Abstract
FLAP (5-lipoxygenase-activating protein) is a protein widely distributed within the central nervous system whose function is to regulate the activation of the 5-Lipoxygenase enzyme. Although previous works show that pharmacological blockade of FLAP improve the amyloidotic phenotype of the Tg2576, its contribution to tau pathology remains to be investigated. In the present paper, we studied the effect of FLAP pharmacological inhibition on the metabolism of endogenous tau in these mice. Total tau levels in the brains of mice receiving MK-591, a selective and specific FLAP inhibitor, were not changed when compared with controls. By contrast, treated animals had a significant reduction of tau phosphorylation at specific sites: Ser396; Ser396/Ser404; and Thr 231/Ser 235. This reduction was associated with a significant decrease in the activity of glycogen synthase kinase-3 beta, but not other kinases. In addition, MK-591-treated mice had a significant increase in the post-synaptic density protein-95 and the dendritic protein microtubule-associated protein 2. These data establish a novel functional role for FLAP in the metabolism of tau, and together with its known Aβ modulatory effect they suggest that its pharmacological inhibition could represent a novel therapeutic opportunity for Alzheimer's disease.
Keywords: Alzheimer's disease, amyloid, FLAP protein, transgenic animal models, tau protein
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by two major pathological hallmark lesions: extracellular accumulation of β-amyloid (Aβ) plaques and intracellular accumulation of insoluble microtubule-associated protein tau as neurofibrillary tangles.1Mutation in the tau gene have been linked to neurofibrillary tangle formation in several neurodegenerative diseases called tauopathies in which no Aβ deposits are detected.2However, no mutations have been found in the tau gene in AD, and the relationship between tau metabolism, amyloid deposition and neurodegeneration is difficult to establish.
Recently, we showed that the enzyme 5-Lipoxygenase (5LO) is a new active player in the neurobiology of AD.3This enzyme is abundantly present in the central nervous system, where its activity is regulated by the presence and availability of another protein, called 5LO-activating protein or FLAP.4From a biochemical point of view they form a functional complex whose integrity is necessary for the full 5LO enzymatic activity. A peculiar aspect of the 5LO-activating protein (FLAP)/5LO pathway is the fact that its expression levels are significantly increased in the CNS with aging, and that this increase is also region-specific as it mainly manifests in the hippocampus, an area vulnerable to neurodegenerative insults. Previously we have reported that FLAP selective pharmacological inhibition significantly reduces Aβ levels and deposition in the amyloid precursor protein (APP) transgenic mice.5However, no data are available on the effect that this therapeutic intervention has on endogenous tau levels and metabolism in the same AD-like mouse model.
To this end, Tg2576 mice were chronically administered with MK-591, a selective FLAP inhibitor,6and the effect on tau metabolism and pathology assessed.
Materials and methods
Mice and treatments
All animal procedures were approved by the Institutional Animal Care and Usage Committee, and in accordance with the National Institute of Health guidelines. The Tg2576 transgenic mice expressing human amyloid precursor protein with the Swedish mutation (K670N/M671L) used in these studies were previously described.5Only female mice were used in the study, they were genotyped by polymerase chain reaction (PCR) analysis using tail DNA and kept in a pathogen-free environment, on a 12-h light/dark cycle and had access to food and water ad libitum. All the experiments presented in this paper were performed with female mice. Starting at 7 months of age, mice were randomized to receive MK-591 (40 mg kg−1 weight) (n=11) or vehicle (n=9) in their chow diet for 8 months until they were 15 months old. Considering that each mouse eats in average 5 g per day of chow diet and the diet is formulated for 320 mg MK-591 per kg diet (Harlan Teklad, WI, USA), the final dose of the active drug was ~40 mg kg−1 weight per day. During the study, mice in both groups gained weight regularly, and no significant difference in weight was detected between the two groups. No macroscopic effect on the overall general health was observed in the animals receiving the active treatment. Post-mortem examination showed no sign of macroscopic pathology in any of the organs considered (that is, spleen liver, thymus and ileum).
After killing, animals were perfused with ice-cold 0.9% phosphate-buffered saline, the brain was removed and dissected in two hemihalves by mid-sagittal dissection. One was immediately stored at −80 °C for biochemistry assays and the other immediately immersed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.6) overnight for immunohistochemistry studies.
Immunohistochemistry
Immunostaining was performed as previously reported by our group.7,8Briefly, serial coronal sections were mounted on 3-aminopropyl triethoxysilane-coated slides. Every eighth section from the habenular to the posterior commissure (8–10 sections per animal) was examined using unbiased stereological principles. The sections were deparaffinized, hydrated, treated with 3% H2O2 in methanol and subsequently retrieve antigen with sodium citrate buffer. Sections were blocked in 2% fetal bovine serum before incubation overnight at 4 °C with the primary antibodies described in the Table 1. Subsequently, sections were incubated with biotinylated anti-mouse immunoglobulin G (Vector Lab., Burlingame, CA, USA) and then developed by using the avidin–biotin complex method (Vector Lab.) with 3,3′-diaminobenzidine as a chromogen. Light microscopic images were used to calculate the integrated optical density (IOD), of the obtained immunoreactivity using the software Image-Pro Plus for Windows version 5.0 (Media Cybernetics, Rockville, MD, USA).7, 8, 9
Table 1. Antibodies used in the study.
| Antibody | Immunogen | Host | Source |
|---|---|---|---|
| Tau-1 | Purified denatured bovine MAP | Mouse | Millipore |
| AT-8 | Peptide containing phospho-S202/T205 | Mouse | Pierce |
| AT-180 | Peptide containing phospho-T231/S235 | Mouse | Pierce |
| AT-270 | Peptide containing phospho-T181 | Mouse | Pierce |
| PHF-13 | Peptide containing phospho-Ser396 | Mouse | Cell Signaling |
| PHF-1 | Peptide containing phospho-Ser396/S404 | Mouse | Dr P Davies |
| GSK-3α/β | aa 1–420 full-length GSK-3β of Xenopus origin | Mouse | Millipore |
| p-GSK-3α/β | aa around Ser21 of human GSK-3α | Rabbit | Cell Signaling |
| JNK2 | aa of human JNK2 | Rabbit | Cell Signaling |
| SAPK/JNK | aa of recombinant human JNK2 fusion protein | Rabbit | Cell Signaling |
| Phospho-SAPK/JNK | aa Thr183/Tyr185 of human SAPK/JNK | Mouse | Cell Signaling |
| Cdk5 | aa C-terminus of Cdk5 of human origin | Rabbit | Santa Cruz |
| P35/P25 | aa C-terminus of p35/25 of human origin | Rabbit | Santa Cruz |
| PP-2A | A 16 residue synthetic peptide corresponding to aa 295–309 of the 36 kDa catalytic subunit of human PP2A | Mouse | Millipore |
| Synaptophysin | aa221–313 of synaptophysisn of human origin | Mouse | Santa Cruz |
| PSD-95 | Purified recombinant rat PSD-95 | Rabbit | Thermo Scientific |
| MAP-2 | Bovine brain microtubule protein | Rabbit | Millipore |
| Actin | aa C-terminus of actin of human origin | Goat | Santa Cruz |
Western blot analyses
Brain tissues were homogenized and extracted in radioimmunoprecipitation assay buffer containing proteases and phosphatases inhibitors, the extracts used for western blot analyses, as previously described.7, 8, 9Samples were electrophoresed on 10% Bis–Tris gels or 3–8% Tris–acetate gel (Bio-Rad, Richmond, CA, USA), according to the molecular weight of the target molecule, transferred onto nitrocellulose membranes (Bio-Rad), and then incubated with appropriate primary antibodies shown in the Table 1. After three washings with Tween-Tris buffered solution, membranes were incubated with IRDye 800CW or IRDye 680CW-labeled secondary antibodies (LI-COR Bioscience, Lincoln, NE, USA) at 22 °C for 1 h. Signals were developed with Odyssey Infrared Imaging Systems (LI-COR Bioscience). Beta-actin was always used as internal loading control.
Sarkosyl insolubility assay
The assay for insoluble tau was performed as described previously.10 Briefly, ultracentrifugation and sarkosyl extraction (30 min in 1% sarkosyl) were used to obtain soluble and insoluble fractions of tau. Insoluble fractions were washed one time with 1% sarkosyl, then immunoblotted with total mouse anti-tau antibody (Table 1).
Data analysis
Data analyses were performed using SigmaStat for Windows version 3.00. Statistical comparisons were performed by Unpaired Student's t-test or the Mann–Whitney rank sum test when a normal distribution could not be assumed. Values represent mean±s.e.m. Significance was set at P<0.05.
Results
Pharmacological blockade of FLAP reduces brain tau phosphorylation
Starting at 7 months of age, Tg2576 mice were randomized to receive MK-591 (40 mg kg−1 weight) (n=11) or vehicle (n=9) for 8 months, then killed and the effect of the treatment on tau level and metabolism assessed.
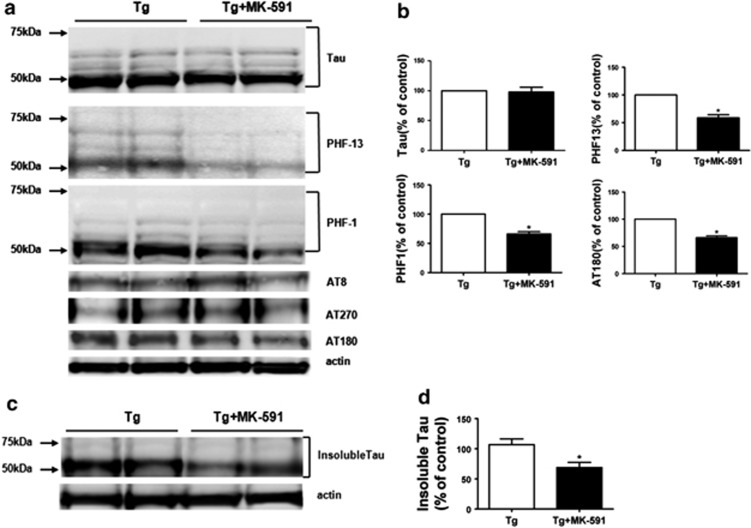
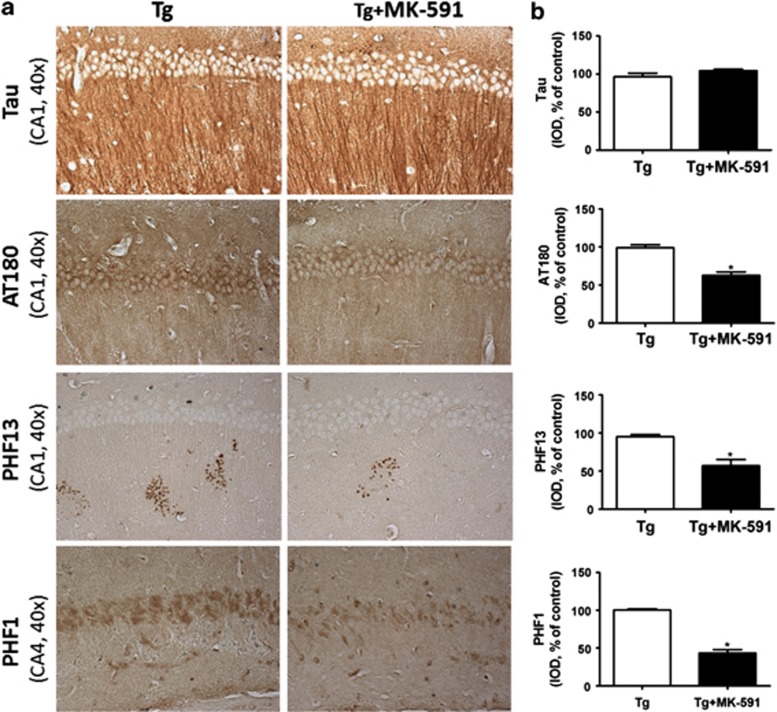
At the end of the study, we observed that compared with controls, mice receiving MK-591 had no change in their brain levels of total endogenous tau (Figures 1a and b). By contrast, we found that the same animals manifested a significant reduction in its phosphorylated forms at Ser396, Ser396/Ser404 and Thr 231/Ser 235 as recognized by the antibody PHF-13, PHF-1 and AT180, respectively (Figures 1a and b). Ratios between phosphorylated and total tau were as follows: PHF-13/tau= 0.68; PHF-1/tau= 0.69; and AT180/tau= 0.73. No significant changes were observed in other phosphorylation epitopes such as Ser202/Thr205 and Thr181, as recognized by the antibodies AT8 and AT270, which are considered early stage phosphorylation changes (Figures 1a and b). In addition, compared with mice receiving vehicle, we observed that treatment with MK-59 significantly reduced the levels of insoluble tau (Figures 1 c and d). Consistent with the immunoblot analysis results, immunohistochemical staining demonstrated a significant decrease in the accumulation of phosphorylated epitopes as recognized by PHF-13, PHF-1 and AT180-positive immunoreactivity in the brains of mice receiving MK-591 when compared with the control group (Figure 2).
Figure 1.
Pharmacologic blockade of (5-lipoxygenase-activating protein, FLAP) decreases tau phosphorylation in the brains of Tg2576 mice. (a) Representative western blot analyses for endogenous total mouse tau, phosphorylated tau as recognized by the antibodies; PHF-1, PHF-13, AT180, AT8, AT270 in brain cortex homogenates of Tg2576 mice receiving MK-591 or placebo. (b) Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (n=9 for control and n=11 for MK-591; *P=0.02). (c) Representative western blot analyses for insoluble tau in brain cortex homogenates of Tg2576 mice receiving MK-591 or placebo (control). (d) Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (*P=0.04). Results are mean±s.e.m.
Figure 2.
Pharmacologic blockade of 5-lipoxygenase-activating protein (FLAP) reduces tau phosphorylation immunoreactivity. (a) Representative sections of brains from Tg2576 mice receiving MK-591 or placebo (control) immunostained for total tau, PHF-13, PHF-1 and AT280-positive areas (x40 magnification). (b) Quantification of the area occupied by the immunoreactivity to the same antibodies shown in the previous panel (*P<0.01).
FLAP regulates tau phosphorylation by modulating GSK-3β activity
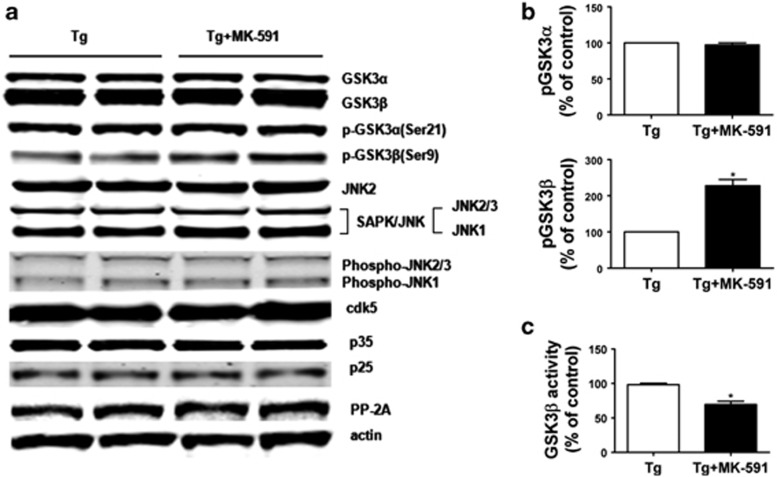
It is known that tau protein is regulated by an array of post-translational modifications, which include phosphorylation changes controlled by different kinases and phosphatases. To investigate the molecular mechanism involved in the effect of MK-591 treatment on tau phosphorylation in vivo, we examined some of the kinases which are considered major regulators of this protein post-translational modification. As shown in Figure 3a, no significant differences in the levels of total or phosphorylated forms of c-Jun N-terminal kinase2 (JNK2) and stress-activated protein kinase/JNK (SAPK/JNK) and cyclin kinase-5 (cdk5) and its two coactivators p35 and p35 were observed between the two groups of mice. By contrast, although we found that brains of Tg2576 mice receiving MK-591 had no changes in total levels of glycogen stynthase kinase-3α (GSK-3α) and GSK-3β, a significant increase in the phosphorylation state of GSK-3β but not GSK-3α was observed, suggesting a decrease in its enzymatic activity (Figures 3a and b). This finding was further confirmed by directly assaying for the kinase activity in the same tissues where we found that compared with controls the GSK-3β activity was significantly decreased in the brains of mice treated with MK-591 (Figure 3c). Finally, no significant changes between the two groups were observed for protein phosphatase (PP)-2A, an enzyme which has been involved in dephosphorylating tau (Figure 3a).
Figure 3.
Pharmacologic blockade of 5-lipoxygenase-activating protein (FLAP) reduces glycogen synthase kinase-3β (GSK-3β) activity. (a) Representative western blot analysis for GSK-3α, GSK-3β, pGSK-3α, pGSK-3β, JNK2, SAPK/JNK, p-JNK1, p-JNK2/3, cyclin kinase-5 (cdk5), p35, p25 and PP-2A in brain cortex homogenates from Tg2576 mice receiving MK-591 or vehicle (control). (b) Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel. (c) Ex vivo GSK-3β activity in brain cortex homogenates of Tg2576 receiving MK-591 or control (*P=0.02). Results are mean±s.e.m.
Pharmacological blockade of FLAP modulates synaptic integrity
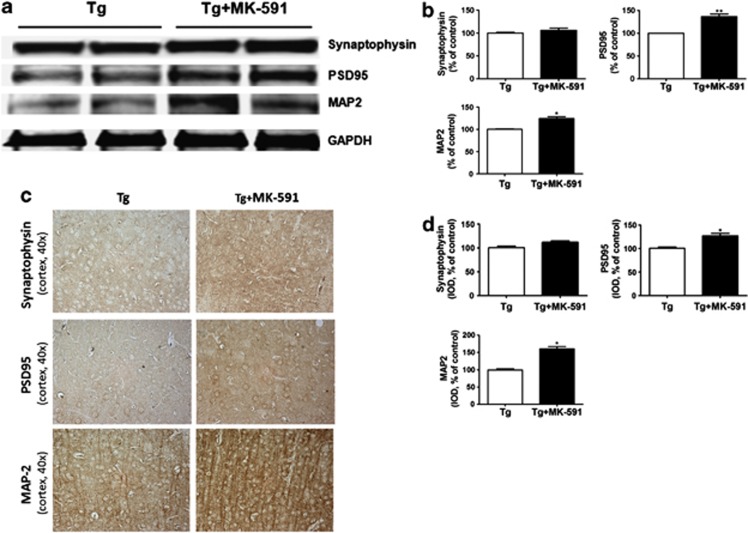
As tau pathology has been correlated with the severity of dementia and memory impairments for which synaptic integrity is an important factor, we next investigated whether pharmacological blockade of FLAP had any effect on this aspect of the AD-like phenotype. As shown in Figure 4, we observed that compared with controls steady state levels of the synaptic protein post-synaptic protein-95 (PSD-95) and the dendritic protein microtubule-associated protein-2 (MAP-2) were significantly increased in mice receiving MK-591.
Figure 4.
Improvement of synaptic integrity by MK-591. (a) Representative western blot analysis for synaptophysin, post-synaptic density protein -95 (PSD-95) and dendritic protein microtubule-associated protein 2 (MAP-2) in brain cortex homogenates of Tg2576 mice treated with MK-591 or placebo (control). (b) Densitometric analyses of the immunoreactivities to the antibodies shown in the previous panel (*P<0.05). (c) Representative sections of brains from Tg2576 mice receiving MK-591 or placebo (control) immunostained for synaptophysisn, PSD-95 and MAP-2 (x40 magnification). (d) Quantification of the area occupied by the immunoreactivity to the same antibodies shown in the previous panel (*P<0.05). Results are mean±s.e.m.
Discussion
The data presented in this study demonstrate that pharmacological blockade of FLAP significantly reduces brain tau phosphorylation in the Tg2576 mouse model of AD and thereby provide experimental evidence that this protein is a novel therapeutic target for modulating tau metabolism in vivo.
A constant feature of AD brain pathology is the intracellular accumulation of insoluble microtubules-associated protein tau in selected brain regions. This type of brain lesion is also present in other neurodegenerative diseases collectively referred to as tauopathies, which include progressive supranuclear palsy, Pick's disease and corticobasal degeneration.11Today we know that in these conditions tau, because of an excessive phosphorylation, acquires a pathological conformation, detaches from the microtubules and accumulates as neurofibrillary tangles. However, the mechanisms involved in these pathological modifications are still unknown. Emerging evidence indicates that a part form the tauopathies secondary to mutations in the tau gene, in the vast majority of the cases tau pathology is probably the results of the interaction between environmental elements and genetic risk factors.12In recent years, our lab has been very interested in the 5LO enzyme, whose activity is strictly dependent on the availability of FLAP.13,14FLAP is an integral membrane protein of 18 kDa with the known function to activate the 5LO by directly associating and presenting to it its natural substrate, arachidonic acid, for the formation of potent biologically active lipids such as leukotrienes.15,16From a biochemical point of view, FLAP and 5LO form a functional complex whose integrity is necessary for the full enzymatic activation of this pathway.17
Previously, we have shown that FLAP pharmacological inhibition results in an amelioration of the amyloidotic phenotype of the APP transgenic mice5 as well as the Aβ and tau neuropathology in a mouse model with plaques and tangles, the 3xTg-AD mice.10However, because the development of tau pathology in the latter mouse model is strictly secondary to the presence of human mutant form of tau protein in their genome, no data are available to date on the effect that FLAP may have on the metabolic fate of endogenous wild-type tau.
In the current study, by using MK-591, an orally available selective and specific FLAP inhibitor, whose binding site partially overlaps with the arachidonic acid-binding site making impossible for this substrate to be oxygenate by the 5LO,18,19we demonstrated that this therapeutic approach results in a significant reduction of brain tau phosphorylation at specific epitopes in the Tg2576 mice.
Thus, by using both a biochemical and immunohistochemical approach we found that mice receiving MK-591 had a selective decrease in tau phosphorylation at Ser396 (detected by antibody PHF-13), Ser396/404 (detected by antibody PHF-1) and Thr 231/Ser 235 (detected by antibody AT180). By contrast, we did not find any significant differences when other phoshoepitopes, as recognized by the antibodies AT8 and AT270, were assayed. Interestingly, previous work showed that at least in AD animal models the latter represent early markers of tau phosphorylation, whereas the PHF1 and PHF-13 reactivity typically represents middle and late stages,20which is compatible with the age of our mice (that is, 15 months old).
In addition to those changes in tau phosphorylation, we also observed a significant increase in two main proteins involved in synaptic integrity, post-synaptic protein-95 and microtubule-associated protein 2, suggesting a beneficial effect of FLAP pharmacological blockade also at the synapse levels.
In an effort to elucidate the mechanisms whereby FLAP pharmacological inhibition induces a selective reduction of tau phosphorylation, we assessed several kinases putatively implicated in this post-translational modification.21,22To this end, we measured the total and activated forms of JNK2 and SAPK/JNK as well as the steady state levels of cyclin kinase-5 (cdk5) and its two activators, p35 and p25. In the current study, we found that FLAP pharmacological inhibition did not alter the activation status of any these kinases. Interestingly, we observed that, although total GSK-3α and GSK-3β steady state levels were unchanged between the two groups of mice, the phosphorylated levels of GSK-3β, but not GSK-3α, were significantly increased in the brains of mice receiving the FLAP inhibitor. Because an increase in phosphorylation of this kinase is considered the biochemical signature of its reduced activity, the result suggests that the reduced tau phoshorylation is secondary to a decrease in GSK-3β activity.23This finding was further supported by the ex vivo assay of this kinase in which brain homogenates from these mice were tested. Taken together these data establish GSK-3β as the mediator of the effect of FLAP on tau phosphorylation.
Because of the known effect of the 5LO/FLAP pathway on Aβ metabolism, it is possible that the effect of MK-591 on tau is somewhat secondary to the one previously reported. However, recently we provided experimental evidence showing that this pathway acts on tau metabolism independently from its effect on Aβ.24
Chronic irreversible pharmacological blockade of FLAP by MK-591 or other similar compounds does not represent any safety issue for several reasons. First, gene deletion of FLAP in mice results in no phenotype, second, in our own study compared with placebo mice chronically treated with MK-591 did not show any changes in their eating pattern, weight gain or macroscopic alterations in their organs at killing.
In summary, our study demonstrates a novel functional role for FLAP in modulating tau phosphorylation in vivo via the GSK-3β pathway and supports the concept that this protein is a novel therapeutic target for AD-like tau neuropathology. Because FLAP inhibitors would target the two most common hallmark lesions of the AD brain (that is, Aβ and tau), our discovery represents a strong biological support for the hypothesis that FLAP pharmacological blockade is an unique and viable therapeutic opportunity for AD.
In conclusion, our studies highlight FLAP as a novel therapeutic target for AD-related tau pathology and represent the successful completion of the initial step in the pipeline for preclinical development of inhibitors of this protein as disease-modifying agents.
Acknowledgments
The study was supported by a grant from the National Institute of Health (NS071096) to DP.
The authors declare no conflict of interest.
References
- Gandy S, DeKosky ST. Toward the treatment and prevention of Alzheimer's disease: rational strategies and recent progress. Annu Rev Med. 2013;64:367–383. doi: 10.1146/annurev-med-092611-084441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX, Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr Alz Res. 2010;7:656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Praticò D. 5-Lipoxygenase as an endogenous modulator of amyloid beta formation in vivo. Ann Neurol. 2011;69:34–46. doi: 10.1002/ana.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- Chu J, Pratico D. Involvement of 5-lipoxygenase activating protein in the amyloidotic phenotype of an Alzheimer's disease mouse model. J Neuroinflammation. 2012;9:127. doi: 10.1186/1742-2094-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant Z, Timmers MC, van der Veen H, Friedman BS, De Smet M, Depre M, et al. The effect of MK-591, a novel 5-lipoxygenase-activating protein inhibitor, on leukotriene biosynthesis and allergen-induced airways responses in asthmatic subjects in vivo. J Allergy Clin Immunol. 1995;95:42–51. doi: 10.1016/s0091-6749(95)70151-6. [DOI] [PubMed] [Google Scholar]
- Joshi YB, Chu J, Pratico D. Stress hormone leads to memory deficits and altered tau phosphorylation in a model of Alzheimer's disease. J Alz Dis. 2012;31:167–176. doi: 10.3233/JAD-2012-120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Pratico D. 5-lipoxygenase pharmacological blockade decreases tau phosphorylation in vivo: involvement of the cyclin-dependent kinase 5. Neurobiol Aging. 2013;34:1549–1554. doi: 10.1016/j.neurobiolaging.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, Chu J, Praticò D. Knockout of 5Lipoxygenase prevents dexamethasone-induced tau pathology in the 3xTg mice. Aging Cell. 2013;12:706–771. doi: 10.1111/acel.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopoulos PF, Chu J, Joshi YB, Sperow M, JG Li, Kirby LG, et al. FLAP reduction ameliorates cognitive deficit, synaptic dysfunction and neuropathology in a mouse model of Alzheimer. Biol Psychiatry. 2013;74 ((5:348–356. doi: 10.1016/j.biopsych.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hernadez F, Avila J. Tauopathies. Cell Mol Life Sci. 2007;64:2219–2233. doi: 10.1007/s00018-007-7220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Tau phosphorylation, tangles, and neurodegeneration: the chicken or the egg. Neuron. 2003;40:457–460. doi: 10.1016/s0896-6273(03)00681-0. [DOI] [PubMed] [Google Scholar]
- Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28:1457–1462. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Praticò D. Adeno-Associated Virus-Mediated Brain Delivery of 5-Lipoxygenase modulates the AD-like phenotype of APP mice. Mol Neurodegen. 2012;7:1. doi: 10.1186/1750-1326-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal AK, Skoch J, Bacskai BJ, Hyman BT, Christmas P, Miller D, et al. The membrane organization of leukotriene synthesis. Proc Natl Acad Sci USA. 2004;101:6587–6592. doi: 10.1073/pnas.0308523101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Gillard JW, Vickers PJ, Sadowski S, Léveillé C, Mancini JA, et al. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990;343:278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- Dixon RAF, Diehl RE, Opas E, Rands E, Vickers PJ, Evans JF, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- Evans JF, Lévillé C, Mancini JA, Prasit P, Thérien M, Zamboni R, et al. 5-Lipoxygenase-activating protein is the target of a quinoline class of leukotriene synthesis inhibitors. Mol Pharmacol. 1991;40:22–27. [PubMed] [Google Scholar]
- Ferguson AD, McKeever BM, Xu S, Wisniewski D, Miller DK, Yamin TT, et al. Crystal structure of inhibitor-bound human 5-Lipoxygenase-activating protein. Science. 2007;317:510–512. doi: 10.1126/science.1144346. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde T, Kayed R, et al. Triple transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Lee MS, Tsai LH. Cdk5: one of the links between senile plaques and neurofibrillary tangles. J Alzheim Dis. 2003;5:127–137. doi: 10.3233/jad-2003-5207. [DOI] [PubMed] [Google Scholar]
- Maldonato H, Ramirez E, Utreras E, Pando MF, Kettlum AM, Chiong M, et al. Inhibition of cyclin-dependent kinase 5 but not glycogen synthase kinase 3β prevents neurite retraction and tau hyperphosphorylation caused by secretable products of human T-cell leukemia virus type I-infected lymphocytes. J Neurosci Res. 2011;89:1489–1498. doi: 10.1002/jnr.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ. Over-expression of glycogen synthase kinase 3 by inhibition of phosphoinositol-3 kinase and protein C leads to hyperphosphorylation of tau and impaired spatial memory. J Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde TE, Praticò D.5-Lipoxygenase gene transfer worsens memory, amyloid and tau brain pathologies in a mouse model of Alzheimer disease Ann Neurol 201272442–454. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]