Abstract
Countermeasures against radiation are critically needed. Ideally, these measures would be easy to store, easy to administer and have minimal toxicity. We used oral delivery of interleukin 11 (IL11) in mice exposed to lethal doses of total-body irradiation (TBI). Animals were given IL11 by gavage at various daily doses beginning 24 h after TBI, which continued for 5 days. At a TBI of 9.0 Gy, mice treated with IL11 had a 70% survival at 30 days compared with control group survival of 25% (P = 0.035). At 10.0 Gy, treated animals had 50% survival at 30 days compared with no survivors in the control group. Treated animals had significant improvement in intestinal mucosal surface area and crypt survival. In addition bacterial translocation of coliform bacteria was significantly less in the treated animals. Systemic absorption of IL11 was low in treated animals and effects on the hematopoietic cells were not seen. Serum citrulline levels rebounded significantly faster after irradiation in the IL11 treated animals, indicating quicker recovery of small intestine health. These data suggest that IL11 given orally protects the intestinal mucosa from radiation damage and that this compound is beneficial as a mitigating agent even when started 24 h after radiation exposure.
INTRODUCTION
The threat of lethal radiation exposure from terrorist attack or nuclear accident continues as a present danger. Countermeasures need to be developed for protection and treatment of the first responders and the general population who maybe exposed to radiation. Ideally, such countermeasures should be easy to deliver, capable of mitigating radiation damage when given after exposure and have minimal toxicity (1). In addition, these countermeasures should be easily stockpiled for rapid distribution after a radiation exposure occurrence. We investigated interleukin 11 (IL11) as an orally delivered countermeasure to radiation injury to the gut in a mouse model.
Interleukin 11 is a multifunctional cytokine of the interleukin 6 family with powerful anti-inflammatory properties (2), hematopoietic proliferative activity (3) and cytoprotective effects on intestinal crypt cells (4–6). Clinically, recombinant human IL11 has been developed and marketed (Neumega, Pfizer Pharmaceuticals) to reduce chemotherapy-induced thrombocytopenia (7). The drug is prescribed as a daily subcutaneous injection and side effects from systemic administration can be severe including fluid retention, tachycardia, atrial flutter and pleural effusion (8). Animal models have shown an improvement in survival after total-body irradiation (TBI) with systemic IL11 administration (9); however, long-term survival is hampered by systemic toxicity (10).
We have previously shown that enteral administration of IL11 as a liquid can reduce local radiation injury to the intestine (11). Local radiation effects to a portion of the small intestine were ameliorated in animals given liquid IL11 directly to the irradiated area and the treated animals had significant reduction in the structural radiation injury of the intestine with improvement in the mucosal surface area of the gut. The current study investigates oral administration of IL11 to mitigate lethal total-body irradiation.
MATERIALS AND METHODS
Chemicals
Recombinant human IL11 (rhIL11) (Neumega/Oprelvekrin) was purchased from Wyeth Pharmaceuticals (Philadelphia, PA) and was stored as a lyophilized powder at 4°C until reconstituted per manufacturer's directions prior to use. All other chemicals, unless otherwise mentioned, were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
The animal experimental protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Central Arkansas Veterans Healthcare System (CAVHS) and University of Arkansas for Medical Sciences. Male CD2F1 mice (Harlan Sprague Dawley, Indianapolis, IN) were used in this study. Mice were housed in conventional cages under standardized conditions with controlled temperature and humidity under a 12-h light/dark cycle and had free access to water and chow (Harlan Teklad laboratory diet 7012, Purina Mills, St. Louis, MO) during the entire period of handling.
Mice 6–7 weeks of age and weighing 22–25 g were selected at the time of initiation of experiments, and a total of 680 mice were used for the experiments reported here. All experiments were performed with different doses of single TBI. Groups comprised of 4–8 mice were sacrificed humanely at set time points (0, 3.5 and 7 days) per the experiment. In lethality studies, mice were observed twice daily during the experimental period and those appearing clearly moribund (exhibiting more than 25% weight loss, lethargy, huddling, shivering, hunched posture or vocalization) were euthanized immediately by CO2 asphyxiation followed by cervical dislocation, in accordance with American Veterinary Medical Association (AVMA) 2007 Guidelines on Euthanasia. Animals in the lethality experiments that survived to 30 days were euthanized at that time.
Irradiation and Dosimetry
Unanesthetized mice were irradiated in a Shepherd Mark I model 25 137Cs irradiator (J. L. Shepherd Associates, San Fernando, CA). Mice were held in a well-ventilated custom-made Plexiglas restrainer, divided into four 9° “pie slice” compartments by vertical dividers made of T-6061 aluminum (machinable grade) with a gold anodized coating. Irradiation occurred at room temperature between 68–75°F, while two chambers were stacked on top of each other and placed on a turntable rotating at 5 rpm in the position furthest away from the radiation source, allowing eight mice to be irradiated at a time. Mice were exposed to uniform TBI at a dose rate of 1.35 Gy per min, after correction for decay.
Drug Treatment
Lyophilized rhIL11 powder vials stored at 4°C were reconstituted in sterile water immediately before treatment. The recommended human dose of IL11 is 50 μg/kg per day as a subcutaneous injection. Our starting dose of IL11 as an oral dose was 100-fold higher (5 mg/kg) to account for differences between the homology of mouse IL11 and human IL11 and due to concerns that much of the IL11 would be degraded in the acidic environment of the stomach. Various daily doses from 2.5–20 mg/kg were utilized with optimum survival seen at 10 mg/kg. This translated to a daily treatment of 250 μg in 0.2 mL water per animal. Daily treatment was given for five days, beginning 24 h after TBI. Vehicle groups received sterile water gavage for the same period of time.
Survival Analysis
Postirradiation survival analysis with and without treatment was performed by exposing mice to graded doses of TBI between 8–12 Gy. The mice were monitored up to 30 days after TBI and the number of dead/moribund mice was recorded twice daily. Subsequent parameters were performed at the LD50/30 dose of 9.0 Gy as calculated by Kaplan-Meier survival curve analysis.
Oral Absorption of IL11
Absorption of orally gavaged human IL11 was measured using an ELISA kit obtained from RayBiotech Inc. (Norcross, GA). Systemic absorption of IL11 from intestine was measured in four different treatment conditions as follows; groups one and two received no TBI and were treated with vehicle and rhIL11 from days 1–4, respectively: groups three and four were exposed to 9.0 Gy TBI and treated with vehicle and rhIL11 from days 1–4, respectively.
All four groups received rhIL11 gavage on day 4 and blood samples were collected at 60 and 120 min post gavage. Plasma was separated as per the manufacturer's guidelines and levels of rhIL11 were measured immediately on a spectrophotometer.
Mucosal Surface Area
A segment of the proximal jejunum was extricated, fixed and vertical sections were stained with H&E. Mucosal surface area on slides was measured using a projection/cycloid method as described by Baddeley et al. (12) with modifications (13).
Intestinal Crypts Survival
The surviving fraction of intestinal crypts was measured by the method of Withers and Elkind (14). Mice were euthanized exactly 3.5 days after TBI (0, 8, 10, 13 and 16 Gy), and segments of proximal jejunum were obtained, fixed and embedded so as to obtain four transverse sections per specimen, cut at 3–5 μm and stained with H&E, a crypt containing 10 or more adjacent, chromophilic non-Paneth cells was counted as a surviving crypt. Four circumferences were scored per mouse and microcolony survival was expressed as the average number of surviving crypts per mouse, with the average of four circumferences considered as a single value for statistical purposes.
Plasma Citrulline
Blood samples were collected on days 0, 3.5 and 7 and at different radiation doses, the samples were centrifuged for 10 min at 12,000g and the plasma was collected. Citrulline concentrations were determined using a high-throughput HILIC-MS/MS method, as previously described by our group (15).
Bacterial Translocation
Radiation-induced translocation of gut bacterial from intestine to organs (liver) was measure on day 12 after TBI at 9.0 Gy. Liver samples were removed aseptically and homogenized immediately. DNA was extracted from samples using Wizard genomic DNA purification kit from Promega (Madison, WI) and quantified spectrophotometrically. Bacterial DNA was quantified by real-time PCR as described by van Minnen et al. (16). Briefly, real-time PCR was performed using Power SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and 16S rRNA gene targeted primers, forward (5′-AAC GCG AAG AAC CTT AC-3′) and reverse (5′-CGG TGT GTA CAA GAC CC-3′). Serially diluted bacterial genomic DNA was used to generate the standard curve. Results were expressed as nanogram bacterial DNA per gram mouse liver tissue.
Blood Cell Counts
Whole blood was collected by retro-orbital bleeding using heparinized micro-hematocrit capillary tubes into EDTA coated microcentrifuge tubes at days 0, 3.5, 7, 14 and 21 after TBI (9.0 Gy). White blood cells (WBC), red blood cells (RBC) and platelet counts were measured using a veterinary hemocytometer (Hematrue System, Heska Corporation, Loveland, CO) according to the manufacturer's instructions.
Spleen Colonies
Spleen colony count is a marker of postirradiation hematopoietic system recovery and indicates the proliferation of single hematopoietic stem cells (25). Spleens were collected at day 12 after TBI (9.0 Gy) and fixed in Bouin's solution. Spleen colonies were clearly visible as yellowish nodules against a dark, smooth background and were counted by two independent observers under magnification.
Statistical Methods
Statistical analyses were performed using NCSS 2004 for Windows (NCSS, Kaysville, UT) and Prism Graphpad 5.0 (La Jolla, CA). Data are presented as means ± SEM, except for survival analysis. Two-tailed t tests were used throughout at P < 0.05. Survival curves were constructed using the Kaplan-Meier method and were compared using the log-rank test. Survival curves for the crypt assay were compared using regression analysis with radiation dose and treatment group as independent variables.
RESULTS
In nonirradiated animals, orally administered IL11 was transported rapidly through the small intestine with high levels detected at 20 and 60 min within the lumen of the distal segment (Table 1). This demonstrates rapid exposure of the luminal surface following gavage.
TABLE 1.
Amount of rhIL11 (pg) Detected in Mouse Intestine (n = 5)
| Oral rhIL11 administration |
||||
|---|---|---|---|---|
| Intestine | Control | 20 min | 60 min | 240 min |
| Proximal | 16.32 ± 15.64 | 71.43 ± 37.29 | 26.12 ± 15.08 | 70.5 ± 52.37 |
| Middle | 38.81 ± 43.83 | 110.78 ± 99.22 | 157.01 ± 284.98 | 46.78 ± 64.85 |
| Distal | 45.25 ± 23.86 | 494.06 ± 323.92 | 220.12 ± 141.63 | 16.52 ± 36.95 |
| Total | 100.38 | 676.28 | 403.26 | 133.81 |
Note. Data are average of 5 mice ± SD.
Survival
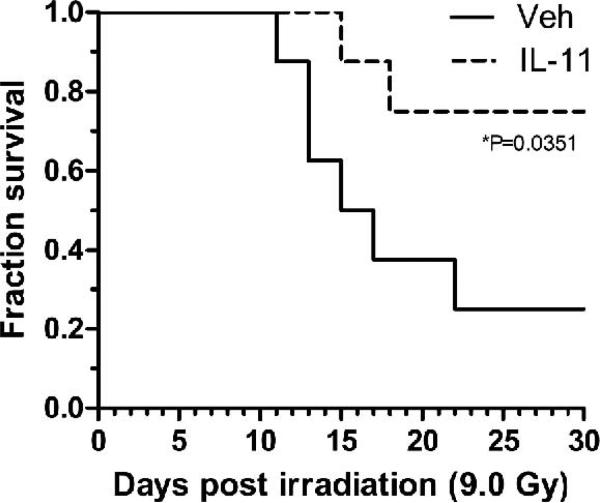
Significant improvement in survival was seen with daily administration of 250 μg IL11 beginning 24 h after lethal irradiation. At a TBI dose of 9.0 Gy, 70% of treated animals survived to 30 days compared with 25% of control animals (P = 0.035) (Fig. 1). Treated animals received five days total of IL11.
FIG. 1.
Survival of mice treated with or without a daily 250 μg dose of IL11 starting 24 h after TBI (9.0 Gy) vs. vehicle-treated animals. P values represent survival compared with controls (vehicle)
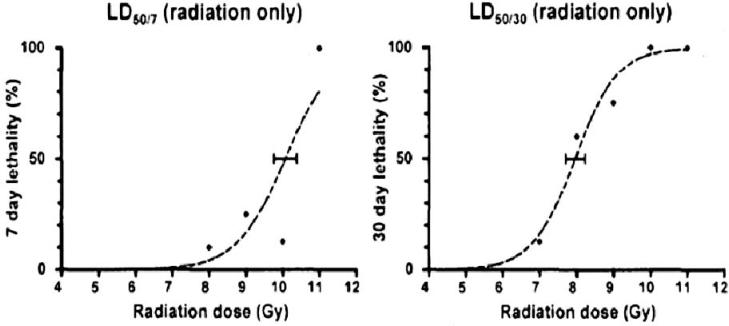
Several different doses of IL11 were given after TBI with 10.0 Gy. This dose of radiation is the LD50/7 in male CD2F1 mice. Death at 7 days is indicative of the gastrointestinal acute radiation syndrome (ARS) and results from lethal fluid and electrolyte loss. In these experiments, 250 μg of IL11 given daily for 5 days produced the best survival with 50% of animals surviving to 30 days versus none of the irradiated vehicle controls (Fig. 2). Most significant at this IL11 dose, 50% of control animals were dead by day 7 and none of the IL11 treated animals died before day 12. Alternative dosing schedules including twice daily treatments and treatments longer than 5 days were utilized but were not superior to the once daily for 5 days schedule (data not shown). Also, treatment beginning 48 h after irradiation did not produce a significant survival benefit. The LD50/7 and LD50/30 survival curves for CD2F1 mice are shown in Fig. 3.
FIG. 2.
Survival of mice treated with or without a daily dose of IL11 starting 24 h after TBI (10 Gy). Doses of IL11 are in μg/day. P values represent survival compared with controls (vehicle).
FIG. 3.
Survival curves of CD2F1 mice after irradiation demonstrating the lethal dose of 50% of animals at 7 days (LD50/7) and the lethal dose of 50% of the animals at 30 days (LD50/30) (Martin Hauer-Jensen, personal communication).
Intestinal Effects in Interleukin 11 Treated Animals
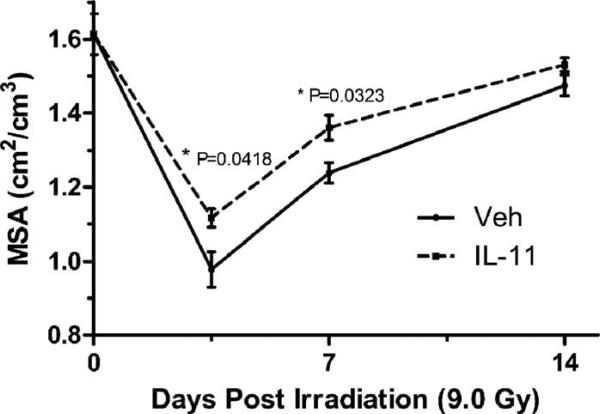
The effect of oral IL11 (250 μg/day beginning 24 h after TBI) on intestinal mucosal injury was assessed at different times after 9.0 Gy TBI by measuring mucosal surface area and plasma citrulline levels. Treatment with IL11 significantly reduced mucosal surface area loss at days 3.5 and 7 after irradiation (Fig. 4). Figure 5 demonstrates the histology of the jejunum 7 days after 9.0 Gy TBI.
FIG. 4.
Mucosal surface area in the proximal jejunum at days 3.5 and 7 after TBI (9.0 Gy). rhIL11 was given at 250 μg/day starting 24 h after irradiation. P values represent mucosal surface area compared with controls (Veh).
FIG. 5.
Histology of mucosa at proximal jejunum 7 days after TBI (9.0 Gy). The images represent from left to right: unirradiated bowel; irradiated and given daily water gavage; irradiated and given 250 μg IL11 daily.
All animals had a significant drop in serum citrulline 3.5 days after irradiation with 9.0 Gy. However, recovery of citrulline levels occurred by day 7 in IL11 treated animals but not until day 14 in the controls (Fig. 6). By day 7 the serum citrulline levels in the IL11 treated animals were significantly higher than in the controls.
FIG. 6.
Serum citrulline levels after TBI (9.0 Gy). rhIL11 treatment was started 24 h after irradiation and the 250 μg doses were continued daily. Controls represent nonirradiated animals. Vehicle (Veh) represents animals gavaged with sterile water. P values represent comparison with nonirradiated controls.
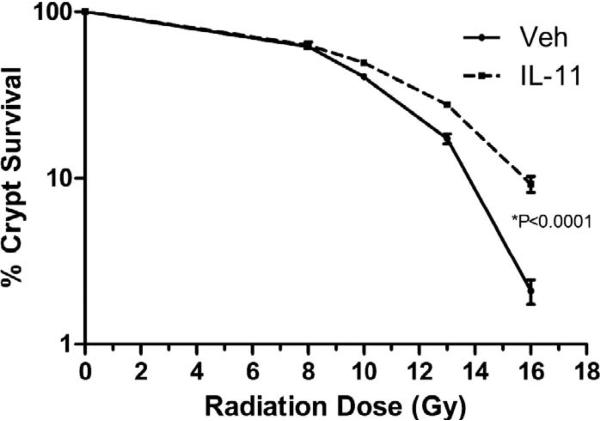
Intestinal crypt survival was improved in IL11 treated animals vs. controls. This improvement became significant for radiation doses above 9.0 Gy (Fig. 7).
FIG. 7.
Crypt survival after irradiation with or without daily rhIL11. Vehicle (Veh) represents animals gavaged with sterile water. P value represents comparison of crypt survival of IL11 treated animals vs. vehicle treated animals. Significance is seen at radiation doses greater than 9.0 Gy.
Systemic Effects
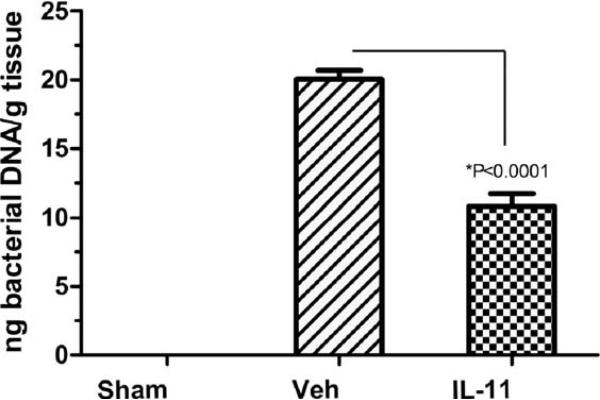
Bacterial translocation as determined by real-time PCR of bacterial DNA in the liver was measured 12 days after TBI (9.0 Gy). IL11 treated animals had a significant reduction in bacterial translocation when compared to vehicle-treated groups (P < 0.0001) (Fig. 8). Sham-irradiated animals had no detectable levels of bacteria in the liver.
FIG. 8.
Bacterial translocation detected in liver after TBI (9.0 Gy) measured at 12 days postirradiation. Sham represents nonirradiated animals. Vehicle (Veh) represents animals gavaged daily with sterile water. IL11 represents animals gavaged daily with 250 μg of IL11 starting 24 h after TBI. P values represent a significant decrease in bacterial translocation in IL11 treated animals compared with vehicle treated animals.
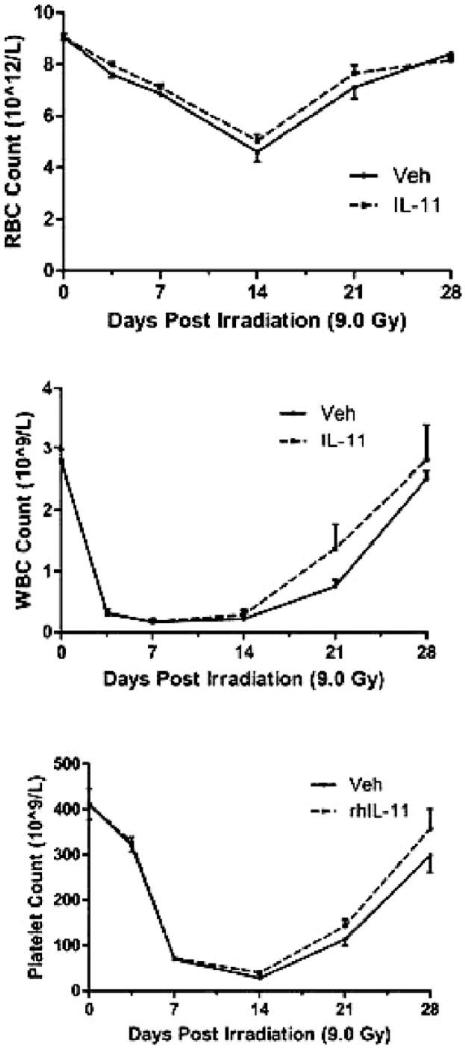
Peripheral blood counts for erythrocytes, neutrophils and platelets declined sharply after irradiation. IL11 administration did not significantly alter either the toxicity or recovery compared with controls (Fig. 9). Of note, IL11 in this model did not appear to influence thrombopoiesis.
FIG. 9.
Peripheral blood cell counts in IL11 treated animals and control animals after TBI (9.0 Gy).
Serum levels of IL11 were measured 60 and 120 min after gavage with 250 lg on day 4 after TBI (9.0 Gy) (Fig. 10). Systemic absorption of rhIL11 from intestine was measured in four different treatment conditions as follows: groups one and two with no TBI, treated with vehicle and rhIL11 from days 1–4, respectively; groups three and four exposed to 9.0 Gy TBI, treated with vehicle and rhIL11 from days 1–4, respectively.
FIG. 10.
Serum rhIL11 levels 60 and 120 min after gavage with 250 μg of rhIL11 on day 4. Sham animals received 4 days of water gavage only after TBI (9.0 Gy). Sham + rhIL11 received 4 days of IL11 without irradiation. Vehicle (Veh) + rhIL11 animals received TBI (9.0 Gy), then 3 days of water gavage only, then IL11 gavage on day 4; rhIL11 treated animals received TBI (9.0 Gy), then daily IL11 on days 1–4. P values represent a significant decreased systemic absorption at 60 and 120 min in animals treated daily with IL11 after irradiation versus those treated with sterile water gavage daily after irradiation.
All four groups received rhIL11 gavage on day 4 and blood samples were collected after 60 and 120 min. Vehicle-treated TBI animals had significantly higher serum levels of IL11 than those levels found in the IL11 treated TBI groups. Results indicate that while rhIL11 absorption was observed after TBI, daily rhIL11 treatment after TBI significantly diminished systemic absorption. This suggests improved integrity of intestinal barrier after rhIL11 treatment.
DISCUSSION
Successful countermeasures against radiation exposure effects continue to be lacking. The recent nuclear disaster in Japan and the continued threat of rogue elements with nuclear weaponry emphasize the critical need for agents that can mitigate the adverse effects of radiation exposure to a large population. The gastrointestinal acute radiation syndrome occurs from destruction of the mucosal lining of the gut with resulting diarrhea, dehydration and transmigration of enteric bacteria into the bloodstream (17). Death from this syndrome typically occurs between 7 and 14 days after exposure. Interventions that can protect the lining of the intestines would have a significant impact on survival after a nuclear disaster. Our data clearly demonstrate that oral IL11 reduces death from TBI in a murine model. Most importantly, this benefit is seen even when IL11 treatment is begun 24 h after radiation exposure. The mechanism for improved survival appears to be preservation of the integrity of the intestinal mucosa.
IL11 has been shown previously to reduce intestinal damage from radiation when given by subcutaneous injection (9). However, systemic use of the compound can result in multiple side effects including fluid retention leading to pulmonary edema and congestive heart failure. Canine experiments with TBI and subcutaneous IL11 treatment led to a high rate of animal death from pneumonitis likely due to a combined pulmonary injury from irradiation and IL11 (10). Oral administration of IL11 in healthy human volunteers resulted in no detectable systemic absorption of the drug (18).
In our study we have shown that IL11 passes quickly through the intestine of the mouse when given as an oral gavage, thereby exposing the mucosa of the entire small intestine to the drug. Systemic absorption of the drug was assessed by measuring serum IL11 60 and 120 min after gavage with 250 μg. Nonirradiated animals had no detectable absorption of the drug, and animals exposed to 9.0 Gy TBI had absorption assessed 4 days later. Those that were treated with interleukin 11 daily after irradiation had an increased in serum IL11 levels after gavage on day 4 but this was significantly less than animals who were not treated with daily IL11. Daily treatment with IL11 after irradiation reduces the damage to the intestinal mucosa (Fig. 3), which probably reduces the systemic absorption of IL11. No animals showed a significant alteration in platelet count after IL11 treatment, therefore, whatever amount of IL11 is systemically absorbed from the irradiated intestine is ineffective in exerting a significant biological effect on the hematopoietic system. It is most likely, therefore, that the protective effect on the intestine by oral IL11 is due predominantly to local effects on the mucosa rather than systemic effects. We have previously shown that exposure of liquid IL11 to irradiated intestine results in locally decreased TGF-β, macrophages and granulocytes, and locally increased mast cells, all of which are associated with decreased acute radiation injury (11). IL11 has also been shown to induce cell cycle arrest in intestinal epithelium that could abrogate the radiation damage to these cells (19).
Survival significantly improved in irradiated animals when started on IL11 24 h after irradiation. At a dose of 9.0 Gy, survival at 30 days was 75% in the treated animals versus 25% in the controls. At a dose of 10.0 Gy, median survival was improved in the treated animals to 17 days vs. 7 days for the control groups. One-half of the IL11 treated animals were alive 30 days after 10.0 Gy total-body irradiation versus none of the nontreated 10.0 Gy irradiated controls. Prolongation of survival following a nuclear disaster could permit time for other interventions such as hematopoietic growth factors, hydration and antibiotics. At the radiation doses used in this study, hematopoietic effects are expected to occur in an individual who survives the acute gastrointestinal effects of irradiation. Other measures will be necessary to counteract the hemapatopoietic effects. IL11 treatment longer than five days and treatment regimens given twice a day did not seem to improve the survival compared with 5 daily treatments (data not shown).
Small bowel preservation is demonstrated by a significant increase in intestinal mucosal surface area in irradiated animals treated with IL11 compared with control groups. Both IL11 treated and control animals displayed some recovery of mucosal surface area by two weeks after irradiation; however, at days 3.5 and 7 after 9.0 Gy TBI, the control animals had significantly more destruction of the mucosal surface than the IL11 treated animals (Fig. 4). The IL11 treated animals also had greater crypt counts indicating better preservation of the regenerating crypt cells. Serum citrulline levels dropped significantly postirradiation in both vehicle and IL11 treated groups at day 3.5, but IL11 treatment resulted in a significantly more rapid return to normal levels from day 7 onwards. The small intestine is the principal source of circulating citrulline and serum levels of citrulline are a strong indicator of small intestine health. In humans and animals, the serum citrulline levels drop proportional to the degree of radiation toxicity to the small bowel (20). Citrulline levels nadir at about day 3.5 after irradiation in a dose-dependent fashion and the decrease in citrulline levels correlates to increased crypt death (21). Higher serum levels are associated with agents that modify radiation response such as glutamine and amifostine (22).
Human studies using IL11 administration in a randomized blinded fashion concurrent with chemotherapy for hemato-logic malignancies showed a significant reduction in sepsis, particularly with intestinal bacteria, in the interleukin 11 treated patients (23). In our study bacterial translocation as measured by coliform DNA in the liver was significantly lower in the IL11 treated animals. This is further evidence of the protection of the integrity of the GI mucosa with liquid IL11. IL11 treated animals began dying 13 days after 10.0 Gy TBI. This is outside the window of death from the acute gastrointestinal radiation syndrome, which is typically between days 5–11 postirradiation. While the level of bacteremia in the treated animals is lower than the control groups (Fig. 6), by day 14 we are seeing the nadir of WBCs, RBCs and platelets in the peripheral blood (Fig. 8). The combined injury to the GI tract and the hematopoietic system may be responsible for the animal deaths after day 11.
The daily dose of IL11 that proved optimal in our study was 250 μg per mouse, begun 24 h after lethal TBI. This is equivalent to 10 mg/kg body weight. Conversion of this to the human equivalent dose using body surface area would be approximately 30 mg for a 60 kg man (24). However, the actual effective dose in humans may be substantially lower than this, because the recombinant human IL11 used in this study shares only about 88% homology with mouse IL11. Potency of the formulation would thus be expected to be greater in humans. Moreover, IL11 is expected to have degraded somewhat in our experiments due to passage through the acidic environment of the stomach and digestion by gastric enzymes. Treatment in larger species could circumvent this problem by encapsulating the drug to avoid exposure to the gastric environment.
It is unlikely that one single therapy will be effective in mitigating both the GI and hematologic acute radiation syndrome. Combined therapies will be necessary that are effective, non-toxic and easy to distribute. Oral IL11 as a countermeasure to protect the GI tract from lethal doses of radiation has several attractive qualities. It is a stable compound on the shelf in its lyophilized form and can be easily reconstituted for rapid local distribution and administration of this oral compound to a large population would not require any special training. There does not appear to be any systemic absorption of this drug when given orally to nonirradiated individuals and no systemic toxicity is observed. More significantly, this agent has the potential to be effective as a mitigating therapy begun 24 h after radiation exposure. This last property has the greatest potential for impact on those exposed when the exact timing of a nuclear disaster cannot be anticipated.
ACKNOWLEDGMENTS
This project was supported by NIH 1 RC1 A1078515-01 (AFB). MJH received support from U19 AI67798 (NIAID) and the Veterans Administration.
REFERENCES
- 1.Augustine AD, Gondre-Lewis T, McBride W, Miller L, Pellmar TC, Rockwell S. Animal models for radiation injury, protection and therapy. Radiat Res. 2005;164:100–109. doi: 10.1667/rr3388. [DOI] [PubMed] [Google Scholar]
- 2.Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia. 1999;13:1307–1315. doi: 10.1038/sj.leu.2401514. [DOI] [PubMed] [Google Scholar]
- 3.Schlerman FJ, Bree AG, Kaviani MD, Nagle SL, Donnelly LH, Mason LE, et al. Thrombopoietic activity of recombinant human interleukin 11 (rHuIL11) in normal and myelosuppressed nonhuman primates. Stem Cells. 1996;14:517–532. doi: 10.1002/stem.140517. [DOI] [PubMed] [Google Scholar]
- 4.Orazi A, Du X, Yang Z, Kashai M, Williams DA. Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest. 1996;75:33–42. [PubMed] [Google Scholar]
- 5.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358–1368. doi: 10.1016/s0016-5085(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 6.Potten CS. Protection of the small intestinal clonogenic stem cells from radiation-induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells. 1996;14:452–459. doi: 10.1002/stem.140452. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs C, Robert NJ, Bailey FA, Schuster MW, Overmoyer B, Graham M, et al. Randomized placebo-controlled study of recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with breast cancer receiving dose-intensive cyclophosphamide and doxorubicin. J Clin Oncol. 1997;15:3368–3377. doi: 10.1200/JCO.1997.15.11.3368. [DOI] [PubMed] [Google Scholar]
- 8.Dykstra KH, Rogge H, Stone A, Loewy J, Keith JC, Jr, Schwertschlag US. Mechanism and amelioration of recombinant human interleukin-11 (rhIL11)-induced anemia in healthy subjects. J Clin Pharmacol. 2000;40:880–888. doi: 10.1177/00912700022009521. [DOI] [PubMed] [Google Scholar]
- 9.Van der Meeren A, Mouthon MA, Gaugler MH, Vandamme M, Gourmelon P. Administration of recombinant human IL11 after supralethal radiation exposure promotes survival in mice: interactive effect with thrombopoietin. Radiat Res. 2002;157:642–649. doi: 10.1667/0033-7587(2002)157[0642:aorhia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Nash RA, Seidel K, Storb R, Slichter S, Schuening FG, Appelbaum FR, et al. Effects of rhIL11 on normal dogs and after sublethal radiation. Exp Hematol. 1995;23:389–396. [PubMed] [Google Scholar]
- 11.Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Cancer Res. 2007;67:9501–9506. doi: 10.1158/0008-5472.CAN-07-0810. [DOI] [PubMed] [Google Scholar]
- 12.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 13.Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–87. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 14.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17:261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- 15.Gupta PK, Brown J, Biju PG, Thaden J, Deutz NE, Kumar S, Hauer-Jensen M, Hendrickson HP. Development of high-throughput HILIC-MS/MS methodology for plasma citrulline determination in multiple species. Anal Meth. 2011;3:1759–1768. [Google Scholar]
- 16.van Minnen LP, Timmerman HM, Lutgendorff F, Verheem A, Harmsen W, Konstantinov SR, et al. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141:470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 17.DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–S44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotreau MM, Stonis L, Strahs A, Schwertschlag US. A multiple-dose, safety, tolerability, pharmacokinetics and pharmacodynamic study of oral recombinant human interleukin-11 (oprelvekin). Biopharm Drug Dispos. 2004;25:291–296. doi: 10.1002/bdd.415. [DOI] [PubMed] [Google Scholar]
- 19.Peterson RL, Bozza MM, Dorner AJ. Interleukin-11 induces intestinal epithelial cell growth arrest through effects on retinoblastoma protein phosphorylation. Am J Pathol. 1996;149:895–902. [PMC free article] [PubMed] [Google Scholar]
- 20.Onal C, Kotek A, Unal B, Arslan G, Yavuz A, Topkan E, et al. Plasma citrulline levels predict intestinal toxicity in patients treated with pelvic radiotherapy. Acta Oncol. 2011;50:1167–1174. doi: 10.3109/0284186X.2011.584557. [DOI] [PubMed] [Google Scholar]
- 21.Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, et al. Citrulline: a physiologic marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol Biol Phys. 2003;57:1067–1074. doi: 10.1016/s0360-3016(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 22.Lutgens L, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: an emerging role for plasma Citrulline. World J Gastroenterol. 2007;13:3033–3042. doi: 10.3748/wjg.v13.i22.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis M, Zwaan F, Hedstrom U, Poynton C, Kristensen J, Jumaa P, et al. Recombinant human interleukin 11 and bacterial infection in patients with [correction of] haematological malignant disease undergoing chemotherapy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361:275–280. doi: 10.1016/s0140-6736(03)12322-7. [DOI] [PubMed] [Google Scholar]
- 24.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 25.Till JE, McCulloch EA. Early repair processes in marrow cells irradiated and proliferating in vivo. Radiat Res. 1963;18:96–105. [PubMed] [Google Scholar]