Abstract
The objective of this study was to evaluate the relationship between environmental arsenic exposure and serum matrix metalloproteinase (MMP)-9, a biomarker associated with cardiovascular disease and cancer. In a cross-sectional study of residents of Arizona, USA (n=215) and Sonora, Mexico (n=163), drinking water was assayed for total arsenic, and daily drinking water arsenic intake estimated. Urine was speciated for arsenic and concentrations were adjusted for specific gravity. Serum was analyzed for MMP-9 using ELISA. Mixed model linear regression was used to assess the relation among drinking water arsenic concentration, drinking water arsenic intake, urinary arsenic sum of species (the sum of arsenite, arsenate, monomethylarsonic acid and dimethylarsinic acid), and MMP-9, controlling for autocorrelation within households. Drinking water arsenic concentration and intake were positively associated with MMP-9, both in crude analysis and after adjustment for gender, country/ethnicity, age, body mass index, current smoking and diabetes. Urinary arsenic sum of species was positively associated with MMP-9 in multivariable analysis only. Using Akaike’s Information Criterion, arsenic concentration in drinking water provided a better fitting model of MMP-9, than either urinary arsenic or drinking water arsenic intake. In conclusion, arsenic exposure was positively associated with MMP-9 using all three exposure metrics evaluated.
Keywords: arsenic, matrix metalloproteinase-9, drinking water, urine, binational study
Introduction
Arsenic exposure is linked to a variety of illnesses including cardiovascular disease (1-5), cancer (1, 2, 5-7), respiratory disease (8-10), diabetes (11, 12) and other diseases (13, 14). However, potential toxic mechanisms by which arsenic exposure causes chronic disease are not well understood.
Proposed mechanisms of arsenic toxicity, with a particular emphasis on cancer, include genotoxicity, oxidative stress, altered gene expression, and changes in cell cycle control, differentiation and apoptosis (15). In cellular and non-human animal models, a partial list of biomarkers of arsenic effect have included down-regulation of antioxidant gene expression (16), altered microRNA expression (17), decreased protein methylation (18), enzyme deactivation (19), altered protein concentrations (20) and altered wound healing (21). Information on biomarkers of effect in humans is more limited. Studies of the effects of ingested arsenic on human peripheral blood lymphocytes have demonstrated a wide array of modifications in gene expression (22), cytogenetic changes (23, 24), and decreased DNA repair (25). Arsenic exposure in drinking water has also been associated with reduced concentration of soluble receptor for advanced glycation end products (sRAGE) in sputum (20).
Matrix metalloproteinase 9 (MMP-9), also known as Collagenase Type IV-B, Collagenase Type IV, 92-Kd, and Gelatinase B, is a secreted zinc metalloprotease which degrades collagen in the extracellular matrix. Many studies link MMP-9 with cardiovascular disease (26-30), cancer (31-34), chronic respiratory disease (35, 36) and diabetes (37, 38). In earlier work, we demonstrated arsenic-related alterations in MMP-9 in cultured lung cells (21) and changes in MMP-9/tissue inhibitor of metalloproteinase-1 concentrations in sputum of arsenic-exposed human populations in Arizona (39).
Studies in the southwestern United States (Arizona) and the contiguous state of Sonora in Mexico identified populations exposed to arsenic in drinking water at concentrations exceeding the current US Environmental Protection Agency (EPA) maximum contaminant level (MCL) of 10 μg/L (39, 40). We hypothesized that among residents in communities predominantly exposed to low to moderate drinking water arsenic concentrations (< 50 ppb), increased arsenic exposure would be accompanied by higher levels of serum MMP-9. A relationship between arsenic exposure and serum MMP-9 would provide a potential mechanism for arsenic-induced chronic disease, including but not limited to cardiovascular disease and cancer. We carried out the present study in Arizona and Sonora to test this hypothesis.
Methods
The study was approved by the University of Arizona Institutional Review Board and all subjects provided informed consent. Detailed methods are described elsewhere (41). In brief, communities with relatively high and low water arsenic levels in Arizona and Sonora were selected for a cross-sectional study and households within these communities randomly selected from within census tracts and neighborhoods, respectively. Subjects were recruited using random digit dial telephone calls in Arizona and door-to-door visits in Sonora. Eligible households had at least one individual 18 years or older who had resided in the household for at least the previous year. Participants completed questionnaires in English or Spanish addressing residential, occupational and health histories, water sources and water usage. Water samples from all reported drinking water sources (e.g., indoor and outdoor tap, well, filtered, bottled, etc.), first morning void urine samples and blood samples were collected, and height and weight were measured.
Water and urine samples were stored at −20°C and transported to the University of Arizona (Tucson, AZ) for analysis. Drinking water samples were subjected to microwave digestion. After digestion, samples were brought up to volume with Milli-Q water for inductively coupled plasma extraction and mass spectroscopy (ICP-MS) analysis for total arsenic, with a detection limit of 0.1 μg/L. Urine total and speciated arsenic analyses were performed based on established methods (42) changing from a gradient to isocratic elution and adjusting the methanol concentration. Urine total arsenic was measured using an Agilent 7500ce ICP-MS (Agilent Technologies, Inc., Santa Clara, CA) and arsenite (AsIII), arsenate (AsV), monomethylarsonic acid V (MMAV) and dimethylarsinic acid V (DMAV) analyzed using an Agilent 1100 HPLC (Agilent Technologies, Inc., Santa Clara, CA) with an anion exchange column (PRP-X100, 10um, 250×4.1mm, Hamilton, Reno, NV). The detection limits were as follows: total arsenic 0.1 μg/L, AsIII 0.12 μg/L, AsV 0.21 μg/L, MMAV 0.12 μg/L, and DMAV 0.12 μg/L.
A weighted average water arsenic concentration was determined from reported usage of all water sources consumed for drinking (e.g., bottled water, filtered water, unfiltered tap water), weighted by the frequency of use (rare, moderate or frequent) of each source (41). Daily arsenic exposure from drinking water (drinking water arsenic intake) was then calculated from the weighted average water arsenic concentration (μg/L) multiplied by the average volume of drinking water consumed per day (L/day), and reported as μg/day. Urinary arsenic sum of species was calculated as the sum of AsIII, AsV, MMAV and DMAV and reported in μg/L. Percent MMA was calculated as MMAV divided by urinary arsenic sum of species multiplied by 100.
Non-fasting blood samples were collected for MMP-9 analysis in 10 mL red-top (serum) tubes (Becton Dickinson, Franklin Lakes, NJ), allowed to clot at room temperature for 15 minutes, and stored at 0-8°C for a maximum of 4 hours before centrifuging for 15 minutes at 1000 × gravity. Serum was separated into 2.0 ml aliquots and stored at −80°C until assayed for MMP-9. For MMP-9 analysis by ELISA (R&D Systems, Minneapolis, MN), standards and controls were assayed in duplicate using an automated microplate reader, Model ELx808 (BioTek, Instruments, Inc., Winooski, VT). The assay limit of detection was 0.156 ng/ml. MMP-9 concentration in samples was determined from the standard curve using a four parameter algorithm for best fit (KC4, BioTEK, Winooski, VT). Testing of all samples was done at a central laboratory in Arizona.
Stata 11.2 (StataCorp, College Station, TX) was used for statistical analyses. The concentration of arsenic in drinking water, drinking water arsenic intake, urinary arsenic sum of species, and serum concentration of MMP-9 were log (10)-transformed for parametric analysis. Pearson chi-square statistic, analysis of variance (ANOVA) using the Bonferroni test for post hoc comparisons, and Kruskal-Wallis tests were used to evaluate differences in population characteristics and risk factors by self-described country/ethnic background. Univariate and multivariable linear mixed models were used to assess the marginal relations between serum MMP-9 and arsenic exposure (weighted average arsenic concentration in drinking water, arsenic intake from drinking water, and urinary arsenic sum of species, adjusted for specific gravity) and other potential risk factors (age, body mass index , gender, country/ethnic background, current smoking status, and diabetes). Likelihood ratio tests were used to compare nested models and Akaike’s information criterion (AIC) was used to assess goodness of fit in non-nested models (43). A P-value < 0.05 was considered statistically significant.
Results
The total study population was composed of 487 participants, including 225 residents of Arizona and 262 residents of Sonora. For the analyses presented here, only those 378 individuals for whom both urine and blood samples were available were included. Characteristics of the study population are shown in Table 1 by country of residence and ethnic background: Mexican Hispanic, US Hispanic, and US non-Hispanic. These categories defined all but five people in the total study population, and these five subjects were excluded from analyses. Participants ranged in age from 19-88 years old, and the mean age was significantly higher in US non-Hispanics than US Hispanics or Mexicans. The study population was 56% female in Arizona and 74% in Sonora. Frequent use of alcohol (> 4x/week) was reported in over 18% of non-Hispanics in the US, but never in Mexico. Reported physician-diagnosed diabetes and BMI were substantially higher in US Hispanics than in the other groups.
Table 1.
Characteristics of the study population by country and self-described ethnicity.
| Total Population | Arizona, USA Non-Hispanic |
Arizona, USA Hispanic |
Sonora, Mexico Hispanic |
P-value† | |
|---|---|---|---|---|---|
| Number of participants (n, %) | 377 | 165 (43.8) | 50 (13.3) | 162 (43.0) | |
| Gender (n, %) | |||||
| Male | 133 (35.2) | 68 (41.2) | 23 (46.0) | 42 (25.8) | |
| Female | 245 (64.8) | 97 (58.8) | 27 (54.0) | 121 (74.2) | 0.007 |
| Age in years (mean, SD) | 50.0 ± 16.3 | 57.2 ± 14.7 | 48.9 ± 15.5 | 43.0 ± 14.9 | <0.001 |
| BMI (mean, SD) | 28.8 ± 5.9 | 28.1 ± 5.9 | 31.6 ± 6.5 | 28.8 ± 5.5 | 0.001 |
| Current smoker | 64 (18.3) | 27 (17.6) | 3 (7.0) | 34 (22.2) | 0.120 |
| Frequent alcohol use (> 4x/wk) | 37 (7.6) | 32 (18.5) | 5 (9.6) | 0 (0) | <0.001 |
| Physician-diagnosed diabetes | 40 (10.6) | 20 (12.2) | 11 (22.0) | 9 (5.8) | 0.017 |
| Drinking water As, geometric mean (95% CI) | |||||
| Weighted concentration# (μg/L) | 7.65 (6.80-8.63) | 8.66 (6.93-10.81) | 4.56 (3.77-5.51) | 7.91 (6.90-9.07) | <0.002 |
| Total volume consumed (L/day) | 0.63 (0.55-0.72) | 1.37 (1.20-1.58) | 1.03 (0.48-1.37) | 0.24 (0.20-0.29) | <0.001 |
| Drinking water intake (μg/day) | 2.47 (1.99-3.07) | 5.83 (4.23-8.02) | 3.37 (2.37-4.80) | 0.93 (0.68-1.28) | <0.001 |
| Urinary arsenic, adjusted for specific gravity (μg/L), geometric mean (95% CI) | |||||
| Total | 41.19 (37.65-45.07) | 33.57 (28.92-38.98) | 24.86 (21.81-28.33) | 59.17 (52.63-66.51) | <0.001 |
| As+3 | 1.10 (0.98-1. 23) | 0.72 (0.60-0.85) | 0.54 (0.42-0.69) | 2.10 (1.86-2.37) | <0.001 |
| As+5 | 0.82 (0.71-0.93) | 0.61 (0.49-0.76) | 0.47 (0.34-0.65) | 1.29 (1.08-1.55) | <0.001 |
| MMA | 2.04 (1.85-2.25) | 1.66 (1.40-1.96) | 1.06 (0.87-1.30) | 3.06 (2.75-3.41) | <0.001 |
| DMA | 13.67 (12.50-14.95) | 10.38 (8.93-12.06) | 7.89 (6.67-9.35) | 21.39 (19.44-23.52) | <0.001 |
| Sum of species | 18.44 (18.86-20.17) | 13.99 (12.01-16.28) | 10.60 (9.05-12.43) | 28.91 (26.31-31.77) | <0.001 |
| Urine specific gravity, geometric mean (95% CI) | |||||
| 1.016 (1.015-1.017) | 1.015 (1.015-1.017) | 1.015 (1.014-1.017) | 1.017 (1.016-1.018) | 0.035 | |
| Serum MMP-9 (ng/mL), geometric mean (95% CI) | |||||
| 295.60 (274.4-318.5) | 347.4 (311.6-387.3) | 250.0 (204.1-306.3) | 264.2 (235.4-296.5) | <0.001 | |
P-values based on chi-square test, analysis of variance, or Kruskal-Wallis test
Weighted concentration = (where n = the number of drinking sources used by the individual, and frequencyi =the frequency of use of each source i).
Weighted average arsenic concentration in household drinking water ranged from 0.132 μg/L to 1,004 μg/L, with a geometric mean concentration of 7.65 μg/L. It was significantly lower in US Hispanic households than in either of the other groups. The Mexican subjects reported consuming substantially less drinking water, contributing to markedly lower estimates of drinking water arsenic intake. However, urinary total arsenic and speciated arsenic concentrations were significantly greater in Mexican than in either US Hispanic or US non-Hispanic subjects, as was urine specific gravity. Serum concentration of MMP-9 ranged from 15-1,503 ng/ml. The geometric mean MMP-9 concentration was significantly higher among US non-Hispanics than in either Mexican or US Hispanic subjects.
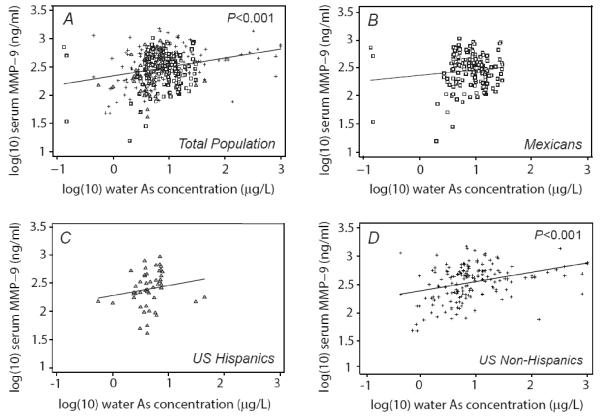
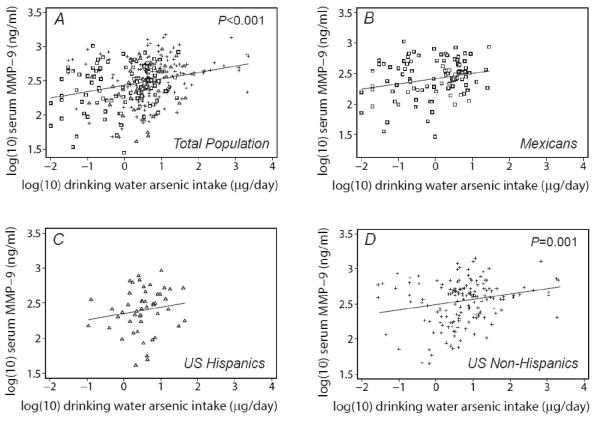
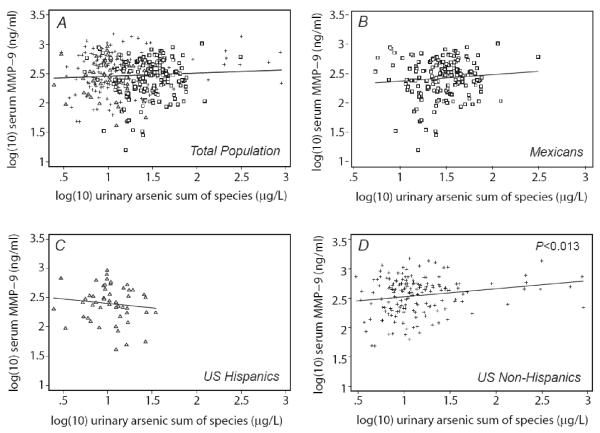
The univariate relation between MMP-9 and exposure to arsenic estimated from 1) weighted mean drinking water arsenic concentration (μg/L), 2) drinking water intake (μg/day), and 3) urinary arsenic sum of species are shown for the total population and stratified by country/ethnic group in Figures 1, 2 and 3, respectively. In the total population, drinking water concentration and intake, but not urinary arsenic, showed a statistically significant positive relationship with MMP-9. All three exposures were each significantly associated with MMP-9 in the US non-Hispanic population, but none showed a significant association in other ethnic subgroups.
Figure 1.
Serum MMP-9 in relation to drinking water arsenic concentration by population subgroup: A) Total population, B) Mexicans, C) U.S. Hispanics, and D) U.S. non-Hispanics. P-values show statistical significance of univariate relationships.
Figure 2.
Serum MMP-9 in relation to drinking water arsenic intake by population subgroup: A) Total population, B) Mexicans, C) U.S. Hispanics, and D) U.S. non-Hispanics. P-values show statistical significance of univariate relationships.
Figure 3.
Serum MMP-9 in relation to urine arsenic sum of species by population subgroup: A) Total population, B) Mexicans, C) U.S. Hispanics, and D) U.S. non-Hispanics. P-values show statistical significance of univariate relationships.
MMP-9 concentration was evaluated against drinking water arsenic concentration, drinking water arsenic intake, urinary arsenic sum of species, gender, country/ethnic group category, age, BMI, smoking status, diabetes and alcohol use through univariate regression analysis (Table 2, column A). Drinking water arsenic concentration and intake, but not urinary arsenic sum of species, were significantly associated with MMP-9. Compared with Mexicans, US non-Hispanics but not US Hispanics had higher MMP-9 concentrations. Gender, age, BMI, current smoking status, diabetes and alcohol use were not significantly associated with MMP-9.
Table 2.
Predictors of serum (log10) MMP-9: A) Univariate models. Multivariable regression models with: B) drinking water arsenic (As) weighted concentration, C) drinking water As intake, and D) urinary As sum of species, adjusted for specific gravity.
| A) Univariate Models |
B) Drinking Water As Concentration Model |
C) Drinking Water As Intake Model |
D) Urinary As Sum of Species Model |
|
|---|---|---|---|---|
|
|
||||
| Predictor variables | Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | Coefficient (SE) |
| Log (10) drinking water As concentration (μg/L) |
0.162 (0.034) ‡ | 0.135 (0.035) ‡ | ||
| Log (10) drinking water As intake (μg/day) |
0.073 (0.020) ‡ | 0.072 (0.022) ‡ | ||
| Log (10) urinary As sum of species (μg/L), adjusted |
0.047 (0.044) | 0.121 (0.050) * | ||
| Country/Ethnicity | ||||
| Mexican/Hispanic | (ref) | (ref) | (ref) | (ref) |
| US/Hispanic | −0.050 (0.054) | 0.003 (0.056) | −0.065 (0.057) | 0.013 (0.060) |
| US/Non-Hispanic | 0.092 (0.037) * | 0.108 (0.040) † | 0.059 (0.044) | 0.155 (0.044) ‡ |
| Male | 0.048 (0.029) | 0.037 (0.031) | 0.034 (0.032) | 0.043 (0.032) |
| Age (years) | −0.001 (0.001) | −0.001 (0.001) | −0.001 (0.001) | −0.002 (0.001) |
| BMI (kg/m2) | −0.005 (0.003) | −0.004 (0.003) | −0.005 (0.003) | −0.004 (0.003) |
| Current smoker | 0.060 (0.042) | 0.047 (0.042) | 0.057 (0.043) | 0.033 (0.043) |
| Diabetes | −0.079 (0.050) | −0.051 (0.052) | −0.058 (0.053) | −0.039 (0.053) |
|
| ||||
| Model fit, AIC (df) | 122.5 (11) | 127.7 (11) | 132.5 (11) | |
Abbreviations: SE, standard error; AIC, Akaike’s Information Criterion; df, degrees of freedom
P<0.05,
P<0.01,
P<0.001
In adjusted linear mixed models, drinking water arsenic concentration and US non-Hispanic ethnicity were statistically significant independent predictors of MMP-9 (Table 2, column B). A separate model using estimates of drinking water arsenic intake, instead of concentration, was also predictive of MMP-9 (Table 2, column C), but this model provided a less parsimonious fit based on the AIC. A fourth model using urinary arsenic sum of species adjusted for specific gravity as a predictor of MMP-9 found that urinary arsenic was also significantly associated with MMP-9, but provided a poorer fit to the data (Table 2, column D). In the urinary arsenic model, US non-Hispanic ethnicity was also significantly related to MMP-9 in the model.
To assess the impact of adjusting urinary arsenic for specific gravity, models of the relation between MMP-9 and unadjusted urinary arsenic sum of species were also analyzed. Urinary arsenic sum of species unadjusted for specific gravity was borderline significant in the univariate model (P=0.080) (not shown), and both adjusted and unadjusted urinary arsenic were statistically significant in the multivariable models. In addition, multivariable models were constructed that included percent urinary MMA as an additional independent variable to evaluate the effect of urinary arsenic methylation, specifically, the formation of MMA, on MMP-9. Percent MMA was not a significant confounder of the relation between drinking water arsenic concentration, water arsenic intake, or urinary arsenic sum of species and MMP-9 (data not shown).
Discussion
In the present study three measures of arsenic exposure, the weighted mean drinking water arsenic concentration, the estimated daily intake from drinking water and the measured urinary arsenic sum of species, were independently associated with serum MMP-9 concentration. These results are consistent with the previously reported alterations in sputum MMP-9/TIMP-1 ratio in an Arizona population exposed to arsenic up to 20 ppb in their tap water (39) and increases in MMP-9 mRNA expression from cultured lung cells exposed to arsenic for five days at concentrations of 60 ppb and above (21). Furthermore, arsenic exposure has been associated with changes in MMP-9 in prostate cells (44) and keratinocytes (45), as well as in the lungs of mice exposed to arsenite in drinking water (46).
The exact mechanism for the association between arsenic exposure and increased MMP-9 is unknown. Possibilities include, but are not limited to, activation of activating protein-1 (AP-1) receptor sites in the promoter region of MMP-9 (47) or altered gene methylation (48). Compared with the limited knowledge concerning the toxic mechanism of arsenic, there are multiple postulated mechanisms by which increased MMP-9 can lead to cardiovascular disease and cancer. MMP-9 activity is increased in atheromatous plaque and increased extracellular matrix metabolism, with its consequent vascular remodeling, may contribute to further plaque development (49). Serum MMP-9 levels are associated with increased arterial stiffness (50). Plasma MMP-9 levels in patients with premature stable cardiovascular disease are positively associated with low density lipoprotein-cholesterol (LDL-C) and negatively associated with high density lipoprotein-cholesterol (HDL-C) (51). MMP-9 can also activate inflammatory mediators, such as interleukin 1-beta (52), and MMP-9 levels have been associated with other inflammatory biomarkers including C-reactive protein (53). For cancer endpoints, MMP-9 facilitates cancer cell growth, migration, invasion, metastasis and angiogenesis (54-56). There are, of course, other mechanisms not directly involving MMP-9 by which arsenic may cause or exacerbate a variety of chronic diseases, including other inflammatory pathways, oxidative stress, altered DNA methylation patterns, inhibition of DNA repair and modulation of signal transduction pathways (57-59).
Although our hypothesis that increased arsenic exposure would be associated with higher MMP-9 concentration was supported by these study results, the original expectation was that measurement of urinary arsenic sum of species would be a better predictor of inorganic arsenic exposure than drinking water arsenic (concentration or intake). This expectation was based on the reasoning that drinking water arsenic concentration would not reflect all sources of arsenic exposure and that estimates of water ingestion are subject to recall bias, while first morning void urinary arsenic concentrations would reflect all sources of exposure and would have more limited variability assuming a steady-state exposure such as consistent daily water arsenic consumption Calderon et al., 1999). One potential explanation for the finding that drinking water arsenic concentration yielded the best fitting model of MMP-9 is that these study populations may not have been at steady-state exposure relative to arsenic. Given the rapid absorption and excretion of arsenic from the body (60), urinary arsenic sum of species measured on a single occasion will only reflect recent intake of inorganic arsenic from water and food. Drinking water arsenic concentration, assuming consistent exposure, might be a better predictor of intermediate biological effects such as increased serum MMP-9. Although in our study drinking water arsenic concentration was a better predictor of serum MMP-9 than estimated intake or urinary arsenic sum of species, additional research is needed to see if such a relationship persists with other biomarkers of toxic effect.
One finding that is difficult to interpret is the elevated mean urinary arsenic sum of species concentration in the Mexican subjects. Even after adjusting for urine specific gravity, and despite low water consumption and therefore low drinking water arsenic intake, urinary arsenic sum of species was greatest in Mexico. Potential explanations for this apparent contradiction include greater arsenic exposure from water used in the preparation of food and beverages, greater dietary arsenic intake, and/or possible underreporting of the quantity of drinking water ingested.
There were a number of limitations to our study. The recruitment methods used in Mexico and the US were different. The proportion of females was markedly higher in the Mexican population as compared with the US, and the mean age was lower. Also, while we adjusted our models for self-reported, physician-diagnosed diabetes, the prevalence of undiagnosed diabetes is a concern. We also sought to determine whether the relation between As exposure and MMP-9 in US Hispanics was more similar to that in Mexicans or in US non-Hispanics, but the number of US Hispanics (n=50) was relatively small, limiting our power to demonstrate a statistically significant association between arsenic exposure and serum MMP-9 in this subpopulation. It was impossible to determine whether our subjects were at steady state in regards to arsenic exposure; however the literature suggests that exposure is fairly stable and significantly correlated over time (61-64), and subjects were eligible only if they had been living in their current residence for a minimum of one year. Because the transport time for the blood samples was relatively long and the ELISA used for MMP-9 analysis measures both the precursor (pro-MMP-9) and active forms, we were unable to determine the concentration of the active form alone. In addition, other variables affecting MMP-9 concentrations were not assessed in this study; including hormone therapy (65, 66), blood lead (67) and weight loss (68). Although alcohol abuse has been linked to high levels of serum MMP-9 and cardiovascular disease (69), alcohol consumption was not associated with MMP-9 in our study. Finally, although the association of arsenic exposure with serum MMP-9 was consistent across populations in both Arizona and Sonora, this association may not be generalizable elsewhere and the study needs to be replicated in other populations.
In summary, our study found an association between increased environmental arsenic exposure and increased concentrations of serum MMP-9 across populations in both Arizona and Sonora. These findings provide support for the hypothesis that alterations in MMP-9 could serve as one mechanism explaining the epidemiologic associations between arsenic exposure and select chronic diseases. In addition, serum MMP-9 was significantly associated with all three estimates of arsenic exposure evaluated, including drinking water arsenic concentration, drinking water arsenic intake and urinary arsenic concentrations.
Acknowledgments
Funding: This project was supported by NIEHS grant ES06694 to the Southwest Environmental Health Sciences Center and NIH/NCI University of Arizona Specialized Program of Research Excellence (SPORE) in GI Cancer #CA95060.
Human Subjects Approval for this project was received from The University of Arizona Office for the Responsible Conduct of Research and Institutional Review Board.
REFERENCES
- 1.Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130(6):1123–32. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 2.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arteriosclerosis, thrombosis, and vascular biology. 1996;16(4):504–10. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 3.Lee MY, Bae ON, Chung SM, Kang KT, Lee JY, Chung JH. Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: a contributing factor to cardiovascular disease. Toxicol Appl Pharmacol. 2002;179(2):83–8. doi: 10.1006/taap.2001.9356. [DOI] [PubMed] [Google Scholar]
- 4.Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201(1):32–9. doi: 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Yuan C, Gao E, He B, Jiang G. Arsenic species and leaching characters in tea (Camellia sinensis) Food Chem Toxicol. 2007;45(12):2381–9. doi: 10.1016/j.fct.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, et al. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995;55(6):1296–300. [PubMed] [Google Scholar]
- 7.Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int J Epidemiol. 1998;27(4):561–9. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- 8.Milton AH, Rahman M. Respiratory effects and arsenic contaminated well water in Bangladesh. Int J Environ Health Res. 2002;12(2):175–9. doi: 10.1080/09603120220129346. [DOI] [PubMed] [Google Scholar]
- 9.Parvez F, Chen Y, Brandt-Rauf PW, Slavkovich V, Islam T, Ahmed A, et al. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: findings from the Health Effects of Arsenic Longitudinal Study (HEALS) Thorax. 2010;65(6):528–33. doi: 10.1136/thx.2009.119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dauphine DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health. 2011;84(6):591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KG, Ross GL. Arsenic, drinking water, and health: a position paper of the American Council on Science and Health. Regul Toxicol Pharmacol. 2002;36(2):162–74. doi: 10.1006/rtph.2002.1573. [DOI] [PubMed] [Google Scholar]
- 12.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:4. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnaike RN. Acute and chronic arsenic toxicity. Postgraduate medical journal. 2003;79(933):391–6. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazumder DN. Effect of drinking arsenic contaminated water in children. Indian Pediatr. 2007;44(2):925–7. [PubMed] [Google Scholar]
- 15.NRC . Arsenic in Drinking Water: 2001 Update. Free Executive Summary. In: National Research Council, editor. Subcommittee to Update the 1999 Arsenic in Drinking Water Report CoT, Board on Environmental Studies and Toxicology. 2001. p. 16. [Google Scholar]
- 16.Bardullas U, Limon-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodriguez VM. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol. 2009;239(2):169–77. doi: 10.1016/j.taap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Meng XZ, Zheng TS, Chen X, Wang JB, Zhang WH, Pan SH, et al. microRNA expression alteration after arsenic trioxide treatment in HepG-2 cells. Journal of gastroenterology and hepatology. 2011;26(1):186–93. doi: 10.1111/j.1440-1746.2010.06317.x. [DOI] [PubMed] [Google Scholar]
- 18.Zarazua S, Rios R, Delgado JM, Santoyo ME, Ortiz-Perez D, Jimenez-Capdeville ME. Decreased arginine methylation and myelin alterations in arsenic exposed rats. Neurotoxicology. 2010;31(1):94–100. doi: 10.1016/j.neuro.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Vahidnia A, van der Voet GB, de Wolff FA. Arsenic neurotoxicity--a review. Hum Exp Toxicol. 2007;26(10):823–32. doi: 10.1177/0960327107084539. [DOI] [PubMed] [Google Scholar]
- 20.Lantz RC, Lynch BJ, Boitano S, Poplin GS, Littau S, Tsaprailis G, et al. Pulmonary biomarkers based on alterations in protein expression after exposure to arsenic. Environ Health Perspect. 2007;115(4):586–91. doi: 10.1289/ehp.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen CE, Liguori AE, Zong Y, Lantz RC, Burgess JL, Boitano S. Arsenic upregulates MMP-9 and inhibits wound repair in human airway epithelial cells. American journal of physiology Lung cellular and molecular physiology. 2008;295(2):L293–302. doi: 10.1152/ajplung.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 2008;116(4):524–31. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YC, Su HJ, Guo YL, Houseman EA, Christiani DC. Interaction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancer. Cancer Causes Control. 2005;16(2):75–81. doi: 10.1007/s10552-004-2235-1. [DOI] [PubMed] [Google Scholar]
- 24.Chen CJ, Hsu LI, Wang CH, Shih WL, Hsu YH, Tseng MP, et al. Biomarkers of exposure, effect, and susceptibility of arsenic-induced health hazards in Taiwan. Toxicol Appl Pharmacol. 2005;206(2):198–206. doi: 10.1016/j.taap.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Andrew AS, Burgess JL, Meza MM, Demidenko E, Waugh MG, Hamilton JW, et al. Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ Health Perspect. 2006;114(8):1193–8. doi: 10.1289/ehp.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferroni P, Basili S, Martini F, Cardarello CM, Ceci F, Di Franco M, et al. Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2003;51(5):295–300. doi: 10.1136/jim-51-05-17. [DOI] [PubMed] [Google Scholar]
- 27.Kalela A, Koivu TA, Sisto T, Kanervisto J, Hoyhtya M, Sillanaukee P, et al. Serum matrix metalloproteinase-9 concentration in angiographically assessed coronary artery disease. Scandinavian journal of clinical and laboratory investigation. 2002;62(5):337–42. doi: 10.1080/00365510260296483. [DOI] [PubMed] [Google Scholar]
- 28.Sundstrom J, Vasan RS. Circulating biomarkers of extracellular matrix remodeling and risk of atherosclerotic events. Current opinion in lipidology. 2006;17(1):45–53. doi: 10.1097/01.mol.0000203891.34890.b5. Epub 2006/01/13. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113(17):2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 30.Eldrup N, Gronholdt ML, Sillesen H, Nordestgaard BG. Elevated matrix metalloproteinase-9 associated with stroke or cardiovascular death in patients with carotid stenosis. Circulation. 2006;114(17):1847–54. doi: 10.1161/CIRCULATIONAHA.105.593483. [DOI] [PubMed] [Google Scholar]
- 31.Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;66(5):888–92. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer metastasis reviews. 2004;23(1-2):101–17. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- 33.Wu CY, Wu MS, Chiang EP, Chen YJ, Chen CJ, Chi NH, et al. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(7):2054–60. doi: 10.1158/1078-0432.CCR-06-2299. [DOI] [PubMed] [Google Scholar]
- 34.IARC Arsenic in drinking water. International Agency for Research on Cancer (IARC) Monographs [Internet] 2004;84(200):229. Available from: http://www.inchem.org/documents/iarc/vol84/84-01-arsenic.html. [Google Scholar]
- 35.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. American journal of respiratory cell and molecular biology. 2003;28(1):12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 36.Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Current drug targets Inflammation and allergy. 2005;4(2):177–81. doi: 10.2174/1568010053586246. [DOI] [PubMed] [Google Scholar]
- 37.Tayebjee MH, Lim HS, MacFadyen RJ, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and -2 in type 2 diabetes: effect of 1 year’s cardiovascular risk reduction therapy. Diabetes Care. 2004;27(8):2049–51. doi: 10.2337/diacare.27.8.2049. [DOI] [PubMed] [Google Scholar]
- 38.Jacqueminet S, Ben Abdesselam O, Chapman MJ, Nicolay N, Foglietti MJ, Grimaldi A, et al. Elevated circulating levels of matrix metalloproteinase-9 in type 1 diabetic patients with and without retinopathy. Clin Chim Acta. 2006;367(1-2):103–7. doi: 10.1016/j.cca.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Josyula AB, Poplin GS, Kurzius-Spencer M, McClellen HE, Kopplin MJ, Sturup S, et al. Environmental arsenic exposure and sputum metalloproteinase concentrations. Environ Res. 2006;102(3):283–90. doi: 10.1016/j.envres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ Res. 2004;96(2):119–26. doi: 10.1016/j.envres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Roberge J, O’Rourke MK, Meza-Montenegro MM, Gutierrez-Millan LE, Burgess JL, Harris RB. Binational Arsenic Exposure Survey: Methodology and Exploration of the Relationship between Estimated Arsenic Intake from Drinking Water and Urinary Arsenic Concentrations. Int J Environ Res Public Health. 2012;9:1051–67. doi: 10.3390/ijerph9041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milstein LS, Essader A, Pellizzari ED, Fernando RA, Raymer JH, Levine KE, et al. Development and application of a robust speciation method for determination of six arsenic compounds present in human urine. Environ Health Perspect. 2003;111(3):293–6. doi: 10.1289/ehp.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown H, Prescott R. Normal Mixed Models. Applied Mixed Models in Medicine. John Wiley & Sons, Ltd.; Chichester, England: 1999. pp. 33–51. [Google Scholar]
- 44.Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94(24):1888–91. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 45.Cooper KL, Myers TA, Rosenberg M, Chavez M, Hudson LG. Roles of mitogen activated protein kinases and EGF receptor in arsenite-stimulated matrix metalloproteinase-9 production. Toxicol Appl Pharmacol. 2004;200(3):177–85. doi: 10.1016/j.taap.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Andrew A, Barchowsky A, Davey J, Soucy N, Mayka D, Lantz RC, et al. In vivo exposure to drinking water arsenic modifies expression of genes in the mouse lung. Society of Toxicology Annual Meeting; March 21-25 (2004).2004. [Google Scholar]
- 47.Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NF-kappaB DNA binding activity and related gene expression. Toxicol Lett. 2002;133(1):33–45. doi: 10.1016/s0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 48.Chanda S, Dasgupta UB, Guhamazumder D, Gupta M, Chaudhuri U, Lahiri S, et al. DNA hypermethylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol Sci. 2006;89(2):431–7. doi: 10.1093/toxsci/kfj030. [DOI] [PubMed] [Google Scholar]
- 49.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. The Journal of clinical investigation. 1994;94(6):2493–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasmin Mc, Eniery CM, Wallace S, Dakham Z, Pulsalkar P, Maki-Petaja K, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(2):372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 51.Noji Y, Kajinami K, Kawashiri MA, Todo Y, Horita T, Nohara A, et al. Circulating matrix metalloproteinases and their inhibitors in premature coronary atherosclerosis. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2001;39(5):380–4. doi: 10.1515/CCLM.2001.060. [DOI] [PubMed] [Google Scholar]
- 52.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161(7):3340–6. [PubMed] [Google Scholar]
- 53.Devaraj S, O’Keefe G, Jialal I. Defining the proinflammatory phenotype using high sensitive C-reactive protein levels as the biomarker. The Journal of clinical endocrinology and metabolism. 2005;90(8):4549–54. doi: 10.1210/jc.2005-0069. [DOI] [PubMed] [Google Scholar]
- 54.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(16):9482–7. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature cell biology. 2000;2(10):737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer research. 2006;26(5A):3579–83. [PubMed] [Google Scholar]
- 57.Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55(2):460–7. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 58.Yamanaka K, Hoshino M, Okamoto M, Sawamura R, Hasegawa A, Okada S. Induction of DNA damage by dimethylarsine, a metabolite of inorganic arsenics, is for the major part likely due to its peroxyl radical. Biochem Biophys Res Commun. 1990;168(1):58–64. doi: 10.1016/0006-291x(90)91674-h. [DOI] [PubMed] [Google Scholar]
- 59.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A. 1997;94(20):10907–12. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki KT, Mandal BK, Ogra Y. Speciation of arsenic in body fluids. Talanta. 2002;58(1):111–9. doi: 10.1016/s0039-9140(02)00260-6. [DOI] [PubMed] [Google Scholar]
- 61.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114(11):1790–6. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pellizzari ED, Clayton CA. Assessing the measurement precision of various arsenic forms and arsenic exposure in the National Human Exposure Assessment Survey (NHEXAS) Environ Health Perspect. 2006;114(2):220–7. doi: 10.1289/ehp.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect. 2009;117(9):1428–33. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rivera-Nunez Z, Meliker JR, Linder AM, Nriagu JO. Reliability of spot urine samples in assessing arsenic exposure. Int J Hyg Environ Health. 2010;213(4):259–64. doi: 10.1016/j.ijheh.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu P, Greendale GA, Palla SL, Reboussin BA, Herrington DM, Barrett-Connor E, et al. The effects of hormone therapy on the markers of inflammation and endothelial function and plasma matrix metalloproteinase-9 level in postmenopausal women: the postmenopausal estrogen progestin intervention (PEPI) trial. Atherosclerosis. 2006;185(2):347–52. doi: 10.1016/j.atherosclerosis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Niessner A, Richter B, Penka M, Steiner S, Strasser B, Ziegler S, et al. Endurance training reduces circulating inflammatory markers in persons at risk of coronary events: impact on plaque stabilization? Atherosclerosis. 2006;186(1):160–5. doi: 10.1016/j.atherosclerosis.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 67.Barbosa F, Jr., Gerlach RF, Tanus-Santos JE. Matrix metalloproteinase-9 activity in plasma correlates with plasma and whole blood lead concentrations. Basic Clin Pharmacol Toxicol. 2006;98(6):559–64. doi: 10.1111/j.1742-7843.2006.pto_392.x. [DOI] [PubMed] [Google Scholar]
- 68.Laimer M, Kaser S, Kranebitter M, Sandhofer A, Muhlmann G, Schwelberger H, et al. Effect of pronounced weight loss on the nontraditional cardiovascular risk marker matrix metalloproteinase-9 in middle-aged morbidly obese women. International journal of obesity. 2005;29(5):498–501. doi: 10.1038/sj.ijo.0802897. [DOI] [PubMed] [Google Scholar]
- 69.Sillanaukee P, Kalela A, Seppa K, Hoyhtya M, Nikkari ST. Matrix metalloproteinase-9 is elevated in serum of alcohol abusers. Eur J Clin Invest. 2002;32(4):225–9. doi: 10.1046/j.1365-2362.2002.00975.x. [DOI] [PubMed] [Google Scholar]