Abstract
Complement is a part of the body’s innate immune system that helps defend the host from microbial infection. It is tightly controlled by a number of cell surface and fluid-phase proteins so that under normal circumstances injury to autologous tissues is avoided. In many pathological settings, such as when the complement regulatory mechanisms are dysfunctional or overwhelmed, complement attack of autologous tissues can occur with severe, sometimes life-threatening consequences. The kidney appears to be particularly vulnerable to complement-mediated inflammatory injury and many kidney pathologies have been linked to abnormal complement activation. Clinical and experimental studies have shown that complement attack can be a primary cause in rare, genetically predisposed kidney diseases or a significant contributor to kidney injury caused by other etiological factors. Here we provide a brief review of recent advances on the activation and regulation of the complement system in kidney disease, with a particular emphasis on the relevance of complement regulatory proteins.
Keywords: anti-GBM glomerulonephritis, atypical hemolytic uremic syndrome, complement, complement regulatory proteins, ischemia-reperfusion injury, membranoproliferative glomerulonephritis type II
Complement is a part of the innate immune system that functions primarily as a first-line host defence against pathogenic infections. It is composed of over 30 plasma and cell surface-associated proteins. Originally discovered at the end of the 19th century as a heat-labile component of plasma, it was so named because scientists found that it ‘complemented’ the bactericidal activity of antibodies.1 Since then, the known biological function of complement in host defence has greatly expanded. More recently, the relevance of complement to many human autoimmune and inflammatory disorders has also become appreciated, and many efforts are currently underway to develop complement-based therapies for these diseases. Among the human diseases that have been linked to complement, several disorders of the kidney have been identified and extensively studied both clinically and experimentally. These works have not only provided insights into pathogenesis of the kidney abnormalities in question, but also contributed significantly to our understanding of complement-mediated human tissue injury in general. In this brief review, we summarize recent advances on the activation and regulation of the complement system in kidney disease, with a particular emphasis on the relevance of complement regulatory proteins.
ACTIVATION AND EFFECTOR FUNCTIONS OF COMPLEMENT
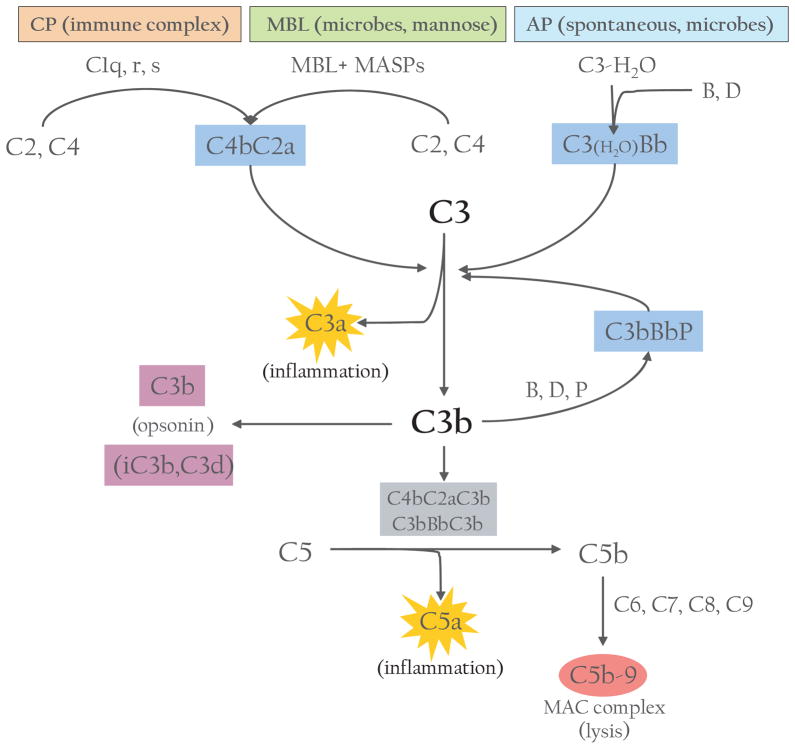
The complement system can be activated by three main pathways: classical, lectin and alternative (Fig. 1).2,3 The classical pathway is triggered by antigen–antibody immune complexes.3 After binding to their cognate antigens, the Fc portion of an IgG or IgM interacts with the collagen-like tail of C1q, a component of C1 complex. This interaction leads to the sequential activation of C1r and C1s, two serine proteases associated with C1q within the C1 complex. The activated C1s then cleaves C4 and C2 to generate the classical pathway C3 convertase C4bC2a, an enzymatic complex that cleaves C3, the central component of the complement cascade, into C3a and C3b. The lectin pathway resembles the classical pathway in that its activation also leads to formation of the C4bC2a enzyme complex. However, instead of relying on antibodies to recognize pathogenic components, the lectin pathway identifies pathogen-associated molecular patterns by members of the collectin family of proteins in the plasma, namely mannose-binding lectins (MBL) and ficolins.2,3 Binding of MBL or ficolin to distinct sugar molecules on the pathogenic surface leads to activation of MBL-associated serine proteases (MASP), which cleave C4 and C2 and generate C4bC2a in a reaction analogous to the classical pathway (Fig. 1).2
Fig. 1.
Overview of the complement system. Activation can be initiated by one of three pathways: classical (CP), mannose-binding lectin (MBL) or alternative (AP). C3 convertases cleave C3 into C3a and C3b. C3b (and further cleavage products) can act as opsonins; C3b also forms additional C3 convertase or C5 convertases, which cleave C5 into C5a and C5b. C5b initiates the terminal complement pathway involving C6, C7, C8 and C9 to form the membrane attack complex (MAC) to cause lysis. C3a and C5a fragments are potent anaphylatoxins that cause inflammation and recruit immune cells to site of injury.
While the classical and lectin pathways are generally activated upon recognition of exogenous materials, the alternative pathway (AP) is constitutively active at low levels in the host.4 This is often referred to as the ‘tickover mechanism’ and allows the system to stay primed for rapid and robust activation.4 The AP is thought to be initiated by the spontaneous hydrolysis of a thioester bond within C3. This leads to a conformational change in the structure of C3, resulting in a form of C3, referred to as C3(H2O), which functions like C3b with regard to its ability to bind factor B (fB). The bound fB then becomes a substrate for the serine protease factor D (fD). Cleavage of fB by fD results in formation of the initial AP C3 convertase C3(H2O)Bb, which, like the classical C3 convertase C4bC2a, can cleave C3 into C3b and C3a. The generation of C3b allows the AP to be fully activated via formation of the bona fide AP C3 convertase C3bBb (Fig. 1). Newly formed C3bBb is stabilized by the plasma protein properdin that binds to the complex and slows its deactivation.4 In fact, it should be noted that while the spontaneously generated C3(H2O)Bb is unique to AP, the C3b fragment generated from any of the pathways can bind to fB and, with the participation of fD, can form the AP C3 convertase C3bBb, which serves as an amplification loop for the entire complement system by rapidly augmenting the conversion of C3 to C3b necessary for full activation of the system and its downstream effects (Fig. 1).4
The cleavage of C3 to C3b is therefore the key step of convergence in the activation of the complement cascade.3 Apart from initiating the AP complement, C3b attaches to cells or immune complexes through covalent bonding; the opsonization of these targets by C3b or its further cleavage fragments facilitates their transportation and disposal through the endoreticular system. Additionally, C3b can associate with either of the C3 convertases to form the C5 convertase that cleaves C5 into C5a and C5b and initiates the terminal complement cascade, ultimately resulting in the formation of the multimeric membrane attack complex (MAC) (Fig. 1). In contrast to the early steps of complement activation, assembly of the cytolytic MAC on the cell surface is a nonenzymatic process, initiated by association of C6 and C7 to C5b and subsequent insertion of the C5b-7 complex into the cell membrane through a hydrophobic domain in C7.5 Further attachment of C8 and multiple copies of C9 to the membrane-residing C5b-7 leads to assembly of the MAC, which creates physical pores in the cell membrane and causes lysis.3,5
Although the above scheme of complement activation is well established, two recent findings have provided novel insight into the activation mechanism of the AP. Biochemical and gene-targeting studies have revealed a critical role of properdin in initiating AP complement activation on some, although apparently not all, susceptible surfaces.6–10 Accumulating evidence supports the conclusion that, in addition to serving as a stabilizer of C3bBb, properdin can function as a pattern recognition molecule to trigger AP complement activation and in some instances such an activity of properdin is indispensible for the AP.6,7 The second notable finding of recent studies is the requirement of MASP1/3 for normal AP complement activity.11 It has been shown that MASP-1/3 cleaves inactive fD zymogen into the active form of fD that is normally present in plasma. When MASP-1/3 is lacking, fD circulates in plasma as the non-processed and inactive zymogen incapable of supporting AP complement activation.11
Activated complement generates three major types of effectors: (i) anaphylatoxins (C3a and C5a), which are potent pro-inflammatory molecules that attract and activate leukocytes through interaction with their cognate G protein-couple receptors, C3a receptor (C3aR) and C5a receptor (C5aR); (ii) opsonins (C3b, iC3b and C3d), which decorate target surface through covalent bonding to facilitate transport and disposal of target cells or immune complexes; (iii) MAC, the terminal assembly of multiple complement proteins that directly lyses targeted (opsonized) pathogens or altered self (Fig. 1). These effectors allow the complement system to fulfil its three major biological functions, i.e. host defence, disposal of immune complexes and cellular ‘wastes’ and priming the adaptive immune systems.2
REGULATORY PROTEINS OF COMPLEMENT
While the complement system is a critical first line of defence against infections, its powerful effector functions also have the potential to harm the host. The activation of classical and lectin pathways is largely dependent on foreign materials, but under certain situations (e.g. tissue ischaemia and reperfusion), both pathways can be activated and cause autologous injury. More relevant to complement-mediated pathologies, deposition of C3b via AP activation and amplification is nondiscriminatory and, if not properly regulated, can rapidly damage host cells.4,12 This is particularly true in the context of pathogenic infection when all three pathways can be activated and bystander injury to host cells may occur more readily. To control unintended complement activation on host cells, humans and mammalian species have developed a variety of inhibitory proteins to regulate the location and efficacy of complement activation. Some of these regulatory proteins are localized on the host cell membrane to provide intrinsic protection. Membrane-bound complement regulators include decay-accelerating factor (DAF/CD55), membrane cofactor protein (MCP/CD46), complement receptor 1 (CR1/CD35) and its rodent analogue CR1-related gene/ protein y (Crry), and CD59.2,13 Others are present in the plasma to limit fluid-phase complement activation but can also protect host cells using specific recognition mechanisms. Key fluid-phase complement regulators include factor H (fH), factor I (fI), C4-binding protein (C4bp)2 and C1 inhibitor. Some of these regulators with relevance to kidney disease will be discussed in more detail in the sections below.
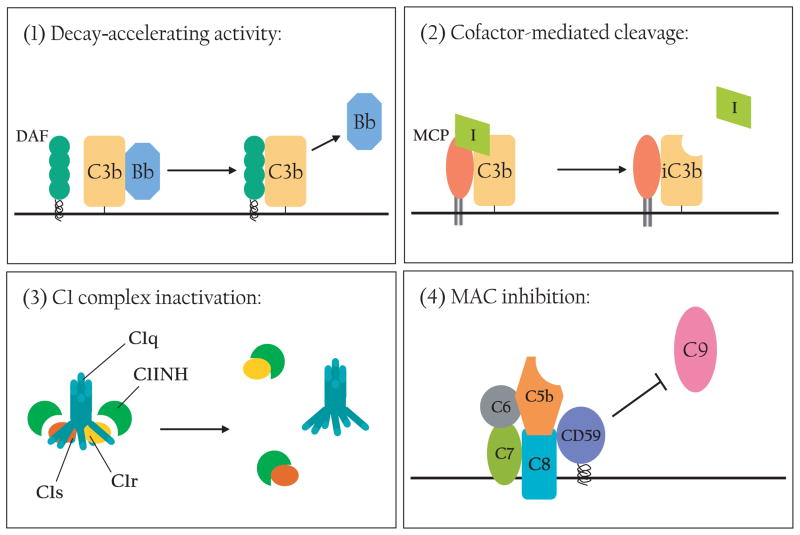
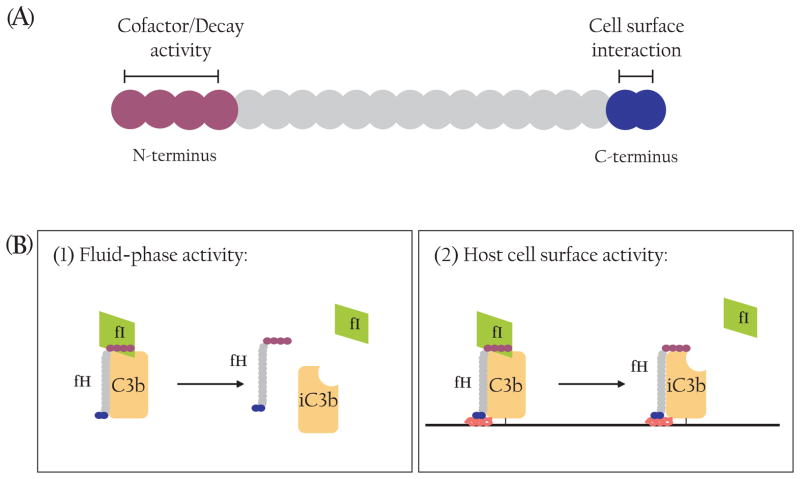
The regulatory proteins work at multiple points along the complement activation cascade (Fig. 2). Given the fact that activation of C3 is the key step in these processes, it is not surprising that several of the regulatory proteins act at the C3 convertase step, often with redundant effects.13 There are two main mechanisms by which C3 convertases are inactivated: (i) accelerated decay, or dissociation, of the C3 convertase components, and (ii) enzymatic cleavage of C3b that prevents its further participation in the cascade (Fig. 2).14 As its name suggests, DAF decreases the stability of the C3 convertases by accelerating the dissociation of C3bBb to C3b and Bb and of C4bC2a to C4b and C2a, respectively.13 MCP, fH and fI participate in the enzymatic inactivation of C3b. MCP or fH binds to C3b as a cofactor to facilitate fI-mediated cleavage of C3b.2,4 Additionally, fH has decay-accelerating activity.15 Both the cofactor and decay-accelerating activities of fH reside in the N-terminal SCR1-4 domains whereas its C-terminal domains (SCR19-20) are believed to be important for host cell surface recognition-(Fig. 3).15 CR1 mainly acts as an immune adherence receptor to facilitate the removal of C3b-opsonized immune complexes and pathogens from circulation, but it also has cofactor and decay-accelerating activities as a complement regulator.13 Crry is a rodent-specific membrane regulator with some homology to human CR1. Like CR1, Crry has both cofactor and decay-accelerating activities, but no immune adherence function has been ascribed to this protein.13 C4bp acts principally as a cofactor for fI to cleave C4b but can also inactivate C3b to a lesser degree.16 Distinct from the above discussed C3 convertase inhibitors, the plasma protein C1 inhibitor irreversibly binds to and inactivates C1r and C1s of the classical pathway and MASP of the lectin pathway and serves to inhibit the initiating steps of these activation pathways.17 The membrane protein CD59 prevents the formation of the MAC and thus works as an inhibitor of the terminal step of all activation pathways (Fig. 2).14
Fig. 2.
Mechanisms of complement regulation. (1) Decay-accelerating activity: DAF (or fH, CR1, Crry) destabilizes C3 convertases and accelerates the dissociation of C3bBb (depicted) and C4bC2a. (2) Cofactor activity: MCP (or fH, CR1, Crry) binds to C3b and serves as a cofactor for fI-mediated cleavage and inactivation of C3b. (3) C1 complex inactivation: C1INH binds to C1r and C1s to inactivate the C1 enzyme complex. (4) MAC inhibition: CD59 inhibits C9 association with C5b-8 to prevent MAC formation.
Fig. 3.
Structure and function of Factor H. (A) Schematic representation of fH functional domains. Each SCR is shown as a filled circle. The four N-terminal SCR contain cofactor and decay-accelerating activities while the two C-terminal SCR are primarily responsible for binding to the host cell surface. (B) Factor H is a unique complement regulator as it inhibits AP complement activity in the fluid-phase (1) as well as on the host cell surface (2). Only the cofactor activity of fH is illustrated here.
COMPLEMENT AND KIDNEY DISEASES
Due to its highly specialized function, the kidney is subject to significant stress from exogenous factors (e.g. pathogens, toxins and cytokines filtered from the bloodstream). Consequently, renal function is dependent on a finely calibrated immune response including proper complement activation and regulation. A critical determinant in complement-mediated kidney injury is the expression and function of complement regulatory proteins. Much work has been carried out to characterize the expression of complement regulators in the kidney of human and experimental animals.18 These studies have demonstrated considerable variation in the level of membrane regulators depending on the cell type (Table 1), suggesting that complement is regulated by distinct inhibitors within different sections of the kidney. There are also significant species differences in the relative abundance and significance of membrane regulators in the kidney. Studies of human and mouse kidneys have shown ubiquitous expression of CD59 on all major cell types within the kidney.19 However, the localization of the other inhibitory proteins is more complex. DAF is likewise ubiquitously expressed in the human kidney, but seems to be particularly abundant in the juxtaglomerular apparatus,20 while in mice DAF is mostly found on podocytes and endothelial cells.18 MCP is expressed throughout human renal tissues, but rodents do not normally express MCP besides spermatozoa.18,19 In humans, CR1 is mostly restricted to erythrocytes and podocytes18 but like MCP, rodents only have limited expression of CR1 that is generated by alternative splicing from the Cr1/2 gene.21 In place of MCP, the rodent-specific complement regulator Crry is expressed ubiquitously in mice (e.g. endothelium, mesangium, tubules)18,19 and is considered a functional homolog of human MCP.13,22
Table 1.
Location and function of complement regulators in kidney
| Name | Mechanism of Action | Sites of expression in kidney
|
|
|---|---|---|---|
| Humans | Rodents | ||
| CD59 | Inhibits MAC complex formation | Ubiquitous | Ubiquitous |
| DAF (CD55) | Decay-accelerating activity against C3 convertases | Ubiquitous | Podocytes, endothelial cells |
| MCP (CD46) | Cofactor for fI inactivation of C3 convertases | Ubiquitous | None (spermatozoa) |
| CR1 (CD35) | Decay-accelerating and cofactor activity against C3 convertases | Podocytes (and erythrocytes) | None (B cells, DC) |
| Crry | Decay-accelerating and cofactor activity against C3 convertases | None (rodents only) | Endothelium, mesangium, tubules |
| Factor H (fH) | Decay-accelerating and cofactor activity against AP C3 convertase | Plasma (binding sites on cells) | Plasma; platelets*, podocytes* (also binding sites on cells) |
| Factor I (fI) | Protease for cofactor inactivation of C3 convertases | Plasma | Plasma |
| C4bp | Cofactor for fI inactivation of CP C3 convertase | Plasma | Plasma |
Adapted from reference.18
Asterisk (*) indicates the regulator also functions as an immune complex receptor on these cells.
Clinically, strong connections between complement and kidney diseases have been provided by cases of deficiency or dysfunction of the fluid-phase complement regulators fH and fI.23–27 Unlike the membrane-bound inhibitors, the fluid-phase inhibitors circulate in the plasma and are largely produced outside the kidney in the liver.15,16,28 However, there is evidence that fH can be synthesized by some phagocytic cells and by murine platelets and podocytes.16,18,29,30 These observations notwithstanding, the current view of fH function, supported by both clinical and animal modelling studies, is that it works principally as a fluid-phase protein to prevent AP complement activation in the plasma as well as on the cell surface (Fig. 3). The latter activity of fH is dependent on its C-terminal domains that bind to surface deposited C3b in the context of host cell-specific polyanionic constituents (Fig. 3).31,32 The identity of the host cell components with which fH interacts has not been positively identified, although heparin has been used frequently as a model ligand in in vitro experiments and several studies have shown that fH can bind to glycosaminoglycans expressed on the cell surface.33,34 Whatever the binding partner(s) may be, it is clear that fH attachment to renal endothelial cells is essential to kidney health, particularly under pathological conditions.32,35
Many of the kidney disorders that have been linked to complement can be attributed to insufficient complement regulation, either as a result of regulator deficiency or dysfunction, or due to exuberant AP complement amplification that overwhelms the normal regulatory mechanisms.36–39 A few of these conditions are highlighted and discussed below.
Ischaemia-reperfusion injury
Ischaemia-reperfusion injury (IRI) is one of the most frequent causes of acute renal failure (ARF) and can have devastating effects on kidney function. Not only does IRI contribute to 50% of intrinsic cases of ARF, but systemic illnesses such as congestive heart failure or sepsis can also reduce renal blood flow and cause ischaemic injury.40 Transplant surgery also involves IRI and can cause ARF from depressed blood flow during anaesthesia on top of the inflammation from the ischaemic tissue being transplanted. When hypoxic conditions exist (i.e. reduced blood flow), cell metabolism is impaired, which generates reactive oxygen species and apoptotic signals.41 While ischaemia causes initial injury, the following reperfusion is far more damaging. Upon restoration of blood flow, cells produce additional reactive oxygen species and cytokines as well as adhesion molecules to attract immune cells, exacerbating tissue injury.41,42
Many studies have demonstrated that complement activation contributes to kidney IRI.43–45 The mechanisms by which complement is triggered during IR and the effectors that are responsible for renal IRI remains to be fully elucidated, but loss or reduced function of complement regulators are likely to play a role. Accordingly, patients with one or more of their regulators deficient or defective may be at increased risk of suffering from IRI. In a study of mice deficient in DAF and CD59, either alone or in combination, Yamada et al. have shown that both regulators are important in preventing catastrophic renal IRI.46 Thus, although DAF-deficient, but not CD59-deficient, mice were significantly more susceptible to renal IRI than wild-type mice, DAF/CD59 double deficiency caused a much greater degree of renal pathology and functional impairment, suggesting that CD59 deficiency in the context of DAF deficiency exacerbated renal injury even though CD59 deficiency alone was inconsequential.46 One of the consequences of ischaemia may be cell membrane disruption, resulting in the transient loss of membrane regulators such as DAF and CD59. Both of these proteins attach to the cell membrane via a GPI anchor and are known to be capable of shedding from and reincorporating into the lipid bilayer of the cell membrane.47 Positional and functional disruption of transmembrane regulators may also occur as has been shown for mouse Crry during renal IR.48 It has been demonstrated that Crry, normally found on the basolateral side of tubular cells along the basement membrane, was sequestered in the tubular lumen upon ischaemic insult, allowing increased complement deposition and injury on these cells.48 Additionally, changes in the cell membrane structural integrity and exposure of neoepitopes may alter the binding kinetics of the fluid-phase complement regulator fH, which can also impact on complement activation and renal IRI.49,50
Although both classical and lectin pathways have been implicated in IRI of other organs, likely through binding of natural antibodies and MBL to neoepitopes exposed on ischaemic cells, most animal modelling studies in mice have suggested that renal IRI is mediated by the AP.43 Nevertheless, there is evidence that CP and MBL activation may be important contributors to IRI in some cases of transplant rejection as renal biopsies from these patients showed numerous deposits of C3d and C4d.51,52 Clinical studies have also shown that while injury can decrease complement regulation in some cells, there are cases where inhibitor expression actually increases in response to injury, which can offer enhanced protection from complement-mediated injury.53–56 A recent study with patients experiencing allograft rejection presented evidence that increased DAF expression correlated with increased allograft survival.51 These studies highlight the relevance of complement regulator to renal IRI and transplantation rejection.
Glomerulonephritis
Glomerulonephritis is one of the most common causes of chronic kidney disease and end-stage renal failure in the world.57 It does not describe a single disease but rather a general phenotype, characterized by glomerular inflammation and cellular proliferation, that produces a number of clinical consequences such as haematuria, proteinuria and reduced glomerular filtration.57 The disease can manifest as a symptom of systemic disorders such as lupus, Goodpasture’s syndrome (anti-glomerular basement membrane (GBM) glomerulonephritis) and anti-neutrophil cytoplasmic autoantibody (ANCA)-induced glomerulonephritis, or a kidney-specific condition as in membranoproliferative glomerulonephritis (MPGN).58
Anti-GBM-induced glomerulonephritis is characterized by immune complex deposition along the GBM. Often, these immune complexes contain autoantibodies against basement membrane proteins such as type IV collagen and neutral endopeptidase.57 Depending on the antigen, these autoantibodies can cause damage outside the kidney, such as lung damage in Goodpasture’s syndrome, or trigger relapses post-transplantation as seen in Alport’s syndrome.57 Many studies have shown that the complement system affects anti-GBM glomerulonephritis in human patients by amplifying antibody-mediated injury through the classical pathway and enhancing the inflammatory response through C5 activation.57–59 The involvement of complement in this disease has also been corroborated by animal modelling studies. The most commonly used experimental model is nephrotoxic serum nephritis, in which IgG antibodies from another species are administered to mice, followed by an injection of antiserum to mouse GBM (generated in the same species as first injection) to cause immune complex deposition and glomerular injury. Initially, it was shown that deficiency of C3 or C4 reduced renal disease,60 confirming complement’s contribution to renal inflammation and injury. Subsequent studies using regulator-deficient mice demonstrated that loss of DAF, Crry, fH and/or CD59 all exacerbated anti-GBM glomerulonephritis,61–64 highlighting the relevance of complement control mechanisms in autoimmune kidney injury.
As in anti-GBM nephritis, ANCA-associated glomerulonephritis is triggered by autoantibodies. However, instead of the antigen being a component of the damaged tissue, the antibodies recognize neutrophil components, usually myeloperoxidase (MPO) or proteinase 3 (PR3).65,66 These antibodies activate neutrophils, which then attack the surrounding vessels and tissues and lead to vasculitis and frequently pauci-immune necrotizing crescentic glomerulonephritis.66,67 Several studies have demonstrated this role of activated neutrophils in ANCA-associated glomerulonephritis in animal models using anti-MPO or anti-proteinase 3 antibodies.67,68 Recently, complement activation, especially the AP, has been implicated as a key step in the development of this disease. Initially, Xiao et al. demonstrated that wild type or C4-deficient mice exhibited symptoms of ANCA-associated glomerulonephritis while C5 or fB-deficient mice did not develop disease.65 Further investigation also demonstrated that this anti-MPO antibody-induced disease could also be prevented by administering a C5 inhibitory antibody.69 The involvement of complement is also supported by several clinical studies that showed the presence of complement components in renal biopsies from ANCA-associated glomerulonephritis patients.70,71 The mechanistic link between ANCA-induced neutrophil activation and initiation of the AP complement system remains to be elucidated, and whether anti-complement therapy might be effective clinically is yet to be established.
Unlike systemic causes of glomerulonephritis, MPGN is defined by mesangial cell proliferation and double contours in the GBM from rapid expansion.72 Subendothelial or intramembranous deposits in glomeruli cause these morphological changes, and the location and contents of these deposits distinguish the subclasses of MPGN.57,72 MPGN type I has subendothelial immune complexes with C1q and is associated with classical pathway complement activation.72,73 Some consider MPGN type III a subset of type I, as it has the same features of type I with additional subepithelial deposits.72 MPGN type II, sometimes called dense deposit disease, does not have immune complexes, but instead is identified by electron-dense intramembranous deposits.74,75 MPGN is a rare disease, observed in USA and western Europe in 2–7% of renal biopsies, but in certain populations of eastern European, African and Asian descent it has been found in up to 30% of renal biopsies.73 Regardless of its incidence, the prognosis for MPGN is poor as treatments are limited and often unsuccessful.
While type I MPGN has been linked to the classical pathway, type II MPGN is associated with overactive AP complement activity,76 often due to the presence of an immunoglobulin termed C3 nephritic factor that binds to the AP C3 convertase and delays its inactivation.72 Interestingly, many cases of MPGNII have also been documented where patients have defective or deficient fH.77,78 Many MPGNII patients also have ocular drusen deposits, which are linked to uncontrolled AP activity and age-related macular degeneration (AMD) pathogenesis.75,78,79 Animal studies have confirmed the role of overactive AP activity in the development of MPGNII. Both pigs with a natural mutation of fH80 and mice engineered by gene targeting to be deficient in fH developed MPGN that resembled the human disease.64 fH knockout mice had low circulating levels of C3 but strong C3 and C9 deposition within the kidney, especially along the capillary walls and mesangium in glomeruli.64 By 8 months the fH knockout mice had spontaneously developed electron-dense deposits similar to those seen in MPGNII patients.64 The fH-deficient mice were also more susceptible to kidney damage when subjected to acute kidney injury by accelerated nephrotoxic serum nephritis.64 Subsequent studies demonstrated that renal injury was prevented in fH knockout mice that were also C5-deficient or when given an inhibitory anti-C5 antibody, suggesting that terminal complement activation contributes to the pathology.59 Interestingly, when fI KO mice were generated they also showed low plasma C3 levels, indicating complement consumption, but unlike fH knockout mice they did not develop MPGN.81 Furthermore, fH/fI double deficient mice also failed to develop MPGN.81 Because fI converts C3b into iC3b and C3d, these data suggest that the development of MPGN may depend more on the forms of activated C3 generated by the AP.
Thrombotic microangiopathies
Thrombotic microangiopathies are a group of diseases characterized by thrombocytopenia, microangiopathic haemolytic anaemia, and either impaired renal or neurologic function.82 Thrombotic thrombocytopenic pupura has varying degrees of renal impairment, but many other organs can be affected, particularly the nervous system. Contrastingly, haemolytic uraemic syndrome (HUS) is another disease in this category, but symptoms are largely restricted to the kidney. There are two types of HUS, distinguished by the presence or absence of diarrhoea caused by Shiga toxin-producing bacteria.82 Diarrhoea-positive, or D+ HUS, is the most common form of HUS and can usually be cured with antibiotics and symptomatic treatment.82 On the other hand, diarrhoea-negative HUS, often referred to as atypical HUS (aHUS), only makes up 5–10% of HUS cases but has a much poorer prognosis.83 Approximately 50% of aHUS patients progress to end-stage renal failure and at least 25% of cases are fatal.84 It is still unclear what triggers aHUS episodes although it is believed to be initiated by endothelial cell injury caused by infection or other exogenous injury.35
While mutations in procoagulant proteins such as thrombomodulin have been found in some aHUS cases,85 the majority of mutations found in aHUS patients have been with AP complement proteins. A multitude of clinical studies over the last decade have demonstrated that at least half of the familial cases of aHUS are caused by mutations in the complement system that lead to uncontrolled AP activation.25,35,86 While a few cases have reported mutations in C3 or fB that tend to produce aberrant C3bBb convertases more resistant to inactivation,87–89 most mutations affect the function of regulatory proteins fH, fI and MCP.35,90,91 In fact, the genes for these proteins are all located on the same region of chromosome 1 (1q32), called the regulators of complement activation gene cluster,92,93 making the latter a ‘hot’ chromosomal spot for aHUS-related mutations. A few cases of dysfunctional C4bp have also been reported,94 but interestingly DAF, another regulators of complement activation gene, has not been linked to any aHUS patients to date.95 The majority of mutations are found in fH (30%), while fI and MCP mutations account for 15% and 10% of aHUS patients, respectively.25,83,91 Most fI and MCP mutations functionally impair their ability to inactivate C3b, but surprisingly the majority of fH mutations are not in the functional N-terminus; instead they cluster in the C-terminal domains (SCR 19-20) that mediate fH binding to the cell surface.35,83 An additional population of aHUS patients (5%) are characterized by the development of autoantibodies to fH that inhibit fH binding to host cells.96 Recent studies have demonstrated that many of these autoantibody-positive patients have deletion or alternative splicing of CFHR1 and CFHR3,97,98 two fH-related genes that encode plasma proteins with 5 SCRs that have homologous C-termini with fH. These findings suggest that lack of CFHR may play a role in fH autoantibody production and aHUS pathogenesis.
Corresponding biochemical and animal studies have bolstered the clinical data and reaffirmed the causal link between increased AP activity and the development of aHUS symptoms. A number of in vitro studies with human fH have demonstrated that loss of fH binding to cells (with intact fluid-phase complement-regulating activity) can cause complement deposition, cell lysis and platelet activation, all characteristics of aHUS.31,99–101 For example, a recombinant protein composed of the two C-terminal SCR domains of human fH and lacking complement regulator function has been shown to compete with native fH for cell binding and, when added to normal human serum, caused AP-dependent erythrocyte lysis.31 The concept that impaired binding to host cells but normal plasma AP complement-regulating activity of fH correlates with aHUS pathogenesis is also supported by a murine model of aHUS.102 While, as discussed above, complete fH deficiency led to depletion of plasma AP complement and the development of MPGN,64 transgenic expression in fH knockout mice of a truncated murine fH protein containing SCR1-16, which lacks the ability to interact with host cells, partially restored plasma AP complement activity.102 Instead of developing MPGN, by 8 weeks of age most of the transgenic mice had spontaneously developed aHUS symptoms – significant haematuria and anasarca, low platelet blood counts and significant kidney tissue remodelling with thrombi throughout the glomeruli.102 The development of this in vivo model of aHUS not only confirmed complement’s contribution to aHUS pathology and shed light on the mechanism of action of fH, but also created a valuable tool with which complement-focused therapies can be tested.
COMPLEMENT-BASED THERAPEUTIC STRATEGIES FOR KIDNEY DISEASE
The kidney diseases discussed above can be life-threatening and most have limited, often unsuccessful, treatment options. Many patients with MPGN and aHUS experience recurrent episodes that eventually lead to end-stage renal failure.40,57,84 Even when kidney transplants are successful, diseases that are caused by systemic factors such as mutated fH, C3 and fB can present again and the outcome is often fatal.72,103 In such situations, combined kidney and liver transplantation may be the only way to correct the underlying defects, and success with such an approach has been described in the literature but the high risk for adverse events in such procedures makes this a less desirable option.104,105 By the same principle, kidney transplantation may be an acceptable option for end-stage aHUS patients whose diseases are attributable to mutations in the membrane regulator MCP.91,106 Given the well-established role of complement in the pathogenesis of these kidney diseases, it is envisioned that systemic or targeted local complement inhibition may represent a promising therapeutic strategy. In this context, the recent approval and successful clinical application of a first-in-class complement inhibitor Eculizumab, a humanized anti-C5 monoclonal antibody,107 for treatment of the complement-mediated disease paroxysmal nocturnal haemaglobinuria108–110 is particularly encouraging. Based on a number of animal studies in which C5 deficiency or C5-blocking antibodies reduced renal injury,59,69,111 it may be anticipated that Eculizumab will prove to be efficacious for some, if not all, complement-mediated kidney disorders as well. Indeed, two case reports on the successful treatments of paediatric aHUS patients with Eculizumab have already appeared in the literature112,113 and clinical trials on the use of Eculizumab in aHUS are currently underway.114
Other complement-based therapeutic strategies include chemical and biological agents that target additional complement components. A chemical inhibitor for C3aR and two antagonists for C5aR, a cyclic hexapeptide and a recombinant C5a analogue, have been developed and shown to effectively block anaphylatoxin-mediated inflammatory injury in a variety in vitro and in vivo studies including models of renal IRI and transplantation.115–118 A synthetic peptide, named Compstatin, with potent human C3-inhibiting activity has also been developed by phage display and shown to effectively shut down human complement activation in several experiments including an ex vivo model of hyperacute rejection of kidney xenotransplantation model.119–121 Compstatin is currently being evaluated in clinical trials for the treatment of AMD, a disease that also implicates abnormal AP complement activation.122 One of the concerns of targeting C3 with agents like Compstatin is that they obliterate the complement system completely, potentially compromising host defence and leaving the patients susceptible to infection. Because the AP complement is principally involved in many of the complement-mediated diseases, efforts have also been made to develop inhibitors that target the AP only. For example, two anti-C3b mAbs that specifically inhibit the AP C3 convertase with no activity on classical and lectin pathway complement activation have been described recently.123,124
A third area of promising research for treating complement-mediated kidney injury is the creation of soluble recombinant forms of complement regulatory proteins. Several studies have shown that administering a soluble form of CR1 or Crry can reduce renal injury125,126 and such proteins have an extended half-life when fused to an Ig Fc domain.127 More recently, strategies have been developed to target the recombinant protein to sites of injury. He et al. targeted recombinant regulatory proteins to the kidney using an Ag-specific single chain Ab fragment.128 In other efforts, the inhibitors were directed to sites of complement activation with the design of a fusion protein consisting the C3d-binding domain of CR2 and a regulatory protein partner, either Crry (CR2-Crry) or the SCR1-5 region of fH (CR2-fH).129 In one study of MRL/lpr mice, which are prone to autoimmune glomerulonephritis and vasculitis, CR2-Crry ameliorated disease symptoms compared with untreated mice.130 Studies with these recombinant proteins have also been performed for other diseases with a strong AP component, including intestinal IRI and collagen-induced arthritis.129,131 These studies demonstrated protection from disease when the complement-targeted fusion proteins were administered, making them excellent candidates to test in additional renal disease models.
CONCLUSION
It is clear that the complement system plays a detrimental role in many kidney diseases and identification and validation of complement inhibitors may provide a promising avenue of drug development for these disorders, which mostly lack effective therapies. The majority of these conditions appear to be mediated by an overactive AP complement system, which can result from mutations in membrane or fluid-phase complement regulators leading to inadequate control of activation or from gain of function mutations in fB or C3 giving rise to a more stable C3bBb enzyme complex. Although some of these diseases are rare in the population, their studies have provided important insight to the pathogenesis of complement-mediated tissue injury as well as new understanding of mechanisms of action of complement regulatory proteins. These advances have also fueled many efforts to develop targeted therapies for these disorders and it is likely that one or more complement-based drugs for kidney diseases will reach the clinic in the near future. Given the fact that complement-mediated kidney pathologies share characteristics with other common diseases such as AMD and rheumatoid arthritis that have been linked to complement and for which intense effort of drug development is also being made, continued translational studies in this field may benefit other areas of investigation of complement biology and therapeutics and vice versa.
SUMMARY AT A GLANCE.
When the complement regulatory mechanisms are dysfunctional or overwhelmed, complement attack of autologous tissues can occur with severe, sometimes life-threatening consequences. This cutting edge review describes recent advances on the activation and regulation of the complement system in kidney disease, with a particular emphasis on the relevance of complement regulatory proteins.
References
- 1.Lachmann P. Complement before molecular biology. Mol Immunol. 2006;43:496–508. doi: 10.1016/j.molimm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Janeway C, Jr, Travers P, Walport M, Schlomchik M. Immunobiology: The Immune System in Health and Disease. 6. New York: Garland Publishing; 2005. [Google Scholar]
- 4.Lachmann PJ, Frederick WA. The Amplification Loop of the Complement Pathways. Adv Immunol. 2009;104:115–49. doi: 10.1016/S0065-2776(08)04004-2. Academic Press. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Eberhard HJ. Transmembrane channel-formation by five complement proteins. Biochem Soc Symp. 1985;50:235–46. [PubMed] [Google Scholar]
- 6.Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol. 2007;179:2600–8. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Miwa T, Zhou L, Song WC. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood. 2008;111:732–40. doi: 10.1182/blood-2007-05-089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira VP, Cortes C, Pangburn MK. Native polymeric forms of properdin selectively bind to targets and promote activation of the alternative pathway of complement. Immunobiology. 2010 doi: 10.1016/j.imbio.2010.02.002. (forthcoming) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemper C, Hourcade DE. Properdin: New roles in pattern recognition and target clearance. Mol Immunol. 2008;45:4048–56. doi: 10.1016/j.molimm.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hourcade DE. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem. 2006;281:2128–32. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M, Ishida Y, Iwaki D, et al. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–37. S1–3. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell Mol Med. 2008;12:1074–84. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–36. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Uszewski MK, Farries TC, Lubun DM, Rooney IA, Atkinson JP, Frank JD. Adv Immunol. Vol. 61. Academic Press; 1996. Control of the complement system; pp. 201–83. [DOI] [PubMed] [Google Scholar]
- 15.Zipfel PF, Skerka C, Hellwage J, et al. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30:971–8. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
- 16.Blom AM, Villoutreix BO, Dahlback B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol. 2004;40:1333–46. doi: 10.1016/j.molimm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Mollnes TE, Kirschfink M. Strategies of therapeutic complement inhibition. Mol Immunol. 2006;43:107–21. doi: 10.1016/j.molimm.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Puri TS, Quigg RJ. The many effects of complement C3- and C5-binding proteins in renal injury. Semin Nephrol. 2007;27:321–37. doi: 10.1016/j.semnephrol.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Nangaku M. Complement regulatory proteins in glomerular diseases. Kidney Int. 1998;54:1419–28. doi: 10.1046/j.1523-1755.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 20.Cosio FG, Sedmak DD, Mahan JD, Nahman NS., Jr Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 1989;36:100–7. doi: 10.1038/ki.1989.167. [DOI] [PubMed] [Google Scholar]
- 21.Prechl J, Erdei A. Immunomodulatory functions of murine CR1/2. Immunopharmacology. 2000;49:117–24. doi: 10.1016/s0162-3109(00)80297-0. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Sallee C, Dehoff M, Foley S, Molina H, Holers VM. Mouse Crry/p65. Characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J Immunol. 1993;151:4295–305. [PubMed] [Google Scholar]
- 23.Pettigrew HD, Teuber SS, Gershwin ME. Clinical significance of complement deficiencies. Ann N Y Acad Sci. 2009;1173:108–23. doi: 10.1111/j.1749-6632.2009.04633.x. [DOI] [PubMed] [Google Scholar]
- 24.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–40. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 25.Kavanagh D, Richards A, Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan M, Erlic Z, Hoffmann MM, et al. Epidemiological approach to identifying genetic predispositions for atypical hemolytic uremic syndrome. Ann Hum Genet. 2010;74:17–26. doi: 10.1111/j.1469-1809.2009.00554.x. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson SC, Kalchishkova N, Trouw LA, Fremeaux-Bacchi V, Villoutreix BO, Blom AM. Mutations in complement factor I as found in atypical hemolytic uremic syndrome lead to either altered secretion or altered function of factor I. Eur J Immunol. 2010;40:172–85. doi: 10.1002/eji.200939280. [DOI] [PubMed] [Google Scholar]
- 28.Kavanagh D, Goodship TH. Membrane cofactor protein and factor I: mutations and transplantation. Semin Thromb Hemost. 2006;32:155–9. doi: 10.1055/s-2006-939771. [DOI] [PubMed] [Google Scholar]
- 29.Alexander JJ, Aneziokoro OG, Chang A, et al. Distinct and separable roles of the complement system in factor H-deficient bone marrow chimeric mice with immune complex disease. J Am Soc Nephrol. 2006;17:1354–61. doi: 10.1681/ASN.2006020138. [DOI] [PubMed] [Google Scholar]
- 30.Alexander JJ, Quigg RJ. The simple design of complement factor H: Looks can be deceiving. Mol Immunol. 2007;44:123–32. doi: 10.1016/j.molimm.2006.07.287. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J Immunol. 2006;177:6308–16. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- 32.Jokiranta TS, Cheng ZZ, Seeberger H, et al. Binding of complement factor H to endothelial cells is mediated by the carboxy-terminal glycosaminoglycan binding site. Am J Pathol. 2005;167:1173–81. doi: 10.1016/S0002-9440(10)61205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira VP, Herbert AP, Cortes C, et al. The binding of factor h to a complex of physiological polyanions and c3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol. 2009;182:7009–18. doi: 10.4049/jimmunol.0804031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pangburn MK. Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacology. 2000;49:149–57. doi: 10.1016/s0162-3109(00)80300-8. [DOI] [PubMed] [Google Scholar]
- 35.Kavanagh D, Goodship TH, Richards A. Atypical haemolytic uraemic syndrome. Br Med Bull. 2006;77–78:5–22. doi: 10.1093/bmb/ldl004. [DOI] [PubMed] [Google Scholar]
- 36.Lihua B, Richard JQ. Complement in lupus nephritis: the good, the bad, and the unknown. Semin Nephrol. 2007;27:69–80. doi: 10.1016/j.semnephrol.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–44. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 38.Klein RJ, Zeiss C, Chew EY, et al. complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–8. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 41.Ryter SW, Kim HP, Hoetzel A, et al. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 42.Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest. 1997;99:2682–90. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thurman JM. Triggers of inflammation after renal ischemia/ reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurman JM, Lenderink AM, Royer PA, et al. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178:1819–28. doi: 10.4049/jimmunol.178.3.1819. [DOI] [PubMed] [Google Scholar]
- 45.Thurman JM, Royer PA, Ljubanovic D, et al. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol. 2006;17:707–15. doi: 10.1681/ASN.2005070698. [DOI] [PubMed] [Google Scholar]
- 46.Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol. 2004;172:3869–75. doi: 10.4049/jimmunol.172.6.3869. [DOI] [PubMed] [Google Scholar]
- 47.Kooyman DL, Byrne GW, McClellan S, et al. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science. 1995;269:89–92. doi: 10.1126/science.7541557. [DOI] [PubMed] [Google Scholar]
- 48.Thurman JM, Ljubanovic D, Royer PA, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006;116:357–68. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman K, Renner B, Ferreira V, et al. Expression of the protein Annexin A2 in the kidney after ischaemia/reperfusion enhances binding of factor H and inhibition of the alternative complement pathway. Mol Immunol. 2008;45:4111. [Google Scholar]
- 50.Trouw LA, Bengtsson AA, Gelderman KA, Dahlback B, Sturfelt G, Blom AM. C4b-binding protein and factor H compensate for the loss of membrane-bound complement inhibitors to protect apoptotic cells against excessive complement attack. J Biol Chem. 2007;282:28540–48. doi: 10.1074/jbc.M704354200. [DOI] [PubMed] [Google Scholar]
- 51.Brodsky SV, Nadasdy GM, Pelletier R, et al. Expression of the decay-accelerating factor (CD55) in renal transplants – a possible prediction marker of allograft survival. Transplantation. 2009;88:457–64. doi: 10.1097/TP.0b013e3181b0517d. [DOI] [PubMed] [Google Scholar]
- 52.Castellano G, Melchiorre R, Loverre A, et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol. 2010;176:1648–59. doi: 10.2353/ajpath.2010.090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abe K, Miyazaki M, Koji T, et al. Expression of decay accelerating factor mRNA and complement C3 mRNA in human diseased kidney. Kidney Int. 1998;54:120–30. doi: 10.1046/j.1523-1755.1998.00961.x. [DOI] [PubMed] [Google Scholar]
- 54.Lin F, Emancipator SN, Salant DJ, Medof ME. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest. 2002;82:563–9. doi: 10.1038/labinvest.3780451. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad SR, Lidington EA, Ohta R, et al. Decay-accelerating factor induction by tumour necrosis factor-B, through a phosphatidylinositol-3 kinase and protein kinase C-dependent pathway, protects murine vascular endothelial cells against complement deposition. Immunology. 2003;110:258–68. doi: 10.1046/j.1365-2567.2003.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibata T, Cosio F, Birmingham D. Complement activation induces the expression of decay-accelerating factor on human mesangial cells. J Immunol. 1991;147:3901–8. [PubMed] [Google Scholar]
- 57.Foster MH. Novel targets for immunotherapy in glomerulonephritis. Biologics. 2008;2:531–45. doi: 10.2147/btt.s2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turnberg D, Cook HT. Complement and glomerulonephritis: new insights. Curr Opin Nephrol Hypertens. 2005;14:223–8. doi: 10.1097/01.mnh.0000165887.75501.24. [DOI] [PubMed] [Google Scholar]
- 59.Pickering MC, Warren J, Rose KL, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA. 2006;103:9649–54. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheerin NS, Springall T, Carroll MC, Hartley B, Sacks SH. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3- and C4-deficient mice. Clin Exp Immunol. 1997;110:403–9. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sogabe H, Nangaku M, Ishibashi Y, et al. Increased susceptibility of decay-accelerating factor deficient mice to anti-glomerular basement membrane glomerulonephritis. J Immunol. 2001;167:2791–7. doi: 10.4049/jimmunol.167.5.2791. [DOI] [PubMed] [Google Scholar]
- 62.Miwa T, Zhou L, Tudoran R, et al. DAF/Crry double deficiency in mice exacerbates nephrotoxic serum-induced proteinuria despite markedly reduced systemic complement activity. Mol Immunol. 2007;44:139–46. doi: 10.1016/j.molimm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 63.Lin F, Salant DJ, Meyerson H, Emancipator S, Morgan BP, Medof ME. Respective roles of decay-accelerating factor and CD59 in circumventing glomerular injury in acute nephrotoxic serum nephritis. J Immunol. 2004;172:2636–42. doi: 10.4049/jimmunol.172.4.2636. [DOI] [PubMed] [Google Scholar]
- 64.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–8. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 65.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falk RJ, Jennette JC. ANCA disease: where is this field heading? J Am Soc Nephrol. 2010;21:745–52. doi: 10.1681/ASN.2009121238. [DOI] [PubMed] [Google Scholar]
- 67.Xiao H, Heeringa P, Liu Z, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfister H, Ollert M, Frohlich LF, et al. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104:1411–18. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- 69.Huugen D, van Esch A, Xiao H, et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71:646–54. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 70.Xing GQ, Chen M, Liu G, et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol. 2009;29:282–91. doi: 10.1007/s10875-008-9268-2. [DOI] [PubMed] [Google Scholar]
- 71.Chen M, Xing GQ, Yu F, Liu G, Zhao MH. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol Dial Transplant. 2009;24:1247–52. doi: 10.1093/ndt/gfn586. [DOI] [PubMed] [Google Scholar]
- 72.Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409–18. doi: 10.1007/s00467-009-1322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benz K, Amann K. Pathological aspects of membranoproliferative glomerulonephritis (MPGN) and haemolytic uraemic syndrome (HUS) / thrombocytic thrombopenic purpura (TTP) Thromb Haemost. 2009;101:265–70. [PubMed] [Google Scholar]
- 74.Noris M, Remuzzi G. Translational mini-review series on complement factor H: therapies of renal diseases associated with complement factor H abnormalities: atypical haemolytic uraemic syndrome and membranoproliferative glomerulonephritis. Clin Exp Immunol. 2008;151:199–209. doi: 10.1111/j.1365-2249.2007.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker PD. Dense deposit disease: new insights. Curr Opin Nephrol Hypertens. 2007;16:204–12. doi: 10.1097/MNH.0b013e3280bdc0f4. [DOI] [PubMed] [Google Scholar]
- 76.Sethi S, Gamez JD, Vrana JA, et al. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75:952–60. doi: 10.1038/ki.2008.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, et al. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15:787–95. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- 78.Montes T, Goicoechea de Jorge E, Ramos R, et al. Genetic deficiency of complement factor H in a patient with age-related macular degeneration and membrano-proliferative glomerulonephritis. Mol Immunol. 2008;45:2897–904. doi: 10.1016/j.molimm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 79.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J Am Soc Nephrol. 2005;16:1392–403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 80.Hogasen K, Jansen JH, Mollnes TE, Hovdenes J, Harboe M. Hereditary porcine membranoproliferative glomerulonephritis type II is caused by factor H deficiency. J Clin Invest. 1995;95:1054–61. doi: 10.1172/JCI117751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–18. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matouk C, Marsden PA. Molecular Insights into the Thrombotic Microangiopathies. Philadelphia; Saunders Elsevier: 2008. [Google Scholar]
- 83.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–77. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caprioli J, Peng L, Remuzzi G. The hemolytic uremic syndromes. Curr Opin Crit Care. 2005;11:487–92. doi: 10.1097/01.ccx.0000176688.10810.30. [DOI] [PubMed] [Google Scholar]
- 85.Delvaeye M, Noris M, De Vriese A, et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–57. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loirat C, Noris M, Fremeaux-Bacchi V. Complement and the atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2008;23:1957–72. doi: 10.1007/s00467-008-0872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fremeaux-Bacchi V, Miller EC, Liszewski MK, et al. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–52. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tawadrous H, Maga T, Sharma J, Kupferman J, Smith RJ, Schoeneman M. A novel mutation in the Complement Factor B gene (CFB) and atypical hemolytic uremic syndrome. Pediatr Nephrol. 2010;25:947–51. doi: 10.1007/s00467-009-1415-3. [DOI] [PubMed] [Google Scholar]
- 89.de Jorge EG, Harris CL, Esparza-Gordillo J, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci. 2007;104:240–45. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Cordoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol. 2008;151:1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caprioli J, Noris M, Brioschi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–79. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodriguez de Cordoba S, Diaz-Guillen MA, Heine-Suner D. An integrated map of the human regulator of complement activation (RCA) gene cluster on 1q32. Mol Immunol. 1999;36:803–8. doi: 10.1016/s0161-5890(99)00100-5. [DOI] [PubMed] [Google Scholar]
- 93.Esparza-Gordillo J, Goicoechea de Jorge E, Buil A, et al. Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet. 2005;14:703–12. doi: 10.1093/hmg/ddi066. [DOI] [PubMed] [Google Scholar]
- 94.Blom AM, Bergstrom F, Edey M, et al. A novel non-synonymous polymorphism (p.Arg240His) in C4b-binding protein is associated with atypical hemolytic uremic syndrome and leads to impaired alternative pathway cofactor activity. J Immunol. 2008;180:6385–91. doi: 10.4049/jimmunol.180.9.6385. [DOI] [PubMed] [Google Scholar]
- 95.Kavanagh D, Burgess R, Spitzer D, et al. The decay accelerating factor mutation I197V found in hemolytic uraemic syndrome does not impair complement regulation. Mol Immunol. 2007;44:3162–7. doi: 10.1016/j.molimm.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 96.Skerka C, Jozsi M, Zipfel PF, Dragon-Durey MA, Fremeaux-Bacchi V. Autoantibodies in haemolytic uraemic syndrome (HUS) Thromb Haemost. 2009;101:227–32. [PubMed] [Google Scholar]
- 97.Jozsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/ CFHR3 deficiency. Blood. 2008;111:1512–14. doi: 10.1182/blood-2007-09-109876. [DOI] [PubMed] [Google Scholar]
- 98.Zipfel PF, Edey M, Heinen S, et al. Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PloS Genet. 2007;3:e41. doi: 10.1371/journal.pgen.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heinen S, Jozsi M, Hartmann A, et al. Hemolytic uremic syndrome: a factor H mutation (E1172Stop) causes defective complement control at the surface of endothelial cells. J Am Soc Nephrol. 2007;18:506–14. doi: 10.1681/ASN.2006091069. [DOI] [PubMed] [Google Scholar]
- 100.Jozsi M, Oppermann M, Lambris JD, Zipfel PF. The C-terminus of complement factor H is essential for host cell protection. Mol Immunol. 2007;44:2697–706. doi: 10.1016/j.molimm.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stahl AL, Vaziri-Sani F, Heinen S, et al. Factor H dysfunction in patients with atypical hemolytic uremic syndrome contributes to complement deposition on platelets and their activation. Blood. 2008;111:5307–15. doi: 10.1182/blood-2007-08-106153. [DOI] [PubMed] [Google Scholar]
- 102.Pickering MC, de Jorge EG, Martinez-Barricarte R, et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204:1249–56. doi: 10.1084/jem.20070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kavanagh D, Goodship TH. Update on evaluating complement in hemolytic uremic syndrome. Curr Opin Nephrol Hypertens. 2007;16:565–71. doi: 10.1097/MNH.0b013e3282f0872f. [DOI] [PubMed] [Google Scholar]
- 104.Saland JM, Shneider BL, Bromberg JS, et al. Successful split liver-kidney transplant for factor H associated hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2009;4:201–6. doi: 10.2215/CJN.02170508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jalanko H, Peltonen S, Koskinen A, et al. Successful liver-kidney transplantation in two children with aHUS caused by a mutation in complement factor H. Am J Transplant. 2008;8:216–21. doi: 10.1111/j.1600-6143.2007.02029.x. [DOI] [PubMed] [Google Scholar]
- 106.Cruzado JM, de Cordoba SR, Melilli E, et al. Successful renal transplantation in a patient with atypical hemolytic uremic syndrome carrying mutations in both factor I and MCP. Am J Transplant. 2009;9:1477–83. doi: 10.1111/j.1600-6143.2009.02647.x. [DOI] [PubMed] [Google Scholar]
- 107.Kaplan M. Eculizumab (Alexion) Curr Opin Investig Drugs. 2002;3:1017–23. [PubMed] [Google Scholar]
- 108.Hillmen P, Hall C, Marsh JC, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–9. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 109.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–47. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 110.Schubert J, Hillmen P, Roth A, et al. Eculizumab, a terminal complement inhibitor, improves anaemia in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2008;142:263–72. doi: 10.1111/j.1365-2141.2008.07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA. Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci USA. 1996;93:8563–8. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davin JC, Gracchi V, Bouts A, Groothoff J, Strain L, Goodship T. Maintenance of kidney function following treatment with eculizumab and discontinuation of plasma exchange after a third kidney transplant for atypical hemolytic uremic syndrome associated with a CFH mutation. Am J Kidney Dis. 2010;55:708–11. doi: 10.1053/j.ajkd.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 113.Nurnberger J, Philipp T, Witzke O, et al. Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med. 2009;360:542–4. doi: 10.1056/NEJMc0808527. [DOI] [PubMed] [Google Scholar]
- 114.Alexion Pharmaceuticals. ‘Alexion Completes Enrollment in All Four Clinical Trials of Soliris® (eculizumab) in Patients with Atypical Hemolytic Uremic Syndrome (aHUS)’. Press release; Apr 20, 2010. Retreived April 23, 2010. Archive copy at http://www.alxn.com/News/article.aspx?relid=461488. 15. 2010. [Google Scholar]
- 115.Tang Z, Lu B, Hatch E, Sacks SH, Sheerin NS. C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J Am Soc Nephrol. 2009;20:593–603. doi: 10.1681/ASN.2008040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gueler F, Rong S, Gwinner W, et al. Complement 5a receptor inhibition improves renal allograft survival. J Am Soc Nephrol. 2008;19:2302–12. doi: 10.1681/ASN.2007111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allegretti M, Moriconi A, Beccari AR, et al. Targeting C5a: recent advances in drug discovery. Curr Med Chem. 2005;12:217–36. doi: 10.2174/0929867053363379. [DOI] [PubMed] [Google Scholar]
- 118.Lewis AG, Kohl G, Ma Q, Devarajan P, Kohl J. Pharmacological targeting of C5a receptors during organ preservation improves kidney graft survival. Clin Exp Immunol. 2008;153:117–26. doi: 10.1111/j.1365-2249.2008.03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sahu A, Soulika AM, Morikis D, Spruce L, Moore WT, Lambris JD. Binding kinetics, structure-activity relationship, and biotransformation of the complement inhibitor compstatin. J Immunol. 2000;165:2491–9. doi: 10.4049/jimmunol.165.5.2491. [DOI] [PubMed] [Google Scholar]
- 120.Nilsson B, Larsson R, Hong J, et al. Compstatin inhibits complement and cellular activation in whole blood in two models of extracorporeal circulation. Blood. 1998;92:1661–7. [PubMed] [Google Scholar]
- 121.Ricklin D, Lambris JD. Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol. 2008;632:273–92. doi: 10.1007/978-0-387-78952-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Magotti P, Ricklin D, Qu H, Wu YQ, Kaznessis YN, Lambris JD. Structure-kinetic relationship analysis of the therapeutic complement inhibitor compstatin. J Mol Recognit. 2009;22:495–505. doi: 10.1002/jmr.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lindorfer MA, Pawluczkowycz AW, Peek EM, Hickman K, Taylor RP, Parker CJ. A novel approach to preventing the hemolysis of paroxysmal nocturnal hemoglobinuria: both complement-mediated cytolysis and C3 deposition are blocked by a monoclonal antibody specific for the alternative pathway of complement. Blood. 2010;115:2283–91. doi: 10.1182/blood-2009-09-244285. [DOI] [PubMed] [Google Scholar]
- 124.Katschke KJ, Jr, Stawicki S, Yin J, et al. Structural and functional analysis of a C3b-specific antibody that selectively inhibits the alternative pathway of complement. J Biol Chem. 2009;284:10473–9. doi: 10.1074/jbc.M809106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Couser WG, Johnson RJ, Young BA, Yeh CG, Toth CA, Rudolph AR. The effects of soluble recombinant complement receptor 1 on complement-mediated experimental glomerulonephritis. J Am Soc Nephrol. 1995;5:1888–94. doi: 10.1681/ASN.V5111888. [DOI] [PubMed] [Google Scholar]
- 126.Bao L, Haas M, Kraus DM, et al. Administration of a soluble recombinant complement C3 inhibitor protects against renal disease in MRL/lpr mice. J Am Soc Nephrol. 2003;14:670–79. doi: 10.1097/01.asn.0000051597.27127.a1. [DOI] [PubMed] [Google Scholar]
- 127.Quigg RJ, Kozono Y, Berthiaume D, et al. Blockade of antibody-induced glomerulonephritis with Crry-Ig, a soluble murine complement inhibitor. J Immunol. 1998;160:4553–60. [PubMed] [Google Scholar]
- 128.He C, Imai M, Song H, Quigg RJ, Tomlinson S. Complement inhibitors targeted to the proximal tubule prevent injury in experimental nephrotic syndrome and demonstrate a key role for C5b-9. J Immunol. 2005;174:5750–57. doi: 10.4049/jimmunol.174.9.5750. [DOI] [PubMed] [Google Scholar]
- 129.Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068–76. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J Immunol. 2008;180:1231–8. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 131.Banda NK, Levitt B, Glogowska MJ, et al. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J Immunol. 2009;183:5928–37. doi: 10.4049/jimmunol.0901826. [DOI] [PMC free article] [PubMed] [Google Scholar]