Abstract
HIV-associated neurocognitive disorders (HAND) are common sequelae of HIV infection, even when viral titers are well controlled by anti-retroviral therapy. Evidence in patients and animal models suggests that neurologic deficits are increased during chronic opiate exposure. We have hypothesized that CNS progenitor cells in both adult and developing CNS are affected by HIV infection, and that opiates exacerbate these effects. To examine this question, neural progenitors were exposed to HIV-1 Tat1-86 in the developing brain of inducible transgenic mice and in vitro. We examined whether Tat affected the proliferation or balance of progenitor populations expressing nestin, Sox2, and Olig2. Disease relevance was further tested by exposing human-derived progenitors to supernatant from HIV-1 infected monocytes. Studies concentrated on striatum, a region preferentially targeted by HIV and opiates. Results were similar among experimental paradigms. Tat or HIV exposure reduced the proliferation of undifferentiated (Sox2+) progenitors and oligodendroglial (Olig2+) progenitors. Co-exposure to morphine exacerbated the effects of Tat or HIV-1SF162 supernatant, but partially reversed HIV-1IIIB supernatant effects. Populations of Sox2+ and Olig2+ cells were also reduced by Tat exposure, although progenitor survival was unaffected. In rare instances, p24 immunolabeling was detected in viable human progenitors by confocal imaging. The vulnerability of progenitors is likely to distort the dynamic balance among neuron/glial populations as the brain matures, perhaps contributing to reports that neurologic disease is especially prevalent in pediatric HIV patients. Pediatric disease is atypical in developed regions, but remains a serious concern in resource-limited areas where infection occurs commonly at birth and through breast-feeding.
Keywords: striatum, neuroAIDs, morphine, Tat, HIV-1, precursor
INTRODUCTION
Morbidity and mortality due to human immunodeficiency virus-1 (HIV-1) infection has been significantly decreased by combined anti-retroviral therapy (cART). cART treatment has also changed the landscape of central nervous system (CNS) disorders. Fewer patients exhibit HIV associated dementia (HAD), but with increased life expectancy and the potential toxic effects of long-term anti-retroviral exposure (Apostolova et al. 2011; Clifford 2002), there is increased prevalence of asymptomatic and mild-to-moderate HIV-associated neurocognitive disorders (HAND) among patients with absence of peripheral viremia (Gonzalez-Scarano and Martin-Garcia 2005; Sacktor 2002; Simioni et al. 2010). Additionally, many HIV therapeutics do not readily penetrate the blood brain barrier, leaving a reservoir of HIV in the CNS.
Microglia are the principal source of active HIV infection in the brain; astroglia can be infected but tend not to produce new virus (Gonzalez-Scarano and Martin-Garcia 2005; Jordan et al. 1991; Kaul 2008). Several studies suggest that CNS neural progenitor cells (NPCs), the undifferentiated precursors of both neurons and glia, are also susceptible to HIV-1 infection (Lawrence et al. 2004; Rothenaigner et al. 2007; Schwartz et al. 2007). Even if NPCs are not readily infected, they may be affected by HIV-1 proteins directly (Buch et al. 2007; Hahn et al. 2010; Krathwohl and Kaiser 2004; Lee et al. 2011; Mishra et al. 2010; Okamoto et al. 2007; Peng et al. 2008), or secondarily by extracellular changes. NPCs are widely distributed in germinative zones during development. Neurons in most brain regions are formed prenatally, but production of astroglia and oligodendroglia continues postnatally in both rodents and humans (Chan et al. 2002; Geha et al. 2010; Lee et al. 2000; Skoff 1990; Skoff and Knapp 1991). NPCs also are present in adult CNS, where neurogenesis occurs in specific regions, including the hippocampal dentate gyrus subgranular zone and the subventricular zone of the lateral ventricles (Doetsch et al. 1997; Eriksson et al. 1998; Kaplan and Hinds 1977; Kornack and Rakic 1999). Glial progenitors throughout the adult CNS undergo constant, slow turnover (Imamoto and Leblond 1978; Kornack and Rakic 1999; Kornack and Rakic 2001; Messier et al. 1958; Sturrock 1979); more active gliogenesis occurs during injury/disease (Burns et al. 2009; Rola et al. 2006; Romanko et al. 2004).
To test whether HIV-1 infection affects the dynamics of cell production during development, we used stage-specific markers to follow changes in the proliferation and populations of NPCs (nestin+ and Sox2+ [SRY (sex determining region Y)-related HMG (high mobility group)-;box gene2]) and their glial progeny both in culture and in intact brains during the perinatal period. NPCs and glial progenitors express functional opioid receptors in adult and neonatal rodent and human CNS (Buch et al. 2007; Hauser et al. 2009; Persson et al. 2003; Reznikov et al. 1999; Rius et al. 1991; Tripathi et al. 2008). Since co-exposure to opiate drugs of abuse enhances HIV-related CNS deficits in patients and experimental models (Anthony and Bell 2008; Bell et al. 2006; Burdo et al. 2006; Byrd et al. 2011; Hauser et al. 2007; Kopnisky et al. 2007), we examined interactive opiate effects. HIV-1 Tat was used to model HIV exposure both in vitro and in vivo. Progenitor responses were tested in a more clinically relevant model by exposing human NPCs (hNPCs) to supernatant from HIV-infected monocytes ± opiates. Tat or morphine alone reduced NPC and glial progenitor proliferation and/or altered the population dynamics of murine progenitors in vitro and in vivo. In certain progenitors, co-exposure to morphine accelerated the timing and extent of Tat effects. Similarly, proliferation of hNPCs was reduced by exposure to Tat, R5 HIV-1SF162, or X4 HIV-1IIIB, and there were interactive opiate-virus effects. We speculate that by affecting expansion of specific CNS progenitors, HIV ± opiate effects might alter the balance of glia and neurons in the CNS of pediatric HIV patients. Since adult progenitors share certain characteristics of neonatal progenitors, our findings may also relate to adult HIV patients. At either age, an altered progenitor pool is predicted to adversely affect CNS function.
METHODS
Protocols conformed to Virginia Commonwealth University Institutional Animal Care and Use Committee and national (PHS) guidelines on the care and ethical use of animals. Experiments were designed to minimize total animals used and their discomfort.
Murine neural progenitor cell cultures
Murine NPCs were cultured from striata of embryonic day 15 (E15) mice (ICR strain; Charles River, Boston, MA) using modifications of published methods (Khurdayan et al. 2004). In brief, striata from 10–12 embryos were dissected from the cortex, minced, and incubated with DNase (0.015 mg/mL, Sigma-Aldrich Co., St. Louis, MO) in Dulbecco’s Modified Eagle’s Medium (DMEM) (15 min, 37°C). Tissue was triturated, centrifuged (5 min, 1,000 rpm), and resuspended in NPC maintenance medium consisting of 1:1 DMEM/F12 (Invitrogen Corp., Carlsbad, CA), 10% Knockout® SR (Invitrogen; a serum replacement product), insulin (25 μg/mL), transferrin (100 μg/mL), progesterone (20 nM), putresceine (60 μM), Na-HCO3 (6 mM), and sodium selenite (30 nM) (all from Sigma-Aldrich). Cells were filtered through a 70-μm nylon cell strainer (BD Falcon, Bedford, MA), centrifuged, and resuspended in the same medium. A total of 5×104 cells were plated on poly-L-lysine coated 15-mm coverslips, and medium was refreshed every 2–3 d until treatment. Cells were grown at 37° C in a humidified atmosphere of 5% CO2. After 5 d, cells were treated with Tat1-86 (Clade B; 100 nM; ImmunoDiagnostics, Woburn, MA), ± morphine sulphate (500 nM; NIDA Drug Supply System, Rockville, MD), ± naloxone (1.5 μM; Sigma-Aldrich) for 12–48 h. Morphine, the major bioactive product of heroin in the brain, is a potent mu-opioid receptor (MOR) agonist; naloxone is a broad spectrum opioid receptor antagonist. These concentrations of Tat1-86 and morphine have been established to elicit functional effects in glia and CNS progenitors in earlier studies (Buch et al. 2007; El-Hage et al. 2005; Hauser et al. 2009; Khurdayan et al. 2004). Naloxone was applied 30 min prior to other treatments, which were added simultaneously in pre-warmed medium. Lastly, 5-bromo-2′-deoxyuridine (10 μM; BrdU, Sigma-Aldrich) was added to medium at 12 h prior to harvest to detect proliferating cells in S phase.
Opiate delivery to prenatal and postnatal Tat transgenic mice in vivo
In vivo, perinatal studies utilized transgenic mice engineered to express the HIV-1 Tat1-86 protein (clade B) under control of a glial fibrillary acidic protein (GFAP) promoter in a doxycycline-inducible manner. As previously described (Bruce-Keller et al. 2008; Fitting et al. 2010; Hauser et al. 2009), mice expressing the tat gene under control of a tet responsive element (TRE) in the pTREX vector (Clonetech, Mountain View, CA) were crossed with mice expressing the reverse tetracycline transactivator (RTTA) driven by the pGFALac-1 human GFAP promoter. Inducible Tat+ transgenic mice express both GFAP-RTTA and TRE-Tat genes; Tat− mice express only the GFAP-RTTA gene. To induce Tat production during the pre- and early postnatal period, pregnant dams were fed chow containing doxycycline (Harlan, Indianapolis, IN; 6 mg/g) from gestational day 15–16 until postnatal day 7. Doxycycline, a water-soluble tetracycline derivative, crosses the placenta and is secreted in breast milk, and reliably induces transgene expression in embryos and neonates via the tet-on system (Fedorov et al. 2001; Perl et al. 2002a; Perl et al. 2002b; Shin et al. 1999). All dams received the same chow, to control for effects of doxycycline unrelated to activation of the tat transgene. Standard PCR procedures were performed on genomic DNA isolated from ear clip samples to confirm genotype. Morphine was delivered by subcutaneous (s.c.) injection at a dose of 2 mg/kg in a 20 μl volume of saline, based on the average daily litter weight. This dose reportedly gave circulating morphine levels approximating those measured in human preterm infants given intermittent or continuous intravenous morphine infusion (Vien et al. 2009). Male, P4 to P7 Tat+ mice received a morphine injection twice daily at 10:00 am and 6:00 pm. Sham pups received 20 μl of saline s.c. twice daily. For BrdU labeling in vivo, 50 μl of 10 mg/ml BrdU (32.5 mM) was injected s.c. in sterile saline twice at 2 h intervals immediately prior to perfusion. There was <5% mortality; death was not related to genotype or administration of morphine or doxycycline. For immunohistochemistry, pups were deeply anaesthetized with isoflurane (Baxter, Deerfield, IL) prior to perfusion with 4% paraformaldehyde. Brains were postfixed overnight, hemisected, rinsed overnight in 15 ml changes of phosphate-buffered saline (PBS), cryopreserved through graded sucrose solutions (10 and 25 %), embedded in Tissue Tek OCT compound (Sacura Finetek, Torrance, CA), and stored at −80°C. 10 μm frozen sections containing striatum were thaw-mounted on SuperFrost Plus slides (VWR Scientific, West Chester, PA).
Immunohistochemistry and quantification
For in vitro studies, NPCs on coverslips were immunolabeled after 12–48 h treatment using antibodies to cell and lineage-specific markers including nestin (1:5000, Aves Labs, Inc., Tigard, OR), Olig2 (oligodendrocyte transcription factor 2; 1:100, Immuno-Biological Laboratories, Minneapolis, MN), and Sox2 (1:200, Millipore, Temecula, CA). Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Double-staining was performed with anti-BrdU (1:200, Abcam, Cambridge, MA) and anti-Sox2 or anti-Olig2 to detect proliferating progenitors. Coverslips were first treated with 1 N HCl in PBS to expose incorporated BrdU. Immunohistochemistry with precursor and BrdU antibodies was done sequentially; all primary antibodies were applied overnight at 4°C and visualized with appropriate fluorescent-conjugated second antibodies (Invitrogen). Immunolabeled coverslips were finally incubated with Hoechst 33342 dye (0.5 μg/ml, 8 min) to identify nuclei (Molecular Probes/Invitrogen), then mounted in ProLong Gold anti-fade reagent (Molecular Probes/Invitrogen). To assess the proportion of different progenitors in cultures, 300–350 Hoechst+ cells were selected randomly per coverslip and assessed for nestin, Sox2, and Olig2 immunoreactivity (n = 6) under oil immersion at 63× using a Zeiss AxioObserver system with integrated MRm camera system (Carl Zeiss, Inc., Thornwood, NY). Cell proliferation was estimated in the overall NPC population by determining the proportion of 300–350 Hoechst+ cells that incorporated BrdU. Cell proliferation was assessed in each progenitor population by counting 200 nestin+, Sox2+, or Olig2+ cells and scoring the proportion of cells with co-localized BrdU. Similar immunodetection methods were applied to 10 μm tissue sections from neonatal brains. The proportion of different cell types in vivo was estimated by averaging the percentage of Sox2+ or Olig2+ cells among 250–350 randomly selected Hoechst+ cells per striatum (left side) in two sections, in n = 6 mice. Proliferation was estimated by scoring BrdU incorporation in 200 cells expressing each marker in two sections per mouse. Double-immunolabeling was performed to assess whether hNPCs expressed the μ opioid receptor (MOR) and C-C chemokine receptor type 5 (CCR5) or C-X-C chemokine receptor type 4 (CCR4) in vitro. MOR (1:250, Antibodies Incorporated, Davis, CA), CCR5 (1:200, BD Pharmingen, Mountain View, CA), and CXCR4 (1:100; BD Pharmingen) antibodies were applied to fixed hNPCs and incubated overnight at 4°C, then visualized as described above, prior to labeling with NPC markers and Hoechst 33342. p24 was detected on hNPCs after exposure to supernatants from HIV-1 infected monocytes using an antibody commonly used to study infective processes (1:50, Dako, Carpinteria, CA) (Eugenin et al. 2011; Hahn et al. 2008; Lawrence et al. 2004). Images were taken under oil immersion at 63×.
Viability assay
Mouse NPCs were cultured on poly-L-lysine coated 48 well plates and treated at 5 d after plating with Tat, morphine and naloxone either alone or in combination as described earlier. After 12–48 h, medium was replaced with PBS containing 4 μM ethidium homodimer-1 (EthD-1) and 5 μg/mL Hoechst 33342 (15 min, at 37°C). The intensity of EthD-1 and Hoechst signals was measured using a PHERAstar FS plate reader (BMG LABTECH, Ortenberg, Germany) in n = 8–10 cultures using appropriate excitation/emission filters (EthD-1: 495/635 nM; Hoechst: 350/510 nM).
RNA Extraction and Reverse transcription PCR
RT-PCR was performed to confirm expression of Tat mRNA in the striatum of 7 d postnatal pups receiving doxycycline through the placenta and lactation as described. Pups were deeply anaesthetized with isoflurane. Striata were rapidly dissected from brains, and stored at −80°C. Total RNA was isolated using TRIzol reagent (Invitrogen). RNA samples were cleaned using GenElute™ Mammalian Total RNA kit (Sigma-Aldrich), after RNase-free DNase I treatment to eliminate genomic DNA. cDNA was synthesized from 2 μg of total RNA using the High-Capacity cDNA Archive Kit (Applied Biosystems, Warrington, UK). PCR was performed to detect Tat mRNA expression using primers previously described (Bruce-Keller et al. 2008; Hauser et al. 2009) with 18S mRNA as a control (Chauhan et al. 2003). PCR products were separated by electrophoresis on 1.5% agarose gels, stained with ethidium bromide.
Viral stock preparation
CCR5- and CXCR4-preferring strains of HIV (HIV-1SF162 and HIV-1IIIB, respectively) were used to test strain specific effects on some outcome measures. The following viral stocks were obtained through the NIH AIDS Research and Reference Reagent Program: HIV-1SF162 from Dr. Jay Levy (Cheng-Mayer and Levy 1988); HIV-1IIIB from Dr. Robert Gallo (Ratner et al. 1985). R5 and X4 strains were propagated in a U937 monocyte cell line (ATCC, Chicago, IL) activated with IL-2 (10 U/ml) and phytohaemagglutinin (5 ng/ml) for 2 d in RPMI with 10% fetal calf serum. Viral stocks containing 500 pg HIV-1 p24, measured by ELISA, were added to U937 cells. The culture supernatant was collected at the peak of infection, passed through a 0.2 μm filter, and stored at −80°C. For FACS analysis, an aliquot of medium containing 500 pg p24 was added to hNPCs grown to 75–80% confluence in each well of 6-well plates, with a final volume of 1.5 ml per well. Viral exposure time was 12 h.
Human progenitor cell line culture and CCR5/CXCR4 blocking assays
These studies used the human neural progenitor ReNcell VM cell line, derived from 14-wk gestation ventral mesencephalon (Millipore). Cells are ≥ 95% Sox2+ and nestin+, and have the potential to produce dopaminergic neurons and glia (Donato et al. 2007). All experiments used cells from passage 4. Frozen cells were thawed and expanded on laminin-coated T75 cm2 culture flasks in ReNcell NSC Maintenance Medium (Millipore) supplemented with epidermal growth factor (20 ng/ml; Millipore) and basic fibroblast growth factor-2 (20 ng/ml; Millipore). Three to four days after plating, at < 80% confluence, cells were passaged by detachment with accutase (Millipore), centrifuged at 300 × g for 5 min and resuspended in fresh maintenance media containing growth factors. For FACS analysis, cells were replated in laminin-coated 6-well plates (30,000 cells/well). Three to four days after plating, cells were treated for 12 h with Tat or HIV+ supernatant ± morphine ± naloxone. For the blocking experiments, non-signaling monoclonal antibodies to CCR5 (2D7; 2 μg/ml; BD Pharmingen) (Lee et al. 1999) and CXCR4 (12G5, 2 μg/ml; BD Pharmingen) (Bleul et al. 1997; Endres et al. 1996), or mouse IgG (2 μg/ml; BD Pharmingen) were applied 1 h prior to treatment. hNPCs were treated with BrdU (10 μM) 2 h prior to harvesting. For p24 immunolabeling, 5,000 cells per well were replated on laminin-coated glass coverslips in 24-well plates and treated with HIV-1 supernatant prior to reaching 80% confluence as described above. p24 immunolabeling was localized in cells fixed at 12 h post-inoculation using a Zeiss LSM 700 confocal laser scanning module configured to an Axio Observer Z1 inverted microscope and 63x (1.40 NA) oil immersion objective. To optimize detection of Alexa 594-labeled anti-human p24 antibodies, 555-nm laser excitation was filtered through a dichroic beam-splitter set at 585 nm. Optical sections were acquired from a single Z plane with acquisition parameters, including the scan step (0.315 μm) and pinhole size (34 μm), set to optimize X-, Y-, and especially Z-plane resolution (Zen 2010 software; Zeiss).
FACS analysis of hNPC proliferation and p24 expression
The APC BrdU Flow Kit (BD Pharmingen) was used to determine the proportion of S-phase hNPCs. Cells were incubated with BrdU (10 μM) for the final 2 h of a 12 h exposure to Tat or to supernatant from HIV-1SF162 or HIV-1IIIB infected monocytes ± morphine. Cells were detached using enzyme-free cell dissociation buffer (Sigma-Aldrich), fixed with BD Cytofix/Cytoperm buffer and treated with DNase to expose incorporated BrdU. An APC-conjugated anti-BrdU antibody and 7-amino-actinomycin D (7-AAD) were used to determine cell cycle phases (Go/G1, S, G2+M). Fluorescent signals for APC (Ex: 650 nm; Em: 615 nm) and 7-AAD/DNA complexes (Ex: 546; Em: 647) were detected on a FACSCanto (BD Biosciences, San Jose, CA) and analyzed using the DIVA FACS data program (BD Biosciences). Single and intact hNPCs were first gated by cell area and width. APC-BrdU fluorescence intensity was assessed after gating on approximately 3 × 105, 7-AAD+ cells. Go/G1 phase cells (APC-BrdU− and 7-AAD+) were defined using appropriate negative (non-specific IgG followed by APC-conjugated secondary antibody only); and positive (7-AAD only; APC-anti-BrdU antibody only) controls. S-phase cells were defined as being APC-BrdU+ and 7-AAD+.
After 12 h exposure to HIV+ supernatant, p24 expression on hNPCs was determined by FACS analysis. Cells were fixed and immunolabeled with p24 antibody and Alexa Fluor 488-conjugated second antibody, followed by incubation in 7-AAD. Single and intact hNPCs were gated as above. p24 immunolabeling intensity was assessed on approximately 3 × 104 intact, 7-AAD+ cells. Single laser excitation (488 nm) and dual fluorescence emission (585/42 band pass; 530/30 band pass) analysis was used to compensate for autofluorescence. Controls included p24 labeling in untreated cells and non-specific IgG followed by second antibody in HIV-treated cells.
Statistical analysis
Significance was assessed by analysis of variance (ANOVA) followed by Duncan’s post-hoc testing if indicated, or main effect testing, using StatSoft software (Statistica, Tulsa, OK).
RESULTS
Effect of HIV-1 Tat and morphine on murine neural progenitors in vitro
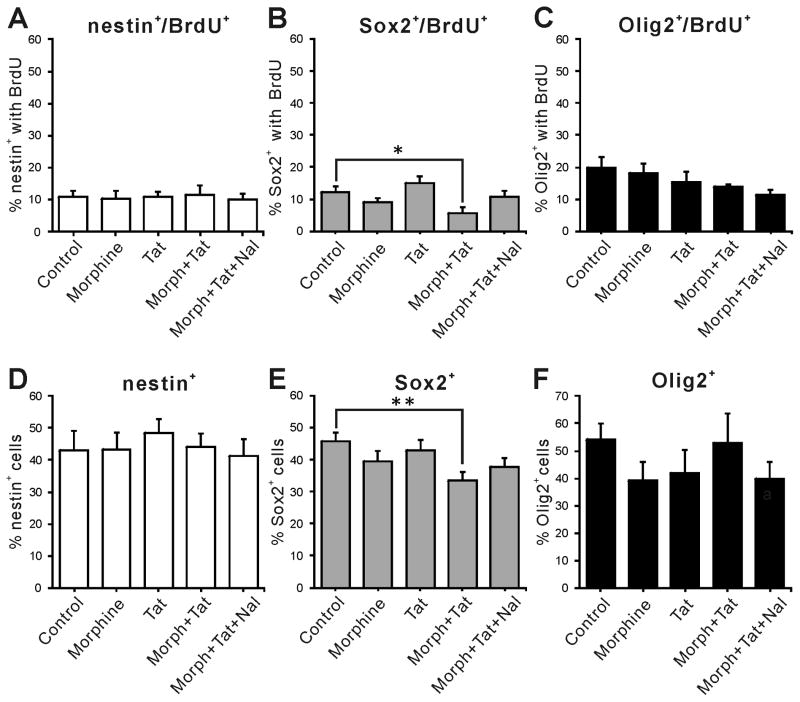
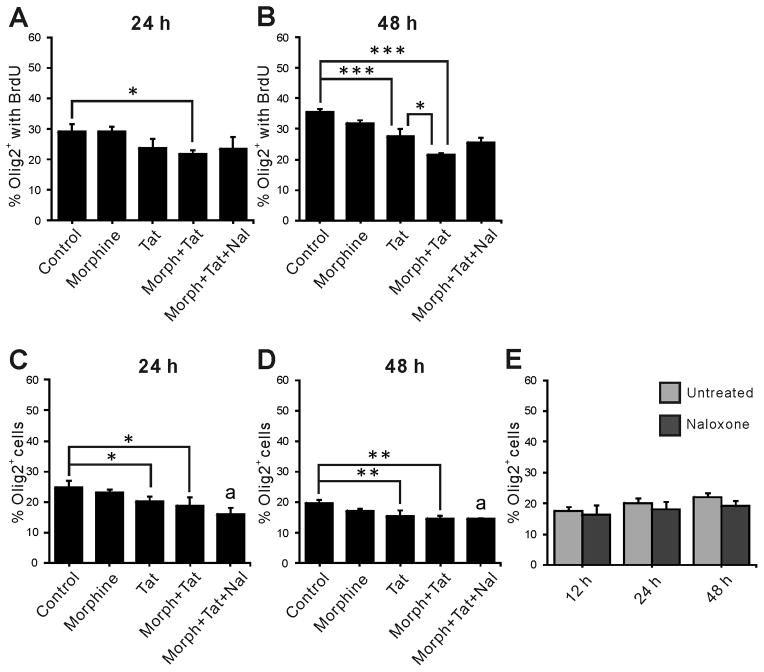
To determine independent or interactive effects of Tat1-86 and morphine on maturation, NPCs from embryonic mouse striata were treated with 100 nM Tat1-86 ± morphine (500 nM) over a period of 48 h, with BrdU added for the final 12 h. Double immunostaining for BrdU and lineage specific markers was performed following 12 h, 24 h and 48 h treatments. Nestin and Sox2 are markers of uncommitted NPCs (Episkopou 2005; Gu et al. 2002; Nunes et al. 2003; Qu and Shi 2009), while Olig2 is a transcription factor expressed in more differentiated cells that develop largely into oligodendrocytes (OLs) and motor neurons (Masahira et al. 2006; Takebayashi et al. 2002). Treatment with Tat ± morphine for 12 h did not alter either the proportion of nestin+ (nestin+/Hoechst+) or Olig2+ (Olig2+/Hoechst+) cells, or the percentage of nestin+ or Olig2+ cells expressing BrdU (Fig. 1A, D vs C, F). There were, however, significant effects on the subset of NPCs expressing Sox2. While neither morphine nor Tat alone had any effect, both the Sox2+/BrdU+ cells and the total Sox2+ cell population were reduced in cells exposed concurrently to Tat and morphine (Fig. 1B, E). Since only Sox2+ cells were affected after 12 h, we hypothesized that the sensitivity of NPCs to Tat ± morphine might depend on differentiation, and that changes in Sox2+ cells might affect their progeny. Experiments were extended to 24 h and 48 h using markers for Olig2 (Fig. 2), and at both times the total Olig2+ population was reduced by Tat alone. The proliferating (Olig2+/BrdU+) population at 24 h was unaffected by either Tat or morphine alone, but Tat and morphine interacted to reduce BrdU uptake (Fig. 2A). By 48 h, Tat alone had reduced the Olig2+/BrdU+ population. Tat and morphine interacted at this time point to further decrease BrdU uptake (Fig. 2B). Thus, interactive effects of Tat and morphine were only observed on proliferative behavior of Olig2+ cells, while Tat by itself affected cell populations. Naloxone partially reversed interactive effects of morphine in proliferating cells, as the groups receiving Tat, morphine, and naloxone were not different from control, but also not different from the group co-exposed to Tat and morphine. Naloxone also appeared to reduce overall Olig2+ cells when given concurrently with Tat and morphine. We conducted the experiments described below to understand these findings.
Figure 1.
Co-exposure to HIV-1 Tat and morphine for 12 h alters the proliferation index and population of Sox2+ cells in vitro. Neither nestin+ (A, D) nor Olig2+ (C, F) populations were affected by any combination of Tat, morphine, or naloxone treatment at 12 h. However, both the percentage of the population that was Sox2+ and the proliferation of cells at this stage of differentiation were decreased by Tat and morphine co-exposure (B, E). (* p<0.05, ** p<0.01; with Duncan post-hoc test, n = 4–6, Morph = Morphine (500 nM), Tat (100 nM), Nal = naloxone (1.5 μM).
Figure 2.
Effects of HIV-1 Tat ± opioids on proliferation and the relative number of Olig2+ cells in vitro. Interactive effects of morphine and Tat reduced Olig2+ cell proliferation at both 24 and 48 h (A, B); Tat by itself also reduced Olig2+ proliferation at 48 h (B), and reduced the percent of Olig2+ cells in the overall population after both 24 h (C) and 48 h (D); there were no additional effects of morphine co-exposure. Morphine by itself did not affect Olig2+ proliferation or populations. Naloxone partially reversed the effect of morphine in both A and B; the percentage of Olig2+/BrdU+ cells in the Morph+Tat+Nal groups was not different from either control or Morph+Tat treatment groups at the same exposure time. Naloxone did not reverse the interactions between morphine and Tat in C and D (indicated as “a”), suggesting that naloxone might directly affect the Olig2+ population. This was not the case at 12 – 48 h exposure (E). Morph, Morphine (500 nM); Tat, HIV-1 Tat1-86 (100 nM); Nal, naloxone (1.5 μM); * p < 0.05, ** p < 0.01, *** p < 0.001, a p < 0.05 vs. control; ANOVA with Duncan’s post-hoc test, n = 4–6.
Effect of naloxone on murine progenitors in vitro
Since naloxone unexpectedly decreased the percentage of Olig2+ cells when combined with morphine and Tat (“a” in Fig. 2C, D), we treated murine NPCs with naloxone alone for 12–48 h and compared the proportion of Olig2+ cells in untreated control and naloxone treated groups. There was no significant reduction in the Olig2+ population (Fig. 2E), suggesting that naloxone by itself is not toxic to Olig2+ cells or their immediate progenitors. Instead, naloxone might interfere with endogenous opioid signals resulting from Tat and morphine treatment. Autocrine opiate signaling pathways are known to play pivotal roles in OL survival (Buch et al. 2007; Hauser et al. 2009; Khurdayan et al. 2004; Knapp et al. 2001) and overall glial cell fate (Gorodinsky et al. 1995; Kim et al. 2006; Persson et al. 2003).
Effect of HIV-1 Tat and morphine on murine neural progenitors: proliferation vs. viability
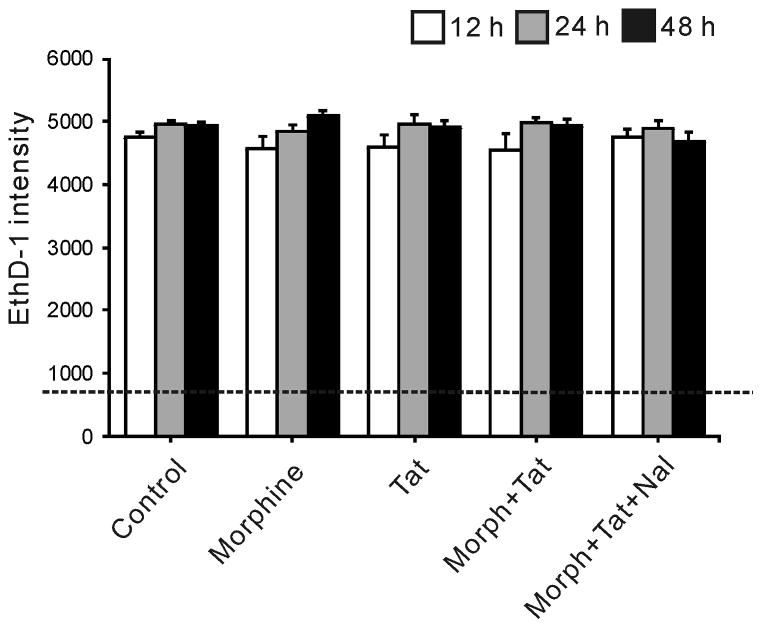
To determine whether cell death might contribute to observed reductions in proliferating or total NPCs, we examined NPC viability at up to 48 h of Tat ± morphine exposure. There was no cell death in response to Tat ± morphine treatment at any time period measured by EthD-1 fluorescence intensity (Fig. 3).
Figure 3.
Neither Tat nor morphine significantly affects NPC death. There was no difference in ethidium homodimer-1 (EthD-1) fluorescence intensity between treatment groups at 12–48 h, indicating no increase in cell death. The dotted line indicates background signal in wells lacking cells. Morph, Morphine (500 nM); Tat, HIV-1 Tat1-86 (100 nM); Nal, naloxone (1.5 μM).
Effects of HIV-1 Tat and morphine on neural progenitors in striatum
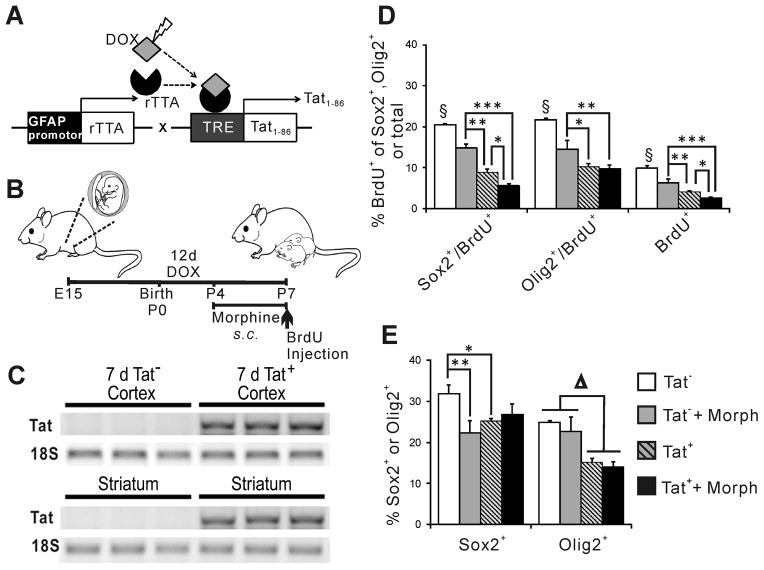
Transgenic mice with inducible expression of HIV-1 Tat in the CNS were used to assess Tat and morphine effects on NPCs in vivo. Detection of Tat mRNA and protein in the brains of these mice was previously reported by our group (Duncan et al. 2008; Fitting et al. 2010; Hauser et al. 2009) and also shown here (Fig. 4A–C). Pregnant dams received chow containing doxycycline from gestational day 15–16 through postnatal day 7 to activate the HIV-1 Tat transgene in fetal and neonatal mice. Reverse transcription PCR confirmed that doxycycline delivered to dams induced Tat mRNA expression in the cortex and striatum of Tat+, but not Tat−, neonatal mice (Fig. 4C).
Figure 4.
Proliferation is reduced in neonatal striatum of Tat-transgenic mice. HIV-1 Tat1-86 was induced via DOX delivered through placenta and lactation (A, B). All dams received chow containing DOX; Tat mRNA transcription was detected by RT-PCR in cortex and striatum of 7 d Tat+ pups (C). 12 d Tat induction and 4 d of s.c. morphine injections significantly reduced proliferation (BrdU+ cells) overall, and in both Sox2+ and Olig2+ cells. Additive effects of Tat and morphine were observed in Sox2+/BrdU+ cells and in the overall BrdU+ population (D, * p < 0.05; ** p < 0.01; § p < 0.05, Tat− vs. all other groups, Duncan’s post-hoc test, n = 6–8). Changes in progenitor proliferation appear to affect overall populations of Sox2+ and Olig2+ cells in the neonatal striatum (E, * p < 0.05; ** p < 0.01 vs. Tat−, One-way ANOVA, Duncan’s post-hoc test, n = 6–8; Δ p < 0.01, main effect Tat− vs Tat+, n =6–8).
All mice used in experiments were male. Some mice received morphine s.c. at postnatal days 4–7 to assess whether co-exposure to HIV-1 Tat and morphine caused effects on NPCs in vivo similar to those in culture. There were several significant effects on proliferating cell populations. Morphine exposure and Tat induction independently reduced BrdU uptake in both Sox2+ and Olig2+ cells in the striatum, and in the overall population of striatal NPCs (Fig. 4D). An interactive effect of morphine and induced Tat was seen both in the Sox2+/BrdU+ population and in the overall population of BrdU+ cells (Fig. 4D). We examined whether this might lead to changes in the overall populations of Sox2+ and Olig2+ cells. Morphine and Tat by themselves each reduced the percentage of the population that was Sox2+ or Olig2+ (Fig. 4E); there was no interactive effect. Additionally, Tat alone, but not morphine alone, reduced the percentage of the population that was Olig2+; again there was no Tat and morphine interaction.
Effects of HIV-1 Tat, HIV, and morphine on proliferation of a human NPC line
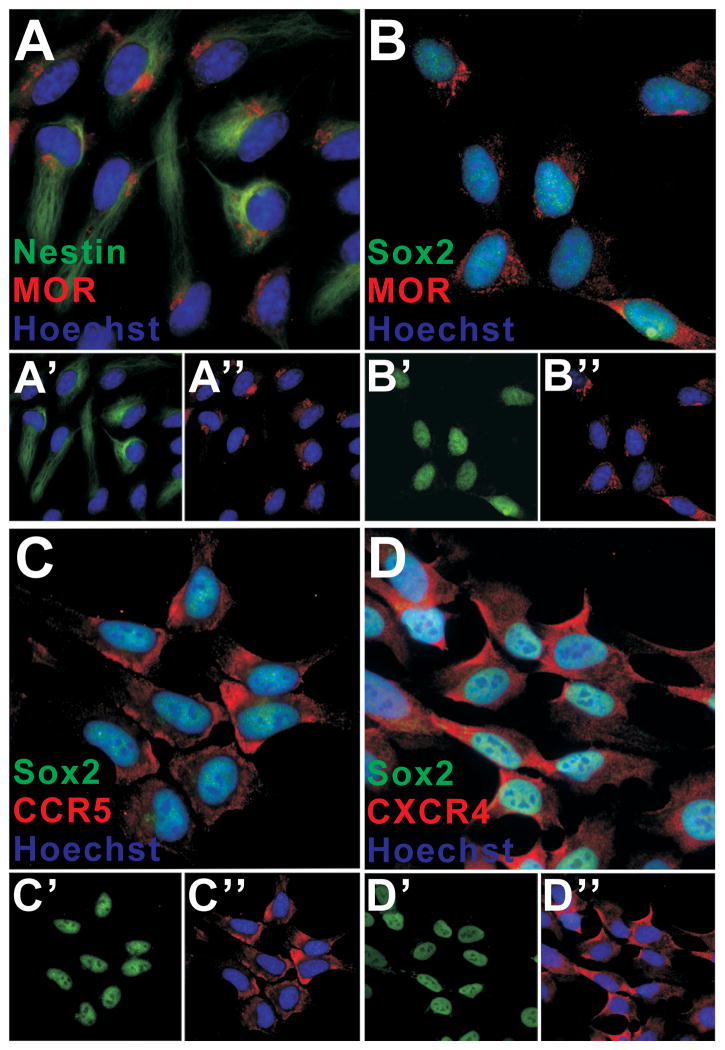
To validate our results in a more clinically relevant system, we exposed human NPCs to HIV-1 Tat1-86, or to supernatant from monocytes infected with R5-tropic HIV-1SF162, or X4-tropic HIV-1IIIB with or without concurrent morphine. Before conducting experiments, we briefly characterized the hNPC cell line by co-localizing MOR, nestin, and/or Sox2 antigenicity. In accordance with previous reports (Breier et al. 2008), more than 95% of hNPCs expressed nestin and Sox2. Approximately 95% of hNPC also showed MOR immunoreactivity (Fig. 5A, B); Olig2 immunofluorescence was not detected (not shown). Since we used both R5-tropic and X4-tropic HIV strains, that respectively use CCR5 or CXCR4 as preferred co-receptors for viral entry, CCR5 and CXCR4 immunoreactivity was co-localized in Sox2+ hNPCs to verify expression (Fig. 5C, D).
Figure 5.
Immunohistochemical characterization of hNPCs. Double-labeling confirmed that 95% of hNPC were nestin+ and Sox2+; 95% also showed immunoreactivity for MOR (A, B). Individual precursor markers are separated in the lower panels (A′: nestin (green) and nuclei (blue); A″: MOR (red) and nuclei (blue); B′: Sox2 (green); B″: MOR (red) and nuclei (blue)). A similar percentage of hNPC express CCR5 (C) and CXCR4 (D). As above, lower panels show individual markers (C′: Sox2 (green); C″: CCR5 (red) and nuclei (blue); D′: Sox2 (green); D″: CXCR4 (red) and nuclei (blue)). The presence of CCR5 and CXCR4 on hNPCs suggests that effectors such as gp120, β-chemokines (RANTES/CCL5, MIP-1α/CCL3, MIP-1β/CCL4), and the α-chemokine (SDF-1/CXCL12), released in response to Tat or contained in viral supernatant, might affect hNPC behavior.
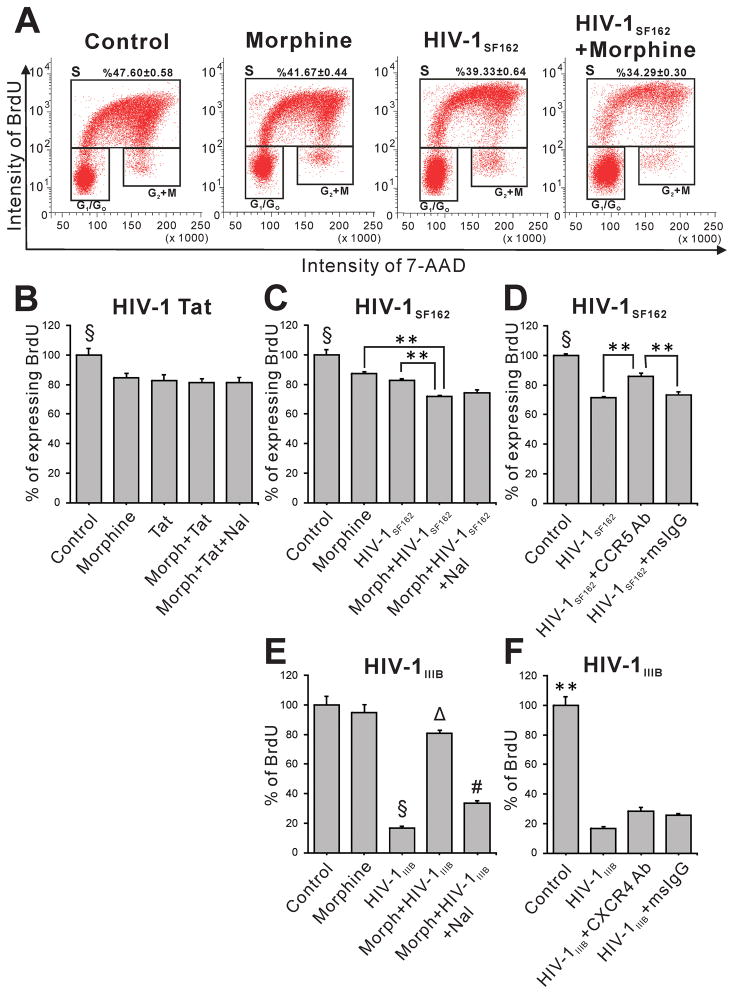
FACS analysis was performed at 12 h following exposure to Tat ± morphine or to supernatant from infected U937 cells ± morphine to assess proliferation (Fig. 6). Due to their rapid cell cycle (Breier et al. 2008), hNPCs were treated with BrdU 2 h prior to harvest. FACS analysis showed that both Tat and morphine independently caused significant reductions in the proportion of S-phase cells as compared to control, with no synergistic or additive effect of combining Tat and morphine (Fig. 6B). Interestingly, hNPCs exposed to supernatant from HIV-1SF162 or HIV-1IIIB infected cells also showed significantly reduced proliferation (Fig. 6C, E). There was an additional reduction in proliferation rate when HIV-1SF162 supernatant was combined with morphine exposure (Fig. 6C). The opposite effect was seen for HIV-1IIIB supernatant, whose effects were partially reversed by morphine co-exposure (Fig. 6E). Specific, non-signaling, CCR5 (2D7) and CXCR4 (12G5) blocking antibodies were applied 1 h before exposure to viral supernatant to test whether some effects might be due to viral entry, or to viral proteins binding to hNPCs. Compared to treatment with control mouse IgG, 2D7 immunoblockade partially reversed the anti-proliferative effect of HIV-1SF162 supernatant (Fig. 6D) while 12G5 immunoblockade was completely ineffective (Fig. 6F).
Figure 6.
Effects of Tat, HIV-1 supernatant, and morphine on proliferation of human NPCs. FACS analyses were performed after 12 h treatment with the indicated Tat, virus, or morphine combinations. BrdU was added during the final 2 h to label S-phase cells. Raw FACS data for an HIV-1SF162 experiment is shown (A). Histograms in B–F reveal that exposure to Tat (B), and supernatant from either HIV-1SF162- or HIV-1IIIB-infected monocytes (C, E) all reduce ReNcell VM progenitor proliferation. Morphine alone variably reduced proliferation (compare C and E). While there was no interactive effect of Tat and morphine (B), BrdU incorporation was additively reduced in cells treated with combined HIV-1SF162 supernatant and morphine (C). A CCR5 blocking antibody (2D7) reduced, but did not abolish, the effect of HIV-1SF162 supernatant (D), suggesting that BrdU uptake was sensitive to multiple factors in the supernatant, only some of which interact with CCR5 (B–D, § p < 0.05, compared to all other groups; ** p < 0.01, one-way ANOVA, Duncan’s post-hoc test, n = 7–8). Surprisingly, morphine reversed HIV-1IIIB-infective supernatant effects, returning proliferation to nearly control values (E) in a naloxone-reversible manner (# p < 0.05 compared to all groups). The 12G5 antibody partially blocks CXCR4 but did not block HIV-1IIIB-supernatant effects on proliferation (F), suggesting that factors reducing BrdU uptake in this supernatant do not signal through CXCR4 (E–F, § p < 0.05, compared to all other groups; Δ p < 0.05, compared to all groups, except morphine; ** p < 0.01 compared to all groups, one-way ANOVA, Duncan’s post-hoc test, n = 4). Morph, Morphine (500 nM); Tat, HIV-1 Tat1-86 (100 nM); Nal, naloxone (1.5 μM); HIVSF162, HIVIIIB, supernatant from U937 monocytes infected with these strains; CCR5 Ab, CCR5 (2D7) antibody (2 μg/ml); CXCR4 Ab, CXCR4 (12G5) antibody (2 μg/ml); msIgG = mouse IgG (2 μg/ml).
p24 expression in hNPCs by FACS analysis and immunolabeling
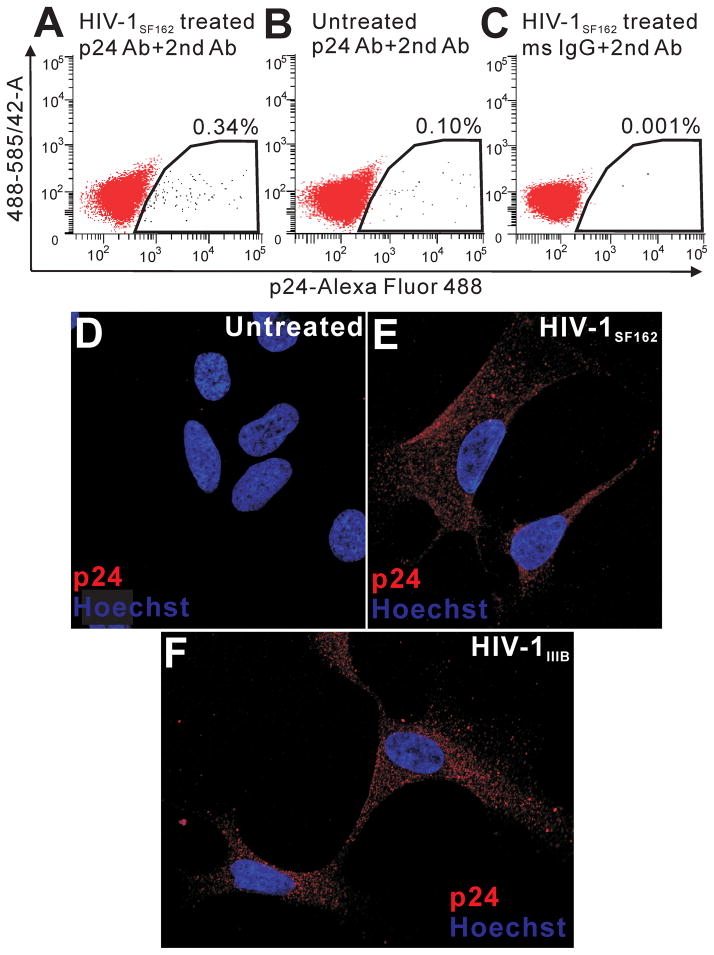
hNPC infection by HIV has been reported by other investigators (Lawrence et al. 2004; Mishra et al. 2010; Schwartz et al. 2007). We assessed hNPCs in the present study for the presence of p24 antigen. hNPCs were exposed for 12 h to HIV-1SF162 or HIV-1IIIb infective supernatant, then fixed and either detached from the plate, immunolabeled with p24 antibody and Alexa Fluor 488–conjugated second antibody, stained with 7-AAD, and subjected to FACS analysis (Fig. 7A–C), or immunolabeled for confocal microscopic analysis of p24 within individual cells (Fig. 7D–F). Immunoreactivity was observed in sporadic hNPCs in cultures with both R5- and X4-infective supernatant, but never in control cultures. p24+ staining appeared as numerous, small foci found throughout the cytoplasm that were not localized in the nucleus (Fig. 7D, E). In FACS studies, approximately 1 × 104 intact, 7-AAD+ cells were assayed using single laser excitation (488 nm) and dual emission (585/42 band pass; 530/30 band pass) analysis to compensate for spectral overlap between p24 and autofluorescence signals. Neither control nor experimental groups showed any evidence of autofluorescence (Fig. 7A–C). A p24+ signal was detected in 0.34% of cells treated with HIV-1SF162 infective supernatant (Fig. 7A). p24 was detected in 0.1% and 0.001% of control populations, respectively.
Figure 7.
Detection of p24 on hNPCs by FACS and confocal microscopy. p24 immunolabeling was assessed on 1 × 104, 7-AAD+ cells using a single laser (488 nm) and two color fluorescence emission (585/42 nm band pass; 530/30 hm band pass) FACS approach (A–C). A very small percentage (0.33%) of hNPCs exposed to supernatant from HIVSF162-infected monocytes were p24+ (A). Labeling indices in control groups, including untreated hNPCs immunolabeled for p24 (B) and HIV-treated hNPCs exposed to control IgG and second antibody (C), were 0.1% and 0.001%, respectively, suggesting that some p24 labeling was specifically related to antigen expression. Autofluorescence (585 > 530 signal) was not detected in any sample. FACS diagrams are representative of n=3 experiments. p24 signal was not detected in individual untreated hNPCs (D). p24 immunoreactivity was found within cytoplasm of cells exposed to both HIV-1SF162 (E) and HIV-1IIIB supernatant (F). Images show 0.32 μM optical sections through the nuclear region. Note the absence of p24 immunolabeling in nuclei.
DISCUSSION
NPCs are present throughout development and in adult brains, although the population and their distribution is substantially reduced with age. Using murine and human embryonic cultures, and an in vivo perinatal model, we determined that HIV-1 Tat or viral exposure dysregulates NPC proliferation and affects cell populations in developing systems. Exposure to Tat, or to infective HIV+ supernatant, both reduced progenitor proliferation. Morphine interacted with both treatments to cause further dysregulation, although developmental stage and HIV strain were critical variables. Results suggest that HIV exposure and opiate co-exposure elicit fundamental changes in the types of NPCs produced that likely contribute to neurologic impairment in both pediatric and adult patients. Morphine by itself affected NPCs in mice exposed during the perinatal period; this may be relevant to children exposed to morphine or heroin before birth.
HIV-1 Tat and opiates alter proliferation/population dynamics of murine NPCs in vitro
Previous studies showed that HIV-1 Tat affects expansion and viability of fetal human or murine NPCs or glial-restricted progenitors in culture, without determining if cells at particular stages of maturation are selectively affected (Buch et al. 2007; Khurdayan et al. 2004; Mishra et al. 2010). We also questioned whether opiate exposure might enhance Tat effects, since opiates can amplify CNS dysfunction in experimental HIV models (Bokhari et al. 2011; Bruce-Keller et al. 2008; Fitting et al. 2010; Hauser et al. 2006; Hu et al. 2005; Kumar et al. 2006; Zou et al. 2011) and in patients (Anthony et al. 2005; Bell et al. 2002; Byrd et al. 2011). We examined striatum, an area that is rich in opiate receptors and extremely vulnerable to HIV-1-induced neuropathology (Berger and Nath 1997; Mirsattari et al. 1998; Nath et al. 2000; Nath et al. 2002). Murine NPCs derived from E14 striatum have been characterized as a mixture of progenitors and very immature cells (Hahn et al. 2010); most proliferate and express MOR (Fig. 5) (Hauser et al. 2009).
We distinguished between cells expressing nestin or Sox2, markers of relatively undifferentiated NPCs, and Olig2, a marker typical of developing OLs. Tat and/or morphine had effects at specific stages of differentiation. As early as 12 h after co-exposure to morphine and Tat, proliferation of Sox2+ cells was reduced and the total Sox2+ population was diminished (Fig. 1B, E). With increasing time, Olig2+ cell proliferation was also affected, by both Tat alone and by Tat-morphine interactions. The gradually increased response of immature cells to Tat ± morphine during the exposure period suggests an increased receptivity of the cells during maturation. By itself, Tat did not affect cell replication until 48 h, and did not affect cell viability at any time studied; however, Tat did reduce the percentage of cells expressing Olig2 at both 24 and 48 h. A potential unexplored explanation is that Tat may influence NPC lineage decisions, perhaps by stalling cells at the Sox2+ stage, or by increasing production of GFAP+ or NG2+ cells. In keeping with this idea, an increase in GFAP+ cell production was noted in human progenitors exposed to HIV-1 (Peng et al. 2008).
Treatment with naloxone partially reversed morphine-Tat interactions in most studies. However, in the Olig2 population studies, naloxone treatment decreased the percentage of Hoechst+ cells expressing Olig2 (“a” in Fig. 2). This effect only occurred for Olig2+ cells, and not for nestin+ or Sox2+ cells. The results are reminiscent of in vivo studies with another non-selective opioid receptor antagonist, naltrexone, which was shown to modulate NPC proliferation in adult rodent hippocampus (Holmes and Galea 2002; Ra et al. 2002) and developing rat brain (Schmahl et al. 1989). However, we found that naloxone did not directly affect the Olig2+ population (Fig. 2E). We previously suggested that naloxone or naltrexone may interfere with endogenous opiate signaling pathways that modify survival and development of OL lineage cells (Hauser et al. 2009; Knapp et al. 2001; Knapp et al. 1998). The present study suggests a more complex phenomenon, since naloxone reversal was only partial, and particularly impaired in the presence of combined Tat and morphine. CCR5, an HIV co-receptor, has well documented interactions with MOR, leading to bi-directional, heterologous cross-desensitization (Chen et al. 2004; Chen et al. 2007; Suzuki et al. 2002; Szabo et al. 2002; Szabo et al. 2003). Tat can elevate β-chemokine ligands that activate CCR5 (RANTES, MIP-1α, and MIP-1β) in multiple cell types including NPCs (Hahn et al. 2010), which may interfere with naloxone activity through the above mechanisms.
HIV-1 Tat and opiates alter proliferation/population dynamics of murine NPCs in vivo
We used inducible HIV-1 Tat1-86 transgenic mice to model Tat and opiate exposure in vivo. While all animal models of a human-specific disease have limitations, these mice have been extensively characterized and shown to exhibit neurologic abnormalities associated with chronic HIV-1 exposure, including glial activation (Bruce-Keller et al. 2008), dendritic damage and reduced spine density in striatal neurons (Fitting et al. 2010), abnormal OLs (Hauser et al. 2009), circadian rhythm (Duncan et al. 2008) and behavioral (Fitting et al. 2012) abnormalities. Since our culture experiments used E14 tissue grown for 7 d in vitro, we performed in vivo studies at similar times. Results may therefore have particular relevance to pediatric HIV patients, but may also pertain to unique progenitor populations in adult brain parenchyma.
We focused on Sox2+ and Olig2+ cells, which are relatively abundant in the striatum compared to nestin+ cells. The in vivo results showed declines that paralleled culture studies in both the proportion of progenitors replicating DNA and the relative number of progenitors, but there were notable differences. Importantly, morphine exposure by itself (Fig. 4D, E) reduced BrdU incorporation in both Sox2+ and Olig2+ cells, as well as in the Sox2+ and overall Hoechst+ populations. This finding, similar to work in vivo by Kornblum et al. (1987), infers that progenitor dysfunction may drive cell population changes in children of opiate-exposed women. Morphine did not affect these parameters in vitro, also in agreement with Kornblum et al. (1987), who postulated an indirect mechanism for morphine-induced changes in progenitor dynamics. Previous findings that morphine reduces neuron generation in neonatal (Hauser 1992; Kornblum et al. 1987; Seatriz and Hammer 1993) and adult rodent brain (Arguello et al. 2008; Eisch et al. 2000; Kornblum et al. 1987) may reflect progenitor dysregulation.
Tat exposure also decreased BrdU uptake into Sox2+ and Olig2+ cells, and within the entire NPC population (Fig. 4D). The greater effect of Tat on BrdU uptake in vivo may reflect the longer Tat exposure time (12 d); BrdU uptake in cultured Olig2+ cells was reduced after 48 h but not earlier. Both Sox2+ and Olig2+ populations were reduced by Tat alone (Fig. 4E), probably due in part to decreased proliferation. As was true in culture, the only interactive effect of morphine and Tat was on BrdU uptake. It seems counter-intuitive that morphine-Tat interactions did not reduce total populations. Neonatal progenitors are a transitory population and it may be more instructive to examine whether their progeny are modified. Small changes in early proliferative rates can translate into biologically significant changes in cell populations over time. To determine if the observed effects of Tat and/or morphine on progenitors adjusts the balance of glia and neurons in the CNS requires a stereological approach. Glial populations may be preferential targets, since their production continues well into the postnatal period in both rodents and humans (Altman 1966; Chan et al. 2002; Price 1994; Skoff and Knapp 1991; Skoff et al. 1976; Sturrock 1979). The present studies predict that OLs are quite vulnerable to HIV-1 Tat, as was observed in the striatum of mature, Tat-transgenic mice, especially with co-exposure to morphine (Hauser et al. 2009).
Tat, HIV-1, and morphine alter proliferation of hNPCs
Findings were re-evaluated in a human NPC line generated from 14-wk gestation human ventral mesencephalon. Over 95% of cells are immunopositive for nestin and/or Sox2, and many also express MOR, CCR5, and CXCR4 (Fig. 5). Tat, HIV-1SF162- or HIV-1IIIB-infective supernatant, and morphine all reduced proliferation after 12 h, with viral supernatant and morphine displaying interactive effects (Fig. 6). In general, these results are in accord with a previous report that 25–100 ng/ml Tat decreased human fetal progenitor proliferation (Mishra et al. 2010). Isolated Tat effects were greater in that study, perhaps reflecting cell heterogeneity, an important consideration, since all nestin+ or Sox2+ cells do not necessarily express equivalent amounts of functional MOR and may not express equivalent MOR splice variants (Dever et al. 2012). Furthermore, nestin+ or Sox2+ cells may not uniformly express molecular targets for Tat, such as αvβ5-integrin (Vogel et al. 1993), mannose (Liu et al. 2004) or NMDA receptors (Li et al. 2008), or the low-density lipoprotein receptor-related protein (Liu et al. 2000). Interestingly, another study showed that supernatant from monocyte-derived macrophages infected with R5 virus and co-stimulated with LPS could occasionally increase progenitor production (Peng et al. 2011; Peng et al. 2008). The presence of LPS makes it difficult to compare these results. Different proliferation assessments may also affect results. In our study, BrdU uptake after 2 h gave an immediate picture of DNA synthesis in individual cells. Exposure to infected supernatant was suggested to cause re-direction of progenitors towards an astroglial fate (Peng et al. 2011; Peng et al. 2008), a possibility also suggested by our findings and by others (Mishra et al. 2010). Although concurrent morphine exposure did not alter Tat effects on hNPCs, there were interactive effects of morphine and HIV-1 supernatant (Fig. 6). Morphine enhanced the anti-proliferative effect of HIVSF162, but unexpectedly reduced the anti-proliferative effect of HIVIIIB. Strain-specific, HIV effects have been observed on other outcomes (El-Hage et al. 2011; Podhaizer et al. 2012). They may be more prominent in complex models, and perhaps especially when viral effects are indirect. Supernatants in the present studies contain virions, viral proteins, and secreted factors from infected cells that may vary with viral strain. Multiple interactions between these factors and morphine may both enhance and reduce viral effects.
FACS analyses after 12 h exposure to infective supernatant showed that < 0.34% of intact hNPCs were p24+ in R5-treated cultures (Fig. 7A), and we very occasionally detected cells exposed to R5 and X4 supernatants that appeared p24+ by immunocytochemistry in 0.32 μM optical sections (Fig. 7E, F). Our experience supports the concept that hNPCs can be infected by HIV-1 (Lawrence et al. 2004; Rothenaigner et al. 2007; Schwartz et al. 2007), but it was not a common event. Most progenitors in the brain appear to respond as bystanders to events triggered by infected cells.
Relationship to HIV Pathology in Humans
Prior to the advent of cART, the prevalence, rate of onset, and severity of HIV-1-associated CNS disease in children was quite high compared to adult patients (Mintz 1994; Schwartz et al. 2007; Schwartz and Major 2006). This is not surprising, since much of CNS development occurs after birth, and the immature brains of children or adolescents exposed to HIV are highly vulnerable to pathologic agents. CNS disease in pediatric HIV patients manifested as static or progressive encephalopathy, with classic symptoms including delay or loss of major motor and mental milestones, and atrophy of cortical/subcortical regions and other abnormalities on CT scans (Drotar et al. 1997; Epstein et al. 1988; Schwartz and Major 2006; Tahan et al. 2006; Ultmann et al. 1985; Van Rie et al. 2007). In developed countries, rates of HIV-1 vertical transmission have been reduced to 1–2%, from 40% in 1995 (Buchholz et al. 2010; Van Dyke 2011). Treatment with CNS-penetrating drugs has reduced the incidence of pediatric and adolescent HIV-associated encephalopathy by ≥ 50%, especially in cases of perinatal acquisition (Patel et al. 2009). It is currently estimated that 10% of HIV+ children on cART therapy will show signs of CNS disease (Chiriboga et al. 2005). The situation in resource-limited settings is quite different. In 2008, an estimated 1,600 children per day became HIV-infected worldwide, 90% of them in sub-Saharan Africa (Joint United Nations Programme on HIV/AIDS. 2009; Joint United Nations Programme on HIV/AIDS. and World Health Organization. 2008). In such regions, mother-to-child transmission via breastfeeding is common in infants not infected at birth, and HIV infection remains a major source of infant mortality (Becquet et al. 2009; Meyers et al. 2007).
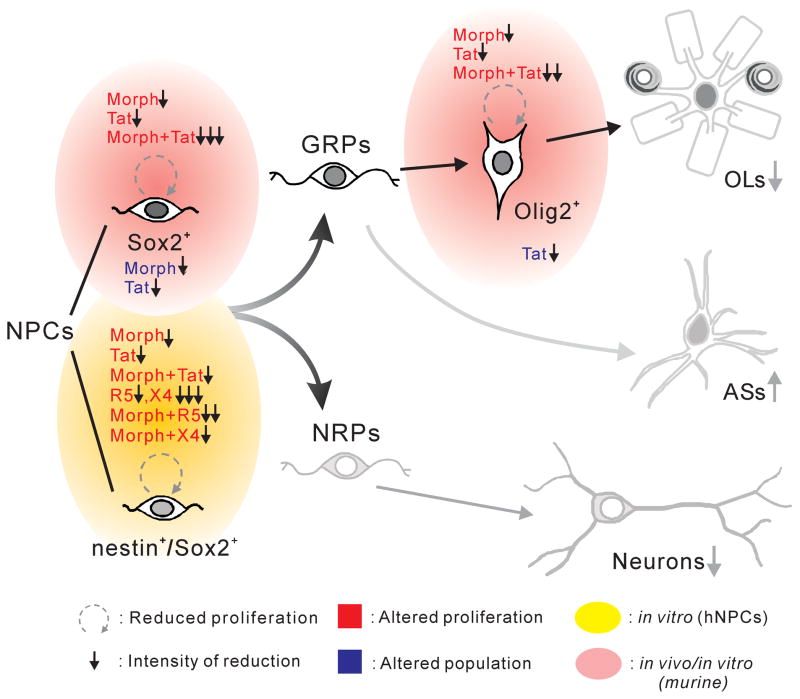
In the context of pediatric HIV, reduced production of Sox2+ progenitors is expected to impact populations of neurons and glia that derive from Sox2+ cells (Fig. 8). We find that immature, Olig2+ OLs are reduced in vivo, and others have reported lower neuron production, possibly through altered cyclin D1 and MAPK signaling (Mishra et al. 2010; Okamoto et al. 2007). Although the hypothesis that Tat exposure can alter lineage decisions remains to be fully tested, a proportional increase in astroglia might enhance interactions between astroglia and HIV-1 infected macrophages/microglia that are deleterious for developing neurons and neural circuitry (Blumberg et al. 1994). Our finding of Tat-morphine and HIV-1-morphine interactive effects is clinically relevant, given the high rate of opioid abuse in HIV patients, and that morphine may initially be used to treat neonatal abstinence syndrome in children of opioid-maintained or non-maintained mothers (Bandstra et al. 2010; Colombini et al. 2008; Ebner et al. 2007; Jansson et al. 2009). (Fig. 8)
Figure 8.
Proposed effects of HIV-1 Tat or HIV-1, and interactions with morphine, on perinatal NPCs. The intermediate filament nestin and the transcription factor Sox2 identify undifferentiated and self-renewing NPCs in the developing CNS, although nestin and Sox2 expression do not entirely overlap. Sox2 is maintained in immature cells destined to become neuroglia (glial-restricted precursors; GRPs) but lost in immature cells destined to become neurons (neural-restricted precursors; NRPs) (Ellis et al. 2004). GRPs further differentiate into astroglia and oligodendroglia; the transcription factor Olig2 is expressed in precursors that largely become oligodendroglia. HIV-1 Tat, supernatant from HIV-1 infected cells, and morphine, all affect proliferation of progenitor subtypes (red lettering). Morphine or Tat reduce the proliferation of Sox2+ progenitors and immature Olig2+ oligodendrocytes, and have an additive effect that further reduces proliferation. Reduced NPC and GRP proliferation is associated with changes in cell populations (blue lettering). Sox2+ cells are reduced by morphine or Tat exposure; Olig2+ cells are reduced by Tat exposure. Proliferation of human Sox2+/nestin+ NPCs is reduced to variable extents by Tat and by supernatant from cells infected with R5 or X4-preferring strains of HIV. Morphine alone can also marginally reduce proliferation. Co-exposure to morphine and HIV supernatant results in strain-specific interactions: morphine enhances the effect of HIV-1SF162 supernatant, but partially counteracts the effect of HIV-1IIIB supernatant. Our findings support a model where the proliferation of both NPCs and GRPs is significantly reduced by HIV, with morphine interactions that can further compromise progenitor production, depending on viral strain. Tat protein clearly reduces proliferation by itself; effects of HIV supernatant reflect combined actions of multiple viral proteins such as gp120 (Lee et al. 2011) and secreted factors. Reduced proliferation predicts overall reduction in progenitor populations, which was observed. Continued effects on proliferation will likely disturb the balance of glia and neurons in the brain, a prediction that remains to be tested. (Arrows and cartoons in grey represent hypothesized outcomes for which we have no direct experimental evidence.)
If the observed effects also occur on progenitors in the adult CNS, the predicted outcome would be fewer NPCs and a tendency towards increased astroglia at the expense of OL production (Fig. 8). Importantly, neural precursors were also reduced in human hippocampal slice cultures by HIV coat proteins and patient CSF (Krathwohl and Kaiser 2004) and in gp120 transgenic mice (Lee et al. 2011; Mishra et al. 2010; Okamoto et al. 2007). Neural precursors were also reportedly reduced in HIV patients with neurological deficits as compared to HIV-1 infected individuals without such deficits and non-infected controls (Krathwohl and Kaiser 2004).
SUMMARY
Consistent findings that Tat and HIV-1 reduce NPC production in a variety of model systems that assess maturation are compelling. By disrupting proliferation and subsequent differentiation of NPCs into mature glia and neurons, HIV-1 infection may increase the severity of neurological impairment in pediatric and adolescent patients. Interactions between Tat/HIV and morphine may exacerbate neurologic problems in young HIV-1 patients receiving opiates prescribed for neonatal abstinence syndrome or pain, as well as HIV-1+ adolescents experimenting with abused opiates. Evidence that some neurologic abnormalities in the pediatric HIV population may be reversible (McCoig et al. 2002) is encouraging from a therapeutic standpoint, and points to the need to fully understand how HIV and opiates individually and interactively affect CNS progenitors. This is especially true in resource-limited environments, where the spectres of perinatal HIV infection and high rates of pediatric disability/mortality have not been diminished.
Acknowledgments
This work was supported by funding from the NIH (DA024461 to PEK). Additional salary support was provided by a K02 Career Scientist Development Award (DA027374 to KFH). The authors are grateful to Dr. Nazira El-Hage and Mr. Ruturaj Masvekar for assistance with viral studies.
References
- Altman J. Proliferation and migration of undifferentiated precursor cells in the rat during postnatal gliogenesis. Exp Neurol. 1966;16(3):263–78. doi: 10.1016/0014-4886(66)90063-x. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Bell JE. The Neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20(1):15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64(6):529–36. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondrial Toxicity in HAART: An Overview of in Vitro Evidence. Curr Pharm Des. 2011;17(36):4047–4060. doi: 10.2174/138161211796904731. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, Eisch AJ. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157(1):70–9. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Mansoor E, Accornero VH. Prenatal drug exposure: infant and toddler outcomes. J Addict Dis. 2010;29(2):245–58. doi: 10.1080/10550881003684871. [DOI] [PubMed] [Google Scholar]
- Becquet R, Ekouevi DK, Arrive E, Stringer JS, Meda N, Chaix ML, Treluyer JM, Leroy V, Rouzioux C, Blanche S, et al. Universal antiretroviral therapy for pregnant and breast-feeding HIV-1-infected women: towards the elimination of mother-to-child transmission of HIV-1 in resource-limited settings. Clin Infect Dis. 2009;49(12):1936–45. doi: 10.1086/648446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1(2):182–91. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and drug misuse in the Edinburgh cohort. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S35–42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40(2–3):122–31. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94(5):1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32(2):253–67. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Hegde R, Callen S, Yao H, Adany I, Li Q, Li Z, Pinson D, Yeh HW, Cheney PD, Buch S. Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques. J Neuroimmune Pharmacol. 2011;6(4):626–39. doi: 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JM, Radio NM, Mundy WR, Shafer TJ. Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol Sci. 2008;105(1):119–33. doi: 10.1093/toxsci/kfn115. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 tat transgenic mice. Glia. 2008;56(13):1414–27. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch SK, Khurdayan VK, Lutz SE, Knapp PE, El-Hage N, Hauser KF. Glial-restricted precursors: patterns of expression of opioid receptors and relationship to human immunodeficiency virus-1 Tat and morphine susceptibility in vitro. Neuroscience. 2007;146(4):1546–54. doi: 10.1016/j.neuroscience.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz B, Hien S, Weichert S, Tenenbaum T. Pediatric aspects of HIV1-infection--an overview. Minerva Pediatr. 2010;62(4):371–87. [PubMed] [Google Scholar]
- Burdo TH, Katner SN, Taffe MA, Fox HS. Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol. 2006;1(1):41–9. doi: 10.1007/s11481-005-9001-3. [DOI] [PubMed] [Google Scholar]
- Burns KA, Murphy B, Danzer SC, Kuan CY. Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57(10):1115–29. doi: 10.1002/glia.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, McCutchan JA, Duarte NA, Simpson DM, McArthur J, Grant I. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58(2):154–62. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Lorke DE, Tiu SC, Yew DT. Proliferation and apoptosis in the developing human neocortex. Anat Rec. 2002;267(4):261–76. doi: 10.1002/ar.10100. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Turchan J, Pocernich C, Bruce-Keller A, Roth S, Butterfield DA, Major EO, Nath A. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J Biol Chem. 2003;278(15):13512–9. doi: 10.1074/jbc.M209381200. [DOI] [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483(2–3):175–86. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;88(1):36–41. doi: 10.1016/j.drugalcdep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C, Levy JA. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Ann Neurol. 1988;23(Suppl):S58–61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART) J Pediatr. 2005;146(3):402–7. doi: 10.1016/j.jpeds.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Clifford DB. AIDS dementia. Med Clin North Am. 2002;86(3):537–50. vi. doi: 10.1016/s0025-7125(02)00005-6. Review. [DOI] [PubMed] [Google Scholar]
- Colombini N, Elias R, Busuttil M, Dubuc M, Einaudi MA, Bues-Charbit M. Hospital morphine preparation for abstinence syndrome in newborns exposed to buprenorphine or methadone. Pharm World Sci. 2008;30(3):227–34. doi: 10.1007/s11096-007-9176-1. [DOI] [PubMed] [Google Scholar]
- Dever SM, Xu R, Fitting S, Knapp PE, Hauser KF. Differential expression and HIV-1 regulation of mu-opioid receptor splice variants across human central nervous system cell types. J Neurovirol. 2012;18(3):181–90. doi: 10.1007/s13365-012-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R, Miljan EA, Hines SJ, Aouabdi S, Pollock K, Patel S, Edwards FA, Sinden JD. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007;8:36. doi: 10.1186/1471-2202-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drotar D, Olness K, Wiznitzer M, Guay L, Marum L, Svilar G, Hom D, Fagan JF, Ndugwa C, Kiziri-Mayengo R. Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics. 1997;100(1):E5. doi: 10.1542/peds.100.1.e5. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Bruce-Keller AJ, Conner C, Knapp PE, Xu R, Nath A, Hauser KF. Effects of chronic expression of the HIV-induced protein, transactivator of transcription, on circadian activity rhythms in mice, with or without morphine. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1680–7. doi: 10.1152/ajpregu.90496.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner N, Rohrmeister K, Winklbaur B, Baewert A, Jagsch R, Peternell A, Thau K, Fischer G. Management of neonatal abstinence syndrome in neonates born to opioid maintained women. Drug Alcohol Depend. 2007;87(2–3):131–8. doi: 10.1016/j.drugalcdep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–84. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Dever SM, Fitting S, Ahmed T, Hauser KF. HIV-1 coinfection and morphine coexposure severely dysregulate hepatitis C virus-induced hepatic proinflammatory cytokine release and free radical production: increased pathogenesis coincides with uncoordinated host defenses. J Virol. 2011;85(22):11601–14. doi: 10.1128/JVI.05239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50(2):91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26(2–4):148–65. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenau-Haggarty B, Choe S, Vance PJ, Wells TN, Power CA, Sutterwala SS, Doms RW, Landau NR, Hoxie JA. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87(4):745–56. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28(5):219–21. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Goudsmit J. Neurological and neuropathological features of human immunodeficiency virus infection in children. Ann Neurol. 1988;23(Suppl):S19–23. doi: 10.1002/ana.410230709. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31(26):9456–65. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov LM, Tyrsin OY, Krenn V, Chernigovskaya EV, Rapp UR. Tet-system for the regulation of gene expression during embryonic development. Transgenic Res. 2001;10(3):247–58. doi: 10.1023/a:1016632110931. [DOI] [PubMed] [Google Scholar]
- Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, Hauser KF. Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur J Pharmacol. 2012;689(1–3):96–103. doi: 10.1016/j.ejphar.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol. 2010;177(3):1397–410. doi: 10.2353/ajpath.2010.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, Chneiweiss H, Daumas-Duport C, Varlet P. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 2010;20(2):399–411. doi: 10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gorodinsky A, Barg J, Belcheva MM, Levy R, McHale RJ, Vogel Z, Coscia CJ. Dynorphins modulate DNA synthesis in fetal brain cell aggregates. J Neurochem. 1995;65(4):1481–6. doi: 10.1046/j.1471-4159.1995.65041481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Wang S, Messam CA, Yao Z. Distribution of nestin immunoreactivity in the normal adult human forebrain. Brain Res. 2002;943(2):174–80. doi: 10.1016/s0006-8993(02)02615-x. [DOI] [PubMed] [Google Scholar]
- Hahn K, Robinson B, Anderson C, Li W, Pardo CA, Morgello S, Simpson D, Nath A. Differential effects of HIV infected macrophages on dorsal root ganglia neurons and axons. Exp Neurol. 2008;210(1):30–40. doi: 10.1016/j.expneurol.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Vo P, Fitting S, Block ML, Hauser KF, Knapp PE. beta-Chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: effects on microglial migration. J Neurochem. 2010;114(1):97–109. doi: 10.1111/j.1471-4159.2010.06744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF. Morphine regulates DNA synthesis in rat cerebellar neuroblasts in vitro. Brain Res Dev Brain Res. 1992;70(2):291–7. doi: 10.1016/0165-3806(92)90210-n. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Nath A, Tyor WR, Bruce-Keller AJ, Knapp PE. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol. 2006;1(1):98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100(3):567–86. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Hahn YK, Adjan VV, Zou S, Buch SK, Nath A, Bruce-Keller AJ, Knapp PE. HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia. 2009;57(2):194–206. doi: 10.1002/glia.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav Neurosci. 2002;116(1):160–8. [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. J Infect Dis. 2005;191(6):886–9. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- Imamoto K, Leblond CP. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978;180(1):139–63. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. Outlook: UNAIDS outlook report. Geneva: Joint United Nations Programme on HIV/AIDS; 2009. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS., World Health Organization. Sub-Saharan Africa: AIDS epidemic update regional summary. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS: World Health Organization; 2008. [Google Scholar]
- Jordan CA, Watkins BA, Kufta C, Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991;65(2):736–42. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–4. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kaul M. HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–94. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, Knapp PE, Turchan-Cholewo J, Nath A, Hauser KF. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19(12):3171–82. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006;281(44):33749–60. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp PE, Itkis OS, Zhang L, Spruce BA, Bakalkin G, Hauser KF. Endogenous opioids and oligodendroglial function: possible autocrine/paracrine effects on cell survival and development. Glia. 2001;35(2):156–65. doi: 10.1002/glia.1080. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: mu and kappa opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22(2):189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kopnisky KL, Bao J, Lin YW. Neurobiology of HIV, psychiatric and substance abuse comorbidity research: workshop report. Brain Behav Immun. 2007;21(4):428–41. doi: 10.1016/j.bbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96(10):5768–73. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294(5549):2127–30. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Loughlin SE, Leslie FM. Effects of morphine on DNA synthesis in neonatal rat brain. Brain Res. 1987;428(1):45–52. doi: 10.1016/0165-3806(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190(2):216–26. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;354(1):192–206. doi: 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78(14):7319–28. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sharron M, Blanpain C, Doranz BJ, Vakili J, Setoh P, Berg E, Liu G, Guy HR, Durell SR, et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274(14):9617–26. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30(2):105–21. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lee MH, Wang T, Jang MH, Steiner J, Haughey N, Ming GL, Song H, Nath A, Venkatesan A. Rescue of adult hippocampal neurogenesis in a mouse model of HIV neurologic disease. Neurobiol Dis. 2011;41(3):678–87. doi: 10.1016/j.nbd.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28(47):12190–8. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6(12):1380–7. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78(8):4120–33. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, Ogawa Y, Nabeshima Y, Alvarez-Buylla A, Shimizu K, Ikenaka K. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293(2):358–69. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- McCoig C, Castrejon MM, Castano E, De Suman O, Baez C, Redondo W, McClernon D, Danehower S, Lanier ER, Richardson C, Keller A, Hetherington S, Saez-Llorens X, Ramilo O. Effect of combination antiretroviral therapy on cerebrospinal fluid HIV RNA, HIV resistance, and clinical manifestations of encephalopathy. J Pediatr. 2002;141(1):36–44. doi: 10.1067/mpd.2002.125007. [DOI] [PubMed] [Google Scholar]
- Messier B, Leblond CP, Smart I. Presence of DNA synthesis and mitosis in the brain of young adult mice. Exp Cell Res. 1958;14(1):224–6. doi: 10.1016/0014-4827(58)90235-0. [DOI] [PubMed] [Google Scholar]
- Meyers T, Moultrie H, Naidoo K, Cotton M, Eley B, Sherman G. Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis. 2007;196(Suppl 3):S474–81. doi: 10.1086/521116. [DOI] [PubMed] [Google Scholar]
- Mintz M. Clinical comparison of adult and pediatric NeuroAIDS. Adv Neuroimmunol. 1994;4(3):207–21. doi: 10.1016/s0960-5428(06)80259-7. [DOI] [PubMed] [Google Scholar]
- Mirsattari SM, Power C, Nath A. Parkinsonism with HIV infection. Mov Disord. 1998;13(4):684–9. doi: 10.1002/mds.870130413. [DOI] [PubMed] [Google Scholar]
- Mishra M, Taneja M, Malik S, Khalique H, Seth P. Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in NeuroAIDS. J Neurovirol. 2010;16(5):355–67. doi: 10.3109/13550284.2010.513028. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14(3):222–7. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–9. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9(4):439–47. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1(2):230–6. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Patel K, Ming X, Williams PL, Robertson KR, Oleske JM, Seage GR., 3rd Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23(14):1893–901. doi: 10.1097/QAD.0b013e32832dc041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Sun L, Jia B, Lan X, Zhu B, Wu Y, Zheng J. HIV-1-Infected and Immune-Activated Macrophages Induce Astrocytic Differentiation of Human Cortical Neural Progenitor Cells via the STAT3 Pathway. PLoS One. 2011;6(5):e19439. doi: 10.1371/journal.pone.0019439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Whitney N, Wu Y, Tian C, Dou H, Zhou Y, Zheng J. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia. 2008;56(8):903–16. doi: 10.1002/glia.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002a;11(1):21–9. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002b;99(16):10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17(6):1159–72. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Podhaizer EM, Zou S, Fitting S, Samano KL, El-Hage N, Knapp PE, Hauser KF. Morphine and gp120 Toxic Interactions in Striatal Neurons are Dependent on HIV-1 Strain. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-011-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. Glial cell lineage and development. Curr Opin Neurobiol. 1994;4(5):680–6. doi: 10.1016/0959-4388(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Qu Q, Shi Y. Neural stem cells in the developing and adult brains. J Cell Physiol. 2009;221(1):5–9. doi: 10.1002/jcp.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ra SM, Kim H, Jang MH, Shin MC, Lee TH, Lim BV, Kim CJ, Kim EH, Kim KM, Kim SS. Treadmill running and swimming increase cell proliferation in the hippocampal dentate gyrus of rats. Neurosci Lett. 2002;333(2):123–6. doi: 10.1016/s0304-3940(02)01031-5. [DOI] [PubMed] [Google Scholar]
- Ratner L, Haseltine W, Patarca R, Livak KJ, Starcich B, Josephs SF, Doran ER, Rafalski JA, Whitehorn EA, Baumeister K, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313(6000):277–84. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Reznikov K, Hauser KF, Nazarevskaja G, Trunova Y, Derjabin V, Bakalkin G. Opioids modulate cell division in the germinal zone of the late embryonic neocortex. Eur J Neurosci. 1999;11(8):2711–9. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- Rius RA, Barg J, Bem WT, Coscia CJ, Loh YP. The prenatal development profile of expression of opioid peptides and receptors in the mouse brain. Brain Res Dev Brain Res. 1991;58(2):237–41. doi: 10.1016/0165-3806(91)90010-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202(1):189–99. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Romanko MJ, Rola R, Fike JR, Szele FG, Dizon ML, Felling RJ, Brazel CY, Levison SW. Roles of the mammalian subventricular zone in cell replacement after brain injury. Prog Neurobiol. 2004;74(2):77–99. doi: 10.1016/j.pneurobio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Rothenaigner I, Kramer S, Ziegler M, Wolff H, Kleinschmidt A, Brack-Werner R. Long-term HIV-1 infection of neural progenitor populations. AIDS. 2007;21(17):2271–81. doi: 10.1097/QAD.0b013e3282f12f27. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–21. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Funk R, Miaskowski U, Plendl J. Long-lasting effects of naltrexone, an opioid receptor antagonist, on cell proliferation in developing rat forebrain. Brain Res. 1989;486(2):297–300. doi: 10.1016/0006-8993(89)90515-5. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DM, Hazra R, Major EO. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol. 2007;13(3):274–83. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4(3):319–27. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- Seatriz JV, Hammer RP., Jr Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull. 1993;30(5–6):523–7. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- Shin MK, Levorse JM, Ingram RS, Tilghman SM. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature. 1999;402(6761):496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–50. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Skoff RP. Gliogenesis in rat optic nerve: astrocytes are generated in a single wave before oligodendrocytes. Dev Biol. 1990;139(1):149–68. doi: 10.1016/0012-1606(90)90285-q. [DOI] [PubMed] [Google Scholar]
- Skoff RP, Knapp PE. Division of astroblasts and oligodendroblasts in postnatal rodent brain: evidence for separate astrocyte and oligodendrocyte lineages. Glia. 1991;4(2):165–74. doi: 10.1002/glia.440040208. [DOI] [PubMed] [Google Scholar]
- Skoff RP, Price DL, Stocks A. Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. II. Time of origin. J Comp Neurol. 1976;169(3):313–34. doi: 10.1002/cne.901690304. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. A quantitative lifespan study of changes in cell number, cell division and cell death in various regions of the mouse forebrain. Neuropathol Appl Neurobiol. 1979;5(6):433–56. doi: 10.1111/j.1365-2990.1979.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280(2):192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A. 2002;99(16):10276–81. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J Leukoc Biol. 2003;74(6):1074–82. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Tahan TT, Bruck I, Burger M, Cruz CR. Neurological profile and neurodevelopment of 88 children infected with HIV and 84 seroreverter children followed from 1995 to 2002. Braz J Infect Dis. 2006;10(5):322–6. doi: 10.1590/s1413-86702006000500004. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12(13):1157–63. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Khurshid N, Kumar P, Iyengar S. Expression of delta- and mu-opioid receptors in the ventricular and subventricular zones of the developing human neocortex. Neurosci Res. 2008;61(3):257–70. doi: 10.1016/j.neures.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Ultmann MH, Belman AL, Ruff HA, Novick BE, Cone-Wesson B, Cohen HJ, Rubinstein A. Developmental abnormalities in infants and children with acquired immune deficiency syndrome (AIDS) and AIDS-related complex. Dev Med Child Neurol. 1985;27(5):563–71. doi: 10.1111/j.1469-8749.1985.tb14127.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke RB. Mother-to-child transmission of HIV-1 in the era prior to the availability of combination antiretroviral therapy: the role of drugs of abuse. Life Sci. 2011;88(21–22):922–5. doi: 10.1016/j.lfs.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11(1):1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Vien TN, Gleason CA, Hays SL, McPherson RJ, Chavkin C, Juul SE. Effects of neonatal stress and morphine on kappa opioid receptor signaling. Neonatology. 2009;96(4):235–43. doi: 10.1159/000220763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel BE, Lee SJ, Hildebrand A, Craig W, Pierschbacher MD, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol. 1993;121(2):461–8. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, Knapp PE. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia. Brain. 2011;134(Pt 12):3616–31. doi: 10.1093/brain/awr281. [DOI] [PMC free article] [PubMed] [Google Scholar]