Abstract
Rhinovirus (genus enterovirus) infections are responsible for many of the severe exacerbations of asthma and chronic obstructive pulmonary disease. Other members of the genus can cause life-threatening acute neurological infections. There is currently no antiviral drug approved for the treatment of such infections. We have identified a series of potent, broad-spectrum antiviral compounds that inhibit the replication of the human rhinovirus, Coxsackie virus, poliovirus, and enterovirus-71. The mechanism of action of the compounds has been established as inhibition of a lipid kinase, PI4KIIIβ. Inhibition of hepatitis C replication in a replicon assay correlated with enterovirus inhibition.
Keywords: antiviral, enterovirus, HCV, inhibitor, PI4KIIIβ
Over the last 15 years, evidence has gathered that viral infections of the respiratory tract are a major cause of exacerbations in both chronic obstructive pulmonary disease (COPD)1 and asthma.2 Of these infections, half to two-thirds are caused by human rhinovirus (HRV), the most well-known etiologic agent of the common cold.3 While these infections are merely inconvenient in otherwise healthy individuals, exacerbation of the symptoms of COPD and asthma are serious, leading to very large numbers of hospitalizations and significant mortality. In fact, the prevalence of COPD is increasing rapidly, and it is predicted to become the third leading cause of death globally by 2030.4 Hence, the identification of compounds that can interfere with rhinovirus replication may lead to drugs that have important clinical value in the management of a growing medical need.
In the past, several drug candidates have been progressed into clinical trials for the treatment of HRV infections. These include Rupintrivir,5 a viral 3C protease inhibitor, and a number of compounds that prevent virus entry into cells by binding to the viral capsid: Pirodavir,6 Pleconaril,7 and BTA798.8 Only the latter of these is currently in clinical development, the others having been terminated due to lack of clinical efficacy or unacceptable side effects.
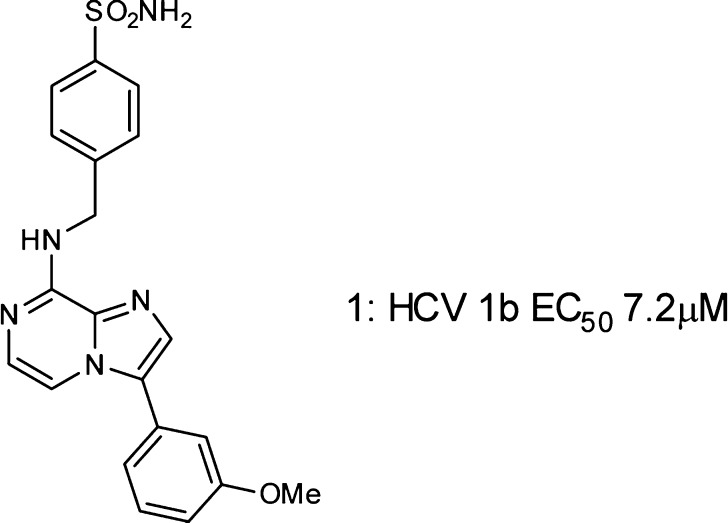
As part of an antiviral program directed toward hepatitis C (HCV), we identified a series of imidazo-pyrazines with modest (micromolar) activity in a genotype 1b replicon screen (Figure 1). These compounds were derived from a BioFocus SoftFocus library (SFK28) initially designed as potential kinase inhibitors.
Figure 1.
HCV 1b replicon screening hit.
Additional screening of the imidazo-pyrazines against a panel of other viruses revealed similar activity against the Enteroviruses, a genus of the Picornaviridae family, including HRV, polio virus (PV), and Coxsackie virus (CV). In most cases, the compounds were 3–5-fold more active against enteroviruses, which, in common with HCV, are positive-sense single-stranded RNA (+ssRNA) viruses. The compounds proved inactive against other RNA, DNA, and retroviruses and were not cytotoxic at antiviral concentrations, indicating a specific and selective antiviral effect.
Enteroviruses present a range of threats to human health, from the common cold to life-threatening infections, including encephalitis and viral meningitis.9 Polio is largely considered to be a disease of the past because of the success of the World Health Organization’s Global Polio Eradication Initiative in reducing the incidence of polio over the past two decades. However, as the initiative moves toward its final goal, it has become apparent that polio antiviral drugs will be required for emergency use in a postvaccination era to deal with the threat of outbreaks caused by circulating vaccine-derived poliovirus (VDPV), including treatment of infections transmitted by immunocompromised patients who chronically shed vaccine-derived poliovirus.10
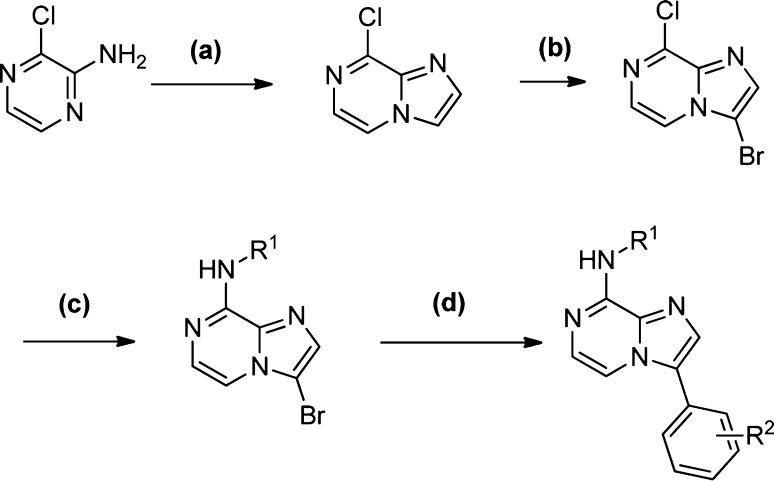
To improve on the antiviral potency of hit compound 1 and its congeners, we explored the effect of varying the substituents on the core scaffold, the aryl ring at the 3-position, and on the 8-position benzylamine. The synthesis of analogues (Scheme 1) followed the methodology developed for the original library production and HRV14 in a Hela Rh cell line was used as the primary target for tracking potency optimization. The synthetic route was sufficiently flexible to readily allow variation of the substituents at both the 3- and 8-position of the core scaffold. Introduction of substituents at the 2-position of the core scaffold was achieved by the use of the appropriate haloacetate (replacing bromoacetaldehyde) under similar reaction conditions. Enhancement in antiviral activity was achieved by varying the position and nature of substituents on the terminal aryl rings, and modification at C2 of the imidazo-pyrazine ring. In particular, polar substituents at the meta position of the benzylamine and H-bond donor/acceptor groups para substituted on the C3 aryl ring were potency enhancing (Table 1). Methylation of the C2 position of the imidazopyrazine generally had no effect on activity or gave a small increase in potency. Methylation at the benzylic position of the benzylamine was also well tolerated with the (racemic) analogue 6 retaining good potency.
Scheme 1. Synthesis of Imidazopyrazines.
Reagents and conditions: (a) BrCH2CHO, iPrOH reflux; (b) NBS, DCM; (c) R1NH2.HCl, iPr2EtN, iBuOH, 108 °C; (d) ArB(OH)2, Pd(0), DMF, microwave.
Table 1. Antiviral Activities against HRV-14.
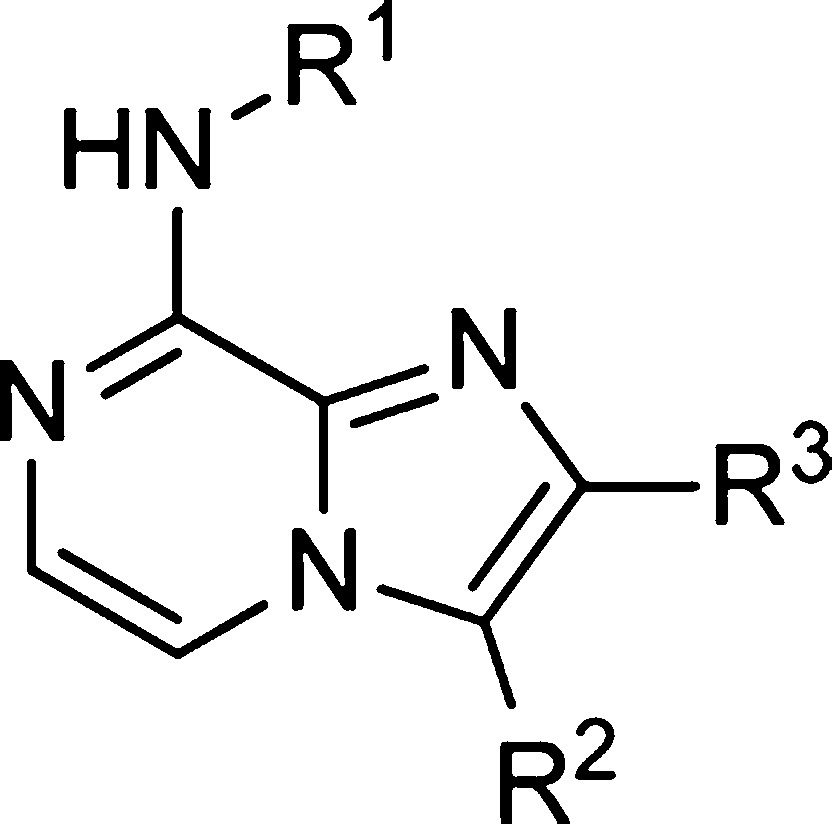
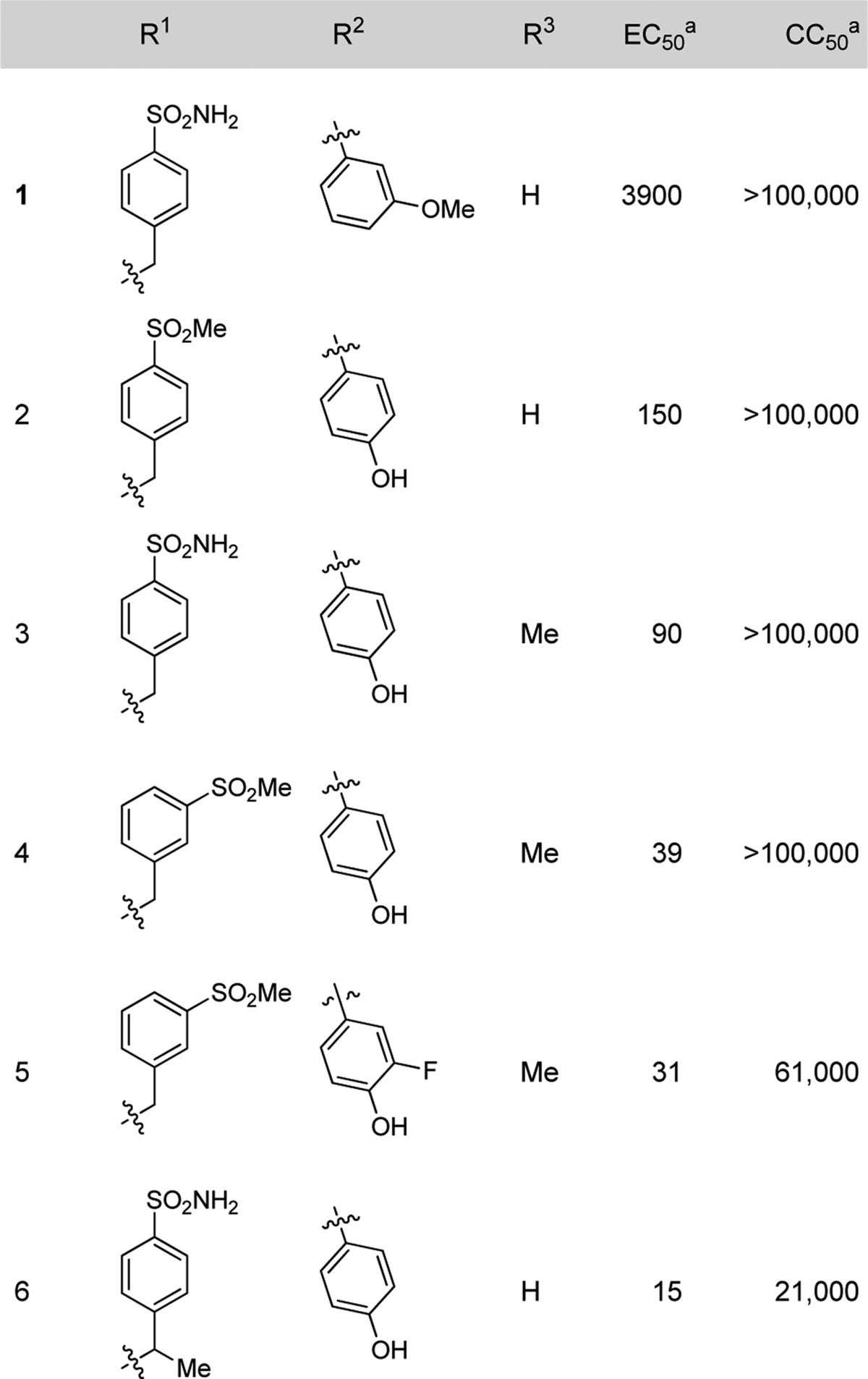
Activity in nM.
Cytotoxicity of the compounds in the Hela Rh cells was assessed in parallel with antiviral efficacy. The compounds were found to have a high selectivity index for antiviral activity relative to cellular toxicity with CC50 values generally >1000-fold higher than the EC50 values.
Comparison of the HRV14 activity of 1–6 against other members of the enteroviruses (Table 2) showed the rank order of potencies is essentially the same regardless of the species. This includes enterovirus-71, an emerging virus that has been the cause of several outbreaks in Asia, resulting in a number of deaths through neurological inflammation.11 The consistency in activity across the enteroviruses suggested that the molecular target of the compounds is either a viral target with very high conservation of sequence within the family or a single common target, most likely a host cell protein essential for the replication of the viruses.
Table 2. Cross-Species Antienteroviral Activitya.
| compd | HRV14 | CVB3 | PV1 | EV71 |
|---|---|---|---|---|
| 1 | 3900 | 3000 | 3000 | 470 |
| 2 | 150 | 550 | 140 | 80 |
| 3 | 90 | 490 | 150 | 34 |
| 4 | 39 | 68 | 38 | 15 |
| 5 | 31 | 15 | 19 | 11 |
| 6 | 15 | 66 |
EC50 values in nM
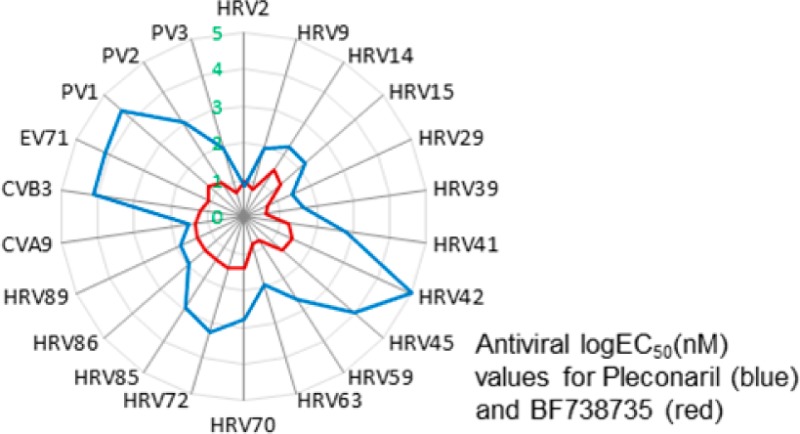
Compound 5 (BF738735) was selected for more extensive antienterovirus profiling and revealed a remarkable consistency in activity across a wide range of species (Table 3). Pleconaril (Figure 2), an anti-HRV agent that acts by preventing viral entry into cells by binding to the viral capsid, had a range of more than 3 orders of magnitude in EC50 values and was essentially inactive against some serotypes.
Table 3. Comparison of the Antienteroviral Activitya of BF738735 with Pleconaril.
| virus | BF738735 | pleconaril |
|---|---|---|
| HRV2 | 9 | 7 |
| HRV9 | 6 | 83 |
| HRV14 | 31 | 177 |
| HRV15 | 21 | 166 |
| HRV29 | 5 | 26 |
| HRV39 | 4 | 44 |
| HRV41 | 17 | 655 |
| HRV42 | 27 | 100000 |
| HRV45 | 25 | 8900 |
| HRV59 | 6 | 471 |
| HRV63 | 6 | 85 |
| HRV70 | 24 | 578 |
| HRV72 | 28 | 1800 |
| HRV85 | 24 | 883 |
| HRV86 | 24 | 89 |
| HRV89 | 24 | 75 |
| CVA9 | 21 | 33 |
| CVB3 | 15 | 13000 |
| EV71 | 11 | 13400 |
| PV1 | 19 | 24200 |
| PV2 | 13 | 1084 |
| PV3 | 5 | 84 |
EC50 values in nM
Figure 2.
Pleconaril.
Pleconaril reached phase III clinical trials but did not progress to registration due to drug–drug interactions with oral contraceptives and insufficient efficacy in treating infections.12 The relatively weak activity of Pleconaril against many of the HRV serotypes may have contributed to its poor clinical efficacy in a community setting where patients presenting with similar symptoms are infected with a spectrum of HRV serotypes. The broad, uniformly potent activity of BF738735 observed in the test panel offers a clear advantage over Pleconaril.
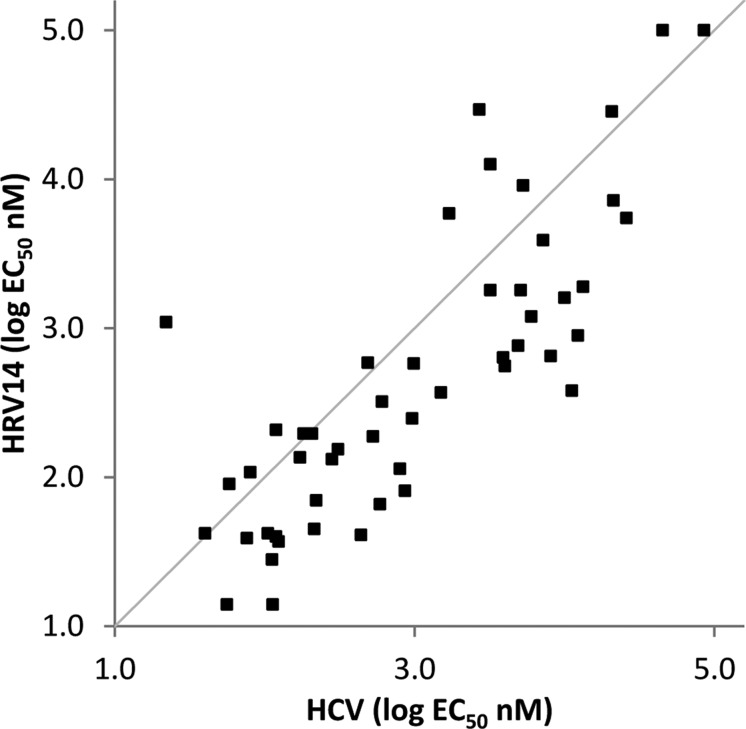
Although the potency of the compounds against HCV was generally slightly lower than that against HRV14 (e.g., BF738735 HCV1b EC50 56 nM), a strong correlation in activity (Figure 3) was observed during the optimization process, again suggesting a common mechanism of action. Because HCV belongs to a different virus family (Flaviviridae), this observation reinforced the likelihood that the target may be a host cell protein rather than a viral enzyme or structural protein.
Figure 3.
Correlation of activity against HRV and HCV. EC50 values were determined for inhibition of HRV replication in Hela Rh cells and against an HCV genotype 1b replicon in Huh 5.2. The correlation coefficient for the line of best fit is 0.81. The straight line drawn is for y = x.
While medicinal chemistry efforts to optimize the potency of this series of compounds were carried out, parallel efforts were undertaken to determine the mechanism of action. Resistance selection experiments showed that, in contrast to Pleconaril, there is a high genetic barrier for HRV to become resistant to the antiviral effect of the compounds. Time of drug-addition studies revealed that, whereas Pleconaril is only effective against HRV14 when added together with, or prior to, the addition of virus, BF738735 is active both when added before or after virus infection, indicating the mechanism is not at an early stage of the virus replication cycle. No significant activity of BF738735 was observed against isolated viral enzymes, which, taken together with the very narrow range of potencies seen within the genus enterovirus, pointed to a mechanism of action involving a host cell factor. The high correlation in activity against HCV suggested the mechanism is shared by this virus.
Representative compounds were selected for broad profiling against a range of enzymes, receptors, and ion channels and showed no significant activity at 1 μM. The highest activity observed in screening against a panel of >400 protein kinases was in the low micromolar range, considerably higher than the nanomolar antiviral activity that was observed in the virus-cell-based assays.
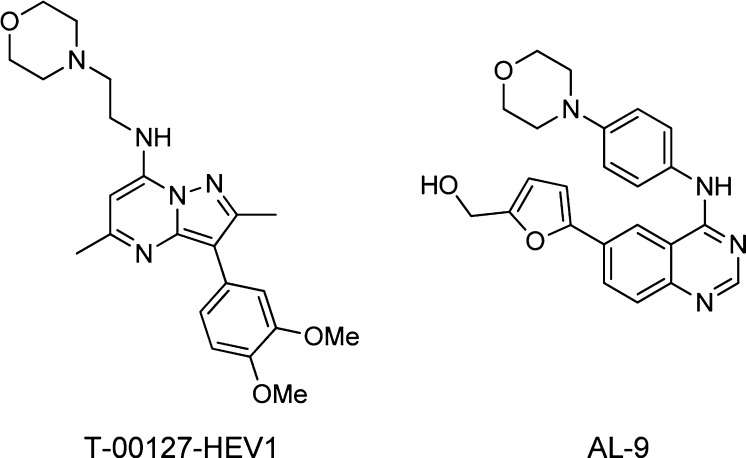
Concurrent with the work detailed here, several publications appeared detailing the results of target identification genomic screening to identify novel host targets involved in HCV replication. From these studies, the lipid kinases phosphatidylinositol-4 kinase III alpha and beta (PI4KIIIα and PI4KIIIβ) emerged as enzymes that appear to be important in the replication of the HCV virus. An siRNA study by Borawski et al.13 showed that knockdown of either PI4KIIIα or PI4KIIIβ led to reduced production of viral RNA for HCV genotypes 1a and 1b. Studies by Reiss et al.14 and Berger et al.15 concluded that only enzymatic activity of PI4KIIIα is required for HCV replication, whereas van Kuppeveld et al.16 demonstrated that PI4KIIIβ mediated enrichment of phosphatidyl inositol-4-phosphate in endoplasmic reticulum membranes is required for HCV replication. Arita et al.17 recently disclosed the discovery of T-00127-HEV1 (Figure 4), which was demonstrated to be a selective PI4KIIIβ inhibitor (IC50 60nM) with micromolar antiviral activity against PV1; T-00127-HEV1 was not active against HCV. In contrast, the dual PI4KIIIα /PI4KIIIβ inhibitor AL-9,18 which has a 5-fold preference for activity against PI4KIIIα (IC50 0.57 vs 3.08 μM), does inhibit HCV replication (EC50 0.29 μM for the 1b genotype) suggesting a greater dependence of this virus on the PI4KIIIα isoform.
Figure 4.
Screening BF738735 against a panel of lipid kinases revealed potent and highly selective inhibitory activity on PI4KIIIβ, with an IC50 of 5.7 nM. Inhibition of the related isoform PI4KIIIα was substantially weaker with an IC50 of 1700 nM, and no activity was found against a range of other lipid kinases (IC50 > 10 000 nM).
In summary, we have discovered a novel series of antiviral compounds with broad applicability for the treatment of enterovirus infections and with a potential for use against HCV in combination with drugs working by complementary mechanisms. The clinical application of PI4KIIIβ inhibitors will depend on the therapeutic index that can be achieved, particularly in nonlife-threatening infections. A recent publication19 has suggested that compounds acting by this mechanism cause reductions in T-cell count as a side effect that potentially will limit the use of this type of drug. Further studies will define the scope under which this may be managed.
Acknowledgments
We would like to acknowledge Stijn Delmotte and Tom Bellon for their excellent assistance in generating the antiviral data.
Glossary
Abbreviations
- COPD
chronic obstructive pulmonary disease
- HRV
human rhinovirus
- PV
poliovirus
- CV
Coxsackie virus
Supporting Information Available
Detailed experimental procedures for the synthesis of compounds and antiviral assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
∥ These authors contributed equally to this work. The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Papi A.; Bellettato C. M.; Braccioni F.; Romagnoli M.; Casolari P.; Caramori G.; Fabbri L. M.; Johnston S. L. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [DOI] [PubMed] [Google Scholar]
- Corne J. M.; Marshall C.; Smith S.; Schreiber J.; Sanderson G.; Holgate S. T.; Johnston S. L. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 2002, 359, 831–834. [DOI] [PubMed] [Google Scholar]
- Mallia P.; Contoli M.; Caramori G.; Pandit A.; Johnston S. L.; Papi A. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr. Pharm. Des. 2007, 13173–97. [DOI] [PubMed] [Google Scholar]
- WHO. World health statistics 2008. http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf.
- Hayden F. G.; Turner R. B.; Gwaltney J. M.; Chi-Burris K.; Gersten M.; Hsyu P.; Patick A. K.; Smith G. J.; Zalman L. S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003, 47123907–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G.; Hipskind G. J.; Woerner D. H.; Eisen G. F.; Janssens M.; Janssen P. A. J.; Andries K. Intranasal Pirodavir (R77,975) treatment of rhinovirus colds. Antimicrob. Agents Chemother. 1995, 392290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G.; Herrington D. T.; Coats T. L.; Kim K.; Cooper E. C.; Villano S. A.; Liu S.; Hudson S.; Pevear D. C.; Collett M.; McKinlay M. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 2003, 36121523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil S. C.; Hamilton S.; Krippner G. Y.; Lin B.; Luttick A.; McConnell D. B.; Nearn R.; Parker M. W.; Ryan J.; Stanislawski P. C.; Tucker S. P.; Watson K. G.; Morton C. J. An orally available 3-ethoxybenzisoxazole capsid binder with clinical activity against human rhinovirus. ACS Med. Chem. Lett. 2012, 34303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma A. M.; Vliegen I.; De Clercq E.; Neyts J. Selective inhibitors of picornavirus replication. Med. Res. Rev. 2008, 286823–884. [DOI] [PubMed] [Google Scholar]
- Barrett S. Polio eradication: strengthening the weakest links. Health Aff. 2009, 2841079–1090. [DOI] [PubMed] [Google Scholar]
- McMinn P. C. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr. Opin. Virol. 2012, 22199–205. [DOI] [PubMed] [Google Scholar]
- Fleischer R.; Laessig K. Safety and efficacy evaluation of pleconaril for treatment of the common cold. Clin. Infect. Dis. 2003, 37121722. [DOI] [PubMed] [Google Scholar]
- Borawski J.; Troke P.; Puyang X.; Gibaja V.; Zhao S.; Mickanin C.; Leighton-Davies J.; Wilson C. J.; Myer V.; Cornellataracido I.; Baryza J.; Tallarico J.; Joberty G.; Bantscheff M.; Schirle M.; Bouwmeester T.; Mathy J. E.; Lin K.; Compton T.; Labow M.; Wiedmann B.; Gaither L. A. Class III phosphatidylinositol 4-kinase alpha and beta are novel host factor regulators of hepatitis C virus replication. J. Virol. 2009, 831910058–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S.; Rebhan I.; Backes P.; Romero-Brey I.; Erfle H.; Matula P.; Kaderali L.; Poenisch M.; Blankenburg H.; Hiet M. S.; Longerich T.; Diehl S.; Ramirez F.; Balla T.; Rohr K.; Kaul A.; Bühler S.; Pepperkok R.; Lengauer T.; Albrecht M.; Eils R.; Schirmacher P.; Lohmann V.; Bartenschlager R. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microb. 2011, 9132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K. L.; Cooper J. D.; Heaton N. S.; Yoon R.; Oakland T. E.; Jordan T. X.; Mateu G.; Grakoui A.; Randall G. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc. Natl. Acad. Sci. U.S.A. 2009, 106187577–7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu N. Y.; Ilnytska O.; Belov G.; Santiana M.; Chen Y. H.; Takvorian P. M.; Pau C.; van der Schaar H.; Kaushik-Basu N.; Balla T.; Cameron C. E.; Ehrenfeld E.; van Kuppeveld F. J.; Altan-Bonnet N. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 2010, 1415799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M.; Kojima H.; Nagano T.; Okabe T.; Wakita T.; Shimizu H. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 2011, 8552364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A.; Reghellin V.; Donnici L.; Fenu S.; Alvarez R.; Baruffa C.; Peri F.; Pagani M.; Abrignani S.; Neddermann P.; De Francesco R. PLoS Pathog. 2012, 83e1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche M. J.; Borawski J.; Bose A.; Capacci-Daniel C.; Colvin R.; Dennehy M.; Ding J.; Dobler M.; Drumm J.; Gaither L. A.; Gao J.; Jiang X.; Lin K.; McKeever U.; Puyang X.; Raman P.; Thohan S.; Tommasi R.; Wagner K.; Xiong X.; Zabawa T.; Zhu S.; Wiedmann B. Anti-hepatitis C virus activity and toxicity of type III phosphatidylinositol-4-kinase beta inhibitors. Antimicrob. Agents Chemother. 2012, 56105149–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.