Abstract
Aims:
To study and compare the efficacy and safety of topical terbinafine hydrochloride 1% cream and eberconazole nitrate 1% cream in localized tinea corporis and cruris.
Methods and Materials:
Patients were randomized after considering various inclusion and exclusion criteria into two groups. Group A (treated with terbinafine 1% cream for 3 weeks) and group B (treated with eberconazole 1% cream for 3 weeks). The sample size was of 30 patients with 15 patients in each group. Assessment of clinical improvement, KOH mount and culture was done weekly up to 3 weeks to assess complete cure.
Results:
On comparison between the two groups, it was observed that eberconazole nitrate 1% cream was as effective as terbinafine hydrochloride 1% cream at the end of first (Non-sisgnificant (NS); P = 0.608, 1.00), second (NS; P = 0.291,0.55), and third (P = 1.00, 1.00) weeks with statistically nonsignificant clinical and mycological values. In both the groups, clinically no significant local side effects were noticed.
Conclusions:
The newer fungistatic eberconazole nitrate 1% cream was as effective as the fungicidal terbinafine hydrochloride 1% cream. Both the drugs showed good tolerability with no adverse effects.
Keywords: Dermatophytosis, eberconazole nitrate 1% cream, terbinafine hydrochloride 1% cream
INTRODUCTION
Dermatophytosis is a superficial fungal infection of skin caused by keratinophilic fungi of trichophyton, epidermophyton, and microsporum species. Tinea corporis and tinea cruris is the dermatophytosis of glabrous skin and groin, respectively.
Topical preparations with good local bioavailability are the commonly used and preferred first line agents in the treatment of localized dermatophytosis. Their improved efficacy aims to shorten the treatment period with fewer side effects. Ease of application, enhanced patient compliance, and minimal recurrences also add to the therapeutic response.[1]
Newer topical antifungal agents like eberconazole, sertaconazole, luliconazole, etc., also belong to azole group of antifungal agents.
Eberconazole is a novel topical broad spectrum fungistatic imidazole derivative with a mode of action similar to that of other azole antifungals, namely inhibition of fungal lanosterol 14-demethylase. It has been shown to have broad antimicrobial spectrum of activity to be effective in dermatophytosis, candidiasis, and infection by other yeasts such as Malassezzia furfur.[2]
Terbinafine hydrochloride is one of the fungicidal allylamine group of drugs with broad spectrum of antifungal activity. It interferes with fungal sterol biosynthesis at an early stage. It also inhibits squalene epoxidase, leading to intracellular accumulation of toxic squalene and fungal cell death.[3]
To the best of our knowledge there is no study available at present that compares the clinical efficacy of topical terbinafine and eberconazole cream in treatment of tinea corporis and tinea cruris. The present study aims to compare the clinical response of topical eberconazole, a fungistatic agent with terbinafine cream, which is fungicidal.
MATERIALS AND METHODS
This randomized control trial with two arms compares the clinical efficacy and side effects of topical terbinafine hydrochloride 1% vs eberconazole nitrate 1% cream in the treatment of localized (<20% involvement) tinea corporis and tinea cruris. The trial was conducted at the Dermatology department of J. N. Medical College & AVBRH, Sawangi during the period December 2010 to November 2011. Patients were randomized into group A (odd numbers) and group B (even numbers): Group A (treated with terbinafine cream) and group B (treated with eberconazole cream). A total of 42 patients were enrolled in the study, 22 in group A and 20 in group B. However, seven patients of group A and five patients of group B were lost to follow-up. Therefore, the final sample size was of 30 patients with 15 patients each in group A and group B. Patients in group A and B were treated with topical 1% terbinafine hydrochloride and 1% eberconazole nitrate cream respectively, twice daily for 3 weeks. The inclusion criteria included untreated patients of dermatophytosis of all age groups, involvement of less than 20% of body surface area, and patients whose diagnosis was confirmed by KOH mount. Exclusion criteria were patients of resolving dermatophytoses, patients already on topical and systemic antifungal treatment, involvement of more than 20% body surface area (BSA), and patients with immunosuppressive disease or on immunosuppressive drugs.
Diagnosis was made on clinical and mycological (KOH and culture) grounds. All the patients had similar demographic features with regards to age, sex, and duration of disease. They were followed up every week to note the efficacy and any adverse effects such as local erythema, swelling, stinging sensation, or increased itching for a total duration of 3 weeks. The patients were graded for improvement in signs and symptoms of each clinical parameter, namely, itching, erythema, papules, pustules, vesicles, and scaling. The overall improvement was graded as grade I (25% improvement), grade II (50% improvement), grade III (75% improvement), and grade IV (100% improvement). KOH mount and culture was done weekly up to 3 weeks to assess mycological cure. Fungal culture was done on Sabouraud's dextrose agar with chloramphenicol and cycloheximide.
We performed the mycological assessment at baseline, at the end of 1st week and also with minimal scales available at the end of 2nd and 3rd week.
Mycological cure was defined as negative KOH and culture. Complete cure was defined as mycological cure with complete absence of clinical signs and symptoms.
Statistical analysis was done using Student's paired and unpaired t-tests from the data obtained.
RESULTS
In both terbinafine and eberconazole groups, a statistically significant complete cure (P <0.05) was observed between baseline to 2nd week, as well as baseline to 3rd week.
But individually in both groups statistically non significant results were observed in complete cure when comparison was done between 2nd to 3rd week (NS, P = 0.317, 0.317; P = 0.083, 0.157).
On comparison between these 2 groups, it was observed that eberconazole nitrate 1% cream [Figures 2a–d] was as effective as terbinafine hydrochloride 1% cream [Figures 1a–d] at the end of 1st (NS. P = 0.608, 1.00), 2nd (NS. P = 0.291,0.55) and 3rd (P = 1.00, 1.00) week with statistically non-significant clinical [Table 1 and Figure 3] and mycological values [Table 2 and Figure 4].
Figure 2.
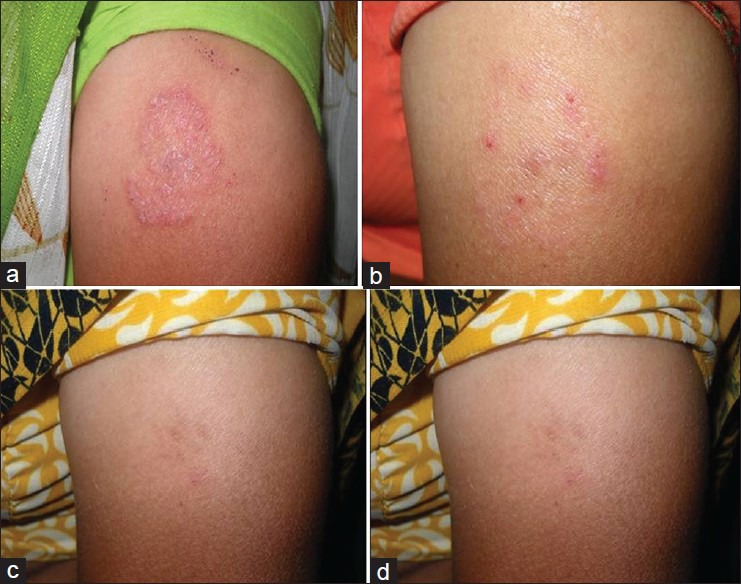
(a) Eberconazole treated (T.Corporis) Baseline. (b) Eberconazole -75% improvement at 1 week. (c) Eberconazole -100% improvement at 2 weeks. (d) Eberconazole-100% improvement at 3 weeks
Figure 1.
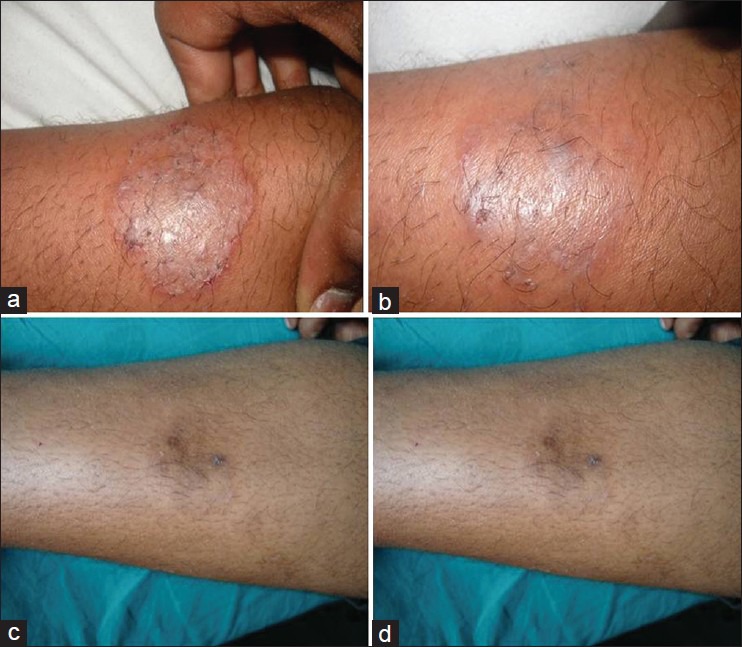
(a) Terbinafine treated (T. Corporis) Baseline. (b) Terbinafine-75% improvement at 1 week. (c) Terbinafine -100% improvement at 2 weeks. (d) Terbinafine -100% improvement at 3 weeks
Table 1.
Comparison of signs and symptoms in both groups at 1st, 2nd and 3rd week
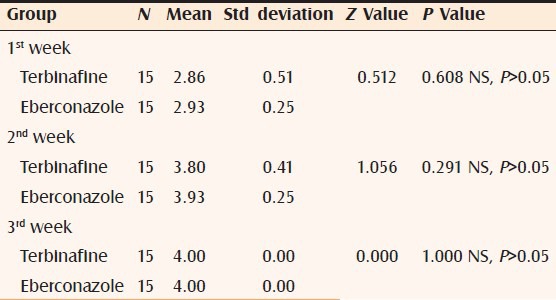
Figure 3.
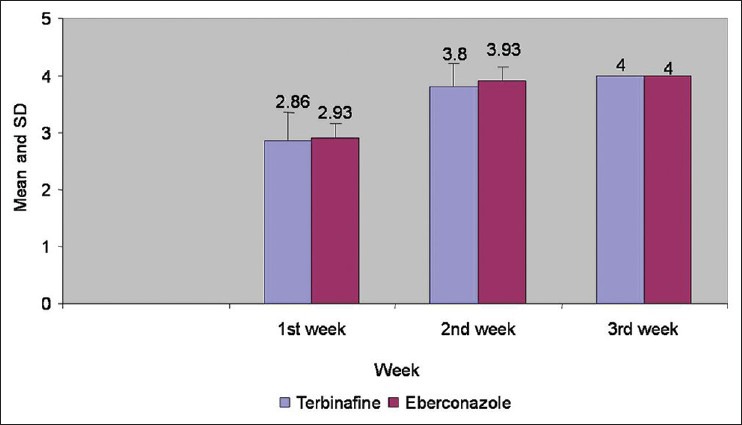
Comparison of signs and symptoms in both groups at 1st, 2nd, and 3rd week
Table 2.
Comparison of mycological assessment in both groups at 1st 2nd and 3rd week
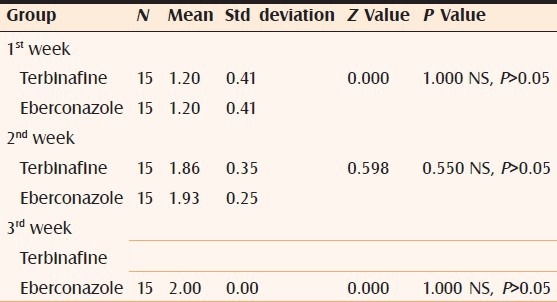
Figure 4.
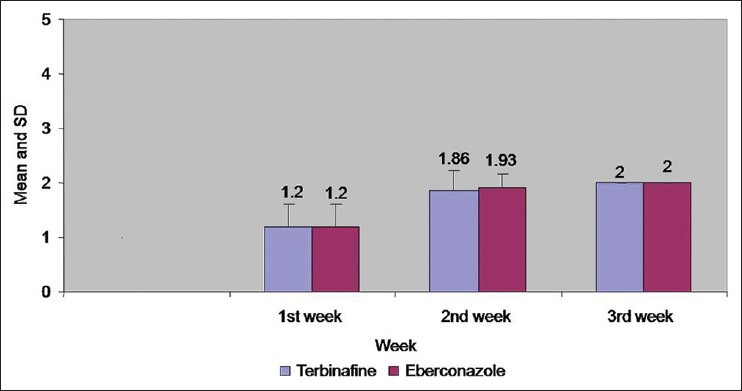
Comparison of mycological assessment in both groups at 1st, 2nd, and 3rd week
However, at the end of 2nd week, complete cure rate for eberconazole was 93.33% as compared to 80% for terbinafine with no statistical significance.
Comparison between both groups for complete cure (clinical and mycological) at the end of 3 weeks showed 100% cure rate.
In both group A and B, no clinically significant side effects such as local erythema, swelling, stinging sensation, or increased itching were noticed. Clinical response with both the topical antifungals was so good that we could hardly see any scales over lesion at the end of 2nd and 3rd week. However, we performed culture with whatever minimal scales available. A majority of our patients in both groups grew Trichophyton rubrum or Epidermophyton floccosum growth on culture. At baseline, in group A, 12 patients showed growth of Trichophyton rubrum, two patients showed growth of Epidermophyton floccosum and one had growth of Trichophyton mentagrophytes. In group B, 11 patients showed growth of Trichophyton rubrum, three patients showed growth of Epidermophyton floccosum and one had growth of Trichophyton mentagrophytes. The therapeutic response was more or less the same with infection by different species.
DISCUSSION
In one of the studies to evaluate the efficacy and safety of the topical 1% gel formulation of terbinafine in the treatment of tinea corporis/cruris, terbinafine gel was applied once daily for 1 week.[4] Complete cure was observed in 59% of patients, against 13% receiving a placebo (P <0.001). The authors concluded that a 1-week course of terbinafine 1% gel was significantly more effective in the treatment of tinea corporis/cruris than placebo gel for complete cure (clinical and mycological).
In another study, 7-day once-daily course of terbinafine cream 1% was significantly more effective than placebo in achieving and maintaining mycological cure (84.2 vs 23.3%, P < 0.001). Terbinafine cream 1% was also significantly more effective than placebo in terms of clinical response, reduction in signs and symptoms scores, and overall efficacy.[5]
In our study, We used a 1% cream formulation of terbinafine, applied twice daily, and 80 and 100% complete cure rates were noted at the end of 2nd and 3rd weeks of treatment period, respectively.
In another study,[6] 60 patients with mycologically proven tinea corporis and tinea cruris were treated with eberconazole cream 1% once daily (group A, 15 patients), 1% twice daily (group B, 15 patients), 2% once daily (group C, 15 patients), and 2% twice daily (group D, 15 patients) for 6 weeks. Eberconazole was effective in 93% of patients in group A, 100% of patients in groups B, and D and 61% of patients in group C at the end of 6 weeks.
In a multicentric, double blind, randomized trial with 1% eberconazole nitrate cream vs miconazole 2% cream applied twice daily for 4 weeks, it was observed that eberconazole 1% cream is an effective treatment for dermatophytosis with a good safety profile (clinical efficacy 76.1% in eberconazole group vs 75% miconazole group).[7]
In a comparative trial of eberconazole 1% vs clotrimazole 1% cream applied twice daily in dermatophyte infections to treat 133 cases for 4 weeks, effective result was seen in 61% patients of eberconazole vs 46% patients of clotrimazole treated group.[8]
In our study, 1% eberconazole cream was used twice daily and had shown 93.33 and 100% complete cure at end of 2nd and 3rd week, respectively.
To the best of our knowledge, there is no study available at present comparing the clinical efficacy of topical terbinafine and eberconazole cream in the treatment of tinea corporis and tinea cruris. Sample size of our study was small; further study with a large sample size in future is needed to support our findings.
In our study eberconazole nitrate 1% cream was as effective as terbinafine hydrochloride 1% cream at the end of 1st, 2nd, and 3rd week with 100% cure rate at the end of 3 weeks. Local side effects such as erythema, swelling, stinging sensation, or itching as mentioned in a few studies were not observed by us.
CONCLUSION
The newer fungistatic drug eberconazole nitrate 1% cream was as effective as terbinafine hydrochloride 1% cream, which is one of the fungicidal drugs. Both drugs showed good tolerability with no adverse effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Moodahadu-Bangera LS, Martis J, Mittal R, Krishnankutty B, Kumar N, Bellary S, et al. Eberconazole-pharmacological and clinical review. Indian J Dermatol Venereol Leprol. 2012;78:217–22. doi: 10.4103/0378-6323.93651. [DOI] [PubMed] [Google Scholar]
- 2.Font E, Freixes J, Julve J. Profile of a new topical antimycotic, eberconazole. Rev Iberoam Micol. 1995;12:16–7. [Google Scholar]
- 3.Ryder NS. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol. 1992;126:2–7. doi: 10.1111/j.1365-2133.1992.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Heerden JS, Vismer HF. Tinea corporis/cruris: new treatment options. Dermatology. 1997;194:14–8. doi: 10.1159/000246177. [DOI] [PubMed] [Google Scholar]
- 5.Budimulja U, Bramono K, Urip KS, Basuki S, Widodo G, Rapatz G, et al. Once daily treatment with terbinafine 1% cream (Lamisil) for one week is effective in the treatment of tinea corporis and cruris. A placebo-controlled study. Mycoses. 2001;44:300–6. [PubMed] [Google Scholar]
- 6.del Palacio A, Cuétara S, Rodríguez Noriega A. Topical treatment of tinea corporis and tinea cruris with eberconazole (WAS 2160) cream 1% and 2%: A phase II dose-finding pilot study. Mycoses. 1995;38:317–24. doi: 10.1111/j.1439-0507.1995.tb00415.x. [DOI] [PubMed] [Google Scholar]
- 7.Repiso Montero T, López S, Rodríguez C, del Rio R, Badell A, Gratacós MR. Eberconazole 1% cream is an effective and safe alternative for dermatophytosis treatment: Multicenter, randomized, double-blind, comparative trial with miconazole 2% cream. Int J Dermatol. 2006;45:600–4. doi: 10.1111/j.1365-4632.2006.02841.x. [DOI] [PubMed] [Google Scholar]
- 8.Del Palacio A, Ortiz FJ, Perez A, Pazos C, Garau M, Font E. A double blind randomized comparative trial: eberconazole 1% cream versus clotriamzole 1% cream twice daily in candida and dermatophyte skin infections. Mycosis. 2001;44:173–80. doi: 10.1046/j.1439-0507.2001.00632.x. [DOI] [PubMed] [Google Scholar]


