Abstract
Lithium (Li) is one of the currently prescribed drugs for bipolar disorders and has many neuro-regulatory and immune-modulating properties. Because many neuro-pathological diseases including bipolar disorders have been associated with some level of inflammation, Li's effect on inflammation may have some crucial consequences. Even though Li has been shown to have proand anti-inflammatory activities in different cell models, mechanisms involved in these effects are not well understood. Moreover, Li's effect on inflammation in the presence of activators of Toll-like receptors (TLRs), especially TLR-2 (that activates MyD88-dependent pathway) and TLR-3 (that activates TRIF-dependent pathway) is not known. Here we tested the role of Li in the presence and absence of TLR2, and TLR3 on MAPK and NFκB pathways and the consequent production of tumor necrosis factor-α (TNFα) in Raw264.7 macrophages. Our results indicate that Li enhances TNFα production both in the absence and presence of TLR stimulation. Interestingly, Li differentially modulates MAPK and NFκB pathways in the absence and presence of TLR2/3 ligands. Our results further indicate that the effect of Li on TNFα occurs at the post-transcriptional level. Together, these studies demonstrate that Li induces TNFα production in macrophages and that it modulates signaling at different levels depending on the presence or absence of TLR2/3 stimulation.
Introduction
Macrophages are innate immune cells with a tremendous ability to be plastic and heterogeneous depending on the tissue environment [Murray and Wynn, 2011]. These cells act as the first line of defense against invading microorganisms and have the capability to modulate a number of different immune functions including innate and adaptive immunity. By virtue of these abilities macrophages are thought to be central players in a number of inflammatory diseases [Sica and Mantovani, 2012]. Macrophages as well as other immune cells express pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns from the microbes as well as certain endogenous protein molecules [Moresco et al., 2011]. Activation of PRRs have been linked to the pathogenesis of a number of diseases caused by microbial as well as non-microbial sources. Among the PRRs, Toll-like receptors play a critical role in inflammatory process in a diverse range of metabolic and non-metabolic diseases. Toll-like receptors (TLRs) are type-I membrane glycoprotein PRRs present on the cell membrane as well as the intracellular membrane compartments. At present, 13 TLRs have been identified in humans and mice combined [Moresco et al., 2011].
Activation of TLRs and inflammatory responses have now been recognized to be important not only for microbial infections but also for many different disease processes including neuro-pathological diseases [Dantzer et al., 2008]. The nervous system has long been shown to influence inflammatory responses via neurotransmitter release and activation of the respective receptors on the surface of immune cells. Alternatively, the immune system has also been shown to influence psychological status including mood and behavior [Dantzer et al., 2008]. Several studies have examined the relationship between inflammatory markers, including cytokines and psychiatric disorders such as major depression and schizophrenia. While studies suggest an association between major depression and enhanced pro-inflammatory cytokines such as TNFα, whether cytokines play causative role remains somewhat controversial [Kenis and Maes, 2002]. In addition, effects of anti-depressants on cytokine levels have not been consistent between different studies [Kenis and Maes, 2002]. Lithium is one such pharmacological agent that has been shown to possess both pro- and anti-inflammatory properties [Beyaert et al., 1991b; Chiu and Chuang, 2010; De Sarno et al., 2008; Kleinerman et al., 1989; Martin et al., 2005; Shenkman et al., 1978].
Lithium is a monovalent cation and is a psychotropic anti-depressant drug that has been the standard pharmacological treatment for bipolar disorder (BPD) for more than five decades [Chiu and Chuang, 2010]. Yet, the precise molecular mechanisms of its therapeutic actions remain elusive. Several in vivo and in vitro studies have demonstrated that lithium mediates a number of effects including regulation of receptors, ion transport, intracellular signaling, hormonal regulation as well as gene expression [Chiu and Chuang, 2010]. Because of its therapeutic effects, Li has been examined for its effects on various cytokines including TNFα in human monocytes as well as other immune cell models. [Kleinerman et al., 1989] [Maes et al., 1999]. [Kucharz et al., 1993; Szuster-Ciesielska et al., 2003; Wu and Yang, 1991]. How Li stimulates TNFα in macrophages and whether this could be modulated by TLR ligands other than lipopolysaccharide (LPS) is still not clear. We demonstrate here that Li-induces TNFα production post-transcriptionally and selectively via the ERK pathway. We further show that in the presence of TLR2 and TLR3 ligands, Li differentially affects MAPK and NFκB pathways and augments TNFα production via multiple MAPK and NFκB pathways.
Materials and Methods
Materials
Ultra pure LPS, PolyI:C, and Pam3CSK4 were from Invivogen. Protease inhibitor cocktail tablet was from Roche. Monoclonal anti-α-tubulin antibody (mouse IgG1 isotype) was from Sigma. pERK1/2, pJNK, pP38, IκBα, pP105 and pMEK antibodies were from Cell signaling. ERK2 antibody was from Santacruz Biotechnology. Lithium chloride and sodium chloride were from Sigma Aldrich. TNFα ELISA kit was from eBiosiences. All other reagents were of the highest quality available.
Cell Culture
Raw264.7 macrophages were from ATCC and were cultured in DMEM with 10% FBS and penicillin-streptomycin at 37 C and 5% CO2. For experiments cells were plates in 12-well plates. Twenty four hours after plating, cells were treated with NaCl or LiCl at the indicated concentrations and time points followed by TLR2 and TLR3 ligands at the indicated concentrations and time points. Samples for ELISA, Western blotting and RNA were obtained accordingly.
Western blotting
After the various treatment protocols as indicated in the figure legends, cells were quickly washed with cold PBS followed by lysis with buffer containing 1% Triton X-100, protease and phosphatase inhibitors. The lysates were then clarified and protein concentration determined using Bradford assay. Equivalent amounts of protein were loaded on the gels for Western blot analysis. Immunoblotting was performed as described previously [Patial et al., 2010]. Secondary antibodies were IR dye conjugated and analyzed by Licor's Odyssey. The bands were quantified using Licor's Odyssey program.
ELISA
TNFα levels in the culture supernatants were analyzed using ELISA kit from eBiosciences following manufacturer's recommendations and as described before [Porter et al., 2010].
RNA and Q-PCR
RNA was isolated from the cells using Qiagen RNeasy mini kit using manufacturer's protocol and as described before [Packiriswamy et al., 2013]. Reverse transcription was performed using 1 μg of RNA with the promega cDNA synthesis kit. Q-RT-PCR was performed with ABI fast 7500 (Applied biosystems) and genes were normalized to HPRT. Following primers were used: TNFα-forward:TCTCATCAGTTCTATGGCCC-3; reverse: GGGAGTAGACAAGCTACAAC; HPRT-forward: AAG CCT AAG ATG AGC GCA AG; reverse: TTA CTA GGC AGA TGG CCA CA.
Analysis of TLR expression by flow cytometry
After the respective treatments, the cells were rinsed with staining buffer (1% FBS and 0.09% sodium azide in PBS) and stained with the following antibodies: anti-TLR4 (MTS510, BD Biosciences, San Jose, CA); anti-TLR3 (11F8, Biolegend) and anti-TLR2 (6C2, eBiosciences). Data were acquired using a LSRII (BD Biosciences) and analyzed using Flowjo software (Tree Star Inc., Ashland, Oregon) as described before [Patial et al., 2011b].
Data analysis
All data are presented as the mean±SEM. Experiments were done in multiple passages. Two group comparisons were performed using Student's t-test and comparisons of more than two groups were done by ANOVA with post-Bonferonni multiple comparison test. All statistical analysis were performed using GRAPHPAD PRISM Software (San Diego, CA) and p<0.05 were considered statistically significant.
Results
Lithium increases TNFα production in mouse macrophages
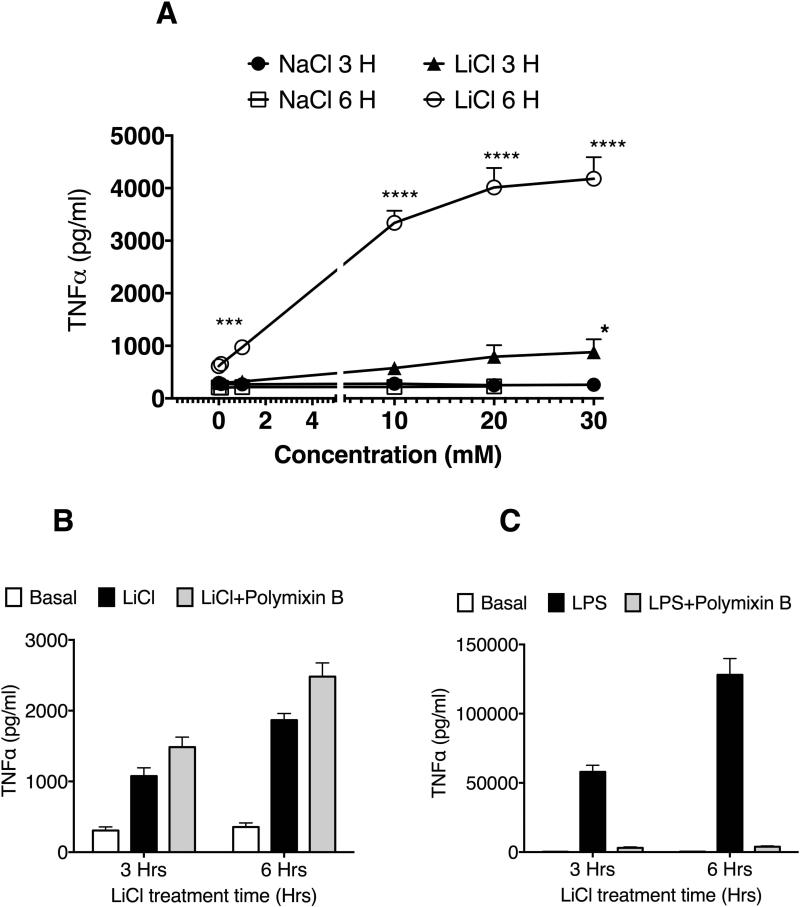
To determine the effect of LiCl on TNFα production in mouse macrophage, Raw264.7 cells were treated with control (NaCl) or LiCl at various concentrations ranging from 0.1 to 30 mM for 3 and 6 hours. LiCl but not NaCl caused a concentration- and time-dependent increase in TNFα release from Raw264.7 cells at both time points tested (Fig 1A). To rule out the presence of endotoxin in LiCl, we treated the cells with LiCl in the presence or absence of polymixin B, an endotoxin inhibitor [Duff and Atkins, 1982]. As shown in Fig 1B, Polymixin B did not affect LiCl's effect on TNFα production either at 3 or 6 hours. Polymixin-B however completely abrogated LPS-induced TNFα production, confirming Polymixin-B's effect in our system (Fig 1C).
Figure 1. Lithium induces a concentration-dependent increase in TNFα production in Raw264.7 macrophages.
Cells were plated in 12 well plates at 1 million cells/well. Twenty four-hours after plating, cells were treated with LiCl or NaCl with the indicated concentrations and time. At the end of the treatment, the supernatants were collected and assayed for TNFα using ELISA kit from eBiosciences. In B and C, cells were treated with polymixin B (50 μg/ml) along with NaCl, LiCl or LPS. N=6. **P<0.01; ***P<0.001; ****P<0.0001; compared between the respective concentrations of LiCl and NaCl.
Lithium enhances TLR-induced TNFα production in mouse macrophages
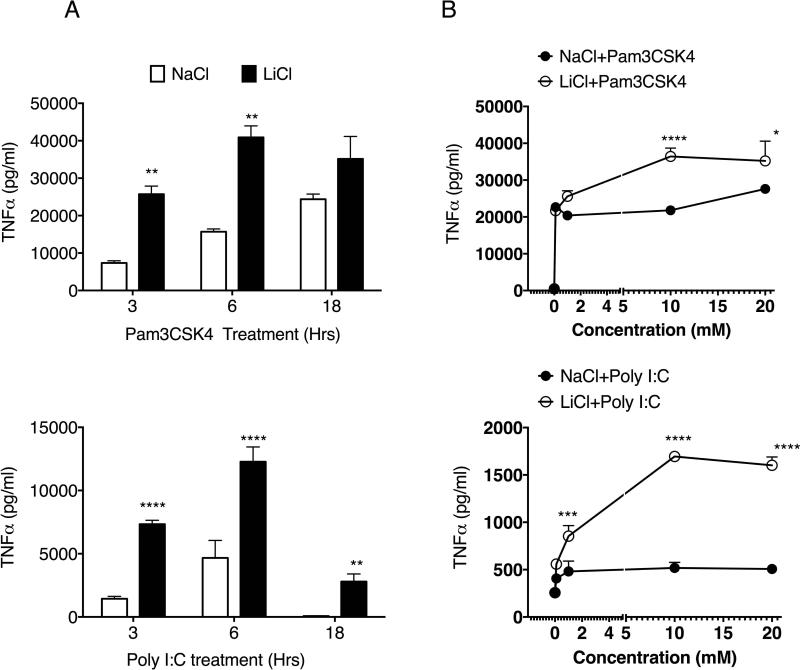
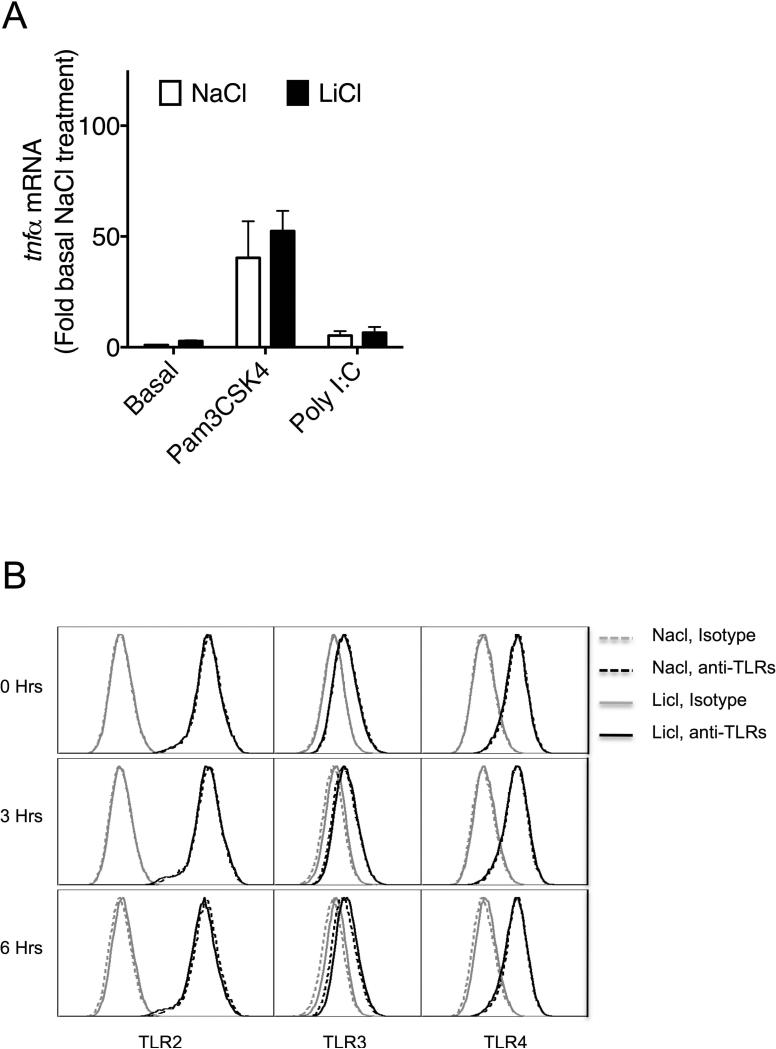
We then tested the effect of LiCl in the presence of TLR2 and TLR3 ligands (Pam3CSK4 and Poly I:C, respectively). Activation of both of these receptors normally stimulates signaling via Myd88-and TRIF-dependent pathways, respectively [Kenny and O'Neill, 2008]. We pretreated Raw264.7 macrophages with NaCl/LiCl for 6 hours and then stimulated the cells with the two TLR ligands for the various times as shown. Pretreatment with LiCl significantly enhanced Pam3CSK4- and Poly I:C-induced TNFα production (Fig 2A). We also pretreated cells with various concentrations of NaCl/LiCl for 6 hours and then stimulated with the two TLR ligands for another 6 hours. As shown in Fig 2B, LiCl enhanced Pam3CSK4- and Poly I:C-induced TNFα production significantly in a dose-dependent manner. Importantly, increasing concentrations of NaCl did not influence TLR2 or TLR3-induced TNFα production. To further determine if the effect of LiCl (without or with TLR stimulation) on TNFα production is mediated at the level of transcription, we examined the mRNA expression of TNFα subsequent to LiCl (±TLR2 or TLR3) treatment. As shown in Figure 3A, TNFα expression was not significantly modulated by LiCl either in the absence or presence of TLR stimulation.
Figure 2. Lithium pretreatment enhances TLR-2 and TLR3-induced TNFα production in mouse macrophages.
Raw264.7 cells were plated in 12-well plate as indicated in Figure 1. In A, Cells were pretreated with NaCl or LiCl (30 mM) for 6 hours and then treated with Pam3CSK4 (1 μg/ml) or Poly I:C (μg/ml) for the indicated time points. Culture supernatants were then assayed for TNFα using ELISA. In B, cells were treated with NaCl/LiCl for 6 hours with the various concentrations as shown and then treated with Pam3CSK4 (1 μg/ml) or Poly I:C (1 μg/ml). Supernatants were then assayed for TNFα using ELSIA.N=3-8 for A and N=6 for B. *P<0.05; **P<0.01; ***P<0.001; compared between the respective concentrations of LICL NaCl.
Figure 3. Lithium's effect on TNFα in mouse macrophages is at the post-transcriptional level and downstream of TLR expression.
(A) Raw264.7 cells were pretreated with NaCl or LiCl (10 mM) for 6 hours followed by treatment TLR ligands as indicated for additional 6 hours. Cells were then processed for RNA extraction and TNFα mRNA expression determined by real time QPCR as described before [Loniewski et al., 2007].(B) Raw264.7 macrophages were treated with NaCl or LiCl (30 mM) for 3 or 6 hours and the expression levels of the different TLRs examined by flow cytometry. A representative experiment from two such experiments is shown
LiCl has been shown to mediate its effects via a number of different biochemical mechanisms, the most prominent one being inhibition of GSK3β [Chiu and Chuang, 2010]. To determine if the effect of LiCl could be mediated via inhibition of GSK3β, we pretreated macrophages with SB216763 (another potent and a more selective GSK3β inhibitor [Coghlan et al., 2000]). SB216763 neither affected basal TNFα production nor TLR-induced TNFα production suggesting that the effect of LiCl on TNFα production is likely independent of GSK3β inhibition (data not shown).
Signaling mechanisms regulating Lithium-induced TNFα production
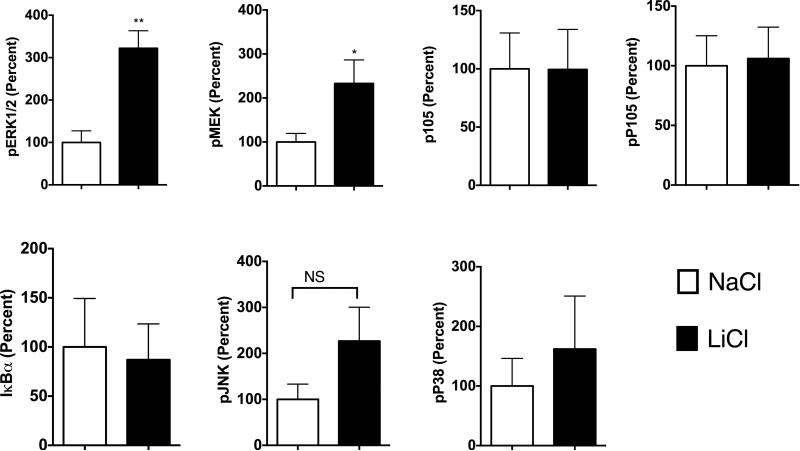
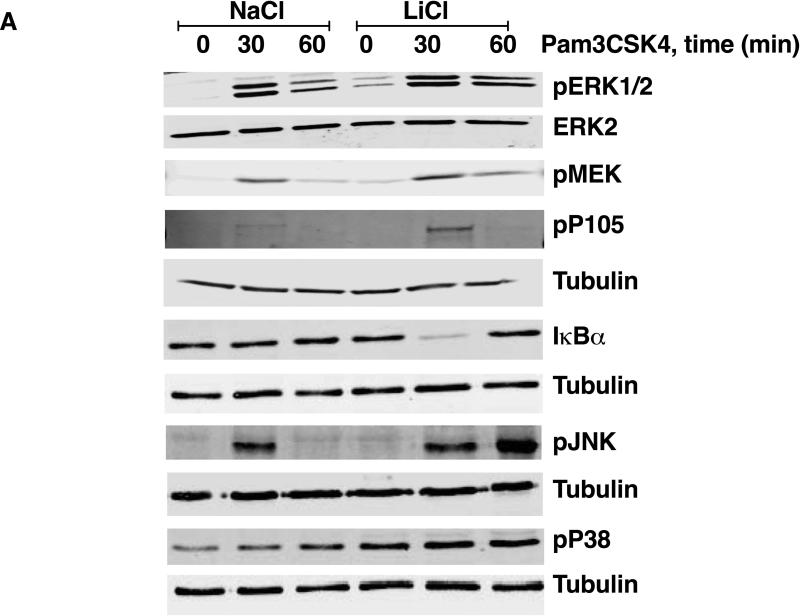
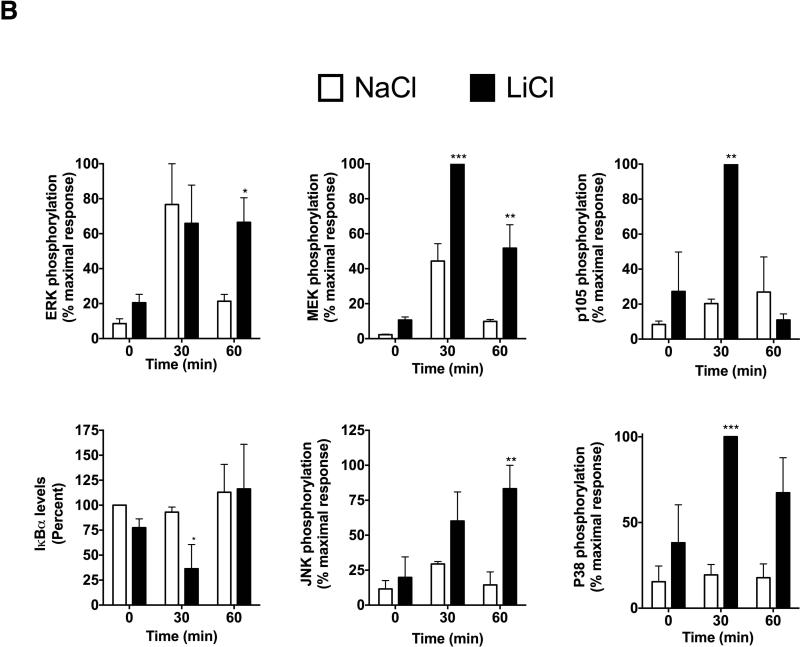
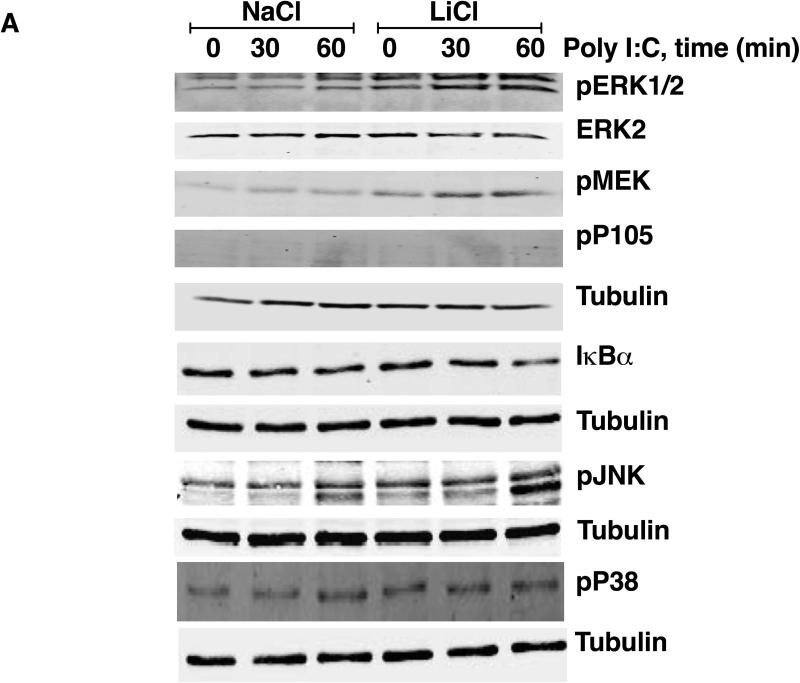
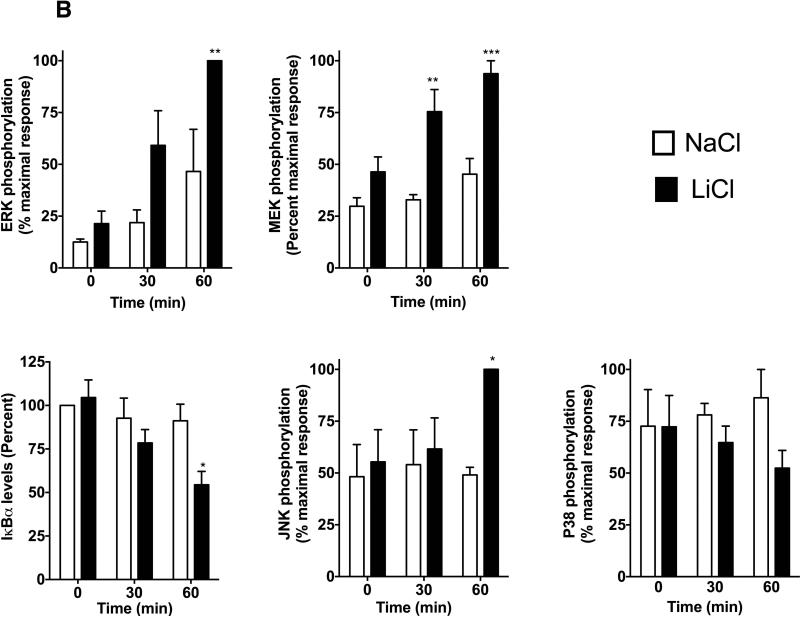
To begin to understand the mechanisms by which LiCl affects TNFα production, we first tested whether LiCl pretreatment enhances the expression levels of TLRs. As shown in Fig 3B, LiCl treatment did not affect the expression levels of any of the TLRs tested including TLR2, TLR3 and TLR4, as determined by flow cytometry. Because the levels of these receptors did not change with LiCl treatment, we focused on the cell signaling pathways stimulated by these TLRs, in the context of TNFα production. Previous studies have shown that TLR-induced ERK pathway plays an important role in the regulation of TNFα production [Coghlan et al., 2000]. Treatment of Raw264.7 macrophages with LiCl significantly induced ERK and MEK phosphorylation (Fig 4). LiCl treatment did not affect either p105 phosphorylation or p105 degradation (one of the upstream regulators of MEK-ERK pathway, especially critical in TLR-induced ERK activation in macrophages) [Gantke et al., 2012; Patial et al., 2011a]. Also, as shown in Fig 4, LiCl did not affect the levels of pP38, pJNK or IκBα.
Figure 4. Lithium induces pERK and pMEK levels in Raw264.7 macrophages.
Raw264.7 macrophages were treated with NaCl or LiCl (30 mM) for 6 hours and the cells were collected and Western blotting performed for the various proteins as shown. N=5. *P<0.05 and **P<0.01 compared to NaCl.
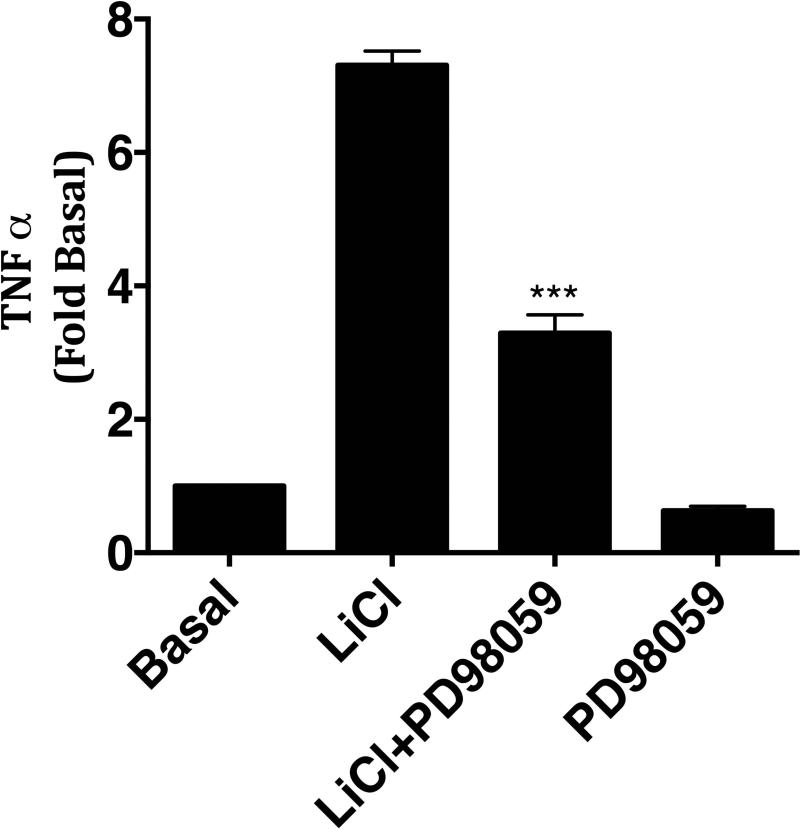
To further confirm that the effect of LiCl on TNFα production is mediated via ERK pathway, we pretreated Raw264.7 macrophages with LiCl in the presence or absence of ERK inhibitor, PD98059 [Davies et al., 2000]. As shown in Fig 5, PD98059 significantly blocked LiCl-induced TNFα production demonstrating that LiCl-induced TNFα is at least in part mediated via ERK activation. Together these data suggest that the effect of Lithium on TNFα production in the absence of TLR ligands occurs in part via regulation of MEK-ERK pathway.
Figure 5. Lithium-induced TNFα production is inhibited by PD98059 in Raw264.7 macrophages.
Raw264.7 cells were treated with LiCl (30 mM) in the presence or absence of MEK-ERK inhibitor, PD98059 (10 μM), for 6 hours and then culture supernatants assayed for TNFα by ELISA. N=3. ***P<0.001 compared to LiCl treatment
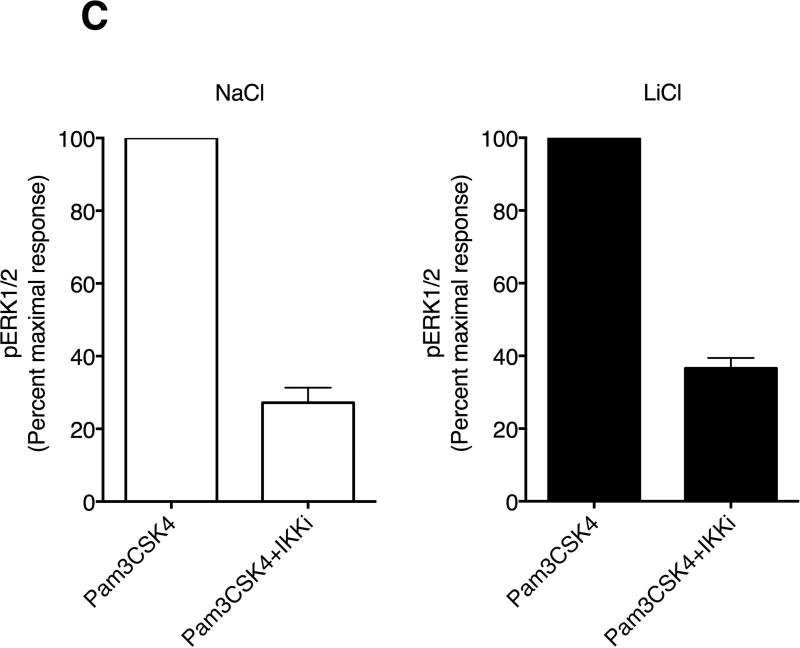
To understand the mechanisms by which LiCl regulates TLR-induced TNFα production, we examined p105-MEK-ERK pathway as well as JNK, p38 and IκBα pathways. LiCl pretreatment significantly enhanced the levels of Pam3CSK4-induced phospho-P105-MEK-ERK pathway compared to the controls (Fig 6). To confirm that p105 phosphorylation is upstream of ERK pathway, we pretreated cells with IKKβ inhibitor prior to Pam3CSK4±LiCl treatment. P105 is phosphorylated by IKKβ and thus inhibition of IKKβ has been shown to abrogate TLR-induced ERK activation [Gantke et al., 2012; Patial et al., 2011a]. As shown in Fig 6C, IKKβ inhibitor (IKK-2 inhibitor IV) significantly blocked TLR2-induced ERK activation both in the absence and presence of LiCl. In addition to the ERK pathway, LiCl pretreatment also enhanced pJNK levels induced by Pam3CSK4. Although IκBα and pP38 levels did not markedly change with Pam3CSK4 alone at the time points tested, LiCl pretreatment significantly enhanced IκBα degradation and pP38 levels.
Figure 6. Lithium pretreatment enhances TLR2-stimulated signaling pathways in mouse macrophages.
Raw264.7 macrophages were plated as described in Fig 1 and then treated with NaCl or LiCl (30 mM) for 6 hours followed by treatment with Pam3CSK4 (1 μg/ml) for the indicated time points. Cells were then processed and Western blotting performed for the various proteins as shown (A and B). N=3. *P<0.05; **P<0.01; ***P<0.001; compared between NaCl and LiCl at the respective time points. In C, effect of IKKβ inhibitor (IKK-2 inhibitor IV, Calbiochem) on Pam3CSK4-induced pERK levels is shown. Cells were treated with NaCl or LiCl followed by IKKβ inhibitor prior to stimulation with Pam3CSK4. PhosphoERK levels were analyzed by Licor Odyss y as described in the methods. N=3.
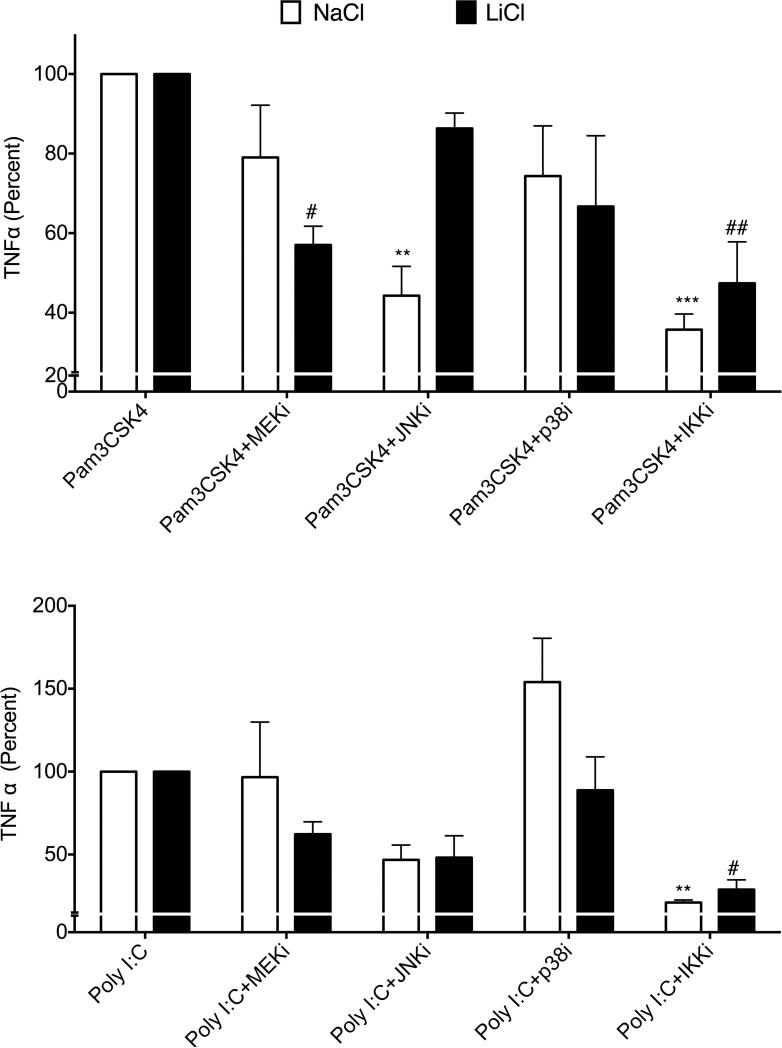
Unlike LiCl's effect on Pam3CSK4-induced signaling, Poly I:C-induced signaling was differentially affected by LiCl. In the absence of LiCl, PolyI:C increased MEK-ERK phosphorylation but did not markedly increase pP105, pP38, pJNK or IκBα degradation at the time points tested (Fig 7). Compared to these effects, LiCl pretreatment significantly enhanced PolyI:C-induced MEK-ERK phosphorylation, JNK phosphorylation as well as IκBα degradation (Fig 7) but did not affect either p38 or p105 phosphorylation. Together these results suggest that LiCl differentially affects TLR2 and TLR3 induced signaling pathways. Thus effects of Li by itself and in the presence of TLR ligands are likely to be different in terms of the signaling mechanisms and the consequent TNFα production that it regulates. To further test the role of these signaling molecules in TNFα production induced by TLR2 or TLR3 in the absence or presence of LiCl, we used selective inhibitors of ERK (PD98059), JNK (SP600125), p38 (SB203580) and IKKβ (IKK-2 inhibitor IV). Interestingly, in the absence of LiCl, JNK and IKKβ inhibitors significantly blocked Pam3CSK4-induced TNFα production, whereas in the presence of LiCl, MEK and IKKβ inhibitors markedly blocked Pam3CSK4-induced TNFα production (Fig 8). Poly I:C-induced TNFα production was inhibited only by the IKKβ inhibitor both in the absence or presence of LiCl (Fig 8). These results suggest that TNFα production is differentially regulated by Pam3CSK4 and Poly I:C in the presence and absence of LiCl.
Figure 7. Lithium pretreatment enhances TLR3-stimulated signaling pathways in mouse macrophages.
Cells were plated and treated with NaCl or LiCl for 6 hours as indicated in Fig 6 and then treated with Poly I:C (1 μg/ml) for the indicated time points. Cells were then processed and Western blotting performed for the various proteins as shown. N=3. *P<0.05; **P<0.01; ***P<0.001; compared between NaCl and LiCl at the respective time points.
Figure 8. Differential effects of MAPK and IKKβ inhibitors on TLR2- and TLR3- induced TNFα production in the absence and presence of LiCl.
Raw cells were plated and treated with NaCl or LiCl (10 mM) for 6 hours followed by the indicated inhibitors 30 min prior to stimulation with Pam3CSK4 (A) or Poly I:C (B) for an additional 6 hours. TNFα levels were determined in the culture supernatants using ELISA as described in the methods. TNFα levels in pg/ml were as follows: Pam3CSK4+NaCl= 21962±4035; Pam3CSK4+LiCl= 41994±3121 and Poly I:C+NaCl= 3397±1102; Poly I:C+LiCl= 21226±6476. N=3. Data were normalized to percent TLR stimulation with or without LiCl and analyzed by ANOVA with post-Bonferroni. **P<0.001; **P<0.0001 compared to TLR ligand treatment with NaCl. #P<0.05; ##P<0.01 compared to TLR ligand treatment with LiCl
Discussion
Lithium is a widely used pharmaceutical in the treatment of neurological diseases such as bipolar disorder. While neurological disorders themselves have been linked to higher levels of inflammatory cytokines, there have been a number of studies examining the role of anti-depressants as well as other neuro-regulatory agents in modulating inflammatory cytokines. In particular role of LiCl in modulating inflammatory cytokines has been examined with variable results depending on the cells used as well as the co-stimulating agents used. For example, using human monocytes, Kleinerman et al [Kleinerman et al., 1989] showed that LiCl stimulates TNFα but not IL-1 secretion. Even though other studies have shown no change in TNFα secretion in the presence of LiCl alone [Maes et al., 1999; Nahman et al., 2012] our studies confirm the basic premise that LiCl indeed is capable of stimulating TNFα secretion from mouse macrophages. Our data also indicate that this occurs at the reported therapeutic concentrations of LiCl [Chiu and Chuang, 2010], suggesting that patients on LiCl, at least acutely are likely able to stimulate TNFα in macrophages. There is clinical evidence that LiCl is able to modulate psoriasis in patients since, in lithium treated patients, psoriasis is triggered or aggravated as a side effect and that Li and TNF are able to synergistically induce IL6 that could play a key role in psoriasis [Beyaert et al., 1991a; Beyaert et al., 1992]. Even though how Li's effect on TNFα could directly influence bipolar treatment outcome is not known, studies have shown that cytokines from the systemic circulation can cross the blood brain barrier to influence the brain [McCusker and Kelley, 2013]. Additionally, systemic cytokines have also been shown to affect the integrity of the blood brain barrier [McCusker and Kelley, 2013]. Li could also immune-modulate glial cells in the nervous system [Nahman et al., 2012]. Together these effects could affect the treatment outcomes.
Our studies suggest that Li induces TNFα production in part via the ERK pathway. Our data also provides evidence that the increase in TNFα is mediated at the post-transcriptional level. TNFα production has previously been shown to be regulated at transcriptional and post-transcriptional levels involving mechanisms such as mRNA stability [Clark, 2000; Dumitru et al., 2000; Kim et al., 2011; Rousseau et al., 2008; Shapira et al., 1994]. Data presented here also confirms that LiCl activates MEK-ERK pathway at or above the level of MEK phosphorylation. Although the mechanisms involved in how LiCl activates MEK is not clear, we have ruled out the role of GSK3 since inhibition of GSK3 using a more selective inhibitor of GSK3 did not induce TNFα production. Further confirming that our results are independent of GSK3β inhibition, are studies showing that TLR-induced TNFα production is diminished in macrophages from GSK3β knockout mice [Martin et al., 2005]. Even though the precise mechanism by which Li modulates ERK pathway is not clear, other studies have shown that Li can influence the ERK pathway in different cell types [Ghribi et al., 2003; Kim et al., 2012; Tsui et al., 2012; Yan et al., 2007]. For example, lithium has been shown to regulate hippocampal neurogenesis via the ERK pathway [Yan et al., 2007]. Interestingly, in SHSY5Y cells, while lithium was shown to increase ERK phosphorylation with wild type DUSP (dual specificity phosphatase), transfection with SNP variants of DUSP6 (dual specificity phosphatase) significantly decreased lithium's effect on ERK phosphorylation[Kim et al., 2012]. In our studies we have ruled out the role of p105, one of the upstream regulators of the ERK pathway [Zhang et al., 2009]. Since Li appears to regulate the ERK at the level of MEK, it is possible that Li might influence phosphatases that regulate this pathway [Chiu and Chuang, 2010; Kim et al., 2012; Zhen et al., 2002].
Li has been shown to have a number of different cellular and biochemical effects that are both dependent and independent of GSK3β inhibition [Chiu and Chuang, 2010]. Even though Li is being widely used as an inhibitor of GSK3β, recent studies have demonstrated that Li has other biochemical effects including inhibition of NMDA receptor-mediated calcium influx, inhibition of various phosphoinositol phosphatases including inositol polyphosphate-1-phosphatase and inositol monophosphatase [Chiu and Chuang, 2010]. In addition to direct inhibition of GSK3β activity, Li has also been shown to indirectly inhibit GSK3β activity via mechanisms such as disruption of β-arrestin-2 signaling complex with PP2A that consequently activates Akt phosphorylation and inhibition of GSK3 [Beaulieu et al., 2008]. One study [Yanagita et al., 2007] found that Lithium selectively inhibits Na+ influx via Na+ channels and subsequent Ca2+ influx and catecholamine secretion independent of GSK3β inhibition, in adrenal chromaffin cells. While many of these effects have been shown in neuronal cell types, mechanisms that regulate inflammatory cytokines such as TNFα in immune cells are not well understood.
One study recently found that treatment of bone-marrow-derived macrophages with Li induces degradation of p105 that subsequently leads to inhibition of LPS-induced ERK pathway and therefore LPS-induced TNFα transcription [Zhang et al., 2009]. Our studies in Raw264.7 macrophages however, suggest that p105 is not regulated by Lithium treatment even after 18 hours of treatment (data not shown). While the reasons for the differences in mechanisms are not clear, macrophages are highly heterogeneous and therefore, different sources of macrophages will likely exhibit different mechanisms of p105 regulation. Interestingly, our studies suggest that LiCl not only regulates TNFα production by itself, it also modulates TNFα production stimulated by two different TLR ligands. The mechanism by which this occurs however is different between the two ligands. This could be because TLR2 activates MyD88-dependent pathway whereas TLR3 activates TRIF-dependent pathway. Whereas TLR2-induced ERK activation is regulated by IKKβ-P105-dependent pathway, TLR3-induced ERK pathway appears to be independent of p105 pathway. Other signaling pathways such as the Ras-Raf pathway has also been shown to be important regulators of the MEK-ERK pathway [Geppert et al., 1994] and thus TLR3-induced ERK activation could be mediated via these mechanisms. Surprisingly, pretreatment of LiCl enhanced all of the major MAPK and NFκB pathways induced by Pam3CSK4 and PolyI:C in these macrophages with particular differences between TLR2 and TLR3 on p105 and p38 phosphorylation. Even though IκBα degradation itself was not evident at the time points tested with TLR2 or TLR3 ligands in the absence of LiCl, IKKβ inhibitor blocked TNFα production in the absence of LiCl with both ligands. IKKβ mediates NFκB via several other IκBs including p105 and thus TLR2 and TLR3-induced TNFα production may be mediated via IκBs independent of IκBα. Furthermore, it is possible that IκBα degradation by TLR2/3 in the absence of LiCl occurred at the time points we did not test. Interestingly function of MEK-ERK and JNK pathways seem to be different in their role in mediating TNFα production in the absence and presence of LiCl especially with TLR2 stimulation. Taken together our studies suggest that LiCl may selectively modulate certain signalosomes of the TLR2 and TLR3 signaling pathways and mediate TNFα production in macrophages.
Li by virtue of also being a GSK3 inhibitor has been used in other settings or diseases where GSK3β has been shown to play an important role. For example, Lithium has been shown to be effective in preventing and suppressing experimental autoimmune encephalomyelitis [De Sarno et al., 2008]. In addition it has been shown to reduce disease severity in other models of diseases including sepsis [Wang et al., 2009], arthritis [Cuzzocrea et al., 2006], colitis [Whittle et al., 2006] and atherosclerosis [Choi et al., 2010]. In addition, lithium has been used as a therapeutic target for bipolar disorder in humans [Chiu and Chuang, 2010]. Thus while lithium may have other effects in addition to GSK3β, its effects on GSK3β has been therapeutically useful. However, understanding how these additional effects of lithium occurs will help further understand some of the side effects of lithium in patients. While a number of studies have examined the role of LiCl in modulating LPS-induced signaling in macrophages as well as other cell types [Kaufmann et al., 2011; Maes et al., 1999; Nahman et al., 2012; Zhang et al., 2009], our studies here in macrophages are the first to examine the role of LiCl in modulating TLR2 and TLR3 signaling. Although the end result on TNFα production is similar between TLR2 and TLR3 activation, the signaling mechanisms modulated by LiCl in these two signal transduction pathways are slightly different. Further elucidation and understanding of these biochemical mechanisms will help us better target neurological diseases without the side effects.
Acknowledgement
We gratefully acknowledge the support from NIH (grants HL095637, AR055726 and AR056680 to N.P.). We would also like to thank Ms. Saloni Shah (Okemos High School Student, Okemos, Michigan) for technical help.
Footnotes
The authors declare no conflict of interest.
References
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–36. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beyaert R, De Potter C, Vanhaesebroeck B, Van Roy F, Fiers W. Induction of inflammatory cell infiltration and necrosis in normal mouse skin by the combined treatment of tumor necrosis factor and lithium chloride. Am J Pathol. 1991a;138:727–39. [PMC free article] [PubMed] [Google Scholar]
- Beyaert R, Schulze-Osthoff K, Van Roy F, Fiers W. Lithium chloride potentiates tumor necrosis factor-induced and interleukin 1-induced cytokine and cytokine receptor expression. Cytokine. 1991b;3:284–91. doi: 10.1016/1043-4666(91)90496-z. [DOI] [PubMed] [Google Scholar]
- Beyaert R, Schulze-Osthoff K, Van Roy F, Fiers W. Synergistic induction of interleukin-6 by tumor necrosis factor and lithium chloride in mice: possible role in the triggering and exacerbation of psoriasis by lithium treatment. Eur J Immunol. 1992;22:2181–4. doi: 10.1002/eji.1830220835. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Chuang DM. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SE, Jang HJ, Kang Y, Jung JG, Han SJ, Kim HJ, Kim DJ, Lee KW. Atherosclerosis induced by a high-fat diet is alleviated by lithium chloride via reduction of VCAM expression in ApoE-deficient mice. Vascul Pharmacol. 2010;53:264–72. doi: 10.1016/j.vph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Clark A. Post-transcriptional regulation of pro-inflammatory gene expression. Arthritis Res. 2000;2:172–4. doi: 10.1186/ar83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Crisafulli C, Mazzon E, Esposito E, Muia C, Abdelrahman M, Di Paola R, Thiemermann C. Inhibition of glycogen synthase kinase-3beta attenuates the development of carrageenan-induced lung injury in mice. Br J Pharmacol. 2006;149:687–702. doi: 10.1038/sj.bjp.0706902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2008;181:338–45. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff GW, Atkins E. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J Immunol Methods. 1982;52:333–40. doi: 10.1016/0022-1759(82)90005-9. [DOI] [PubMed] [Google Scholar]
- Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Gantke T, Sriskantharajah S, Sadowski M, Ley SC. IkappaB kinase regulation of the TPL-2/ERK MAPK pathway. Immunol Rev. 2012;246:168–82. doi: 10.1111/j.1600-065X.2012.01104.x. [DOI] [PubMed] [Google Scholar]
- Geppert TD, Whitehurst CE, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1:93–103. [PMC free article] [PubMed] [Google Scholar]
- Ghribi O, Prammonjago P, Herman MM, Spaulding NK, Savory J. Abeta(1-42)-induced JNK and ERK activation in rabbit hippocampus is differentially regulated by lithium but is not involved in the phosphorylation of tau. Brain Res Mol Brain Res. 2003;119:201–6. doi: 10.1016/j.molbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kaufmann L, Marinescu G, Nazarenko I, Thiele W, Oberle C, Sleeman J, Blattner C. LiCl induces TNF-alpha and FasL production, thereby stimulating apoptosis in cancer cells. Cell Commun Signal. 2011;9:15. doi: 10.1186/1478-811X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–12. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kenny EF, O'Neill LA. Signalling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–9. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Kim JK, Lee SM, Suk K, Lee WH. A novel pathway responsible for lipopolysaccharideinduced translational regulation of TNF-alpha and IL-6 expression involves protein kinase C and fascin. J Immunol. 2011;187:6327–34. doi: 10.4049/jimmunol.1100612. [DOI] [PubMed] [Google Scholar]
- Kim SH, Shin SY, Lee KY, Joo EJ, Song JY, Ahn YM, Lee YH, Kim YS. The genetic association of DUSP6 with bipolar disorder and its effect on ERK activity. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:41–9. doi: 10.1016/j.pnpbp.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Kleinerman ES, Knowles RD, Blick MB, Zwelling LA. Lithium chloride stimulates human monocytes to secrete tumor necrosis factor/cachectin. J Leukoc Biol. 1989;46:484–92. doi: 10.1002/jlb.46.5.484. [DOI] [PubMed] [Google Scholar]
- Kucharz EJ, Sierakowski SJ, Goodwin JS. Lithium in vitro enhances interleukin-2 production by T cells from patients with systemic lupus erythematosus. Immunopharmacol Immunotoxicol. 1993;15:515–23. doi: 10.3109/08923979309019728. [DOI] [PubMed] [Google Scholar]
- Loniewski KJ, Patial S, Parameswaran N. Sensitivity of TLR4- and -7-induced NF kappa B1 p105-TPL2-ERK pathway to TNF-receptor-associated-factor-6 revealed by RNAi in mouse macrophages. Mol Immunol. 2007;44:3715–23. doi: 10.1016/j.molimm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin AH, Pioli R, Kenis G, Kubera M, Bosmans E. In vitro immunoregulatory effects of lithium in healthy volunteers. Psychopharmacology (Berl) 1999;143:401–7. doi: 10.1007/s002130050965. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–84. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker RH, Kelley KW. Immune-neural connections: how the immune system's response to infectious agents influences behavior. J Exp Biol. 2013;216:84–98. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–93. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahman S, Belmaker RH, Azab AN. Effects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cells. Innate Immun. 2012;18:447–58. doi: 10.1177/1753425911421512. [DOI] [PubMed] [Google Scholar]
- Packiriswamy N, Lee T, Raghavendra PB, Durairaj H, Wang H, Parameswaran N. G-Protein-Coupled Receptor Kinase-5 Mediates Inflammation but Does Not Regulate Cellular Infiltration or Bacterial Load in a Polymicrobial Sepsis Model in Mice. J Innate Immun. 2013 doi: 10.1159/000347002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. G-protein-coupled-receptor kinases mediate TNFalpha-induced NFkappaB signalling via direct interaction with and phosphorylation of IkappaBalpha. Biochem J. 2010;425:169–78. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Saini Y, Parvataneni S, Appledorn DM, Dorn GW, 2nd, Lapres JJ, Amalfitano A, Senagore P, Parameswaran N. Myeloid-specific GPCR kinase-2 negatively regulates NF-kappaB1p105-ERK pathway and limits endotoxemic shock in mice. J Cell Physiol. 2011a;226:627–37. doi: 10.1002/jcp.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Shahi S, Saini Y, Lee T, Packiriswamy N, Appledorn DM, Lapres JJ, Amalfitano A, Parameswaran N. G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced NFkappaB activation in primary macrophages and modulates inflammation in vivo in mice. J Cell Physiol. 2011b;226:1323–33. doi: 10.1002/jcp.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KJ, Gonipeta B, Parvataneni S, Appledorn DM, Patial S, Sharma D, Gangur V, Amalfitano A, Parameswaran N. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by beta-arrestins. J Cell Physiol. 2010;225:406–16. doi: 10.1002/jcp.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau S, Papoutsopoulou M, Symons A, Cook D, Lucocq JM, Prescott AR, O'Garra A, Ley SC, Cohen P. TPL2-mediated activation of ERK1 and ERK2 regulates the processing of pre-TNF alpha in LPS-stimulated macrophages. J Cell Sci. 2008;121:149–54. doi: 10.1242/jcs.018671. [DOI] [PubMed] [Google Scholar]
- Shapira L, Takashiba S, Champagne C, Amar S, Van Dyke TE. Involvement of protein kinase C and protein tyrosine kinase in lipopolysaccharide-induced TNF-alpha and IL-1 beta production by human monocytes. J Immunol. 1994;153:1818–24. [PubMed] [Google Scholar]
- Shenkman L, Borkowsky W, Holzman RS, Shopsin B. Enhancement of lymphocyte and macrophage function in vitro by lithium chloride. Clin Immunol Immunopathol. 1978;10:187–92. doi: 10.1016/0090-1229(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuster-Ciesielska A, Tustanowska-Stachura A, Slotwinska M, Marmurowska-Michalowska H, Kandefer-Szerszen M. In vitro immunoregulatory effects of antidepressants in healthy volunteers. Pol J Pharmacol. 2003;55:353–62. [PubMed] [Google Scholar]
- Tsui MM, Tai WC, Wong WY, Hsiao WL. Selective G2/M arrest in a p53(Val135)-transformed cell line induced by lithium is mediated through an intricate network of MAPK and beta-catenin signaling pathways. Life Sci. 2012;91:312–21. doi: 10.1016/j.lfs.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang WC, Wang CY, Tsai CC, Chen CL, Chang YT, Kai JI, Lin CF. Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. Br J Pharmacol. 2009;157:1004–13. doi: 10.1111/j.1476-5381.2009.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle BJ, Varga C, Posa A, Molnar A, Collin M, Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta. Br J Pharmacol. 2006;147:575–82. doi: 10.1038/sj.bjp.0706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Yang XH. Enhancement of interleukin 2 production in human and Gibbon T cells after in vitro treatment with lithium. Proc Soc Exp Biol Med. 1991;198:620–4. doi: 10.3181/00379727-198-43298. [DOI] [PubMed] [Google Scholar]
- Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–95. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Yanagita T, Maruta T, Uezono Y, Satoh S, Yoshikawa N, Nemoto T, Kobayashi H, Wada A. Lithium inhibits function of voltage-dependent sodium channels and catecholamine secretion independent of glycogen synthase kinase-3 in adrenal chromaffin cells. Neuropharmacology. 2007;53:881–9. doi: 10.1016/j.neuropharm.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Zhang M, Jin W, Zhou X, Yu J, Lee AJ, Sun SC. Deregulation of Tpl2 and NF-kappaB signaling and induction of macrophage apoptosis by the anti-depressant drug lithium. Cell Signal. 2009;21:559–66. doi: 10.1016/j.cellsig.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Torres C, Friedman E. Lithium regulates protein tyrosine phosphatase activity in vitro and in vivo. Psychopharmacology (Berl) 2002;162:379–84. doi: 10.1007/s00213-002-1126-y. [DOI] [PubMed] [Google Scholar]