Abstract
Objective
To determine whether phonophobia and dynamic mechanical (brush) allodynia are associated in episodic migraine (EM).
Methods
Adult patients with EM were prospectively recruited. A structured questionnaire was used to obtain demographic and migraine related data. Phonophobia was tested quantitatively using a real time sound processor and psychoacoustic software. Sound stimuli were pure tones at frequencies of 1000 Hz, 4000 Hz and 8000 Hz, delivered to both ears at increasing intensities, until an aversive level was reached. Allodynia was assessed by brushing the patient’s skin with a gauze pad at different areas. Patients were tested both between and during acute attacks. Sound aversion thresholds (SATs) in allodynic and non-allodynic patients were compared.
Results
Between attacks, SATs were lower in allodynic compared with non-allodynic patients, with an average difference of −5.7 dB (p=0.04). During acute attacks, the corresponding average SAT difference (allodynic-non-allodynic) was −15.7 dB (p=0.0008). There was a significant negative correlation between allodynia scores and SATs, both within and between attacks.
Conclusions
The results support an association between phonophobia and cutaneous allodynia in migraine.
Migraine patients often report on aversion to various sensory stimuli during an acute attack. These include aversion to light (photophobia), sound (phonophobia), odours (osmophobia) and mechanical or thermal stimuli to the skin (cutaneous allodynia).1–3 Cutaneous allodynia has been studied extensively in migraine.4–6 There are less data on the characteristics and mechanisms of phonophobia, photophobia and osmophobia in this disease.7 8 The occurrence of aversion to stimuli of various sensory modalities in migraine raises the question of whether they are associated in this disease or, alternatively, are independent phenomena. There are currently few data regarding this question.
Phonophobia may be defined as aversion to normally non-aversive sounds. This symptom has been reported in 70–80% of migraine patients during an acute attack.7 9 The International Headache Society (IHS) lists phonophobia (along with photophobia) as one of the diagnostic criteria of migraine.10 However, the IHS does not provide a quantitative definition of this symptom. The mechanisms of phonophobia in migraine, and its relation to cutaneous allodynia, are poorly understood.
We developed a method to evaluate quantitatively aversion to sound in human subjects, as a measure of phonophobia. Using this method, we have recently shown that migraine patients exhibit increased aversion to sound when tested between attacks, and that this sound aversion is further increased during an acute attack.11
The aim of the present study was to determine whether phonophobia and dynamic mechanical (brush) allodynia are associated in migraine. We hypothesised that the two phenomena would be associated in migraine subjects.
METHODS
Patients
We prospectively recruited adult women and men, 18–65 years old, who had a diagnosis of episodic migraine with or without aura, as defined by the IHS Headache Classification Committee.10 Patients were recruited from the outpatient clinic of Jefferson Headache Center and from the population of the Philadelphia area through advertising. Included patients were required to have at least one migraine attack per month, but less than 15 headache days per month, for 6 months prior to enrolment, and to have a normal audiogram (defined as a hearing threshold of ≤20 dB at the tested sound frequencies). Exclusion criteria were: any other headache diagnosis except for episodic tension-type headache, as defined by the IHS; use of any medication, vitamin or supplement considered or suspected to be a headache preventive within 90 days prior to enrolment; and treatment with occipital nerve block or any other nerve block in the head or neck area within 30 days prior to enrolment. Patients were recruited and examined from August 2006 to August 2007. The study was approved by the Thomas Jefferson University Institutional Review Board for Human Research. All participants signed an informed consent form prior to enrolment according to the Declaration of Helsinki.
Study protocol
An audiogram was performed at the Jefferson Center for Balance and Hearing when patients were not experiencing an acute headache. Eligible patients with a normal audiogram proceeded with the study protocol. Other than the audiogram, all study procedures took place at the Jefferson Headache Center. Patients were examined when they were not experiencing an acute attack (nor within 72 h from the time they had had an attack) (visit 1) and when they were experiencing an acute untreated attack (visit 2). During visit 1, patients were interviewed using a structured questionnaire to obtain demographic data and data on migraine characteristics (frequency and duration of attacks, head pain severity during an attack and number of years they suffered from migraine). Patients were then tested for cutaneous allodynia and, thereafter, for phonophobia. For visit 2, patients were instructed not to take any medication for their acute migraine attack. During that visit, patients were asked about the characteristics of their current attack. Subsequently, patients were tested for allodynia and thereafter for phonophobia. After completion of all study procedures at visit 2, patients were given a rescue migraine treatment by one of the investigators (AA). Patients were instructed to inform the investigator if, at any time, they wanted to terminate the testing due to discomfort or for any other reason. The duration of testing at each visit was approximately 45 min.
Phonophobia testing
Phonophobia testing was performed with the subject sitting in a sound-proof booth (Eckel Industries, Morrisburg, Ontario, Canada). We used a RP2.1 enhanced real time processor (Tucker-Davis Technologies, Alachua, Florida, USA) and PSYCHRP psychoacoustic software (Tucker-Davis Technologies) for the test. Sound stimuli were pure tones at frequencies of 1000 Hz, 4000 Hz and 8000 Hz, delivered simultaneously to both ears using calibrated ER3 insert earphones (Etymotic Research, Elk Grove Village, Illinois, USA). We chose these sound frequencies as the test stimuli because the human auditory system is most sensitive to sounds at around 4000 Hz.12 Each sound stimulus lasted 1.5 s and the inter-stimulus onset interval was 3 s. Initial sound intensity was 50 dB and it was increased gradually by increments of 5 dB/3s until an unpleasant or painful intensity was reached, at which time the subject pressed and held a response box button, causing the sound intensity to decrease gradually. Once a tolerable sound intensity was reached, the subject released the response box button, causing the sound intensity to increase again until an unpleasant or painful intensity was reached, repeating the testing cycle once more. A sound aversion threshold (SAT, measured in dB) was determined as the average of the unpleasant or painful sound intensities at the two cycles. For safety reasons, at no time did the delivered sound intensity exceed 110 dB. Each subject was tested for sound aversion at the three different frequencies, starting at 1000 Hz, then at 4000 Hz and finally at 8000 Hz. There was a 10 min between test interval for the different frequencies for each subject. We adhered to the limits of sound exposure safety, as recommended by the Occupational Safety and Health Administration Noise Exposure Standards.13
Allodynia testing
We tested patients for the presence of dynamic mechanical (brush) allodynia (BA) by lightly brushing a 4×4 inch gauze pad over a 2 inch area of skin, at a rate of 2/s, for a total of 10 times, as we described previously.5 The tested skin areas for BA were: frontal, posterior cervical and medial forearm. BA testing was done sequentially on both sides for each skin area. Patients were asked to assess the degree of pain or unpleasantness (if any) evoked by the gauze pad brushing, using a 100 mm visual analogue scale. Allodynia score (mm) at each area ranged from 0 to 100, and the total allodynia score ranged from 0 to 600 (100 (maximal allodynia score at each skin area × 6 (number of tested areas)). We have previously shown that healthy subjects who do not suffer from headaches do not exhibit BA when tested using this method.5 Therefore, in this study, a total visual analogue scale allodynia score greater than zero was considered to reflect the presence of BA. To avoid a potential bias of the examiner, allodynia testing was performed before the computerised testing of phonophobia.
Statistical analysis
Patient demographics were summarised using frequencies and percentages for categorical variables and medians and ranges for continuous variables. Mixed effects linear regression was used to model SATs. Fixed effects were included for time of SAT testing (visit 1 (between attacks) or visit 2 (during attacks)), sound frequency (1000, 4000 or 8000 Hz), allodynia status (present or absent) and all possible interactions. For each visit, we used the mixed effects model to compare SATs in allodynic with those in non-allodynic patients at each frequency. Mixed effects linear regression was used to assess the relationship between SATs and allodynia scores by visit and frequency. For purposes of regression, allodynia scores were transformed using the equation Anew=log10 (A+1). We calculated the Spearman rank correlation coefficient between SATs and headache severity.
A p value of <0.05 was considered to reflect statistical significance.
RESULTS
Patient demographics and migraine characteristics
Of 60 recruited patients, 38 (63%) completed the two study visits (30 (79%) women and eight (21%) men; median age 25.5 (range 18–51) years) (table 1). Twelve (32%) had migraine with aura and 26 (68%) had migraine without aura. Median disease duration was 8.5 years (range 1–38 years). Median attack frequency was 3.5 per month (range 1–9 per month) and median headache severity during an acute untreated attack was 8 (range 4–10) on a 0–10 point verbal scale. There were no significant differences with regard to age, gender or migraine characteristics between patients who completed the two visits and those who did not.
Table 1.
Demographics and disease characteristics of the patients who completed the two visits
| Allodynic | Non-allodynic | Total | p Value | |
|---|---|---|---|---|
| Gender (n (%)) | ||||
| Men | 6 (20.0) | 2 (25.0) | 8 (21.1) | 1.00 |
| Women | 24 (80.0) | 6 (75.0) | 30 (78.9) | |
| Age (years) | 26.5 (18, 51) | 26.5 (18, 50) | 25.5 (18, 51) | 0.86 |
| Disease duration (years) | 9 (1, 35) | 10 (4, 38) | 8.5 (1, 38) | 0.72 |
| Attack frequency (per month) | 3.3 (1, 9) | 4 (1, 9) | 3.5 (1, 9) | 0.28 |
| Headache severity during an attack (scale: 0—10) | 8 (4, 10) | 6.5 (4, 10) | 8 (4, 10) | 0.19 |
| Aura (n (%)) | 11 (36.7) | 1 (12.5) | 12 (31.6) | 0.39 |
Data on continuous variables are presented as medians (min, max).
Patients were defined as allodynic or non-allodynic based on their allodynia status during the tested attack.
The mean time interval between attack onset (defined as the onset of the first attack related symptom) and testing at visit 2 was 4.2±2.6 h (range 1–11 h) while the mean time interval between onset of throbbing pain and testing at visit 2 was 3.3±2.7 h (range 0–9 h). Between attacks, 18/60 (30%) patients were allodynic while during an acute attack 30/38 (79%) patients exhibited allodynia.
SATs between attacks and during attacks in allodynic and in non-allodynic patients
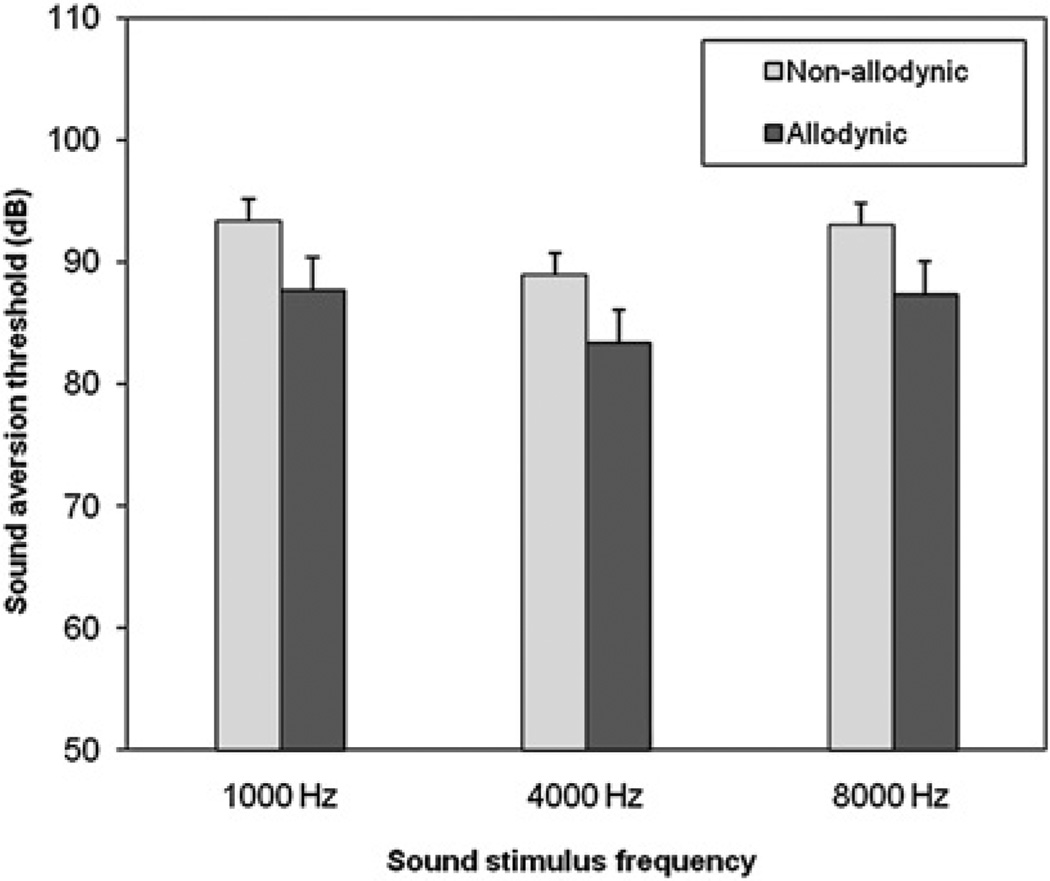
Between attacks, SATs were lower in allodynic compared with non-allodynic patients at all sound frequencies (figure 1). The SAT difference between allodynic and non-allodynic patients, when tested between attacks (averaged for the three frequencies), was −5.7 dB (p=0.04) (at 1000 Hz: 87.6±2.7 vs 93.3±1.8 dB, p=0.07; at 4000 Hz: 83.3±2.7 vs 88.9±1.8 dB, p=0.07; and at 8000 Hz: 87.3±2.7 vs 93.0±1.8, p=0.06).
Figure 1.
Sound aversion thresholds (mean (SEM)) between attacks in non-allodynic and allodynic patients.
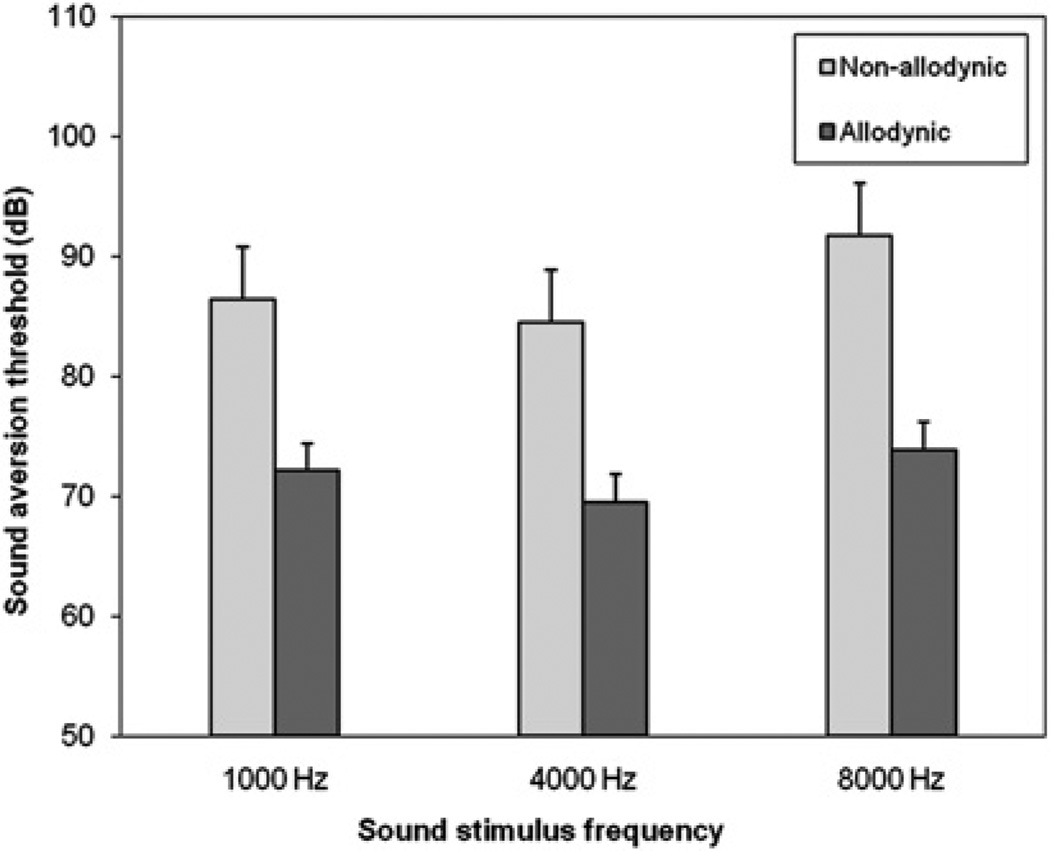
During an acute attack, SATs were again lower in allodynic compared with non-allodynic patients at all sound frequencies (figure 2). The SAT difference between allodynic and non-allodynic patients, when tested during an acute attack, averaged for the three frequencies, was −15.7 dB (p=0.0008). This difference was highly significant for all tested sound frequencies (at 1000 Hz: 72.1±2.3 vs 86.4±4.4 dB, p=0.004; at 4000 Hz: 69.5±2.3 vs 84.5±4.4 dB, p=0.003; at 8000 Hz: 71.8±2.3 vs 87.5±4.4 dB, p=0.0004).
Figure 2.
Sound aversion thresholds (mean (SEM)) during an acute attack in non-allodynic and allodynic patients.
SATs versus allodynia scores
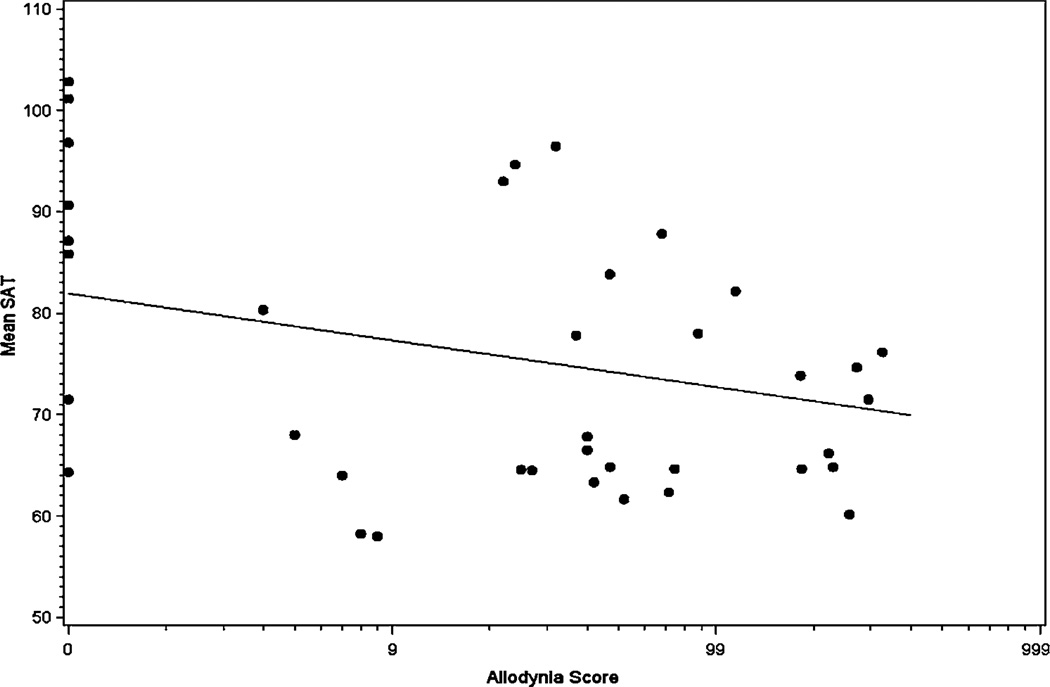
There was a significant negative correlation between allodynia scores and SATs both during and between attacks. Figure 3 shows the SAT scores, averaged for the three frequencies, as a function of allodynia scores at visit 2. During an acute attack, for every 1 unit increase in the transformed allodynia score, the mean SAT decreased by 4.6 units (p=0.01) (figure 3). Similarly, between attacks, for every 1 unit increase in the transformed allodynia score, the mean SAT decreased by 5 units (p=0.01).
Figure 3.
Allodynia scores versus sound aversion thresholds (SATs) during acute attacks.
Spatial distribution of allodynia and SATs
Of the 30 patients who were allodynic during the tested attack, all had allodynia at the frontal area, 22 (73%) had allodynia at the posterior cervical area and 15 (50%) had allodynia at the medial forearm (since patients may have had allodynia at more than one area, the sum of these numbers exceeds 100%). We looked at the average SATs in patients who had allodynia at the frontal area only (n=6), those who had allodynia at the frontal and the posterior cervical area but not at the medial forearm (n=9) and those who had allodynia at all tested skin territories (frontal, posterior cervical and medial forearm, n=13; two patients did not belong to any of these groups and were not included in this analysis). The average SATs in these groups were 70.8±13.2 dB, 72.4±14.2 dB and 73.8±8.4 dB, respectively, with no significant difference among the groups (Kruskal-Wallis test, p=0.59).
Subjective report of phonophobia, photophobia and osmophobia in allodynic and in non-allodynic patients during the acute attack
During the acute attack, 90% of allodynic and 75% of non-allodynic patients reported on phonophobia (Fisher’s exact test, p=0.29). At the same time, 97% of allodynic and 88% of non-allodynic patients reported on photophobia, (p=0.39) and 35% of allodynic, compared with 25% of non-allodynics, reported on osmophobia (p=0.69).
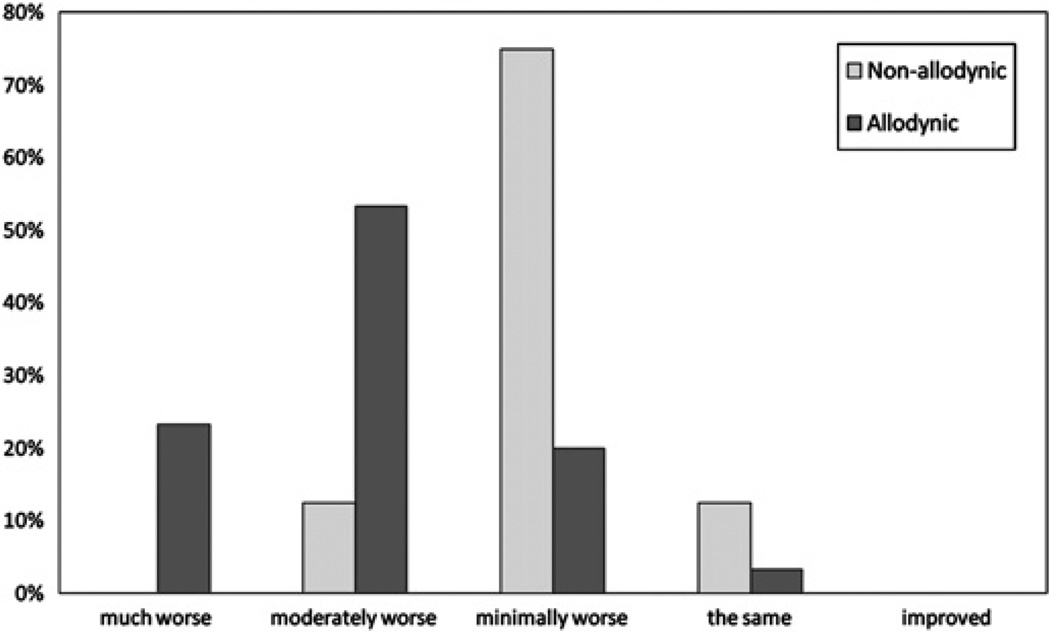
We further evaluated subjective phonophobia by asking the patients to rate their sound aversion during the tested attack in relation to that between attacks as ‘much worse’, ‘moderately worse’, ‘minimally worse’, ‘the same’, ‘minimally improved’, ‘moderately improved’ or ‘much improved’. Of the 30 patients who were allodynic during the tested attack, seven (23.3%) rated their sound aversion as ‘much worse’, 16 (53.3%) rated it as ‘moderately worse’, six (20%) as ‘minimally worse’ and one (3.3%) as ‘the same’ (figure 4). Conversely, of the eight patients who were non-allodynic during the tested attack, none rated their sound aversion as ‘much worse’, one (12.5%) rated it as ‘moderately worse’, six (75%) as ‘minimally worse’ and one (12.5%) as ‘the same’ (figure 4). No patient in either group rated their sound aversion during the acute attack as improved compared with that between attacks. Converting these ratings to numerical values (from 1 = ‘much improved’ to 7 = ‘much worse’), the median subjective phonophobia scores of allodynic and non-allodynic patients during the tested attack were 6.0 and 5.0, respectively (Wilcoxon rank sum test, p=0.001).
Figure 4.
Subjective rating of phonophobia during the acute attack, in relation to that between attacks, in non-allodynic and allodynic patients.
SATs versus migraine characteristics
SATs were non-significantly lower in patients with migraine with aura compared with those who had migraine without aura, both between attacks (88.4±1.2 vs 92.7±1.7, p=0.08) and within attacks (73.7±1.9 vs 77.7±1.8, p=0.18). During attacks, there was a non-significant negative correlation between SATs and headache severity: the average acute attack SAT decreased by 1.8 dB for every unit increase in acute headache severity (p=0.28).
DISCUSSION
We found that migraine patients who exhibited brush allodynia during an acute attack were also more aversive to sound (ie, phonophobic), as demonstrated by lower SATs, compared with non-allodynic patients. These results suggest an association between phonophobia and allodynia in migraine.
Phonophobia has long been recognised in migraine.9 14 In a study by Kayan and Hood, phonophobia was subjectively reported by 81% of 200 migraine patients during an acute attack.9 The authors suggested a peripheral mechanism to this symptom—specifically, a transient disturbance of function of the cochlear receptors. This hypothesis has been challenged by others.7 8 Few studies have examined phonophobia in migraine in a quantitative manner.2 7 8 Vanagaite Vingen et al found increased aversion to sound in migraineurs compared with controls, both between and during attacks.7 The authors suggested a central mechanism for phonophobia in migraine, possibly mediated by noradrenergic or serotonergic pathways.15 In accordance with these results, Main et al reported on increased hearing discomfort in migraine patients between attacks.2 Woodhouse and Drummond found an increase in both auditory and visual discomfort in migraineurs during attacks.8 They suggested a centrally mediated hyperexcitability of the special senses during attacks as the underlying mechanism. In none of these studies was the association between phonophobia and cutaneous allodynia examined.
The presence of increased aversion to stimuli of multiple sensory modalities (eg, sound, light, touch) in migraine raises the hypothesis that they are associated through a central integrative mechanism rather than generated separately by dysfunction of multiple distinct afferent pathways. Our demonstration of association between two of these phenomena (phonophobia and allodynia) lends support to this hypothesis. It is interesting that the reported prevalence of phonophobia in migraine is remarkably similar to that of allodynia (up to 80% in some studies), further supporting (albeit not proving) their association.9 16
To test for phonophobia, we used sound stimuli at frequencies that are often encountered in daily life. The human auditory system is most sensitive to sounds at a frequency of approximately 4000 Hz.12 In an attempt to simulate a real life situation, we chose sound stimuli that were at, and around, this frequency.
During an acute migraine attack, SATs in allodynic patients were, on average, 15.7 dB lower than those of non-allodynic patients. Since the sound intensity decibel scale is logarithmic (ie, a decrease of 10 dB in SAT corresponds to a 10-fold decreased intensity of the aversive sound), this is a substantial difference.17 18 Between attacks, the average SAT difference between allodynic and non-allodynic patients was smaller (5.7 dB). The biological significance of this SAT difference at the interictal state remains to be determined.
We found that the average acute attack SAT decreased by 1.8 dB for every unit increase in acute headache severity (p=0.28). These data are in accordance with the findings of Kelman and Tanis who showed in a large clinic based study a correlation between headache intensity and a number of associated symptoms, including phonophobia.19
The mechanisms of phonophobia in migraine are poorly understood. Electrophysiological studies suggest aberrant processing of auditory stimuli at the cortical level in these patients.20 In these studies, that used auditory evoked potential recordings, migraine patients exhibited lack of habituation, and even potentiation, of the cortical response to repeated auditory stimuli in the interictal state.21 Interestingly, lack of habituation was also found in migraineurs in response to visual and somatosensory stimuli.20 22 23 Data from a neuroimaging study further support a cortical role in the development of phonophobia in migraine: Weiller et al found in a PET study increased blood flow in the auditory and visual association cortices (in addition to brainstem activation) in patients with migraine without aura during an acute attack.24 The authors suggested that these cortical findings were related to the presence of phonophobia and photophobia in migraine.
Allodynia in migraine is thought to result from sensitisation of second order neurons in the trigeminal nucleus caudalis.25 As phonophobia is probably mediated through mechanisms that involve higher brain centres (as discussed above), it is difficult to explain an association between allodynia and phonophobia based on a brainstem mechanism of allodynia alone. Rather, our results suggest that the mechanisms of allodynia in migraine are more complex, possibly involving neuronal dysfunction at higher levels as well. These may be either thalamic or cortical, or both. Neuroimaging studies support this notion. Witting et al, in a PET study that used a model of capsaicin evoked pain and allodynia, have shown that brush evoked allodynia, but not capsaicin induced pain, activated the posterior parietal cortex, suggesting the involvement of distinct cortical areas in the processing of allodynia.26 In another PET study, Lorenz et al found that the pattern of cortical representation of heat allodynia was distinct from that of normal heat pain.27
Our study was limited to the evaluation of the subjective experience of phonophobia, as reported by the subjects. The objective correlates of this experience should be examined in future studies. Other limitations of our study are: (1) the investigators were not blinded to the tested subject group. However, this probably did not have a significant impact on our results, for the following reasons: (a) allodynia testing was performed before the testing of phonophobia and therefore the examiner was not aware of subjects’ phonophobia status when examining them for allodynia; (b) phonophobia testing was entirely automated. (2) Determination of allodynia status of the subjects was based on an examination during a single attack. (3) Due to the high rate of allodynia at visit 2, the number of non-allodynic subjects at that visit was small. (4) It has been shown that depression and anxiety are associated with altered perception of auditory stimuli.7 28 As these psychiatric disorders are common in migraine, their occurrence may have affected our results. Although we cannot rule out such an effect, this is unlikely to be a major factor in our study as none of our patients was clinically depressed or using antidepressant drugs. (5) We examined our patients for allodynia using brush testing, as previously described.5 We did not test the patients for allodynia to other sensory modalities (eg, heat, cold or pressure). In previous studies, our method for brush allodynia testing has proved reliable and reproducible.5 29 Furthermore, the calculated prevalence of allodynia in our study (79%) agrees with results obtained in studies that used quantitative sensory testing for this purpose.4 16
In summary, our results suggest an association between phonophobia and brush allodynia in migraine. This association raises the possibility that the two phenomena may share common mechanisms in migraine. Further studies are needed to clarify these mechanisms and to examine whether similar associations exist among other symptoms of increased sensory aversion in migraine, such as photophobia and osmophobia.
Acknowledgements
We thank Dr Benjamin Leiby for his help with data analysis and Dr James W Shaw for his helpful advice on the study design.
Funding
This work was supported by Merck Inc, IISP grant No 31402 (to AA) and by NIH grant No NS061571 (to MLO)
Footnotes
Competing interests
None.
Ethics approval This study was conducted with the approval of the Institutional Review Board for the Study of Human Subjects, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatry. 1960;23:23–32. doi: 10.1136/jnnp.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–495. doi: 10.1046/j.1526-4610.1997.3708492.x. [DOI] [PubMed] [Google Scholar]
- 3.Zanchin G, Dainese F, Trucco M, et al. Osmophobia in migraine and tension-type headache and its clinical features in patients with migraine. Cephalalgia. 2007;27:1061–1068. doi: 10.1111/j.1468-2982.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 4.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the developing allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazi A, Sholtzow M, Shaw JW, et al. Identifying cutaneous allodynia in chronic migraine using a practical clinical method. Cephalalgia. 2007;27:111–117. doi: 10.1111/j.1468-2982.2006.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanagaite Vingen J, Pareja JA, Storen O, et al. Phonophobia in migraine. Cephalalgia. 1998;18:243–249. doi: 10.1046/j.1468-2982.1998.1805243.x. [DOI] [PubMed] [Google Scholar]
- 8.Woodhouse A, Drummond PD. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia. 1993;13:417–421. doi: 10.1046/j.1468-2982.1993.1306417.x. [DOI] [PubMed] [Google Scholar]
- 9.Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107:1123–1142. doi: 10.1093/brain/107.4.1123. [DOI] [PubMed] [Google Scholar]
- 10.Headache Classification Committee. The International Classification of Headache Disorders. Cephalalgia. (2nd edn) 2004;24:1–160. [Google Scholar]
- 11.Ashkenazi A, Mushtaq A, Yang I, et al. Ictal and interictal phonophobia in migrained—a quantitative controlled study. Cephalalgia. 2009;29:1042–1048. doi: 10.1111/j.1468-2982.2008.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinsler LE, Frey AR, Coppens AB, et al. Noise, signal detection, hearing, and speech. In: Kinsler LE, Frey AR, Coppens AB, et al., editors. Fundamentals of acoustics. Hoboken: Wiley; 2000. pp. 302–332. [Google Scholar]
- 13. [accessed 18 oct 09];Occupational noise exposure. 2007 http://www.osha.gov.
- 14.Gowers WR. A manual of diseases of the nervous system. Philadelphia: P Blakiston, Son & Co; 1888. [Google Scholar]
- 15.Marriage J, Barnes NM. Is central hyperacusis a symptom of 5-hydroxytryptamine (5-HT) dysfunction? J Laryngol Otol. 1995;109:915–921. doi: 10.1017/s0022215100131676. [DOI] [PubMed] [Google Scholar]
- 16.Jakubowski M, Silberstein S, Ashkenazi A, et al. Can allodynic migraine patients be identified interictally using a questionnaire? Neurology. 2005;65:1419–1422. doi: 10.1212/01.wnl.0000183358.53939.38. [DOI] [PubMed] [Google Scholar]
- 17.Kinsler LE, Frey AR, Coppens AB, et al. The acoustic wave equation and simple solutions. In: Kinsler LE, Frey AR, Coppens AB, et al., editors. Fundamentals of acoustics. Hoboken: Wiley; 2000. pp. 113–148. [Google Scholar]
- 18.Kinsler LE, Frey AR, Coppens AB, et al. Environmental acoustics. In: Kinsler LE, Frey AR, Coppens AB, et al., editors. Fundamentals of acoustics. Hoboken: Wiley; 2000. pp. 359–389. [Google Scholar]
- 19.Kelman L, Tanis D. The relationship between migraine pain and other associated symptoms. Cephalalgia. 2006;26:548–553. doi: 10.1111/j.1468-2982.2006.01075.x. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosini A, Schoenen J. The electrophysiology of migraine. Curr Opin Neurol. 2003;16:327–331. doi: 10.1097/01.wco.0000073945.19076.1f. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosini A, Rossi P, De Pasqua V, et al. Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain. 2003;126:2009–2015. doi: 10.1093/brain/awg206. [DOI] [PubMed] [Google Scholar]
- 22.Ozkul Y, Uckardes A. Median nerve somatosensory evoked potentials in migraine. Eur J Neurol. 2002;9:227–232. doi: 10.1046/j.1468-1331.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 23.Afra J, Proietti CA, Sandor PS, et al. Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin Neurophysiol. 2000;111:1124–1129. doi: 10.1016/s1388-2457(00)00271-6. [DOI] [PubMed] [Google Scholar]
- 24.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 25.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 26.Witting N, Kupers RC, Svensson P, et al. Experimental brush-evoked allodynia activates posterior parietal cortex. Neurology. 2001;57:1817–1824. doi: 10.1212/wnl.57.10.1817. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz J, Cross D, Minoshima S, et al. A unique representation of heat allodynia in the human brain. Neuron. 2002;35:383–393. doi: 10.1016/s0896-6273(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 28.Katzenell U, Segal S. Hyperacusis: review and clinical guidelines. Otol Neurotol. 2001;22:321–326. doi: 10.1097/00129492-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 29.LoPinto C, Young WB, Ashkenazi A. Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia. 2006;26:852–856. doi: 10.1111/j.1468-2982.2006.01121.x. [DOI] [PubMed] [Google Scholar]