Abstract
Recent genetic studies have linked mental illness to alterations in disrupted in schizophrenia 1 (DISC1), a multifunctional scaffolding protein that regulates cyclic adenosine monophosphate (cAMP) signaling via interactions with phosphodiesterase 4 (PDE4). High levels of cAMP during stress exposure impair function of the prefrontal cortex (PFC), a region gravely afflicted in mental illness. As stress can aggravate mental illness, genetic insults to DISC1 may worsen symptoms by increasing cAMP levels. The current study examined whether viral knockdown (KD) of the Disc1 gene in rat PFC increases susceptibility to stress-induced PFC dysfunction. Rats were trained in a spatial working memory task before receiving infusions of (a) an active viral construct that knocked down Disc1 in PFC (DISC1 KD group), (b) a ‘scrambled' construct that had no effect on Disc1 (Scrambled group), or (c) an active construct that reduced DISC1 expression dorsal to PFC (Anatomical Control group). Data were compared with an unoperated Control group. Cognitive performance was assessed following mild restraint stress that had no effect on normal animals. DISC1 KD rats were impaired by 1 h restraint stress, whereas Scrambled, Control, and Anatomical Control groups were unaffected. Thus, knocking down Disc1 in PFC reduced the threshold for stress-induced cognitive dysfunction, possibly through disinhibited cAMP signaling at neuronal network synapses. These findings may explain why patients with DISC1 mutations may be especially vulnerable to the effects of stress.
Keywords: disrupted in schizophrenia 1, prefrontal cortex, schizophrenia, stress, working memory.
Introduction
Psychiatric disorders such as schizophrenia involve profound dysfunction of the prefrontal cortex (PFC).1, 2, 3, 4, 5, 6 The PFC uses working memory to provide top-down modulation of behavior, thought and affect7, 8, 9, and its function is weakened by exposure to even mild, uncontrollable stress.10 Psychiatric symptoms are often precipitated or worsened by stress,11, 12, 13, 14 causing descent from cognitive coherence to debilitating illness. Thus, it is critical to understand the molecular influences that modulate PFC function during stress in order to develop intelligent medications for psychiatric disorders. Unfortunately, there are currently few pharmacological treatments that ameliorate PFC cognitive deficits, and the challenge of developing effective cognitive enhancers is compounded by the unique neurochemical needs of PFC.
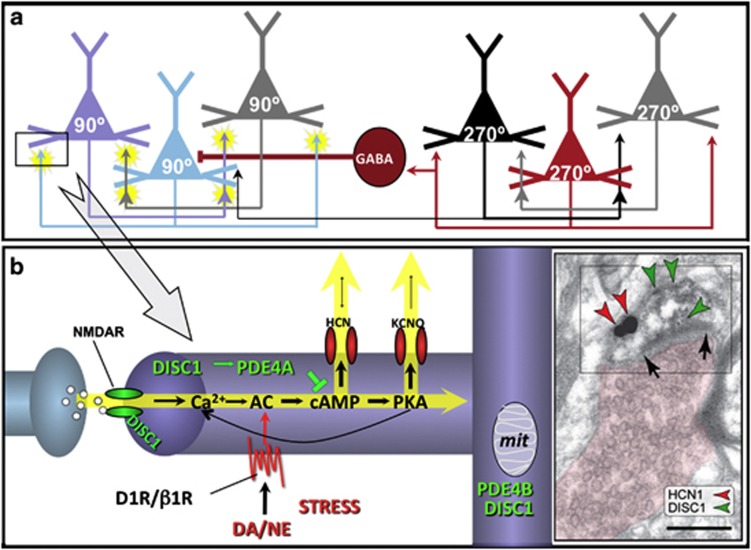
The cellular circuitry underlying spatial working memory has been identified by Goldman-Rakic.7 Spatial working memory is maintained by networks of pyramidal cells that interconnect at dendritic spines in layer III of dorsolateral PFC (Figure 1a). Recurrent excitation via N-methyl-D-aspartate receptor synapses maintains persistent firing across a delay period when a spatial position is held in working memory.7,15 The efficacy of these network connections is dynamically modulated at spines by intracellular mechanisms collectively termed, dynamic network connectivity16,17 (Figure 1b). Generation of cyclic adenosine monophosphate (cAMP) increases the open state of nearby potassium channels in dendritic spines, for example, hyperpolarization-activated cyclic nucleotide-gated (HCN) channels,18,19 and reduces network firing. Although this mechanism allows for rapid flexibility in sculpting the contents of working memory, it is also vulnerable to a variety of genetic and environmental insults, for example, in psychiatric disorders and during stress. In particular, increased release of catecholamines in PFC during even mild stress exposure increases cAMP signaling, which reduces PFC neuronal firing and impairs working memory.11
Figure 1.
Circuit basis for spatial working memory7 and molecular mechanisms of dynamic network connectivity at dendritic spines. (a) Spatial working memory is maintained in the dorsolateral prefrontal cortex (DLPFC) by N-methyl-D-aspertate-receptor (NMDAR)-mediated recurrent excitation among networks of pyramidal neurons with shared stimulus inputs (for example, 270°). The spatial tuning is enhanced by lateral inhibition of non-preferred inputs (for example, 90°) from gamma-aminobutyric acidergic (GABAergic) interneurons.64,65 (b) Working model of molecular mechanisms that modulate PFC network connectivity. Dynamic network connectivity signaling proteins are typically localized in long, thin spines with narrow spine necks in layer III monkey DLPFC. During stress, dopamine (DA) and norepinephrine (NE) stimulation of D1 (D1R) and β1 receptors (β1R), respectively, increase cyclic adenosine monophosphate (cAMP). Cyclic AMP/protein kinase A (PKA) then increase the open probability of hyperpolarization-activated cyclic nucleotide-gated (HCN) and KCNQ potassium channels, as well as regulate feedforward calcium–cAMP signaling, to weaken network connectivity. Disrupted in schizophrenia 1 (DISC1) interacts with the phosphodiesterase 4A (PDE4A) isoform (Paspalas & Arnsten, unpublished data) to reduce cAMP and strengthen network connectivity. (c) Dual immunoelectron microscopy for HCN1 channels (red arrowheads) and DISC1 (green arrowheads) in monkey DLPFC. HCN1 channels and DISC1 are colocalized in dendritic spines. The postsynaptic density is also DISC1-labeled, but HCN1 channels are invariably asynaptic. These findings are yet to be verified in rats. AC, adenylyl cyclase; mit, mitochondria; PDE4B, phosphodiesterase 4B. Scale bar, 200 nm.
Genetic insults associated with mental illnesses often affect stress-induced signaling pathways in PFC, and may explain why patients are especially vulnerable to the effects of stress.2,11 A major genetic risk factor is the disrupted in schizophrenia 1 (DISC1) gene. Initially discovered to have a balanced chromosomal translocation mutation in a Scottish pedigree with a high incidence of psychiatric disorders,20, 21, 22 it has since been found to be a susceptibility gene for a variety of psychiatric disorders in populations worldwide.21,23, 24, 25, 26, 27, 28, 29, 30, 31 DISC1 protein acts as a scaffold for many interacting molecules, for example, phosphodiesterase 4 (PDE4),32, 33, 34 and is involved in a variety of cellular functions such as intracellular signaling, neurodevelopment, and synaptogenesis.23,24,35, 36, 37, 38, 39, 40, 41, 42
DISC1 regulates intracellular levels of cAMP under conditions of high cAMP production through its interactions with PDE4 enzymes32,33 (Figure 1b). In primate PFC, DISC1 is colocalized with HCN1 channels in layer III dendritic spines19 (Figure 1c), and is thus in an ideal location to modulate recurrent PFC networks. That is, DISC1 may anchor PDE4 to the correct subcellular location and act like a ‘molecular brake' to restore normal cAMP levels following stress exposure, thus maintaining PFC network connectivity. Conversely, loss of DISC1 function in PFC would likely prevent proper PDE4 function, leading to a dysregulated build-up of cAMP in spines, excessive opening of HCN channels, and network dissociation. Consistent with this hypothesis, inhibition of PDE4 in PFC weakens network firing via opening of HCN channels in primates engaged in a working memory task,18 and impairs working memory in mice with Disc1 mutations.37,43,44 In addition, various DISC1 haplotypes in humans are associated with impaired working memory44, 45, 46, 47, 48 and reduced PFC gray matter.44,46,48, 49, 50, 51, 52
The current study examined the role of DISC1 in stress-induced PFC cognitive dysfunction. We explored whether knocking down Disc1 in rat PFC would lower the threshold for stress-induced cognitive dysfunction. Understanding the basic mechanisms underlying PFC function is key to understanding the etiology of many psychiatric disorders, and will hopefully provide rational therapeutic targets for treating cognitive impairment.
Materials and Methods
All procedures were approved by the Yale Institutional Animal Care and Use Committee.
Subjects
Forty-seven young, male Sprague–Dawley rats (2 months old at the beginning of the study; Taconic, Germantown, NY, USA) were housed individually under standard laboratory conditions. They were kept on a 12 h light/dark cycle, and behavioral experiments were conducted during the light phase. Highly palatable rewards (chocolate chips) were used during the experiments to minimize the need for dietary regulation. Water was provided ad libitum, and animals were fed 12–16 g of autoclaved rat chow (Purina Mills, Gray Summit, MO, USA) immediately following testing. They were weighed weekly, and weights were maintained at 400–450 g. The rats were habituated to all procedures, and tested by a single experimenter who was blind to experimental conditions.
Delayed alternation spatial working memory task
Rats were trained individually in a delayed alternation spatial working memory task in a T-shaped maze, as previously described.53 Further details are provided in the Supplementary Methods.
Once trained, rats were screened for normal sensitivity to stress exposure by assaying their response to 3 mg kg−1 of the pharmacological stressor, FG7142 (Tocris, Ellisville, MO, USA). This dose had no effect in most adult, male rats in a pilot study. Eleven rats that showed impaired performance, and thus inferred to have an exaggerated stress response, were removed from the study. The 36 remaining rats were assigned to the DISC1 knockdown (DISC1 KD; N=9), Scrambled (N=8), Control (N=12), and Anatomical Control (N=7) groups.
Production of viral constructs
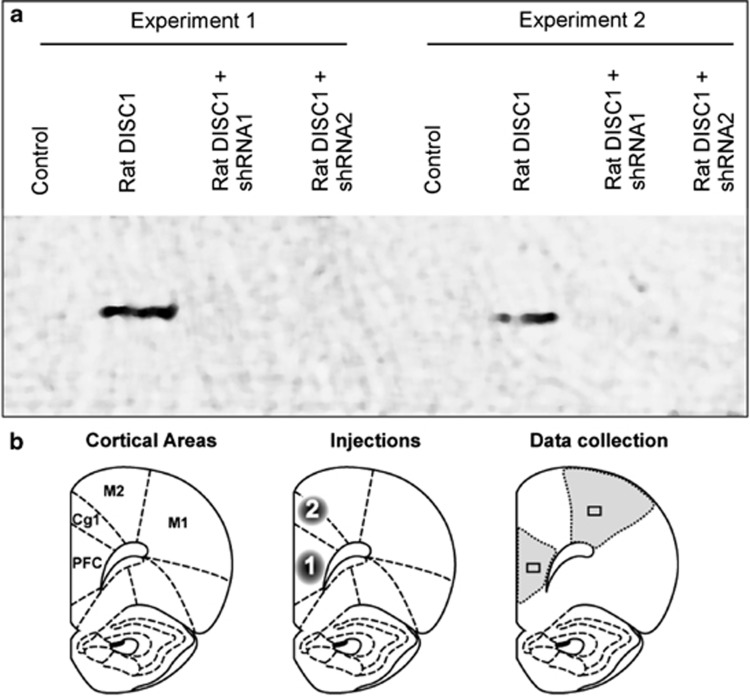
We initially generated five viral constructs designed to knock down Disc1 expression, Disc1-short hairpin-expressing RNA1–5 (Disc1-shRNA1-5). Briefly, a shRNA was designed to target a sequence within exon 1 of rat Disc1. The promotor, hairpin sequence, and terminator sequences were ligated in an adeno-associated virus (AAV) plasmid. Viral constructs were then produced in HEK293 cells by transfecting them with pDG plasmids (kindly provided by Drs Mark Kay and Dirk Grimm, Stanford University, CA). Western blots verified effective KD of Disc1 expression in HEK293 cells by two of the constructs (Figure 2a); of these, Disc1-shRNA1 was more effective, and was thus used for the behavioral experiments. In addition, a ‘scrambled' control construct (scrDisc1-shRNA) was designed to target a sequence that was not homologous to any known mammalian gene. Further details are provided in the Supplementary Methods.
Figure 2.
Viral constructs knocked down disrupted in schizophrenia 1 (DISC1) expression in rat prefrontal cortex (PFC). (a) Western blots verified that the Disc1–short hairpin-expressing RNA1 (shRNA1) and Disc1–shRNA2 constructs effectively knocked down expression of full-length rat DISC1 in HEK293 cells in two separate trials (Experiments 1 and 2). Disc1–shRNA1 was subsequently used in the behavioral experiments. (b) Rats received viral infusions in PFC (AP −3.2 mm; ML±0.75 mm; DV −4.2 mm) (1) or dorsal to PFC in cingulate cortex area 1 (Cg1)/secondary motor cortex (M2) (Cg1/M2; AP −3.2 mm; ML±0.75 mm; DV −2.0 mm) (2) (left, middle), and DISC1 labeling in these regions was compared with that in primary motor cortex (M1). Shaded areas indicate regions compared for stereology (right). Small rectangles represent regions compared for optical densitometry and % area of DISC1 labeling (right); actual sites and orientation varied from field to field.
Viral infusion surgery
The DISC1 KD, Scrambled, Control, and Anatomical Control groups were counterbalanced by baseline cognitive ability to ensure that preoperative performance was equal between the groups. Surgery was performed for the DISC1 KD, Scrambled, and Anatomical Control rats under Equithesin (pentobarbital-chloral hydrate, 4.32 mg g−1) anesthesia using aseptic methods.
DISC1 KD rats received infusions of the active construct to KD Disc1 expression in PFC, whereas Scrambled rats received the ‘scrambled' construct in PFC (Figure 2b). Guide cannulae were directed immediately dorsal to PFC (AP −3.2 mm; ML±0.75 mm; DV −4.2 mm), and infusion needles reached DV −4.5 mm. Two microliters of viral constructs were infused bilaterally into PFC at 0.25 μl min−1, and the cannulae were left in place for 5 min following the infusions. Pilot studies determined the appropriate parameters for the viral infusion procedure to ensure that only PFC was affected.
Rats in the Anatomical Control group received viral infusions in a region just dorsal to PFC that covered anterior cingulate cortex area 1 and motor area M2 (Cg1/M2) (Figure 2b). Guide cannulae were directed dorsal to PFC (AP −3.2 mm; ML±0.75 mm; DV −1.7 mm), and infusion needles reached DV −2.0 mm.
The viral constructs were allowed to express for 20 days following surgery before further behavioral data were collected. The Control group received no viral infusions. Testers were blind to group assignments.
One-hour restraint stress
Following viral expression, the rats were re-trained in the delayed alternation task. After reaching a stable baseline, they underwent 1 h restraint stress immediately before testing. Previous studies confirmed that 2 h, but not 1 h, of restraint stress impaired working memory performance in control rats.10 Thus, we investigated whether 1 h would be sufficient to impair DISC1 KD rats, but not Scrambled, Control, or Anatomical Control rats.
Restraint stress was performed by placing the rat in a restraint device (Harvard Apparatus, Holliston, MA, USA) in a separate room from the usual testing room. This procedure was done within 20–200 days following the viral infusion surgery. Infusions were less effective outside this period, likely because the viral constructs required enough time to express, but not enough for possible compensatory mechanisms to develop in the brain, for example, upregulation of PDE4. Rats were monitored continuously during the restraint period to ensure that there was no undue distress.
Behavioral data analysis
Cognitive performance without stress was compared before and after the viral infusion surgery, as well as between groups. Cognitive performance was defined as a product of the delay and mean pre- or postsurgery score. For the presurgery cognitive performance, scores from three consecutive testing days just prior to surgery were used. For the postsurgery cognitive performance, scores from three consecutive testing days 20–30 days after surgery were used, when the rats' performance appeared to have stabilized. If the rats underwent 1 h restraint stress during this time, the scores from three consecutive testing days just prior to the restraint stress were used. The pre- and postsurgery cognitive scores were compared using paired t-tests within each group. We also compared the difference between the pre and postsurgery cognitive scores between groups using a one-way analysis of variance (ANOVA) with a between-subjects factor of group (Control, Scrambled, DISC1 KD, Anatomical Control).
Working memory performance before and after 1 h restraint stress was analyzed using a 2-way mixed-design ANOVA with a between-subjects factor of group (Control, Scrambled, DISC1 KD, Anatomical Control) and a within-subjects factor of stress (baseline, stress). User-defined contrasts were then performed to compare stress scores for DISC1 KD vs Control, Scrambled and Anatomical Control groups, and baseline vs stress for each group.
Finally, errors in individual trials following 1 h restraint stress were examined, for example, incorrectly returning to an arm following a correct choice in ‘Win-stay' trials, and perseverating further by repeatedly choosing the same arm in the T-maze. In addition, in ‘time-out' or omitted trials, the rats failed to make a choice for 2 min, which could indicate an inability to make a choice or ‘freezing,' a severe stress response. The number of such error trials following stress was compared between the DISC1 KD and Control groups using Wilcoxon Signed-Rank tests. Statistical analyses were performed using SPSS (IBM Corporation, Armonk, NY, USA).
DISC1 immunohistochemistry
After completion of behavioral testing, viral injection sites were verified with DISC1 immunohistochemistry. For quantitative assessments, we primarily focused on DISC1 expression in dendrites, as we were interested in its role in modulating the connectivity of PFC networks at dendritic spines. To analyze DISC1 expression in PFC, we used stereology, optical densitometry, and % area of DISC1 labeling. Data collection was performed with an Axioskop microscope (Carl Zeiss, Thornwood, NY, USA) interfacing with a Dell personal computer via a Microfire camera (Optronics, Goleta, CA, USA). Further details are provided in the Supplementary Methods.
Quantification of DISC1 staining using isotropic virtual planes-based stereology
The total length and length density of DISC1-stained dendrites were quantified in the regions of interest using StereoInvestigator (MicroBrightField, Williston, VT, USA). Specifically, the Isotropic Virtual Plane probe was used in a systematic random sampling scheme. To avoid over or under-sampling, 4 sections from each cortical area were analyzed for each rat, chosen based on the quality of tissue. This number was statistically sufficient according to the low coefficients of error (<0.05).
The length density in PFC was normalized to that in motor cortex within each group, and compared using a 1-way ANOVA with a between-subjects factor of group (Control, Scrambled, DISC1 KD, Anatomical Control). User-defined contrasts compared DISC1 KD vs Control, Scrambled and Anatomical Control groups. We visually verified that the active viral construct knocked down DISC1 in Cg1/M2 in the Anatomical Control group. Statistical analyses were performed using SPSS.
Results
Basal working memory performance following KD of Disc1 in PFC
To minimize stress under basal conditions, rats were fully adapted to all conditions prior to infusion of viral constructs. When pre- and postsurgery cognitive scores were compared within each group using paired t-tests, there were no significant differences (DISC1 KD: P=0.76; Scrambled: P=0.35; Control: P=0.20; Anatomical Control: P=0.34). When the difference between the pre- and postsurgery cognitive scores were compared between groups with a one-way ANOVA, there were no significant differences (F[3,32]=0.41, P=0.75).
Stress-induced cognitive dysfunction following KD of Disc1 in PFC
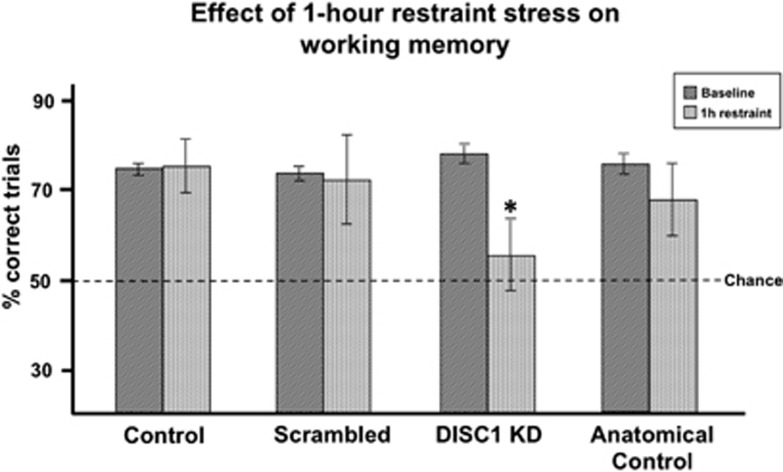
One-hour restraint stress impaired working memory performance in DISC1 KD rats, but not Scrambled, Control, or Anatomical Control rats (Figure 3). These results agreed with a previous finding that 1 h restraint stress did not impair working memory in control rats.10 A two-way ANOVA revealed a trend toward a significant main effect of stress (F[1,32]=3.917, P=0.056), a non-significant main effect of group (F[3,32]=0.877, P=0.463), and a non-significant interaction between group and stress (F[3,32]=2.016, P=0.131). User-defined contrasts revealed that 1 h restraint stress significantly impaired performance relative to baseline in DISC1 KD rats (P=0.045), but not in Scrambled (P=0.869), Control (P=0.916), or Anatomical Control (P=0.334) rats. DISC1 KD exacerbated stress-induced working memory deficits in rats tested shortly after transfection (mean of 50% correct), as well as those tested >100 days after transfection (mean of 56.7% correct), consistent with a stable reduction in DISC1 expression over this time period (see below). The normal performance of the Anatomical Control rats, in which DISC1 was reduced dorsal to PFC in Cg1/M2, verified the importance of DISC1 within PFC and not nearby areas for the regulation of working memory.
Figure 3.
One-hour restraint stress impaired working memory performance relative to baseline in disrupted in schizophrenia 1 (DISC1) knockdown (KD) rats (*P=0.045), but not in Control (P=0.916), Scrambled (P=0.869), or Anatomical Control rats (P=0.334).
Trial-by-trial error types following 1 h restraint stress
A number of analyses were performed to determine if there were qualitative differences in the patterns of response between baseline and stress conditions in each group. Specifically, the following variables were examined: the maximum number of errors to one side, the number of win–stay trials, and the number of omitted trials. The first two measures are signs of perseverative responding, whereas the third measure can be a reflection of freezing behavior characteristic of a more severe stress response.
No significant differences were observed in any of these measures between stress and non-stress conditions for any group. These findings suggest that the impaired performance following stress in the DISC1 KD group was mainly due to quantitative differences in the number of correct trials, rather than to qualitative changes in any particular type of error, and are consistent with the very mild stressor used in the study.
DISC1 immunohistochemistry
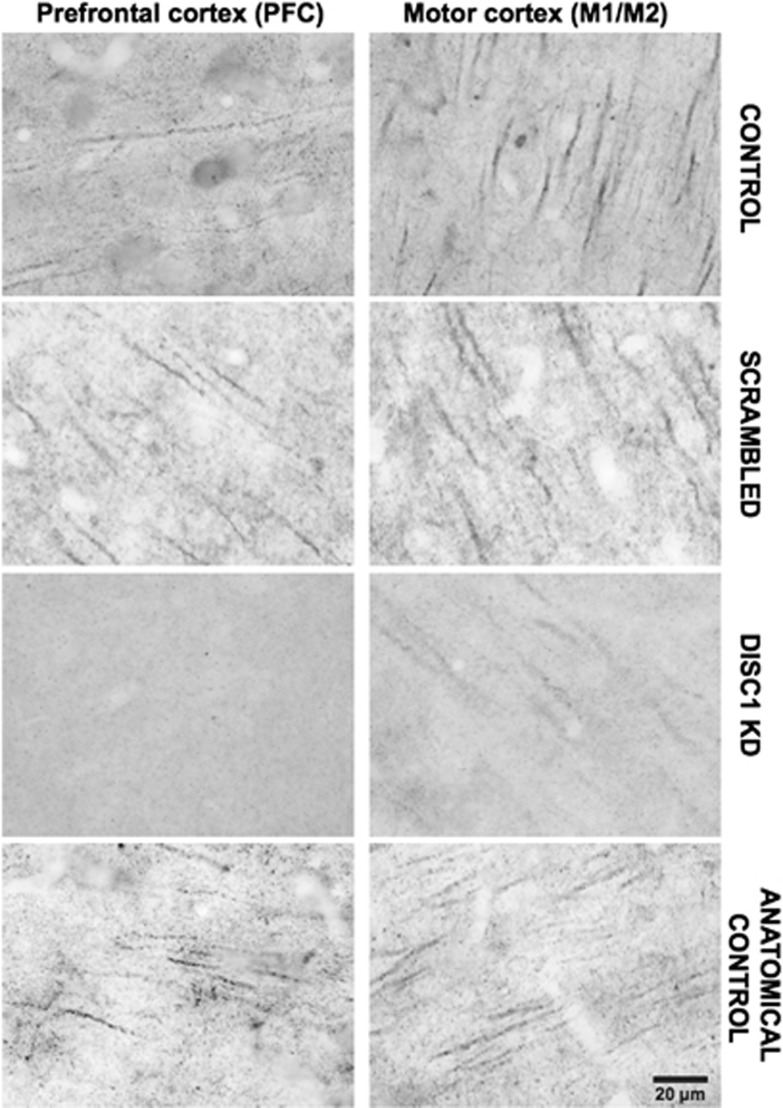
Following the behavioral experiments, we confirmed whether the viral constructs successfully knocked down DISC1 expression in PFC using immunohistochemistry. Figure 4 shows sample images of DISC1 labeling in the PFC and motor cortex in each group. For comparison, sample images of Cg1/M2 in the Anatomical Control and Control groups are shown in Supplementary Figure S1, and verification of the DISC1 antibody in DISC1 knockout rats (SAGE Labs, St Louis, MO, USA) is shown in Supplementary Figure S4.
Figure 4.
Examples of photographs taken of disrupted in schizophrenia 1 (DISC1) labeling in PFC and motor cortex are shown according to experimental group. Actual photograph site and orientation varied from field to field. Scale bar applies to all images.
Analysis using stereology, optical densitometry, and % area of DISC1 labeling revealed that DISC1 expression was reduced in PFC in the DISC1 KD group relative to motor cortex, as well as to PFC in the other groups. Further details are provided in the Supplementary Results.
Quantification of DISC1 labeling using isotropic virtual planes-based stereology
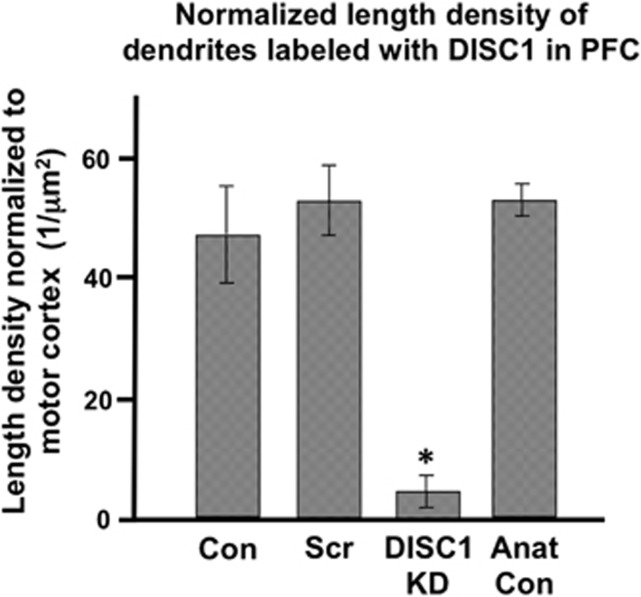
Length density of DISC1-labeled dendrites was measured in PFC relative to motor cortex, and compared between groups. A one-way ANOVA revealed a significant main effect of group (F[3,12]=13.535, P<0.0005). User-defined contrasts revealed that the DISC1 KD group showed reduced length density in PFC relative to the Control (P=0.003), Scrambled (P=0.001), and Anatomical Control (P=0.001) groups (Figure 5). DISC1 KD was still effective 100–200 days after the viral infusions, as these animals had no DISC1 in PFC but maintained DISC1 in nearby motor cortex (Supplementary Figure S3).
Figure 5.
Disrupted in schizophrenia 1 (DISC1) knockdown (KD) virus reduced DISC1 expression in the prefrontal cortex (PFC) in DISC1 KD rats. Length density of DISC1-labeled dendrites was measured in PFC using stereology and normalized to that in motor cortex. The length density in PFC was reduced in the DISC1 KD group relative to that in the Control (Con; *P=0.003), Scrambled (Scr; *P=0.001), and Anatomical Control (Anat Con; *P=0.001) groups.
Discussion
Summary of findings
In this study, we have demonstrated that reducing DISC1 function in PFC increased vulnerability to the impairing effects of acute stress on working memory performance. Under non-stress conditions, KD of Disc1 had no significant effect on working memory. However, rats that received an active viral construct that knocked down Disc1 in PFC were significantly impaired by 1 h restraint stress, a very mild stressor that had no effect on cognitive performance in control animals.10 Rats that received a construct with a ‘scrambled' sequence or received the active construct dorsal to PFC were also unaffected by this very mild stress. Thus, reduction of DISC1 expression in the adult rat PFC increased susceptibility to stress-induced PFC dysfunction. Koike et al.43 and Kvajo et al.37 have shown that mice with a truncation mutation in Disc1 showed impaired performance in a challenging test of working memory. Our study adds to their findings by investigating the effects of a DISC1 lesion restricted to PFC in adult animals, free from developmental effects of the Disc1 mutation. These data suggest that mutations that reduce DISC1 function may continue to impair PFC cognitive function in the adult cortex during stress exposure.
Potential mechanisms
DISC1 serves as a scaffolding protein for a large number of proteins, including interactions with a variety of PDE4s to regulate cAMP levels.32,33,54 Thus, it is possible that KD of DISC1 aggravates stress-induced cognitive deficits through dysregulation of cAMP signaling. Both physiological and behavioral studies have shown that acute stress impairs PFC network firing and working memory performance via increased cAMP–HCN channel signaling.18,55,56 Although in vitro tissue culture studies have shown that elevation of intracellular cAMP levels causes the release of PDE4D3 and PDE4C2 isoforms from DISC1, it does not affect DISC1 binding to PDE4B1 and PDE4A5 isoforms under these conditions.54 Thus, DISC1 interactions with PDE4s and cAMP signaling appear to be heterogeneous.
Immunoelectron microscopy data indicate that DISC1 appears to be crucial for the proper localization of PDE4s within PFC neurons, anchoring them to precise subcellular locations.19 Thus, loss of DISC1 would lead to spatially dysregulated cAMP signaling. For example, PDE4A and DISC1 are both found near HCN channels in spines (Figure 1c), and unanchoring of PDE4A may lead to excessive cAMP opening of HCN channels and reduced PFC network firing. (We were not able to investigate the role of HCN channels in the current study, as these experiments would require cannulation procedures that are incompatible with restraint stress.)
DISC1 also anchors PDE4A to the spine apparatus (endoplasmic reticulum), where it is positioned to regulate feedforward cAMP-calcium signaling.19 Unanchoring of PDE4A from this key location may lead to increased calcium and cAMP–protein kinase A signaling, which may reduce neuronal firing by opening SK and KCNQ potassium channels, and closing canonical transient receptor potential channels. KCNQ channels are localized on PFC spines,17 are opened by protein kinase A signaling,57 and have been shown to interact with human DISC1 in vitro.58 Physiological recordings have also shown that DISC1 KD in rat PFC hyperpolarizes layer V pyramidal neurons and reduces their firing, at least in part, through cAMP-induced reductions in transient receptor potential channels currents and increases in calcium-activated SK currents (El Hassar & Yeckel, unpublished data). Thus, dysregulated DISC1–cAMP signaling can lead to a number of changes that reduce PFC neuronal excitability. Furthermore, loss of DISC1 may impair energy regulation by unanchoring PDE4B from mitochondria in PFC dendrites;19 this mechanism may be particularly problematic under conditions of stress exposure.
Finally, although the current study focused on the contribution of DISC1 to working memory in the adult PFC, DISC1 likely also affects PFC function via developmental changes in the PFC circuitry. Disc1-mutant mouse models show altered dendritic structure and spine density in PFC pyramidal cells.41,59 Furthermore, many models show reduced immunoreactivity for parvalbumin-positive interneurons in PFC.59, 60, 61 Such DISC1-related changes would likely affect working memory performance both during development and as adults.
Role of various DISC1 isoforms
The DISC1 KD virus used here was designed to target all known rodent DISC1 isoforms, and thus our results likely reflected reduction or absence of total DISC1 protein in PFC. This manipulation is not identical to the translocation mutation observed in the Scottish pedigree in which DISC1 was initially discovered.20, 21, 22 However, our results are still relevant, as the translocation mutation has been proposed to result in ‘haploinsufficiency' due to a loss of the whole protein,32 or in the expression of a truncated protein with dominant-negative function.28,58,62,63 Thus, these families may have a ‘functional KO' of DISC1 actions.
Relevance to psychiatric disorders
The current findings may extend to a range of psychiatric disorders associated with dysregulated cAMP signaling, where patients often show precipitation or exacerbation of symptoms with stress.11, 12, 13, 14 For example, schizophrenia is associated with a number of alterations that either reduce the regulation of cAMP signaling, or aggravate the induction of cAMP-calcium signaling.17 DISC1 may normally protect PFC cognitive function under mild stress by acting as a molecular ‘brake' on stress-induced increases in cAMP. However, patients with insults to DISC1 may show increased sensitivity to the effects of stress due to impaired interactions with PDE4 and excessive build-up of cAMP, which in turn may weaken network synapses. Understanding these intracellular pathways in PFC may help us to develop rational strategies for novel psychiatric treatments and cognitive enhancers.
Acknowledgments
This research was supported by NINDS T32 grant NS07224 to NJG, and a NARSAD Distinguished Investigator Award, PO1 grant AG030004 and 1RL1AA017536 within U54RR024350 to AFTA. We would like to thank Drs Mark Kay and Dirk Grimm (Stanford University, CA) for the AAV viral constructs. This work has been presented in abstract form at the DISC1 2010 meeting in Edinburgh, Scotland in September 2010; Society for Neuroscience Meeting in San Diego, CA in November 2010; New York Academy of Sciences Advancing Drug Discovery for Schizophrenia meeting in New York, NY in March 2011; and the Biological Psychiatry meeting in Philadelphia, PA in May 2012.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of ‘representational knowledge': a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17 (Suppl 1:i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Wolkowitz O, Pickar D.In: Tamminga C and Schult S (eds).Schizophrenia Research Raven Press, Ltd.: New York; 1991 [Google Scholar]
- Hammen C, Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of disorder. Am J Psychiatry. 1997;154:856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- Mazure CM.Does stress cause psychiatric illnessIn: Spiegel D (ed.). Progress in Psychiatryvol. 46. American Psychiatric Press: Washington, DC; 1995. p. 270. [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic network connectivity: a new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Wang M, Arnsten AF. Constellation of HCN Channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: potential substrate for working memory deficits in schizophrenia. Cereb Cortex. 2012;23:1643–1654. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- St Clair D, Blackwood D, Muir W, Carothers A, Walker M, Spowart G, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1—an emerging role in psychosis and cognition. Biol Psychiatry. 2006;60:123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Macgregor S, Visscher PM, Knott SA, Thomson P, Porteous DJ, Millar JK, et al. A genome scan and follow-up study identify a bipolar disorder susceptibility locus on chromosome 1q42. Mol Psychiatry. 2004;9:1083–1090. doi: 10.1038/sj.mp.4001544. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Li W, Noltner K, Yan J, Green E, Grozeva D, et al. Identification of high risk DISC1 protein structural variants in patients with bipolar spectrum disorder. Neurosci Lett. 2010;486:136–140. doi: 10.1016/j.neulet.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Harris SE, Hennah W, Thomson PA, Luciano M, Starr JM, Porteous DJ, et al. Variation in DISC1 is associated with anxiety, depression and emotional stability in elderly women. Mol Psychiatry. 2010;15:232–234. doi: 10.1038/mp.2009.88. [DOI] [PubMed] [Google Scholar]
- Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R, et al. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry. 2008;13:187–196. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Millar JK, Mackie S, Clapcote SJ, Murdoch H, Pickard BS, Christie S, et al. Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness. J Physiol. 2007;584:401–405. doi: 10.1113/jphysiol.2007.140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, James R, Christie S, Porteous DJ. Disrupted in schizophrenia 1 (DISC1): subcellular targeting and induction of ring mitochondria. Mol Cell Neurosci. 2005;30:477–484. doi: 10.1016/j.mcn.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR.Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence Mol Psychiatry 20051040–68., image 5. [DOI] [PubMed] [Google Scholar]
- Hennah W, Porteous D. The DISC1 pathway modulates expression of neurodevelopmental, synaptogenic and sensory perception genes. PLoS ONe. 2009;4:e4906. doi: 10.1371/journal.pone.0004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci USA. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, et al. Disc1 point mutations in mice affect development of the cerebral cortex. J Neurosci. 2011;31:3197–3206. doi: 10.1523/JNEUROSCI.4219-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci USA. 2006;103:3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci USA. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennah W, Tuulio-Henriksson A, Paunio T, Ekelund J, Varilo T, Partonen T, et al. A haplotype within the DISC1 gene is associated with visual memory functions in families with a high density of schizophrenia. Mol Psychiatry. 2005;10:1097–1103. doi: 10.1038/sj.mp.4001731. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunen M, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Hodgkinson CA, Szeszko PR, Lencz T, Ekholm JM, Kane JM, et al. DISC1 and neurocognitive function in schizophrenia. Neuroreport. 2005;16:1399–1402. doi: 10.1097/01.wnr.0000175248.25535.f6. [DOI] [PubMed] [Google Scholar]
- Carless MA, Glahn DC, Johnson MP, Curran JE, Bozaoglu K, Dyer TD, et al. Impact of DISC1 variation on neuroanatomical and neurocognitive phenotypes Mol Psychiatry 2011161096–1104., 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Hodgkinson CA, Robinson DG, Derosse P, Bilder RM, Lencz T, et al. DISC1 is associated with prefrontal cortical gray matter and positive symptoms in schizophrenia. Biol Psychol. 2008;79:103–110. doi: 10.1016/j.biopsycho.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Tsunoda M, Maeno N, Kawasaki Y, Zhou SY, et al. The Disrupted-in-Schizophrenia-1 Ser704Cys polymorphism and brain morphology in schizophrenia. Psychiatry Res. 2009;172:128–135. doi: 10.1016/j.pscychresns.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Long R, Greenstein D, Clasen L, Addington A, et al. Common functional polymorphisms of DISC1 and cortical maturation in typically developing children and adolescents. Mol Psychiatry. 2011;16:917–926. doi: 10.1038/mp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, et al. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, et al. D1 Pdopamine receptor-induced prefrontal cortical dysfunction during stress: Interactions with HCN channel (in submission).
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, et al. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci USA. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Genetics. Two genes link two distinct psychoses. Science. 2005;310:1128–1129. doi: 10.1126/science.1121114. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol. 1999;81:1903–1916. doi: 10.1152/jn.1999.81.4.1903. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.