Abstract
Cardiovascular magnetic resonance (CMR) and hepatic magnetic resonance imaging (MRI) has become a reliable non-invasive tool to monitor iron excess in thalassemia major (TM) patients. However, long-term studies are lacking. We reviewed CMR and hepatic MRI T2* imaging on 54 TM patients who had three or more annual measurements. They were managed on various chelation regimens. Patients were grouped according to their degree of cardiac siderosis: severe (T2*<10msec), mild to moderate (T2*=10-20 msec) and no cardiac siderosis (T2*>20msec). We looked at the change in cardiac T2*, liver iron concentration (LIC) and left ventricular ejection fraction (LVEF) at years 3 and 5. In patients with severe cardiac siderosis, cardiac T2* (mean±SD) improved from 6.9±1.6 at baseline to 13.6±10.0 by year 5, mean ΔT2*=6.7 (p-value 0.04). Change in cardiac T2* at year 3 was not significant in the severe group. Patients with mild to moderate cardiac siderosis had mean cardiac T2* of 14.6±2.9 at baseline which improved to 26.3±9.5 by year 3, mean ΔT2*=11.7 (p-value 0.01). At baseline, median LIC (mg/gm dw) in patients with severe, mild-moderate and no cardiac siderosis was 3.6, 2.8 and 3.3 while LVEF (mean±SD) (%) was 56.3±10.1, 60±5 and 66±7.6 respectively. No significant correlation was noted between Δ cardiac T2* and Δ LIC, Δ cardiac T2* and Δ LVEF at years 3 and 5. Throughout the observation period, patients with no cardiac siderosis maintained their cardiac T2* above 20msec. The majority of patients with cardiac siderosis improve cardiac T2* over time with optimal chelation.
Keywords: thalassemia, cardiac siderosis, chelation
Introduction
Patients with thalassemia major (TM) who are transfusion dependent develop considerable iron excess in various organs of the body. (1) Cardiac siderosis is a major cause of mortality in these patients. Chelation therapy has been shown to remove cardiac iron and is successful in prolonging survival. (2-4) Life threatening complications like heart failure and arrhythmias occur late in the course of the disease. There is a paucity of predictors for worsening in cardiac function. Indicators like ferritin and hepatic iron concentration correlate poorly with cardiac function and elevated brain-natriuretic peptide only occurs late in the course of the disease. (5-7) Left ventricular dysfunction as noted on echocardiography is more evident in patients with severe cardiac siderosis. (8) Once symptomatic heart failure develops mortality is very high. (9) It is highly desirable to identify these patients early in the course of cardiac iron deposition and aggressively treat with iron chelation. Studies have shown that long-term subcutaneous deferoxamine infusions have prolonged life but did not prevent progressive cardiac iron deposition resulting in cardiac failure. (10) This may be partly due to poor compliance (11, 12) and the lack of ability to monitor cardiac iron load. Electrocardiography (13) and 2D-echocardiography (14) have been used to correlate cardiac function with iron deposition with some success. Recently, CMR has allowed accurate measurement of iron excess in the heart and can be a better guide for managing iron chelation therapy with single or a combination of chelators. (6, 15) However, extended monitoring of cardiac iron status in thalassemia major patients is lacking. We reviewed CMR and hepatic MRI scans in our thalassemia clinic patients for 6 years to know the trend of cardiac T2*, LIC and LVEF with optimal chelation therapy.
Methods
We retrospectively reviewed 54 beta-thalassemia major patients 18 Male (M) 36 Female (F) with a mean age of 35.1 years (yrs) (range: 11-61 yrs). All received regular red blood cell transfusions to maintain pre transfusion Hb levels of 9 to 10 gm/dl and all received iron chelation initially with deferoxamine (DFO) and subsequently treated with deferasirox (DFX) or deferiprone (DFP) in combination with DFO. CMR and hepatic MRI T2* was monitored for each patient yearly for iron excess. During the course of the observation their iron chelation therapy was optimized to reduce serum ferritin levels < 1500 µg/dl and to reduce or maintain liver iron concentration (LIC) ≤ 7 mg/gm dw. As a part of routine clinical management, patients had monthly evaluations of liver and renal function, serum ferritin, complete blood count (or weekly if on DFP), urinalysis, annual electrocardiogram, echocardiogram, hearing and vision testing and endocrine evaluations. The institutional review board approved this study and written consent was obtained from patients prior to participation.
All MRI examinations were performed on a 1.5 Tesla MRI (GE Signa HDx 1.5T MRI, GE Healthcare, Waukesha, WI). A multi-echo fast gradient echo (MFGRE) sequence was performed for both cardiac and hepatic iron assessment. Up to 16 echos were obtained for both myocardial and hepatic images; region of interest (ROI) measurements were utilized to calculate T2* values. For cardiac values, ROI was drawn in the septum of a mid-ventricular slice. Hepatic and myocardial iron was obtained from the T2* value using the method described by Wood et al (16) and analysis was performed using a commercial GE workstation via ReportCARD software. The mean signal intensity was plotted against echo time and an exponential decay curve was fitted. The following curve fit equation was used: (T2*)= a*exp(-TE/T2*) + C. Since we used 2 parameter fit, C=0 was applied in the equation. Liver iron concentration was calculated using the equation, Fe (mg/gm dry weight) = 0.0254 R2* + 0.202 (16) and cardiac iron concentration (CIC) was derived using the equation, CIC (mg/gm dry weight) = 45 × T2* (-1.22). (15)
The patients were grouped according to the severity of cardiac siderosis: severe cardiac siderosis (T2*<10msec), mild to moderate (T2*=10-20 msec) and no cardiac siderosis (T2*>20msec). Liver iron concentration was defined as mild, moderate and severe according to MRI T2* mg Fe/gm dry weight (dw) of liver: mild<3, moderate=3-15 and severe >15. Cardiac volumetric and functional (LVEF) measurements were performed using an established automated segmentation program (LV-METRIC), for which high inter- and intra-observer reproducibility has previously been reported. (17, 18). We looked at years 3 and 5 to evaluate changes from baseline. We used cardiac R2* (R2*=1000/T2*) for correlation analysis, since R2* is linearly proportional to CIC.
Statistical analysis
The normal distribution of the patient population was verified using Shapiro-Wilk normality test. Difference in means between baseline and subsequent measurements at years 3 and 5 was compared using student T test. Differences among the means of various parameters in the three groups were tested using one-way ANOVA. Differences among the medians of LIC and ferritin levels in the three groups were tested using Kruskal-Wallis test. Chi-square test or Fisher’s exact test was used to evaluate differences for categorical variables. Spearman correlation coefficient was used to evaluate correlation between change percent change (% Δ) in cardiac R2* versus initial LIC, final LIC and % Δ LIC at years 3 and 5. Linear regression analysis and pearson correlation coefficient were used to know the correlation between cardiac T2* and LVEF in mod-severe and severe cardiac siderosis patients. Observed and predicted occurrence of heart failure events based on historical data (19) in severe cardiac siderosis patients was shown by Kaplan-Meier curves. A p-value of <0.05 was considered significant for all analyses. All statistical tests were done using GraphPad Prism, GraphPad Software, Inc, CA, USA.
Patient population
Out of all thalassemia major patients enrolled in our program, 54 had three or more annual MRIs, of which 33 patients had cardiac T2* >20, 8 patients had cardiac T2*=10-20 and 13 patients had cardiac T2*<10msec at baseline. The characteristics of the three groups are listed in Table I. The chelation regimens for the three groups during the study period are as follows. No cardiac siderosis: 28 out of 33 patients were on DFX of which 3 switched to DFP and 1 patient to DFO+DFP; Out of the remaining patients, 4 were on DFO and 1 had DFO+DFP. Mild-moderate cardiac siderosis: 7 out of 8 patients were on DFX of which 1 changed to DFP and 1 to DFO; 1 patient was on DFO but changed to DFP subsequently. Severe cardiac siderosis: 10 out of 13 were on DFX of which 1 changed to DFP and 1 to DFO+DFP; 3 were on DFO of which 2 switched to DFX. Changes in the chelation were made due to intolerance or non-compliance to the medications or due to patient’s choice.
Table I.
Characteristics of patients with severe, mild to moderate and no cardiac siderosis at baseline.
| Characteristic | Severe cardiac siderosis (n=13) | Mild-moderate cardiac siderosis (n=8) | No cardiac siderosis (n=33) | p-value |
|---|---|---|---|---|
| Mean Age (yrs) (range) | 34.7(21-61) | 39.3(27-50) | 31.3(11-54) | 0.28 |
| Gender (Male:Female) | 3:10 | 3:5 | 12:21 | 0.70 |
| Mean transfusion burden (ml/kg/yr)±SD | 128.1±14.2 | 114.8±14.7 | 120.3±45.6 | 0.815 |
| Median baseline ferritin (mg/dl) (range) | 1759(388-17489) | 1254(523-2661) | 1026 (342-3322) | 0.418 |
| Mean baseline cardiac T2* (msec)±SD | 6.9±1.6 | 14.6±2.9 | 36.3±7.3 | <0.0001 |
| Median baseline LIC (mg Fe/gm dry weight) (range) | 4.3(0.4-46) | 2.2(1.2-16) | 3.3(0.4-32) | 0.523 |
| Mean LVEF (%)±SD | 56.3±10.1 | 60±5 | 66±8.2 | 0.003 |
LIC- liver iron concentration, LVEF- left ventricular ejection fraction
LIC was calculated from liver T2* value measured by MRI
Results
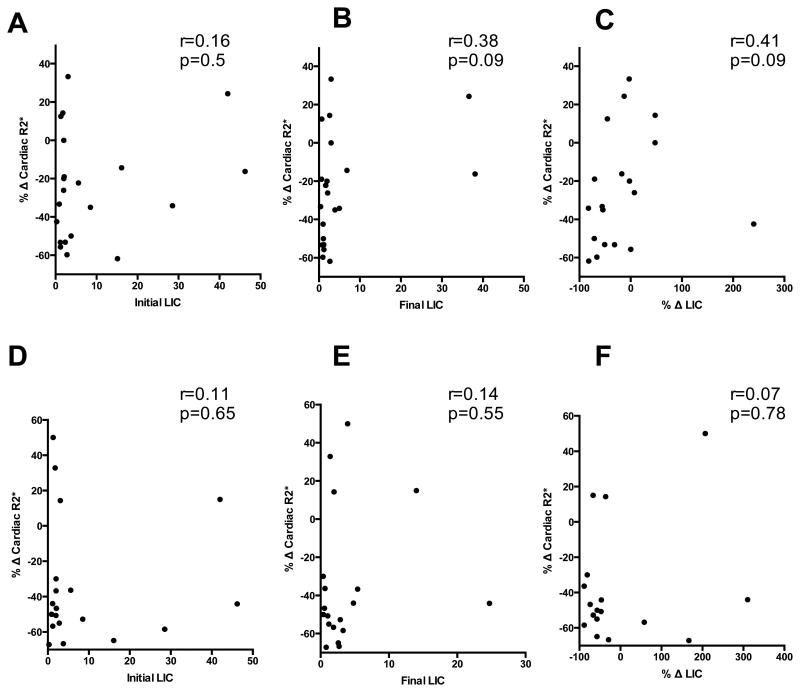
Baseline characteristics of the patients in the three groups- severe, mild to moderate and no cardiac siderosis, including transfusion burden, mean cardiac T2*, LIC, LVEF, and median ferritin values are shown in Table I. There was no significant difference in the age, baseline transfusion burden (cc/kg/yr), ferritin levels and LIC among the three groups at baseline. Patients with severe cardiac siderosis had a mean cardiac T2*(msec)±SD=6.9±1.6 at baseline which consistently improved over the treatment course of 6 yrs. At year 5, significant improvement was noted when cardiac T2* reached a level of 13.6±10 (p-value 0.04) (Table II). Those patients who at baseline had a mild to moderate cardiac siderosis with mean cardiac T2*(msec)±SD of 14.6±2.9 achieved significant improvement by year 3, when the level reached 26.3±9.5 (p-value 0.01). Patients with no cardiac siderosis had stable levels of 36.3±7.3 at baseline and 35.4±7 at year 3 (Table II). In severe cardiac siderosis group, 30.8% (4/13 patients) improved by year 3, whereas 58.3% (7/12) improved by year 5. In mild-moderate cardiac siderosis group, 62.5% (5/8) improved by year 3 and all patients improved by year 5. At baseline, 6 out of 13 (46%) patients with severe cardiac siderosis and 6 out of 8 (75%) patients with mild-moderate cardiac siderosis had LIC <3 mg/gm dw. In no cardiac siderosis group, 19 out of 33 (58%) patients had LIC >3 mg/gm dw. For both severe and mild-moderate cardiac siderosis groups, Spearman correlation between percent change in cardiac T2* and LIC parameters including initial LIC, final LIC or percent change in LIC did not show significance when compared at years 3 and 5. (Figure 1)
Table II.
Baseline and mean change in cardiac T2* noted at years 3 and 5 in the three groups of cardiac siderosis.
| Baseline | Year 3 | Year 5 | |||
|---|---|---|---|---|---|
| Cardiac siderosis | Mean T2*±SD | Mean ΔT2* (95%CI) | P value | Mean ΔT2* (95%CI) | P value |
| Severe | 6.9±1.6 | 2.3(-0.5 to 5.0) | 0.1 | 6.7(0.3 to 13.1) | 0.04 |
| Mild-mod | 14.6±2.9 | 11.7(3.7 to 19.7) | 0.01 | 19.4 (12.1-26.7) | 0.001 |
| None | 36.3±7.3 | -0.8(-4.1 to 2.4) | 0.59 | 3 (-6 to 12) | 0.28 |
Figure 1.
Percent change in cardiac R2* was plotted against initial LIC, final LIC and percent change in LIC at year 3 (A, B, C) and year 5 (D, E, F) using spearman correlation. P-value was not significant in any of the above correlations.
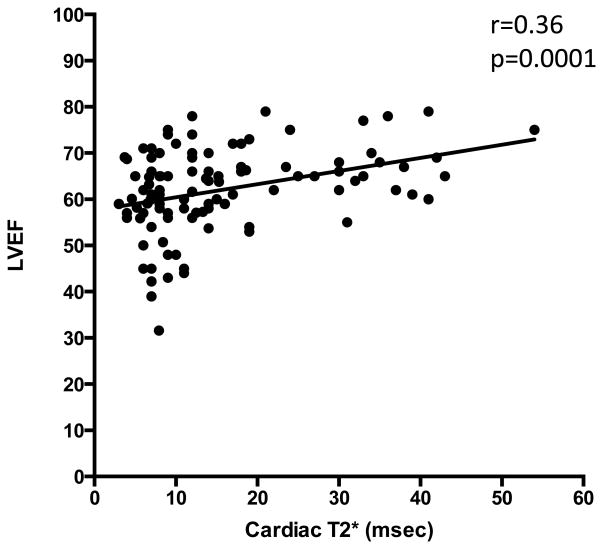
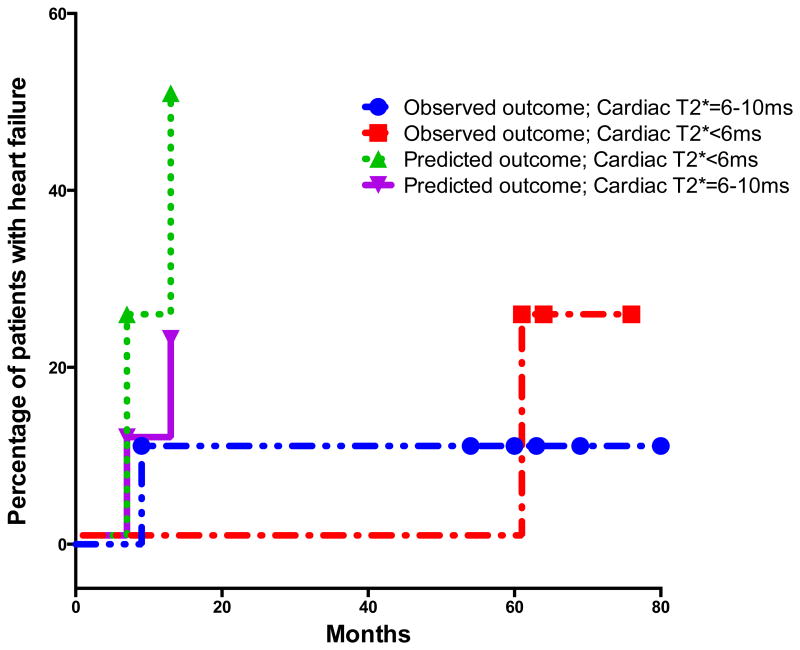
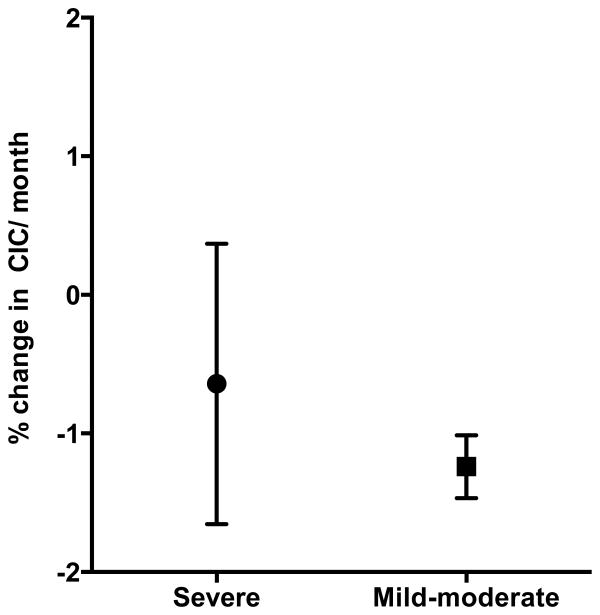
There was a significant difference in baseline MRI LVEF among the three groups (p-value 0.003) as shown in Table I. Serial measurements show that LVEF improves in patients with severe and mild to moderate cardiac siderosis. The mean ±SD of LVEF in patients with severe cardiac siderosis improved from 56.3 ±10.1 to 62.6 ±8.9 at 5 years (p-value 0.03). In patients with mild-moderate cardiac siderosis, mean ±SD of LVEF improved from 60 ±5 to 65.5 ±6.4 at 3 years (p-value 0.045). Regression analysis of cardiac T2* and LVEF in patients with mild-moderate and severe cardiac siderosis is shown in figure 2. Pearson correlation (r=0.36) showed significant correlation (p=0.0001) between cardiac T2* and LVEF in patients with cardiac siderosis. However, there were many patients with abnormal cardiac siderosis who had normal LVEF by the standards for general population. At baseline, only 3 out of 13 (23%) patients with severe cardiac siderosis and 2 out of 8 (25%) patients with mild-moderate cardiac siderosis had LVEF<55%. Spearman correlation did not show significant correlation between change in cardiac T2* and change in LVEF when noted at years 3 and 5 among the three groups. None of the patients in the mild-moderate cardiac siderosis group developed heart failure or arrhythmias. Two patients in the severe cardiac siderosis group developed symptomatic heart failure and arrhythmias at 9 and 60 mo after their baseline scan (Figure 3). Cardiac iron concentration (CIC) in mg/gm dry weight was calculated from cardiac T2* values at baseline and final scan. Mean (± SD) percent change in CIC per month in patients with severe and mild-moderate cardiac siderosis was -0.64 (± 1.01) and -1.24 (±0.23) (Figure 4).
Figure 2.
Pearson correlation of cardiac T2* and LVEF in patients with mild-moderate and severe cardiac siderosis.
Figure 3.
Kaplan-Meier curves showing the proportion of patients developing cardiac failure in the severe cardiac siderosis group (<6ms and 6-10ms) in our cohort. We calculated the expected number of patients that would develop cardiac failure based on the historical data by Kirk et al, to compare the changing trends with aggressive chelation.
Figure 4.
Mean (± SD) percent change in cardiac iron concentration (CIC) per month in patients with mild-moderate and severe cardiac siderosis.
Discussion
Myocardial iron deposition has been shown to be poorly correlated with serum ferritin, labile plasma iron, liver iron grading and even myocardial biopsy in earlier studies. (6, 20-22) However, CMR T2* appears to be in agreement with iron measurements by myocardial biopsy and in post-mortem hearts as shown in recent studies in patients with TM. (15, 23) It is now possible to non-invasively monitor MRI cardiac iron serially in patients with transfusion dependent thalassemia. We report the longest series of annual cardiac MRI T2* measurements in TM patients. During our study, patients were on different iron chelation regimens to optimize chelation effectiveness. The majority of our patients were on chelation with DFX- 17 of 21 (81%) patients with cardiac siderosis and 28 out of 33 (85%) patients with no cardiac siderosis. A previous retrospective study of TM patients treated on various single chelation agents suggested that patients treated with oral deferiprone showed less myocardial iron burden and higher EF than those on deferasirox chelation for 2±1 yrs. (24) The kinetics of iron clearance from heart has been shown to be slow. (3, 25) Therefore, prospective longitudinal studies are necessary to evaluate the efficacy of cardiac iron chelators. In our 6 yr annual MRI assessments of TM patients, we have seen the normalization of cardiac T2* at year 3 in patients with mild-moderate cardiac siderosis and improvement at year 5 in majority of those with severe cardiac siderosis (Table II). Only one patient with severe cardiac siderosis developed cardiac failure within the first year of observation and another patient at 60 mo. This experience improves up on the previously reported rates of heart failure, where in 47.2% of patients with cardiac T2*<6 msec and 21.4% of patients with T2* 6-10 msec developed heart failure within one year. (19) Perhaps strict monitoring, aggressive management and optimization of chelation were responsible for decrease in heart failure rates in our patients with severe cardiac siderosis (Figure 3). The limitation of our study is small number of patients in mild-moderate and severe cardiac siderosis groups. Recently others have documented improvement in cardiac siderosis on DFX for one to three years of continued treatment. (26-29) In our study, percent mean change in CIC per month was 0.64% and 1.24% in patients with severe and mild-moderate cardiac siderosis respectively. Our findings are comparable to previously reported percent change in CIC per month of 0.8% and 0.7% in trials of only 18-36mo duration. (26, 30) There were 2 patients with severe cardiac siderosis who did not show improvement after 5 years of variable DFX chelation, who were switched to alternative regimens. We noted that 46% of our patients with severe cardiac siderosis and 75% of those with mild-moderate cardiac siderosis had LIC < 3 mg/gm dw at baseline. Others have also suggested that cardiac iron clearance is often delayed and lags behind clearance from liver. (31) Therefore, as previously shown, LIC is unlikely to be an accurate indicator of cardiac iron status. (5)
A multicenter, open label single arm study (US04 trial) showed that percent change in cardiac R2* correlated with initial LIC, final LIC and percent change in LIC. (30) However, we did not see a signifcant correlation between percent change in cardiac R2* and various LIC parameters- iniial LIC, final LIC and percent change in LIC at 3 or 5 year measurements in our cohort (Figure 1).
In our study, mean EF improved during the course of treatment along with increasing cardiac T2* measurements. Regression analysis showed a significant correlation between LVEF and cardiac T2* in patients with mild-moderate and severe cardiac siderosis as shown in figure 2, similar to earlier studies (6, 32). However, we did not see a significant correlation between percent improvement in cardiac T2* and percent change in LVEF. This could be related to the small numbers in our study as well as the fact that most had near-normal LVEF. It has been noted that the mean right ventricular and left ventricular EF in patients with TM is relatively higher than in the normal population, therefore, appropriate reference ranges should be used for determining cardiac function in these patients. (33, 34) While LVEF may show improvement with continued chelation therapy it may not accurately reflect an improvement in cardiac T2*.
MRI cardiac and hepatic imaging is now available and serial monitoring of organ iron excess serves as a guide for managing iron chelation with single or combination iron chelators. Annual monitoring allows for optimization of chelation strategies in dosing or use of alternative therapies.
Acknowledgments
We would like to thank Ms. Ya-Lin Chiu, Biostatistician, Weill Cornell Medical College for helping with statistical analysis of our data.
Funding: This study was supported in part by CTSC grant number: UL1 RR 024996 and Children’s Cancer and Blood Foundation.
Footnotes
Authorship and disclosures: PJG was the principal investigator and SRA participated in patient care, did statistical analysis and wrote the paper. They both take the primary responsibility of the paper. RER worked on the data analysis and edited the paper. DAK participated in patient care and edited the paper. KM and JWW performed imaging studies and edited the paper.
The authors report no potential conflicts of interest.
Contributor Information
Srikanth R. Ambati, Department of Pediatrics, Memorial Sloan-Kettering Cancer Center, NY, USA.
Rachel E. Randolph, Department of Pediatrics, Weill Cornell Medical College, NY, USA.
Kevin Mennitt, Department of Radiology, Weill Cornell Medical College, NY, USA.
Dorothy A. Kleinert, Department of Pediatrics, Weill Cornell Medical College, NY, USA.
Jonathan W. Weinsaft, Cardiac MR/CT Imaging Program, Division of Cardiology, Weill Cornell Medical College, NY, USA.
Patricia J. Giardina, Department of Pediatrics, Weill Cornell Medical College, NY, USA.
References
- 1.Buja LM, Roberts WC. Iron in the heart. Etiology and clinical significance Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 2.Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–578. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 3.Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 4.Telfer P, Constantinidou G, Andreou P, et al. Quality of life in thalassemia. Annals of the New York Academy of Sciences. 2005;1054:273–282. doi: 10.1196/annals.1345.035. [DOI] [PubMed] [Google Scholar]
- 5.Tanner MA, Galanello R, Dessi C, et al. Myocardial iron loading in patients with thalassemia major on deferoxamine chelation. J Cardiovasc Magn Reson. 2006;8:543–547. doi: 10.1080/10976640600698155. [DOI] [PubMed] [Google Scholar]
- 6.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham MJ, Macklin EA, Neufeld EJ, Cohen AR. Complications of beta-thalassemia major in North America. Blood. 2004;104:34–39. doi: 10.1182/blood-2003-09-3167. [DOI] [PubMed] [Google Scholar]
- 8.Aessopos A, Giakoumis A, Fragodimitri C, et al. Correlation of echocardiography parameters with cardiac magnetic resonance imaging in transfusion-dependent thalassaemia major. European journal of haematology. 2007;78:58–65. doi: 10.1111/j.1600-0609.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 9.Engle MA, Erlandson M, Smith CH. Late Cardiac Complications of Chronic, Severe, Refractory Anemia with Hemochromatosis. Circulation. 1964;30:698–705. doi: 10.1161/01.cir.30.5.698. [DOI] [PubMed] [Google Scholar]
- 10.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 11.Trachtenberg F, Vichinsky E, Haines D, et al. Iron chelation adherence to deferoxamine and deferasirox in thalassemia. American journal of hematology. 2011;86:433–436. doi: 10.1002/ajh.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobota A, Yamashita R, Xu Y, et al. Quality of life in thalassemia: a comparison of SF-36 results from the thalassemia longitudinal cohort to reported literature and the US norms. American journal of hematology. 2011;86:92–95. doi: 10.1002/ajh.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detterich J, Noetzli L, Dorey F, et al. Electrocardiographic consequences of cardiac iron overload in thalassemia major. American journal of hematology. 2012;87:139–144. doi: 10.1002/ajh.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggio A, Vitrano A, Lucania G, et al. Long-term use of deferiprone significantly enhances left-ventricular ejection function in thalassemia major patients. American journal of hematology. 2012;87:732–733. doi: 10.1002/ajh.23219. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter JP, He T, Kirk P, et al. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123:1519–1528. doi: 10.1161/CIRCULATIONAHA.110.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Codella NC, Weinsaft JW, Cham MD, et al. Left ventricle: automated segmentation by using myocardial effusion threshold reduction and intravoxel computation at MR imaging. Radiology. 2008;248:1004–1012. doi: 10.1148/radiol.2482072016. [DOI] [PubMed] [Google Scholar]
- 18.Codella NC, Cham MD, Wong R, et al. Rapid and accurate left ventricular chamber quantification using a novel CMR segmentation algorithm: a clinical validation study. Journal of magnetic resonance imaging : JMRI. 2010;31:845–853. doi: 10.1002/jmri.22080. [DOI] [PubMed] [Google Scholar]
- 19.Kirk P, Roughton M, Porter JB, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood JC, Glynos T, Thompson A, et al. Relationship between labile plasma iron, liver iron concentration and cardiac response in a deferasirox monotherapy trial. Haematologica. 2011;96:1055–1058. doi: 10.3324/haematol.2010.032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noetzli LJ, Carson SM, Nord AS, et al. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barosi G, Arbustini E, Gavazzi A, et al. Myocardial iron grading by endomyocardial biopsy. A clinico-pathologic study on iron overloaded patients. European journal of haematology. 1989;42:382–388. doi: 10.1111/j.1600-0609.1989.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 23.Westwood MA, Sheppard MN, Awogbade M, et al. Myocardial biopsy and T2* magnetic resonance in heart failure due to thalassaemia. Br J Haematol. 2005;128:2. doi: 10.1111/j.1365-2141.2004.05234.x. [DOI] [PubMed] [Google Scholar]
- 24.Pepe A, Meloni A, Capra M, et al. Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: cardiac iron and function comparison determined by quantitative magnetic resonance imaging. Haematologica. 2010;96:41–47. doi: 10.3324/haematol.2009.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musallam KM, Taher AT. Deferiprone or deferasirox for cardiac siderosis in beta-thalassemia major. Haematologica. 2011;96:e5–6. doi: 10.3324/haematol.2010.036061. author reply e7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennell DJ, Porter JB, Cappellini MD, et al. Deferasirox for up to 3 years leads to continued improvement of myocardial T2* in patients with beta-thalassemia major. Haematologica. 2012;97:842–848. doi: 10.3324/haematol.2011.049957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwiatkowski JL, Kim HY, Thompson AA, et al. Chelation use and iron burden in North American and British thalassemia patients: a report from the Thalassemia Longitudinal Cohort. Blood. 2012;119:2746–2753. doi: 10.1182/blood-2011-04-344507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennell DJ, Porter JB, Cappellini MD, et al. Efficacy of deferasirox in reducing and preventing cardiac iron overload in beta-thalassemia. Blood. 2010;115:2364–2371. doi: 10.1182/blood-2009-04-217455. [DOI] [PubMed] [Google Scholar]
- 29.Pennell DJ, Porter JB, Cappellini MD, et al. Continued improvement in myocardial T2* over two years of deferasirox therapy in beta-thalassemia major patients with cardiac iron overload. Haematologica. 2011;96:48–54. doi: 10.3324/haematol.2010.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood JC, Kang BP, Thompson A, et al. The effect of deferasirox on cardiac iron in thalassemia major: impact of total body iron stores. Blood. 2010;116:537–543. doi: 10.1182/blood-2009-11-250308. [DOI] [PubMed] [Google Scholar]
- 31.Wood JC. Cardiac iron across different transfusion-dependent diseases. Blood Reviews. 2008;22:S14–S21. doi: 10.1016/S0268-960X(08)70004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonardi B, Margossian R, Colan S, Powell A. Relationship of Magnetic Resonance Imaging Estimation of Myocardial Iron to Left Ventricular Systolic and Diastolic Function in Thalassemia. JACC: Cardiovascular Imaging. 2008;1:572–578. doi: 10.1016/j.jcmg.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter JP, Alpendurada F, Deac M, et al. Right ventricular volumes and function in thalassemia major patients in the absence of myocardial iron overload. J Cardiovasc Magn Reson. 2010;12:24. doi: 10.1186/1532-429X-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westwood MA, Anderson LJ, Maceira AM, et al. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. Journal of Magnetic Resonance Imaging. 2007;25:1147–1151. doi: 10.1002/jmri.20915. [DOI] [PubMed] [Google Scholar]