Abstract
In vitro treatment of Nf2-deficient cells with epidermal growth factor receptor (EGFR) inhibitors can inhibit cellular proliferation. We retrospectively assessed the effect of erlotinib (150 mg daily) on eleven consecutive NF2 patients with progressive VS who were poor candidates for standard therapy. A radiographic response was defined as ≥ 20% decrease in tumor volume compared to baseline. A hearing response was defined as a statistically significant increase in word recognition score (WRS) compared to baseline; a minor hearing response was defined as a 10 dB improvement in pure-tone average with stable WRS. Before treatment, the median and mean annual volumetric growth rate for eleven index VS were 26% and 46%, respectively. Among 10 evaluable patients, the median time-to-tumor progression was 9.2 months. Three patients with stable disease experienced maximum tumor shrinkage of 4%, 13%, and 14%. Nine patients underwent audiologic evaluations. One experienced a transient hearing response, two experienced minor hearing responses, three remained stable, and two developed progressive hearing loss. The median time-to-progressive hearing loss was 9.2 months and to either tumor growth or progressive hearing loss was 7.1 months. Adverse treatment effects included mild-to-moderate rash, diarrhea, and hair thinning, with 2 episodes of grade 3 toxicity. Erlotinib treatment was not associated with radiographic or hearing responses in NF2 patients with progressive VS. Because a subset of patients experienced prolonged stable disease, time-to-progression may be more appropriate than radiographic or hearing response for anti-EGFR agents in NF2-associated VS.
Keywords: neurofibromatosis, vestibular schwannoma, acoustic neuroma, erlotinib, hearing
Introduction
Neurofibromatosis 2 (NF2) is a progressive neurogenetic disorder characterized by the predisposition to multiple benign tumors including schwannomas, meningiomas, and ependymomas. The hallmark of NF2 is bilateral vestibular schwannomas (VS), which present earlier in life than sporadic, unilateral vestibular schwannomas.1, 2 Progressive growth of these bilateral tumors place NF2 patients at high risk for complete hearing loss. Although surgery and radiation can be effective in controlling NF2 vestibular schwannoma growth, patients often suffer hearing loss in treated ears as a consequence of the treatments.3-5 Disease-specific mortality is high in NF2: greater than 90% of NF2 patients die of their disease or from complications of its treatment.6 Despite a clear need, there is currently no approved medical treatment for NF2-related or sporadic VS. Although a recent consensus conference recognized that rationally designed phase II trials were a realistic short-term goal in NF2-related VS,7 actual clinical experience with drug treatments for these tumors to date has been limited.8, 9
Much evidence implicates human epidermal growth factor (HER) receptors, including EGFR/HER1, ErbB2/HER2, and ErbB3/HER3 in vestibular schwannoma growth.10-15 Some studies have demonstrated that Merlin, the NF2 gene protein product, controls surface availability of the EGFR, ErbB2, and ErbB3 receptors in human and Drosophila models.11, 16 Pathological specimens of sporadic and NF2-related vestibular schwannomas demonstrate upregulation of EGFR and ErbB2 mRNA and protein in most, but not all, studies.12, 13, 15, 17 However, amplification of EGFR is not present in human tumor specimens.17 Cell culture models of mouse embryo fibroblasts (MEFs) support the role of EGFR in NF2-associated tumorigenesis. Nf2-deficient cells in culture lack contact-dependent inhibition of growth and continue to grow in confluent cultures.11 This effect appears to be mediated by EGFR signaling.11 Treatment of Nf2-deficient cells with EGFR inhibitors such as gefitinib can restore contact-dependent inhibition,11 suggesting that this class of drugs might be useful for NF2 patients with progressive vestibular schwannomas. A recent consensus conference on NF2 clinical trial design identified the ErbB family of receptors as perhaps the most compelling candidate targets for novel drug treatment testing in this disease.7
Erlotinib is an oral EGFR tyrosine kinase inhibitor approved by the Food and Drug Administration (FDA) for treatment of non-small cell lung cancer and pancreatic cancer.18, 19 Preclinical and clinical studies indicate that erlotinib crosses the blood-brain barrier in clinically active concentrations.20, 21 It is not known whether erlotinib crosses the blood-nerve barrier or whether vestibular schwannomas have an intact blood-nerve barrier. Multiple phase I and II clinical studies of erlotinib have been performed for high-grade glioma and these studies suggest that the drug is well tolerated in brain tumor patients.22-24,21-23 These observations suggest that erlotinib is a promising candidate for early phase drug testing in NF2 VS.7 However, pilot data on responses of NF2-related VS to drug treatment may be valuable in assisting future phase II trial design. For example, NF2-related VS may show radiographic responses consistent either with significant volumetric shrinkage9 or prolonged tumor stability,8 situations which demand different phase II designs in formal drug testing.
Here, we report treatment of 11 NF2 patients who were not candidates for surgery or radiation treatment on a compassionate-use basis using erlotinib. Our results show that erlotinib was not associated with radiographic or hearing responses, although a subset of patients experienced prolonged tumor stability or improved pure tone hearing thresholds. Thus, time to progression may be a more appropriate endpoint than radiographic response in future phase II trials of erlotinib in progressive NF2-related vestibular schwannoma.
Materials and Methods
Patient selection
We offered erlotinib on a compassionate-use basis to NF2 patients with progressive vestibular schwannomas who were poor candidates for surgery and radiation therapy or who refused these treatments. All patients met the following criteria: (1) fulfilled National Institutes of Health clinical diagnostic criteria for NF225; (2) were at risk either for hearing loss in their only hearing ear, or for brainstem compression due to progressive growth of a vestibular schwannoma; (3) were considered poor candidates for or refused treatment with surgery or radiation. Consecutive patients who met these criteria and who received at least one dose of erlotinib between October 1, 2006, and August 31, 2009, were included in the analysis.
Clinical evaluations
The intervals for clinical evaluations were determined before treatment of the first patient. Baseline MRI and audiological testing were performed within 3 months before starting treatment. Clinical evaluation including a physical examination, complete blood count, chemistry panel, and urinalysis was performed monthly during the first year of treatment and then every 2 to 3 months. Tumor response was monitored using serial MRI scanning at clinical visits at months 3, 6, 9, and every 3 months thereafter. Volumetric analysis of vestibular schwannomas was performed as described previously.26 In brief, semiautomated segmentation software was used to outline tumors on post-contrast T1-weighted images and the volume calculated by multiplying the outlined area by the slice thickness and then summing the values across slices.
Hearing response was monitored using serial audiologic evaluations including determination of pure tone thresholds and word recognition scores. Thresholds were determined at frequencies of 250, 500, 1000, 2000, 3000, 4000, and 8000 Hz and pure tone average was calculated as the average of thresholds at 500, 1000, 2000 and 3000 Hz.27 Word Recognition was tested using the 50-item, recorded CID-W22 (Central Institute for the Deaf) monosyllable wordlists.28
Toxicity
All patients who received treatment were included in the toxicity results reported. Toxicity data were recorded monthly during clinic visits and were graded using the NIH Common Terminology Criteria for Adverse Events version 3.0 (CTCAE 3.0).
Definition of radiographic and hearing response
Response criteria for phase 2 studies of NF2-related vestibular schwannoma have been published 29 and used successfully in previous studies. 9 These criteria differ from the MacDonald response criteria for malignant gliomas30 by basing radiographic response on volumetric MRI analysis and hearing response on changes in word recognition score. A radiographic response was defined as a decrease in tumor volume of 20% or more in the index tumor compared to the pretreatment baseline.29 Patients were eligible for assessment of radiographic response if they completed one or more cycles of treatment and underwent MRI scanning. Baseline word recognition scores were used to determine the critical difference at the p=0.05 level. There are two critical difference scores, one above and one below the baseline score. A hearing response was defined as a statistically significant increase in word recognition score (p < 0.05) above the 95% critical difference for the baseline score.29, 31 A patient was considered eligible for a hearing response if their pretreatment word recognition score allowed the possibility of hearing improvement above the upper limit of the critical difference threshold – that is, if their baseline word recognition score was 94% or below. Patients with surgically absent auditory nerves were not eligible for hearing response. Published tables32 of significant differences between word recognition scores were used to compare posttreatment tests for each patient to baseline values. A minor hearing response was defined as an improvement in the pure-tone average audiometric threshold of 10 dB over the baseline value in the setting of stable word recognition scores (i.e., within the 95% critical difference compared to baseline). In addition, responses were measured according to the American Academy of Otolaryngology (AAO) criteria for hearing preservation to facilitate comparison with previous studies.33 All patients signed informed consent for treatment; the retrospective chart review was approved by the Institutional Review Board at the Massachusetts General Hospital.
Results
Patients
The baseline clinical characteristics of the 11 consecutive patients treated using erlotinib for NF2-related vestibular schwannoma are shown in Table 1. All patients were poor candidates for standard treatment: eight patients were at high risk for complete hearing loss from surgery or radiation, and three patients refused surgery to relieve critical brainstem compression. No patients received previous chemotherapy or radiotherapy. The “index” tumor was defined as the VS causing hearing loss in an only hearing ear or brainstem compression. One patient was reported previously.8
Table 1.
Patient baseline characteristics and outcomes after treatment using erlotinib.
| Patient | Age | Gender | Inheritance | Indication for Treatment | Baseline Tumor size (cc) | Baseline Annual Volumetric growth rate (% per year) | Baseline Word recognition (%) | Treatment Duration (months) | Max Change in index VS volume | Max Change In Contralateral VS volume | Last word recognition | Radiographic Response | Hearing Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | M | Familial | Brainstem compression | 17.3 | 11% | 0% | 33 | −13% | 69% | 2% | Stable disease | Stable hearing |
| 2 | 22 | F | Founder | Hearing loss | 8.5 | 75% | 94% | 7 | 22% | −13% | 42% | Progressive disease | Progressive hearing loss |
| 3 | 26 | F | Founder | Hearing loss | 1.8 | 26% | 50% | 9 | 22% | n/a | 22% | Progressive disease | Progressive hearing loss |
| 4 | 32 | M | Founder | Hearing loss | 2.2 | 14% | 96% | 12 | 14% | n/a | 96% | Stable disease | Stable hearing |
| 5 | 16 | M | Familial | Brainstem compression | 9.8 | 118% | 0% | 0.7 | n/a | n/a | 0% | n/a | Not eligible |
| 6 | 23 | M | Founder | Hearing loss | 13.5 | 121% | 42% | 7 | 44% | 143% | 36% | Progressive disease | Stable disease |
| 7 | 32 | M | Familial | Brainstem compression | 27.0 | 53% | n/a | 8 | 43% | 0% | n/a | Progressive disease | not eligible |
| 8 | 18 | F | Founder | Hearing loss | 13.9 | 65% | 100% | 7 | 108% | n/a | 96% | Progressive disease | Stable hearing |
| 9 | 44 | F | Founder | Hearing loss | 2.3 | 9% | 50% | 4 | −4% | n/a | 28% | Stable disease | Progressive hearing loss |
| 10 | 42 | M | Founder | Hearing loss | 0.7 | 28% | 94% | 19 | 43% | 67% | 80% | Progressive disease | Progressive hearing loss |
| 11 | 36 | M | Founder | Hearing loss | 10.7 | 6% | Not tested | 19+ | −14% | −23% | Not tested | Stable disease | Not tested |
Abbreviations: M—male; F—female; progressive disease (increase in tumor volume of 20% or more compared to the pretreatment baseline; stable disease (change in tumor volume between −19% and +19% compared to pretreatment baseline); progressive hearing loss (decrease in word recognition score below the critical difference threshold compared to baseline); stable hearing (changes in word recognition score within the critical difference threshold); (+) indicates patient continues treatment at the time of analysis; n/a—not applicable.
Treatment
Seven men and 4 women with a median age of 31 years (range, 16 to 63 years) received erlotinib 150 mg daily by mouth. A cycle was defined as 28 days of treatment. The mean and median annual tumor volume growth rates of index tumors before treatment were 46% and 26%, respectively (range, 6% to 121%). At baseline, tumors demonstrated slow growth in 4 patients (which we defined as annual volumetric growth rate < 20% per year), intermediate growth in 3 patients (annual volumetric growth rate 20-60% per year), and rapid growth in 5 patients (annual volumetric growth rate > 60% per year).
The median duration of treatment was 7.9 months (range, 0.7 to 33 months) with four patients treated for one year or longer. One patient continues on treatment using erlotinib 19 months after starting treatment as of the time of writing. Ten patients have discontinued treatment: 5 due to tumor growth; 2 due to tumor growth and hearing loss; and 1 each due to progressive hearing loss, patient preference, and toxicity (rash, nausea).
Toxic effects of erlotinib
Forty-three grade 1 or 2 adverse events and two grade 3 adverse events (rash and elevated bilirubin) occurred during treatment (Table 2). The most common adverse event was skin rash, often affecting the scalp. Symptomatic patients were treated using oral antibiotics and topical corticosteroids with modest clinical benefit; no dose interruption was required. Ten episodes of hair thinning (grade 1) were reported; some female patients reported that this symptom was a significant source of distress. Two patients with baseline cranial nerve dysfunction—one with combined trigeminal and facial nerve dysfunction, one with facial nerve dysfunction only—developed grade 2 corneal keratopathy due to curling of eyelashes. This symptom mandated dose interruption for 3 months in patient 7; he restarted treatment at a reduced dose of 100 mg daily. Grade 1 nail changes were noted in 3 patients and led to non-urgent medical evaluations.
Table 2.
Drug-related toxicity observed in study patients.
| Adverse Event | Grade 1/2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Rash | 10 | 1 | 0 |
| Hair thinning | 10 | 0 | 0 |
| Nail changes | 3 | 0 | 0 |
| Diarrhea | 9 | 0 | 0 |
| Keratopathy | 2 | 0 | 0 |
| Bilirubin | 2 | 1 | 0 |
| Alkaline phosphatase | 4 | 0 | 0 |
| SGPT | 1 | 0 | 0 |
| Lipase | 1 | 0 | 0 |
| TSH | 1 | 0 | 0 |
Diarrhea was common: 9 patients experienced grade 1 or 2 diarrhea. Patient 2 experienced grade 2 diarrhea and discontinued erlotinib after 2 weeks because he did not wish to take supportive medication and did not agree to dose reduction. Laboratory toxicity was primarily hepatic with 1 episode of grade 3 hyperbilirubinemia. No hematologic toxicity was noted.
Radiographic response to erlotinib
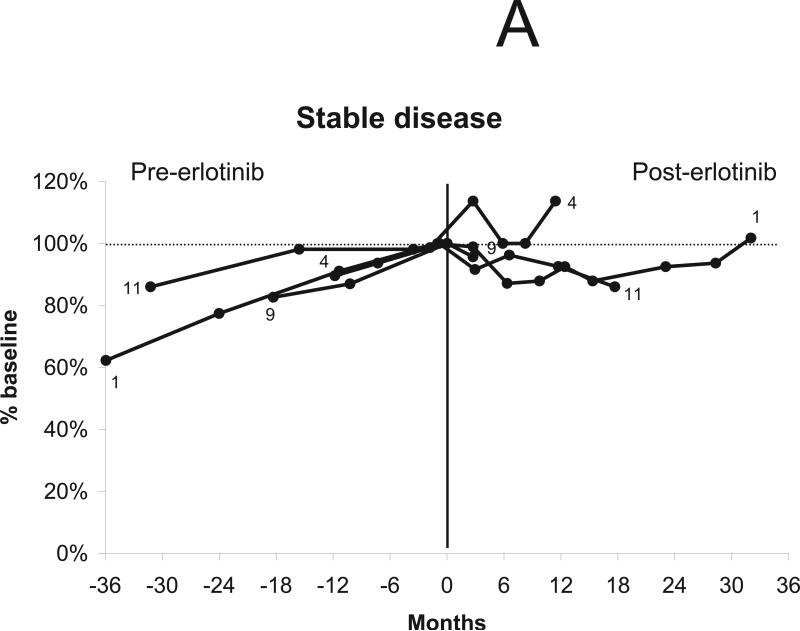
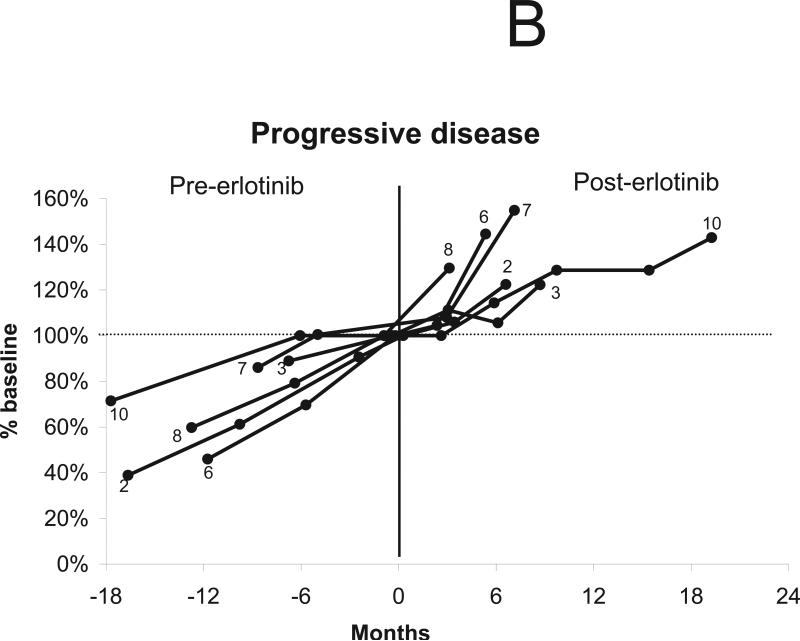
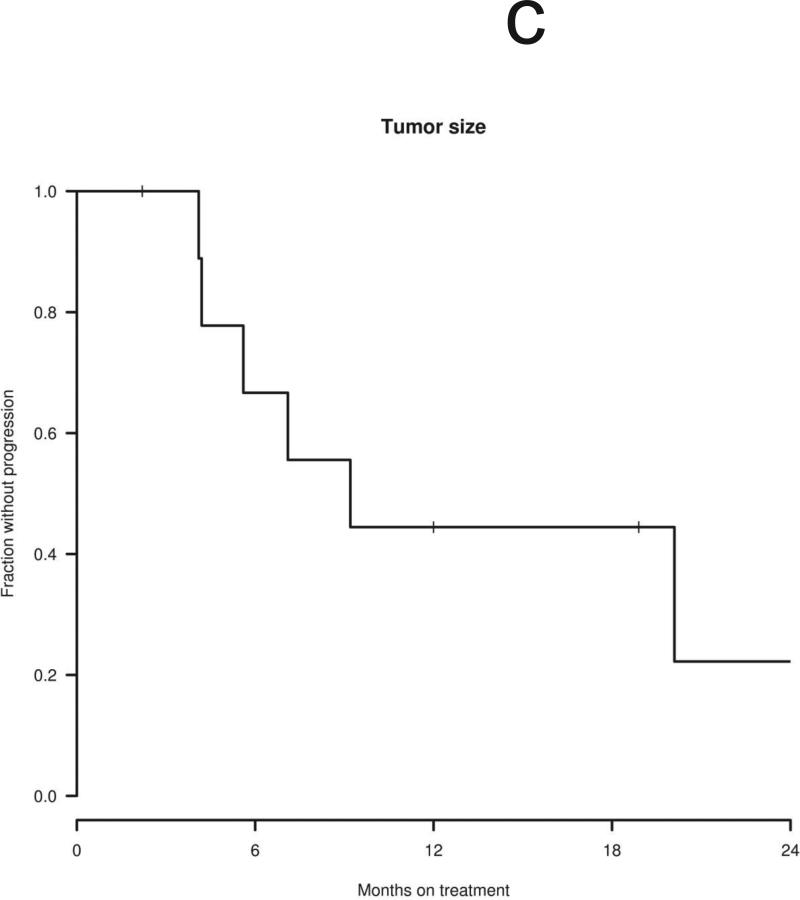
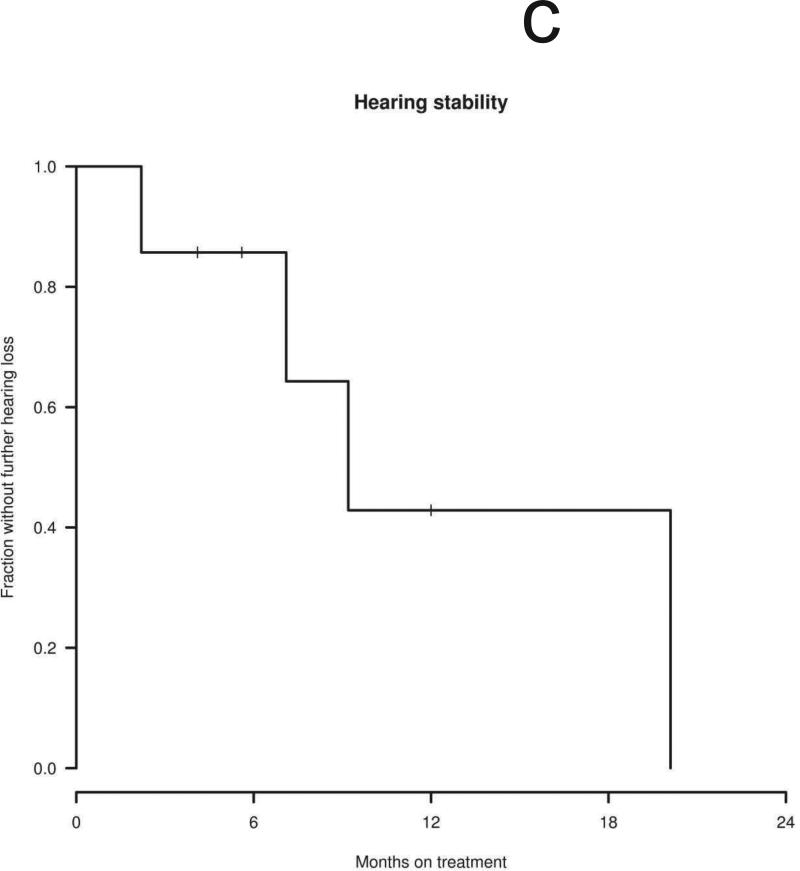
Ten patients were considered eligible for a radiographic response, excluding the patient who discontinued treatment after 2 weeks. Three of eleven index tumors shrank after treatment with erlotinib (maximum tumor shrinkage of 4%, 13%, and 14% compared to baseline volume), with no tumors achieving a radiographic response by volumetric or Macdonald criteria (Table 1). Overall, four of 10 patients’ tumors remained stable (i.e., less than 20% volumetric growth or shrinkage compared to baseline) during erlotinib treatment. Of the four stable tumors, all had demonstrated slow growth before treatment (annual volumetric growth rate 0% to 20% per year). In 3 cases, minor shrinkage was noted between 3 to 6 months after starting treatment with erlotinib (Figs. 1a and 1b), as previously reported with bevacizumab. The median time to tumor progression (i.e., > 20% volumetric growth from baseline) was 9.2 months with the earliest failure noted at 4.1 months and the latest at 20.1 months (Fig. 1c). The 12-month progression-free survival for tumor growth was 44%. Of the six progressive tumors, four had demonstrated rapid growth before erlotinib treatment (annual volumetric growth rate > 60%) and two had demonstrated intermediate growth (annual volumetric growth rate 20% to 60% per year).
Figure 1.
Changes in tumor volume after treatment using erlotinib 150 mg daily. “0” indicates initiation of treatment. The vertical line indicates the start of treatment (day 0). (a) Four tumors were stable during treatment, defined as change in tumor volume between 19% growth and 19% shrinkage compared to the baseline volume. (b) Six tumors progressed during treatment, defined as an increase in tumor volume of greater than or equal to 20% compared to the baseline volume. (c) Time to tumor progression in 10 evaluable patients.
Five patients had contralateral vestibular schwannomas that were suitable for volumetric measurement during treatment. Two of five contralateral tumors shrank after treatment with erlotinib (maximum tumor shrinkage of 13% and 23% compared to baseline volume). One tumor remained stable during treatment (no change in volume) and three tumors enlarged significantly during treatment (table 1)
Hearing response to erlotinib
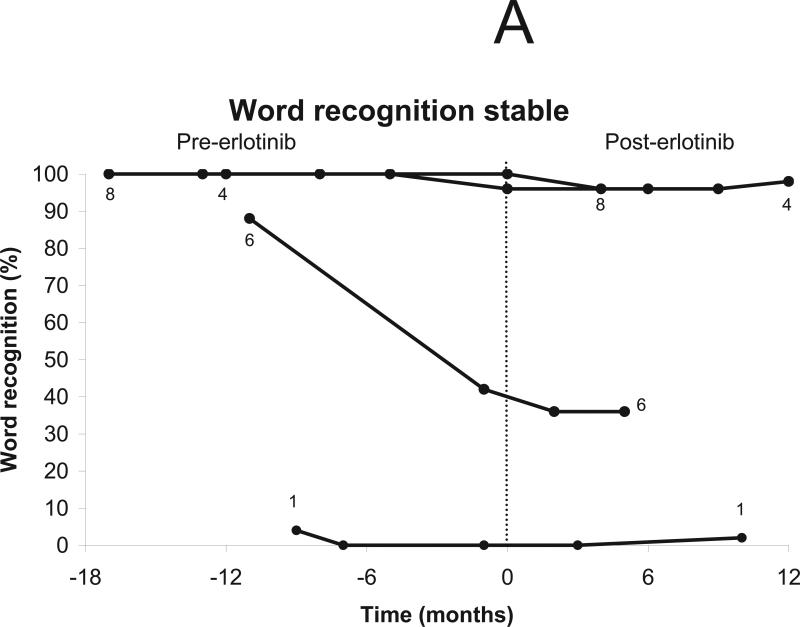
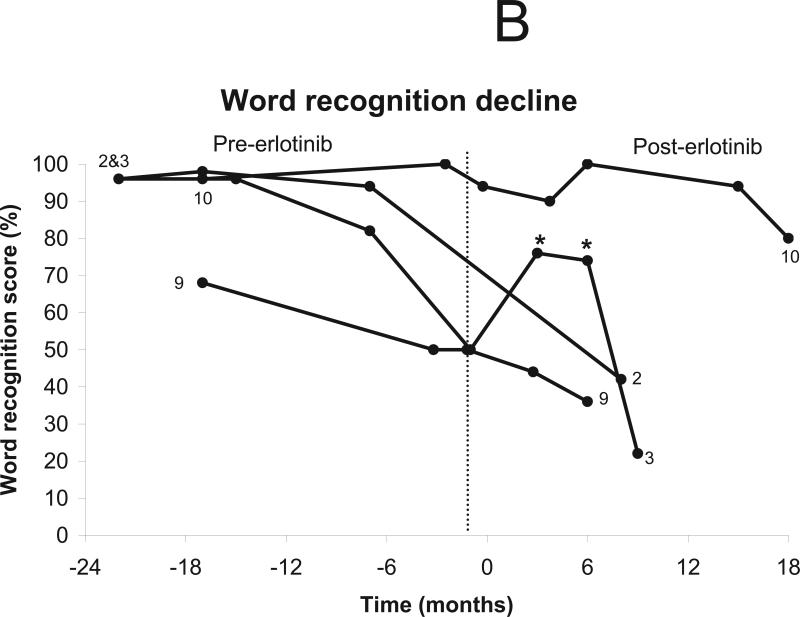
Six patients were eligible for a hearing response by word recognition score -- of the other five patients, 3 had normal hearing ipsilateral to index vestibular schwannomas at baseline (and thus could not improve), 1 had undergone surgical resection of both auditory nerves, and 1 spoke Italian and was tested for pure-tone thresholds but not for word recognition. A hearing response was observed in one of the 6 eligible patients lasting 6 months (Figs. 2a-b).
Figure 2.
Change in word recognition after treatment using erlotinib 150 mg daily. The vertical line indicates the start of treatment (day 0). Patients who did not undergo audiologic evaluation (n=1) and those with surgical resection of both auditory nerves (n=1) were excluded from analysis. One patient was tested only with pure tone audiometry. (a) Stable word recognition scores were documented in five patients during treatment. (b) Progressive hearing loss was documented in 3 patients, including one patient with transient hearing improvement. * indicate timepoints qualifying as a hearing response. (c) Time to progressive hearing loss in 7 evaluable patients.
Seven patients were eligible for progressive hearing loss by word recognition score -- of the other 4 patients, 2 had no word recognition hearing ipsilateral to index vestibular schwannomas at baseline (and thus could not decline), another had undergone surgical resection of both auditory nerves, and 1 spoke Italian and was tested for pure-tone thresholds but not for word recognition. Stable hearing was noted in 3 patients and progressive hearing decline was noted in 4 patients at 8, 9, 9, and 18 months after starting treatment (Figs. 2a-b). The median time to progressive hearing loss was 9.2 months with 12-month progression-free survival of 43% (Fig. 2c).
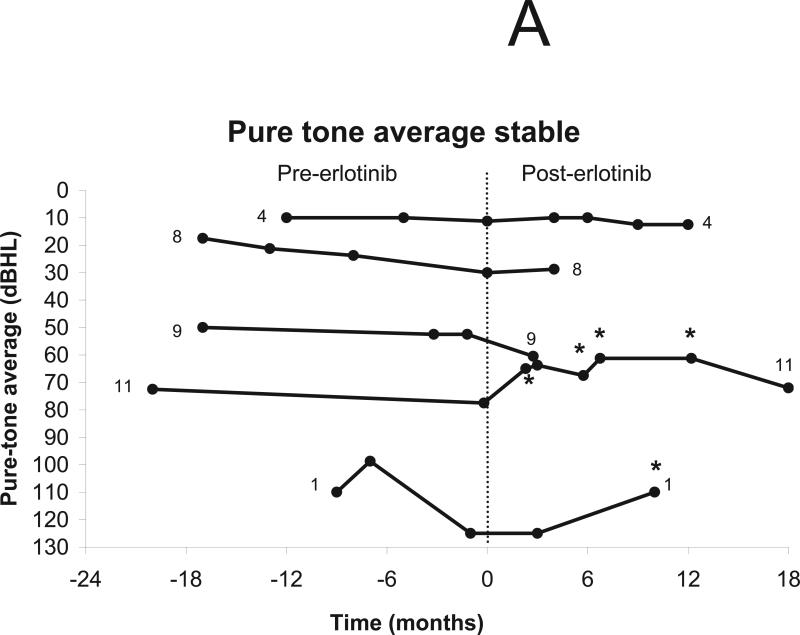
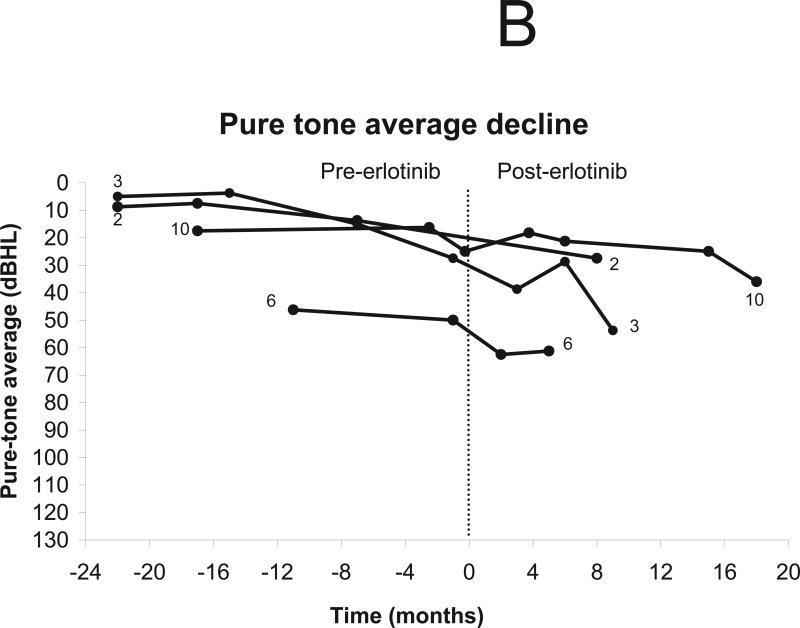
Nine of 11 patients underwent testing for pure-tone thresholds, excluding 1 patient who had undergone surgical resection of both auditory nerves and 1 who discontinued treatment after 2 weeks (Fig 2b). Six patients were eligible for improvement in pure-tone thresholds and seven were eligible for decline. Improvement was noted in two patients, including one reported previously.34 Stability in pure-tone thresholds was noted in four patients and thresholds worsened in 3 patients (Figs. 3a-b). No patients improved their hearing class as defined by AAO criteria.33
Figure 3.
Change in pure-tone thresholds after treatment using erlotinib 150 mg daily. The vertical line indicates the start of treatment (day 0). Patients who did not undergo audiologic evaluation (n=1) and those with surgical resection of both auditory nerves (n=1) were excluded from analysis. (a) Minor response (defined as increase in pure tone average of 10 dBHL from baseline in the setting of stable word recognition) was noted in two patients and stable hearing in three patients. (b) Progressive hearing decline was documented in 3 patients.
Discussion
Preclinical and clinical data suggest that EGFR is an attractive target for treatment of NF2-associated vestibular schwannomas.7, 8, 11 In this report, we describe a pilot experience with erlotinib treatment in a small number of NF2 patients with progressively enlarging vestibular schwannomas in their only hearing ears or causing brainstem compression.
We found that erlotinib given orally on a daily basis was generally well tolerated by NF2 patients, with only one patient discontinuing treatment due to toxicity and another due to patient preference; another two required a dose reduction. These results are similar to those reported recently in patients with meningiomas, another NF2-associated intracranial tumor.35 The main side effects of treatment in our patients included the expected findings of skin rash and diarrhea, but two patients experienced corneal keratopathy related to curling of the eyelashes. This toxicity has special importance in NF2, as these patients frequently have trigeminal or facial nerve pareses that can allow rapid development of corneal opacity from minor trauma. Our patients did not experience decline in visual acuity but required close ophthalmological follow-up to manage this toxicity. This finding and reports of eardrum perforation after erlotinib highlight the importance of careful monitoring of NF2 patients for visual, auditory, and facial function during clinical trials.36, 37 The occurrence of the expected toxicities of rash, hair thinning and diarrhea in nearly all the patients we treated suggests achievement of systemic drug levels comparable to those seen with erlotinib treatment at comparable doses in other cancers.18, 19
Although no patient achieved a formal radiographic response in this series, three patients (27%) with baseline annual tumor growth rates of 6%, 11%, and 28% experienced prolonged stable disease during treatment. This result is consistent with preclinical studies in which erlotinib exhibits a cytostatic, rather than cytotoxic effect, on NF2-deficient cells, and with recently reported results after erlotinib treatment of meningiomas.35 Interestingly, an NF2 patient with extensive peripheral schwannomas (who had undergone resection of both vestibular schwannomas) who has been treated with erlotinib showed a more robust response to treatment with a 26% reduction in total body tumor volume (S; Plotkin, unpublished results). However, tumor stability is difficult to ascribe with certainty to a treatment effect in our patients, given the lack of extensive data on progression of untreated tumors matching our entry criteria, and the correlation between slow pretreatment tumor growth and stability during treatment in our patients urges extra caution in interpretation.
Two of six eligible patients achieved minor hearing responses that lasted 19+ and 24 months. The one patient who experienced both radiographic shrinkage (not meeting criteria for formal response) and a minor hearing response was reported previously.34 These radiographic and hearing findings suggest that antitumor effects may be durable in patients treated using erlotinib.
Although the formal response rate for these patients treated with erlotinib was low, other anti-angiogenic therapies such as bevacizumab, an anti-VEGF antibody, may be active against NF2-associated vestibular schwannomas – as shown by radiographic shrinkage of tumors and significant hearing improvement.9 In this context, minor responses and stable disease may merit further investigation. Time to progression (TTP) is an alternate endpoint for clinical trials in NF2, although NF2 treatment trials using this endpoint have not yet been reported. Prolonged stable disease is a desirable goal in NF2, because these patients are young when they lose functional hearing due to tumor growth or from complications of surgical or radiation treatment.7 Currently, nearly all patients with NF2 undergo resection of a vestibular schwannoma at some point and ultimately develop tumor progression in the contralateral vestibular schwannoma. These patients are at high risk for complete hearing loss. Historically, treatment options for NF2-related vestibular schwannomas have included surveillance by MRI scan or treatment with surgery or radiation. Our results suggest that erlotinib may induce prolonged tumor stability in some such patients, and given the permanent nature of hearing loss after either surgery or radiation, erlotinib or other HER inhibitors may merit further formal testing in this patient population.
Erlotinib was the first drug tested in our clinic for possible activity against vestibular schwannomas. We have subsequently tested bevacizumab treatment in NF2 patients with similar clinical characteristics to those in this report, with results reported separately.9 Five patients reported in this study whose tumors progressed during erlotinib therapy were subsequently treated with bevacizumab.9 Four of these patients achieved either a radiographic or hearing response with bevacizumab treatment, suggesting that multiple pathways are involved in vestibular schwannoma growth and hearing loss, and also suggesting that bevacizumab has much more consistent activity in this patient population than erlotinib. Combination therapy targeting the VEGF and EGFR pathways may be an attractive approach for treating these tumors, and in vivo studies of AZD6474, a VEGFR2/EGFR inhibitor, in mouse VS models have provided preliminary evidence for anti-tumor activity.38 The options for drugs that show promise in treating these tumors will likely expand rapidly as our knowledge of receptor tyrosine kinases expressed on vestibular schwannomas grows. Table 3 lists some receptor-targeted drugs that are approved for use in solid tumors and that are candidates for treatment of vestibular schwannomas., Prioritizing testing of various agents in rare diseases can be difficult, and may call for multicenter collaboration on clinical trials in NF2, potentially using novel trial designs.29
Table 3.
Examples of approved receptor-targeted drugs that are candidates for treatment of vestibular schwannomas.
| Receptor | Drug | Model system |
|---|---|---|
| VEGF receptor 2 | Sunitinib, pazopanib, sorafenib | Mouse models, humans (REF) |
| EGF receptor (erbB1) | Erlotinib, gefitinib, lapatinib, cetuximab | Cell culture, humans |
| erbB2, erbB3 | Lapatinib, trastuzumab | Cell culture, mouse models |
| PDGF receptors/c-KIT | Sunitinib, pazopanib, imatinib, dasatinib, sorafenib | Cell culture (guha) |
There are a number of possible reasons why erlotinib did not show greater activity in our patients. First, preclinical studies indicate that inhibition of EGFR is associated with a cytostatic, rather than a cytotoxic, effect on Nf2-deficient cell lines.11 Thus, the predicted effect of erlotinib in these patients would be to decrease rather than reverse tumor growth. The endpoints we defined for formal radiologic and audiologic responses required tumor shrinkage and hearing improvement, respectively. Second, most patients in this study had large tumors that were growing rapidly. The biology of vestibular schwannomas is not fully understood and it is likely that aggressive tumors have acquired additional molecular alterations leading to accelerated growth. These rapidly-progressing tumors may be less dependent on EGFR-mediated mechanisms of growth and thus would be less sensitive to inhibition of the EGFR pathway as a single treatment. Finally, vestibular schwannomas show overexpression of ErbB2 and ErbB3 receptors in addition to EGFR receptors.12, 13, 15, 17 Previous studies have shown that escape from EGFR and HER2 inhibition is mediated through downstream activation of ErbB339, 40 and erlotinib does not inhibit this related pathway. So-called “phase 0” trials, in which the endpoints include efficacy in inhibiting molecular targets within the tumor, could be valuable in studying these questions.7
A limitation of this study is that patients were treated on compassionate-use basis. The lack of pre-defined inclusion and exclusion criteria likely resulted in a more heterogenous patient population that would be expected in a traditional prospective phase 2 clinical trial. While this may have biased the results of the study, we believe that the current results provide important information about the activity of erlotinib in typical NF2 patients who are seen in tertiary care centers in the US.
In summary, our study shows that treatment with erlotinib is associated with a possible modest treatment response (stability in tumor size and hearing) in some NF2 patients with progressive vestibular schwannoma. As in a pilot study of bevacizumab treatment for NF2-associated vestibular schwannomas,9 we found that our patients were willing to accept modest toxicity from erlotinib treatment and that a relatively short period of treatment time (< 6 months) was necessary to distinguish progressive VS tumors from those with stability potentially related to treatment. These findings have important implications for the design of future early phase clinical trials for NF2 patients. Small pilot studies may be necessary to define the type of response to be expected from individual drugs used for NF2-related VS. Drugs causing tumor shrinkage or hearing improvement in pilot studies, such as bevacizumab,9 may be further tested in single-arm phase II trials that measure response rates and durability of response in a larger population. Drugs associated with tumor and hearing stability in pilot studies may require phase 0 testing, in which tumor drug levels and molecular endpoints are measured after drug administration.7 Phase II testing of these agents will likely require direct comparison to a carefully matched group of (at least initially) untreated patients, as present data on natural history in this patient population is too limited to use published experience to define treatment success. Such trials will necessarily be larger than single-arm phase II trials that test for tumor shrinkage or hearing improvement, an important consideration in this comparatively rare disease. Our experience in this small pilot study leads us to suggest that additional, carefully designed clinical trials of HER-family tyrosine kinase inhibitors are certainly warranted, particularly in subjects with slowly growing NF2-related VS who are at risk for complete hearing loss.
Acknowledgement
Supported by the Harvard Medical School Center for Neurofibromatosis and Allied disorders and DOD NF050202 (Plotkin). We thank Marybeth Singh, NP, for her help with patient care. This data was presented previously at ASCO annual meeting in 2008.
Reference List
- 1.Evans DG, Huson SM, Donnai D, et al. A genetic study of type 2 neurofibromatosis in the United Kingdom. II. Guidelines for genetic counselling. J Med Genet. 1992 Dec;29(12):847–52. doi: 10.1136/jmg.29.12.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DG, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005 Jan;26(1):93–7. doi: 10.1097/00129492-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Brackmann DE, Fayad JN, Slattery WH, III, et al. Early proactive management of vestibular schwannomas in neurofibromatosis type 2. Neurosurgery. 2001 Aug;49(2):274–80. doi: 10.1097/00006123-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Combs SE, Volk S, Schulz-Ertner D, Huber PE, Thilmann C, Debus J. Management of acoustic neuromas with fractionated stereotactic radiotherapy (FSRT): long-term results in 106 patients treated in a single institution. Int J Radiat Oncol Biol Phys. 2005 Sep 1;63(1):75–81. doi: 10.1016/j.ijrobp.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Samii M, Matthies C, Tatagiba M. Management of vestibular schwannomas (acoustic neuromas): auditory and facial nerve function after resection of 120 vestibular schwannomas in patients with neurofibromatosis 2. Neurosurgery. 1997 Apr;40(4):696–705. doi: 10.1097/00006123-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Baser ME, Friedman JM, Aeschliman D, et al. Predictors of the risk of mortality in neurofibromatosis 2. Am J Hum Genet. 2002 Oct;71(4):715–23. doi: 10.1086/342716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans DG, Kalamarides M, Hunter-Schaedle K, et al. Consensus Recommendations to Accelerate Clinical Trials for Neurofibromatosis Type 2. Clinical Cancer Research. 2009 Aug 15;15(16):5032–9. doi: 10.1158/1078-0432.CCR-08-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotkin SR, Halpin C, McKenna MJ, Batchelor TT, Loeffler JS, Barker FG. Treatment of progressive neurofibromatosis type 2-related vestibular schwannoma with erlotinib. J.Clin.Oncol. 2008;15(Suppl pt I):100s. Ref Type: Abstract. [Google Scholar]
- 9.Plotkin SR, Stemmer-Rachamimov AO, Barker FG, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009 Jul 23;361(4):358–67. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008 Feb;28(4):1274–84. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007 Jun 4;177(5):893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty JK, Ongkeko W, Crawley B, Andalibi A, Ryan AF. ErbB and Nrg: potential molecular targets for vestibular schwannoma pharmacotherapy. Otol Neurotol. 2008 Jan;29(1):50–7. doi: 10.1097/mao.0b013e31815d4429. [DOI] [PubMed] [Google Scholar]
- 13.Sturgis EM, Woll SS, Aydin F, Marrogi AJ, Amedee RG. Epidermal growth factor receptor expression by acoustic neuromas. Laryngoscope. 1996 Apr;106(4):457–62. doi: 10.1097/00005537-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly BF, Kishore A, Crowther JA, Smith C. Correlation of growth factor receptor expression with clinical growth in vestibular schwannomas. Otol Neurotol. 2004 Sep;25(5):791–6. doi: 10.1097/00129492-200409000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Stonecypher MS, Chaudhury AR, Byer SJ, Carroll SL. Neuregulin growth factors and their ErbB receptors form a potential signaling network for schwannoma tumorigenesis. J Neuropathol Exp Neurol. 2006 Feb;65(2):162–75. doi: 10.1097/01.jnen.0000199575.93794.2f. [DOI] [PubMed] [Google Scholar]
- 16.Lallemand D, Manent J, Couvelard A, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009 Feb 12;28(6):854–65. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- 17.Prayson RA, Yoder BJ, Barnett GH. Epidermal growth factor receptor is not amplified in schwannomas. Ann Diagn Pathol. 2007 Oct;11(5):326–9. doi: 10.1016/j.anndiagpath.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JR, Cohen M, Sridhara R, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005 Sep 15;11(18):6414–21. doi: 10.1158/1078-0432.CCR-05-0790. [DOI] [PubMed] [Google Scholar]
- 19.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007 May 20;25(15):1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 20.Meany HJ, Fox E, McCully C, Tucker C, Balis FM. The plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite (OSI-420) after intravenous administration of erlotinib in non-human primates. Cancer Chemother Pharmacol. 2008 Aug;62(3):387–92. doi: 10.1007/s00280-007-0616-3. [DOI] [PubMed] [Google Scholar]
- 21.Katayama T, Shimizu J, Suda K, et al. Efficacy of Erlotinib for Brain and Leptomeningeal Metastases in Patients with Lung Adenocarcinoma Who Showed Initial Good Response to Gefitinib. J Thorac Oncol. 2009 Aug 18; doi: 10.1097/JTO.0b013e3181b62572. [DOI] [PubMed] [Google Scholar]
- 22.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009 Mar 10;27(8):1268–74. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prados MD, Lamborn KR, Chang S, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neuro Oncol. 2006 Jan;8(1):67–78. doi: 10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009 Feb 1;27(4):579–84. doi: 10.1200/JCO.2008.18.9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvihill JJ, Parry DM, Sherman JL, Pikus A, Kaiser-Kupfer MI, Eldridge R. NIH conference. Neurofibromatosis 1 (Recklinghausen disease) and neurofibromatosis 2 (bilateral acoustic neurofibromatosis). An update. Ann Intern Med. 1990 Jul 1;113(1):39–52. doi: 10.7326/0003-4819-113-1-39. [DOI] [PubMed] [Google Scholar]
- 26.Harris GJ, Plotkin SR, MacCollin M, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008 Jun;62(6):1314–9. doi: 10.1227/01.neu.0000333303.79931.83. [DOI] [PubMed] [Google Scholar]
- 27.Monsell EM. New and revised reporting guidelines from the Committee on Hearing and Equilibrium. American Academy of Otolaryngology-Head and Neck Surgery Foundation, Inc. Otolaryngol Head Neck Surg. 1995 Sep;113(3):176–8. doi: 10.1016/S0194-5998(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch IJ, DAVIS H, SILVERMAN SR, REYNOLDS EG, ELDERT E, BENSON RW. Development of materials for speech audiometry. J Speech Hear Disord. 1952 Sep;17(3):321–37. doi: 10.1044/jshd.1703.321. [DOI] [PubMed] [Google Scholar]
- 29.Plotkin SR, Halpin C, Blakely JO, et al. Suggested response criteria for phase II antitumor drug studies for neurofibromatosis type 2 related vestibular schwannoma. J Neurooncol. 2009;93:61–77. doi: 10.1007/s11060-009-9867-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990 Jul;8(7):1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 31.Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978 Sep;21(3):507–18. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- 32.Halpin C, Rauch SD. Using audiometric thresholds and word recognition in a treatment study. Otol Neurotol. 2006 Jan;27(1):110–6. doi: 10.1097/00129492-200601000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995 Sep;113(3):179–80. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 34.Plotkin SR, Singh MA, O'Donnell CC, Harris GJ, McClatchey AI, Halpin C. Audiologic and radiographic response of NF2-related vestibular schwannoma to erlotinib therapy. Nat Clin Pract Oncol. 2008 Aug;5(8):487–91. doi: 10.1038/ncponc1157. [DOI] [PubMed] [Google Scholar]
- 35.Norden AD, Raizer JJ, Abrey LE, et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol. 2009 Jun 28; doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SM, Buchler T, Joseph T, Lai C. Bilateral eardrum perforation after long- term treatment with erlotinib. J Clin Oncol. 2008 May 20;26(15):2582–4. doi: 10.1200/JCO.2007.15.3387. [DOI] [PubMed] [Google Scholar]
- 37.Braiteh F, Kurzrock R, Johnson FM. Trichomegaly of the Eyelashes After Lung Cancer Treatment with the Epidermal Growth Factor Receptor Inhibitor Erlotinib. J Clin Oncol. 2008 Jul 10;26(20):3460–2. doi: 10.1200/JCO.2008.16.9391. [DOI] [PubMed] [Google Scholar]
- 38.Wong HK, Lahdenranta J, Kamoun WS, et al. Anti-vascular endothelial growth factor therapies as a novel therapeutic approach to treating neurofibromatosis- related tumors. Cancer Res. 2010 May 1;70(9):3483–93. doi: 10.1158/0008-5472.CAN-09-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007 May 18;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 40.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007 Jan 25;445(7126):437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]