morin D, Fleschner CA, Buckpitt AR, Plopper CG. Inhaled naphthalene causes dose dependent Clara cell cytotoxicity in mice but not in rats. Toxicol. Appl. Pharmacol. 2001;173(2):114–119. doi: 10.1006/taap.2001.9151. [DOI] [PubMed] [Google Scholar]
- Willems BAT, Melnick RL, Kohn MC, Portier CJ. A physiologically based pharmacokinetic model for inhalation and intravenous administration of naphthalene in rats and mice. Toxicol. Appl. Pharmacol. 2001;176(2):81–91. doi: 10.1006/taap.2001.9269. [DOI] [PubMed] [Google Scholar]
- Williams KE, Carver TA, Mrianda JJL, Kautienen A, Vogel JS, Dingley K, Baldwin MA, Turteltaub KW, Burlingame AL. Atto mole detection of in vivo protein targets of benzene in mice. Mol. Cell. Proteomics. 2002;1(11):885–895. doi: 10.1074/mcp.m200067-mcp200. [DOI] [PubMed] [Google Scholar]
- Wilson AS, Davis CD, Williams DP, Buckpitt AR, Pirmohamed M, Park BK. Characterization of the toxic metabolite(s) of naphthalene. Toxicology. 1996;114:233–242. doi: 10.1016/s0300-483x(96)03515-9. [DOI] [PubMed] [Google Scholar]
- Wolf O. Krebserkrankungen bei chemiearbeitern einer ehemaligen naphthalinreinigung [Cancer diseases in chemical workers in a former naphthalene cleaning plant.] Dtsch. Gesundheits-Wesen. 1976;31:996–999. [Google Scholar]
- Wolf O. Arbeitshygiene und arbeitsschutz. [Industrial hygiene and industrial safety] Z. Ges. Hyg. 1978;24:737–739. [Google Scholar]
- Yu D, Berlin JA, Penning TM, Field J. Reactive oxygen species generated by PAH o-quinones cause change-in-function mutations in p53. Chem. Res. Toxicol. 2002;15:832–842. doi: 10.1021/tx010177m. [DOI] [PubMed] [Google Scholar]
- Zheng J, Cho M, Jones AD, Hammock BD. Evidence of quinone metabolites of naphthalene covalently bound to sulfur nucleophiles of proteins of murine Clara cells after exposure to naphthalene. Chem. Res. Toxicol. 1997;10(9):1008–1014. doi: 10.1021/tx970061j. [DOI] [PubMed] [Google Scholar]