Abstract
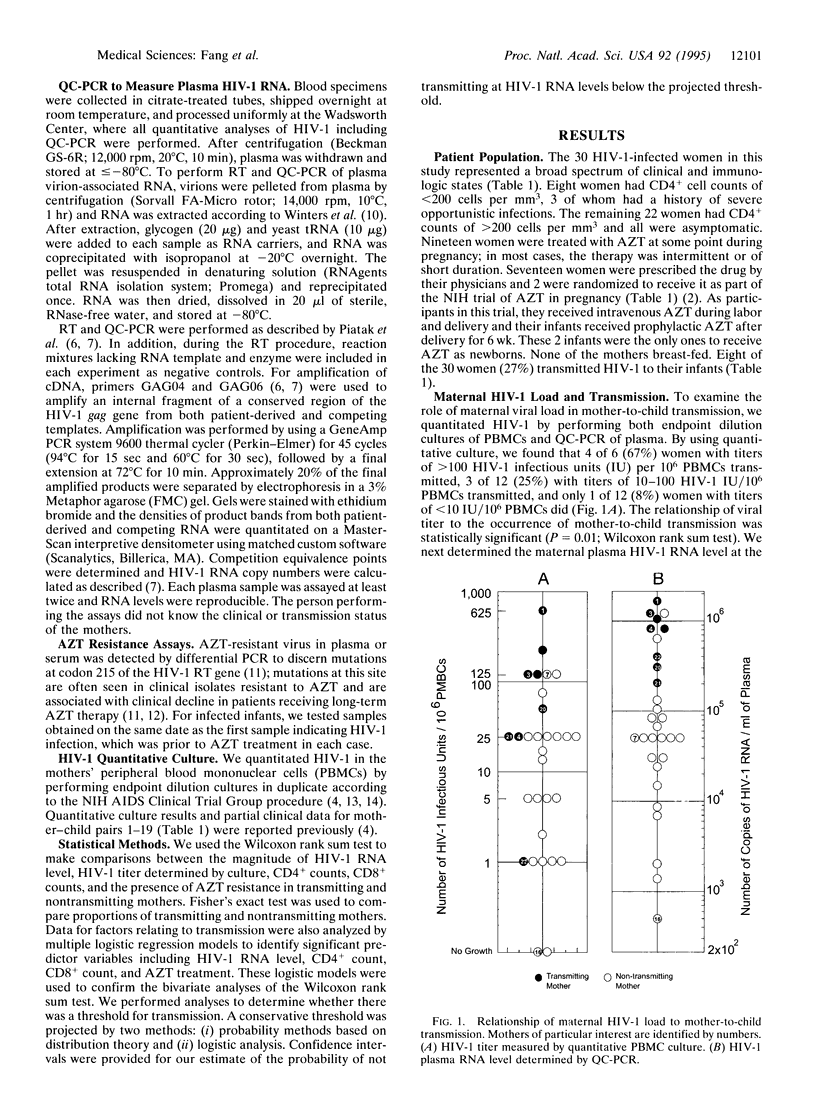
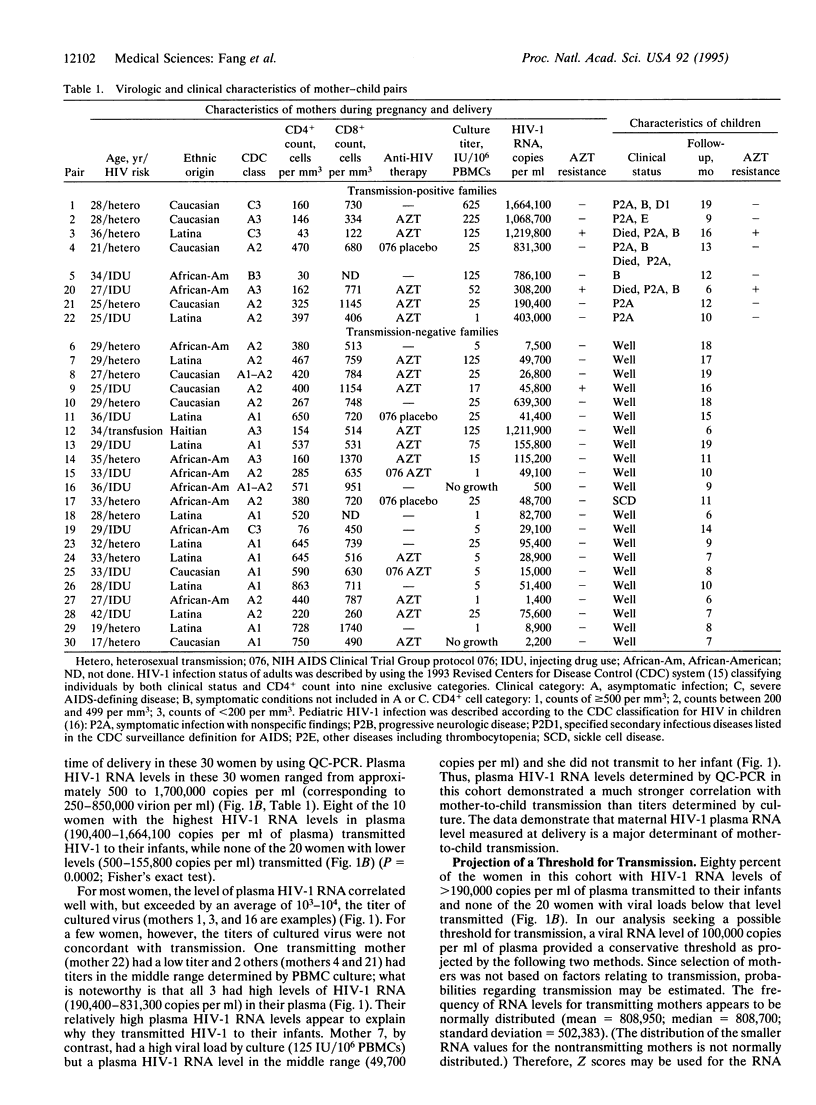
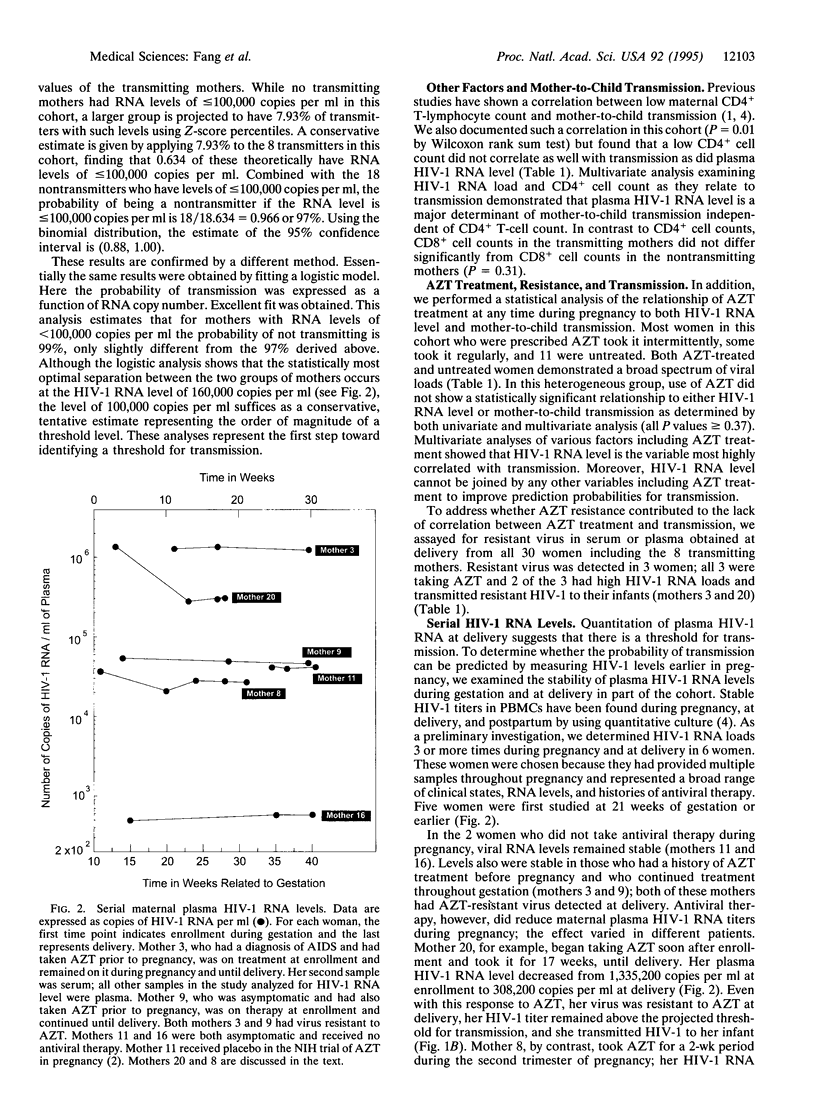
To prevent mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission, it is important to identify its determinants. Because HIV-1 RNA levels can be reduced by antiviral therapy, we examined the role of maternal plasma HIV-1 RNA level in mother-to-child transmission. We used quantitative competitive PCR to measure HIV-RNA in 30 infected pregnant women and then followed their infants prospectively; 27% of the women transmitted HIV-1 to their infants and maternal plasma HIV-1 RNA level correlated strikingly with transmission. Eight of the 10 women with the highest HIV-1 RNA levels at delivery (190,400-1,664,100 copies per ml of plasma) transmitted, while none of the 20 women with lower levels (500-155,800 copies per ml) did (P = 0.0002). Statistical analysis of the distribution of HIV-1 RNA loads in these 30 women projected a threshold for mother-to-child transmission in a larger population; the probability of a woman with a viral RNA level of < or = 100,000 copies per ml not transmitting is predicted to be 97%. Examination of serial HIV-1 RNA levels during pregnancy showed that viral load was stable in women who did not initiate or change antiviral therapy. These data identify maternal plasma HIV-1-RNA level as a major determinant of mother-to-child transmission and suggest that quantitation of HIV-1 RNA may predict the risk of transmission.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borkowsky W., Krasinski K., Cao Y., Ho D., Pollack H., Moore T., Chen S. H., Allen M., Tao P. T. Correlation of perinatal transmission of human immunodeficiency virus type 1 with maternal viremia and lymphocyte phenotypes. J Pediatr. 1994 Sep;125(3):345–351. doi: 10.1016/s0022-3476(05)83274-3. [DOI] [PubMed] [Google Scholar]
- Boyer P. J., Dillon M., Navaie M., Deveikis A., Keller M., O'Rourke S., Bryson Y. J. Factors predictive of maternal-fetal transmission of HIV-1. Preliminary analysis of zidovudine given during pregnancy and/or delivery. JAMA. 1994 Jun 22;271(24):1925–1930. [PubMed] [Google Scholar]
- Goedert J. J., Duliège A. M., Amos C. I., Felton S., Biggar R. J. High risk of HIV-1 infection for first-born twins. The International Registry of HIV-exposed Twins. Lancet. 1991 Dec 14;338(8781):1471–1475. doi: 10.1016/0140-6736(91)92297-f. [DOI] [PubMed] [Google Scholar]
- Hammer S., Crumpacker C., D'Aquila R., Jackson B., Lathey J., Livnat D., Reichelderfer P. Use of virologic assays for detection of human immunodeficiency virus in clinical trials: recommendations of the AIDS Clinical Trials Group Virology Committee. J Clin Microbiol. 1993 Oct;31(10):2557–2564. doi: 10.1128/jcm.31.10.2557-2564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger F. B., Bremer J. W., Myers L. E., Gold J. W., McQuay L. Standardization of sensitive human immunodeficiency virus coculture procedures and establishment of a multicenter quality assurance program for the AIDS Clinical Trials Group. The NIH/NIAID/DAIDS/ACTG Virology Laboratories. J Clin Microbiol. 1992 Jul;30(7):1787–1794. doi: 10.1128/jcm.30.7.1787-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliks S. C., Wara D. W., Landers D. V., Levy J. A. Features of HIV-1 that could influence maternal-child transmission. JAMA. 1994 Aug 10;272(6):467–474. [PubMed] [Google Scholar]
- Kline M. W., Hollinger F. B., Rosenblatt H. M., Bohannon B., Kozinetz C. A., Shearer W. T. Sensitivity, specificity and predictive value of physical examination, culture and other laboratory studies in the diagnosis during early infancy of vertically acquired human immunodeficiency virus infection. Pediatr Infect Dis J. 1993 Jan;12(1):33–36. doi: 10.1097/00006454-199301000-00009. [DOI] [PubMed] [Google Scholar]
- Kozal M. J., Shafer R. W., Winters M. A., Katzenstein D. A., Merigan T. C. A mutation in human immunodeficiency virus reverse transcriptase and decline in CD4 lymphocyte numbers in long-term zidovudine recipients. J Infect Dis. 1993 Mar;167(3):526–532. doi: 10.1093/infdis/167.3.526. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kellam P., Kemp S. D. Zidovudine resistance predicted by direct detection of mutations in DNA from HIV-infected lymphocytes. AIDS. 1991 Feb;5(2):137–144. doi: 10.1097/00002030-199102000-00002. [DOI] [PubMed] [Google Scholar]
- Luzuriaga K., McQuilken P., Alimenti A., Somasundaran M., Hesselton R., Sullivan J. L. Early viremia and immune responses in vertical human immunodeficiency virus type 1 infection. J Infect Dis. 1993 May;167(5):1008–1013. doi: 10.1093/infdis/167.5.1008. [DOI] [PubMed] [Google Scholar]
- Mole L., Margolis D., Carroll R., Todd J., Holodniy M. Stabilities of quantitative plasma culture for human immunodeficiency virus, RNA, and p24 antigen from samples collected in VACUTAINER CPT and standard VACUTAINER tubes. J Clin Microbiol. 1994 Sep;32(9):2212–2215. doi: 10.1128/jcm.32.9.2212-2215.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatak M., Jr, Luk K. C., Williams B., Lifson J. D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. Biotechniques. 1993 Jan;14(1):70–81. [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Grimes J. M., Lagakos S. W. Effect of stage of disease and drug dose on zidovudine susceptibilities of isolates of human immunodeficiency virus. J Acquir Immune Defic Syndr. 1990;3(8):743–746. [PubMed] [Google Scholar]
- Rogers M. F., Jaffe H. W. Reducing the risk of maternal-infant transmission of HIV: a door is opened. N Engl J Med. 1994 Nov 3;331(18):1222–1223. doi: 10.1056/NEJM199411033311810. [DOI] [PubMed] [Google Scholar]
- Scarlatti G., Hodara V., Rossi P., Muggiasca L., Bucceri A., Albert J., Fenyö E. M. Transmission of human immunodeficiency virus type 1 (HIV-1) from mother to child correlates with viral phenotype. Virology. 1993 Dec;197(2):624–629. doi: 10.1006/viro.1993.1637. [DOI] [PubMed] [Google Scholar]
- Weiser B., Nachman S., Tropper P., Viscosi K. H., Grimson R., Baxter G., Fang G., Reyelt C., Hutcheon N., Burger H. Quantitation of human immunodeficiency virus type 1 during pregnancy: relationship of viral titer to mother-to-child transmission and stability of viral load. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8037–8041. doi: 10.1073/pnas.91.17.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters M. A., Tan L. B., Katzenstein D. A., Merigan T. C. Biological variation and quality control of plasma human immunodeficiency virus type 1 RNA quantitation by reverse transcriptase polymerase chain reaction. J Clin Microbiol. 1993 Nov;31(11):2960–2966. doi: 10.1128/jcm.31.11.2960-2966.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


