Abstract
Vitiligo is an acquired pigmentary disorder, clinically characterized by depigmented macules caused by destruction of melanocytes in the affected skin. Half of all patients develop the disease in childhood and adolescence before the age of 20 years, making vitiligo an important skin disease of childhood. There are numerous studies in the literature that suggest the efficacy of topical tacrolimus in vitiligo, without serious adverse effects. We describe a case of vitiligo in a pediatric patient who developed hyperpigmentation in the periorbital lesions of vitiligo with the use of topical tacrolimus. To the best of our knowledge, this is only the second such reported occurrence in a patient with vitiligo.
Keywords: Hyperpigmentation, tacrolimus, tacrolimus induced hyperpigmentation, vitiligo
INTRODUCTION
Vitiligo is characterized clinically by the development of depigmented macules that correspond histologically to decreased or absent melanocytes in the affected skin. Half of all patients develop the disease in childhood and adolescence before the age of 20 years, making vitiligo an important skin disease of childhood.[1] The main mechanism of melanocyte destruction is hypothesized to be an autoimmune lymphocytic attack on melanocytes.[2] Tacrolimus is an immunomodulator which has the ability to interfere with multiple immune as well as inflammatory pathways, and this may explain its effect on vitiligo.[3] Tacrolimus exerts its biological activity after binding to the cytosolic 12-kd macrophilin FK506 binding protein (FK-BP). The tacrolimus/FK-BP complex inhibits calcineurin-mediated phosphorylation of the transcription factor, that is, nuclear factor of activated T cells (NFAT); and thereby the expression of several inflammatory T-cell cytokines.[4,5] In general, topical tacrolimus is well tolerated by most patients and does not have any serious adverse effects. This makes it an excellent alternative for persons with vitiligo, who have poor compliance to phototherapy or have a fear of side effects of long term topical steroid use.[3] The common adverse effects include pruritus and burning sensation at application site, which are mild and transient.[6] There are few reports of tacrolimus-induced localized hyperpigmentation in the literature. To the best of our knowledge, there exists only one such report in a case of vitiligo.
CASE REPORT
A 10-year-old boy presented with depigmented macules over his scalp, periorbital regions, elbows, thighs, legs, and feet for the past 1.5 years. The disease was stable and he was prescribed topical tacrolimus 0.03% once daily application at bedtime over all the depigmented macules. No photoprotection was advised. At first follow-up visit after 2 months, there was near complete repigmentation over the periorbital macules; however, it was hyperpigmented as compared to surrounding normal skin [Figure 1]. There was some perifollicular repigmentation in most of the other vitiligo lesions [Figure 2]. Topical tacrolimus was stopped and the patient was advised photoprotection along with use of sunscreens. Additionally, the patient was started on topical psoralen + ultraviolet A (PUVAsol) for the treatment of vitiligo over other sites. On his next follow-up visit a month later, there was a mild reduction in the periorbital hyperpigmentation; while the other lesions continued to show perifollicular repigmentation.
Figure 1.
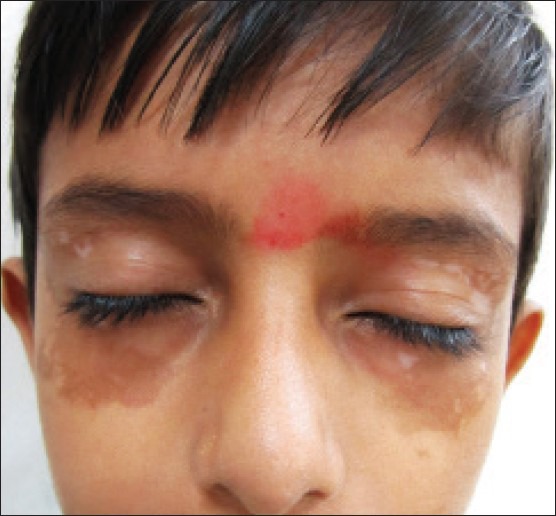
Well-defined hyperpigmented macules at sites of tacrolimus application for periorbital lesions of vitiligo
Figure 2.
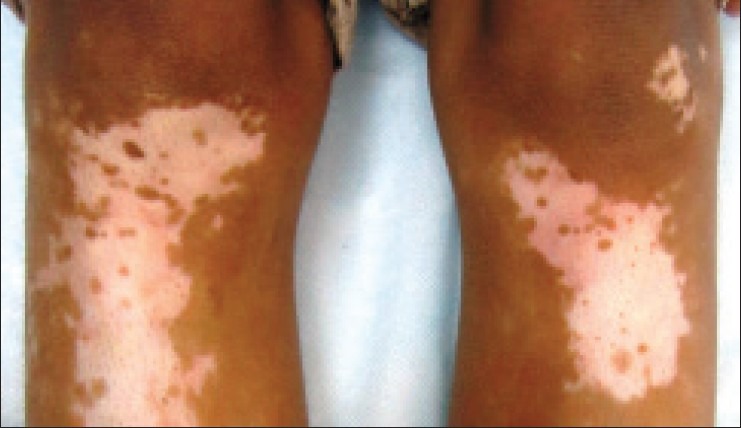
Perifollicular repigmentation in vitiligo lesions over the knees
DISCUSSION
Topical tacrolimus is currently approved by the US Food and Drug Administration (FDA) for the treatment of atopic dermatitis. Its use in vitiligo is off-label. It is indicated in both 0.03 and 0.1% strengths for adults, and is indicated only in the 0.03% strength for children aged 2-15 years. Its safety and efficacy have been repeatedly demonstrated in many clinical trials. The adverse effects include pruritus, burning sensation, and erythema which generally subside spontaneously.[7] Though long-term human data have not demonstrated any increase in photocarcinogenicity with topical tacrolimus, protection from the sun is generally recommended during treatment.
Though hyperpigmentation is often seen following photochemotherapy for lesions of vitiligo, reports relating to tacrolimus-induced hyperpigmentation have only recently started emerging in the literature. Shen and Pedvis-Leftick first reported staining of gingiva, labial mucosa, and vestibule after topical use of tacrolimus to treat oral lichen planus.[8] Fricain et al., reported the case of oral mucosal pigmentation due to an increased local number of melanocytes and increased melanogenesis after oral lichen planus treatment with topical tacrolimus.[9] In both these cases, the pigmentation was temporary and completely disappeared within months of stopping treatment. Hickey et al., reported three cases of lentiginosis after topical application of tacrolimus for atopic dermatitis.[10] De and Kanwar reported a case of hyperpigmentation in a photo exposed patch of vitiligo with use of topical tacrolimus. The hyperpigmentation was temporary, with return of depigmentation within 1 month of stopping treatment.[11] Strikingly, the site of hyperpigmentation was the periorbital area which was similar to our patient. This may be related to the greater efficacy of tacrolimus in repigmenting facial lesions of vitiligo. In fact, in a study on the efficacy of tacrolimus in 57 pediatric patients of vitiligo, the fastest and most complete response was seen in eyelid lesions. This may relate to the high vellus hair density over the eyelids.[2] As in the case reported by De and Kanwar, hyperpigmentation in our patient was observed only in the photo exposed site and was temporary. Lo et al., felt that those vitiligo patients who received UV radiation incidentally while using tacrolimus, responded better than the patients who did not.[3]
The mechanism of repigmentation in vitiligo by tacrolimus is elucidated by the molecular in vitro studies of Lan et al., and Kang and Choi. Lan et al., reported that the interaction between keratinocytes and tacrolimus create a favorable milieu for melanocyte growth and migration.[12] Kang and Choi further explained the increase in pigmentation, as due to the stimulatory action of tacrolimus on tyrosinase activity and its expression.[13] This may have been responsible for the hyperpigmentation seen in our patient.
CONCLUSION
In our patient, the hyperpigmentation was seen only in the photo exposed site, while vitiligo lesions on other sites showed follicular pigmentation. The hyperpigmentation was temporary and started reducing upon discontinuing tacrolimus and observance of photoprotective measures.
Because UV light might have a synergistic action with tacrolimus in causing hyperpigmentation, we would like to suggest regular monitoring of patients on tacrolimus for the development of this side effect. In addition, sunscreen use or photoprotection may be recommended, especially if the lesions are on photo-exposed sites, with special caution for lesions in the periorbital area.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Plettenberg H, Assmann T, Ruzicka T. Childhood vitiligo and tacrolimus: Immunomodulating treatment for an autoimmune disease. Arch Dermatol. 2003;139:651–4. doi: 10.1001/archderm.139.5.651. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg NB, Lin P, Travis L, Farley-Li J, Mancini AJ, Wagner AM, et al. Tacrolimus ointment promotes repigmentation of vitiligo in children: A review of 57 cases. J Am Acad Dermatol. 2004;51:760–6. doi: 10.1016/j.jaad.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Lo YH, Cheng GS, Huang CC, Chang WY, Wu CS. Efficacy and safety of topical tacrolimus for the treatment of face and neck vitiligo. J Dermatol. 2010;37:125–9. doi: 10.1111/j.1346-8138.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 5.Assmann T, Homey B, Ruzicka T. Topical tacrolimus for the treatment of inflammatory skin diseases. Expert Opin Pharmacother. 2001;2:1167–75. doi: 10.1517/14656566.2.7.1167. [DOI] [PubMed] [Google Scholar]
- 6.Koo JY, Fleischer AB, Jr, Abramovits W, Pariser DM, McCall CO, Horn TD, et al. Tacrolimus ointment is safe and effective in the treatment of atopic dermatitis: Results in 8000 patients. J Am Acad Dermatol. 2005;53(Suppl 2):S195–205. doi: 10.1016/j.jaad.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 7.Zabawski EJ, Costner M, Cohen JB, Cockerell CJ. Tacrolimus: Pharmacology and therapeutic uses in dermatology. Int J Dermatol. 2000;39:721–7. doi: 10.1046/j.1365-4362.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen JT, Pedvis-Leftick A. Mucosal staining after using topical tacrolimus to treat erosive oral lichen planus. J Am Acad Dermatol. 2004;50:326. doi: 10.1016/s0190-9622(03)02479-4. [DOI] [PubMed] [Google Scholar]
- 9.Fricain JC, Sibaud V, Campana F, Lepreux S, Taïeb A. Mucosal pigmentation after oral lichen planus treatment with topical tacrolimus. Dermatology. 2005;210:229–32. doi: 10.1159/000083516. [DOI] [PubMed] [Google Scholar]
- 10.Hickey JR, Robson A, Barker JN, Smith CH. Does topical tacrolimus induce lentigines in children with atopic dermatitis? A report of three cases. Br J Dermatol. 2005;152:152–4. doi: 10.1111/j.1365-2133.2004.06280.x. [DOI] [PubMed] [Google Scholar]
- 11.De D, Kanwar AJ. Tacrolimus-induced hyperpigmentation in a patch of vitiligo. Skinmed. 2008;7:93–4. doi: 10.1111/j.1751-7125.2008.07558.x. [DOI] [PubMed] [Google Scholar]
- 12.Lan CC, Chen GS, Chiou MH, Wu CS, Chang CH, Yu HS. FK506 promotes melanocytes and melanoblast growth and create a favourable milieu for cell migration via keratinocytes: Possible mechanisms of how tacrolimus ointment induces repigmentation in patients with vitiligo. Br J Dermatol. 2005;153:498–505. doi: 10.1111/j.1365-2133.2005.06739.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang HY, Choi YM. FK506 increases pigmentation and migration of human melanocytes. Br J Dermatol. 2006;155:1037–40. doi: 10.1111/j.1365-2133.2006.07467.x. [DOI] [PubMed] [Google Scholar]


