Abstract
COPD represents a major respiratory disorder, causing significant morbidity and mortality throughout the world. While therapies exist for COPD, they are not always effective, and many patients experience exacerbations and morbidity despite current therapies. Study of the molecular mechanisms involved in the underlying physiological manifestations of COPD has yielded multiple new targets for therapeutic intervention. In this review, we discuss signaling pathways involved in COPD pathogenesis and review clinical studies of p38 MAPK inhibitors, TNFα inhibitors, and IKK2 inhibitors as potential COPD therapies.
Keywords: airways disease, inflammation, irreversible airway obstruction, airway remodeling
Introduction
Treatments for COPD include inhaled steroids, anticholinergics, and β2-adrenergic receptor agonists. Inhaled long-acting β2-agonists (LABAs) and long acting anticholinergics improve lung function and health-related quality of life [1], while treatment with inhaled steroids decreases the frequency and severity of exacerbations but has little effect on decline in lung function [2]. Many patients however experience morbidity and frequent exacerbations despite therapy with LABAs and inhaled steroids; the molecular mechanisms underlying COPD remain unclear and novel therapeutics to treat inflammation are being examined [3]. With the underlying causes of COPD being diverse, recent approaches attempt to personalize COPD management using novel anti inflammatory drugs, as seen in Table 1. Multiple basic science studies suggest that the p38 mitogen activated protein kinase (MAPK) inhibitors, TNFα inhibitors, and IKK2 inhibitors may modulate the physiologic changes seen in COPD. In this review, we address signaling pathways that are activated in effector cells in the lungs of COPD patients and identify emerging therapeutics providing targeted bronchodilation and anti-inflammatory therapy.
Table 1.
Possible effects of new treatments on physiological changes seen in COPD. Review of the literature [47–54] suggests that p38 MAPK inhibitors, TNFα inhibitors, and IKK2 inhibitors may inhibit many of the physiological changes seen in COPD and could serve as useful therapies for this disease.
| COPD Manifestations | P38 MAPK inhibitors | TNFα inhibitors | IKK2 Inhibitors |
|---|---|---|---|
| Airflow Limitation | +[47] | +[48] | +[49] |
| Mucus Hypersecretion | +[50] | unknown | unknown |
| Alveolar Destruction | unknown | +[51] | +[49] |
| Pulmonary Vasoconstriction | +[52] | unknown | unknown |
| Skeletal Muscle Weakness | +[53] | +[54] | +[54] |
p38 MAP kinase
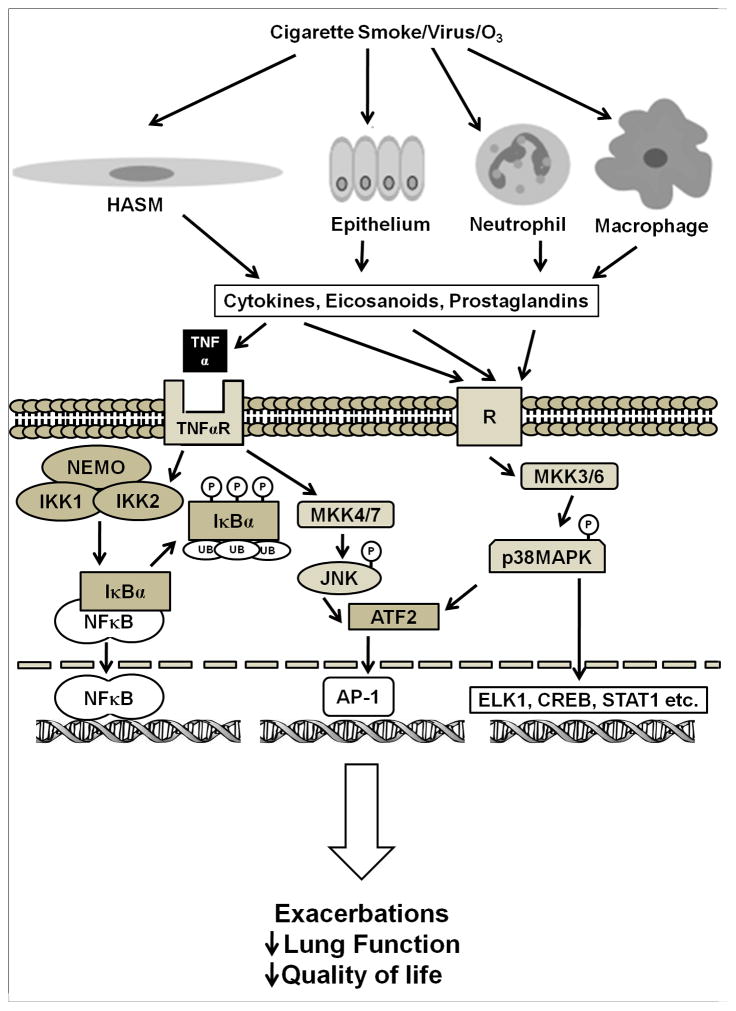
Patients with COPD have chronic airway inflammation and suffer from exacerbations, that increase morbidity. The inflammatory mediators involved in COPD are complex, and since relative glucocorticoid insensitivity is a hallmark of COPD, new therapies that target the inflammatory diathesis are being evaluated. The p38 mitogen activated protein kinase (MAPK) family consists of four isoforms of p38 MAPK, α, β, γ, and δ, which are expressed in different tissues and regulate activation of different kinases and phosphorylation of different substrates, causing diverse and often opposing effects. The α isoform of p38 MAPK is expressed in airway smooth muscle cells, epithelial cells, and immune cells and the study of p38 MAPK inhibitors for COPD have focused on this isoform [4]. As seen in Figure 1, the p38 MAPK signaling pathway is activated in response to multiple inflammatory signals, including inflammatory cytokines, oxidative stress, and growth factors. The p38 MAPK signaling pathway also interacts with other signaling pathways to modulate inflammation and cell proliferation. Patients with COPD have increased p38 MAPK activation in both macrophages and other cells within the alveolar wall when compared to nonsmokers or smokers without COPD. Additionally, p38 MAPK activation correlated with the degree of lung function impairment and alveolar wall inflammation [5]. Patients with COPD also manifest increased oxidative stress in the airways [6], and exposure to either ozone or cigarette smoke extract induces p38 MAPK activation [4, 7]. Bacterial or viral infection is a common trigger for COPD exacerbations, and exposure to LPS induces p38 MAPK activation in rat peritoneal macrophages and dendritic cells as well as an increase expression of inflammatory mediators [8, 9]. Activation of p38 MAPK is also implicated in inhibition of glucocorticoid function induced by pro-inflammatory cytokines such as TNFα, IL-1α and IL-13 [10-13]. Collectively, these studies suggest that p38 MAPK inhibitors may be an effective treatment for COPD. Isoform-selective p38 MAPK inhibitors have been developed that selectively inhibit the α and β isoforms by binding the ATP-binding site, including first generation inhibitor SB203580 and subsequently developed inhibitors SD282 (an indole-5-carboxamide), VX745, SCIO469, SD0006 (a diarylpyrazole), SB681323 (dilmapimod), PH797804 (identified from a series of N-aryl pyridinones), BMS582949, R1503 and AW814141 [4]. Inhibitors of p38 MAPK decrease airway inflammation [14], and the p38 MAPK inhibitor SB706504 decreased LPS-induced release of TNFα from macrophages in vitro [15]. In vivo the p38 MAPK inhibitor SD-282 inhibited tobacco smoke-induced increases in number of bronchoalveolar lavage (BAL) neutrophils and macrophages in mice, while steroids had little effect [16]. Additionally, in bleomycin-treated rats, treatment with p38 MAPK inhibitor SB239063 decreased bleomycin-induced synthesis of hydroxyproline, associated with fibrosis, collagen synthesis, and right ventricular hypertrophy. In guinea pigs, SB 239063 inhibited LPS-induced numbers in of neutrophils and IL-6 levels in the BAL [17]. Currently, p38 MAPK inhibitors are aslo considered to target inflammation in other diseases such as hyperlipidemia and rheumatoid arthritis. Trials of p38 MAPK inhibitors in humans have also demonstrated that p38 MAPK inhibitors decreases serum levels of both TNFα and IL-6 after LPS administration as well as acute phase reactants associated with inflammation, serum erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) [18]; a trial of an oral p38 MAPK inhibitor SCIO-469 in patients with rheumatoid arthritis demonstrated a decrease in ESR and CRP. Over the 24 weeks of the trial, however, there was little change in levels of acute phase reactants or arthritis symptoms when compared to placebo [19]. Another study showed that patients with coronary artery disease given p38 MAPK inhibitor SB-681323 prior to stent placement manifested decreased CRP levels compared to placebo [20]; patients with hyperlipidemia demonstrated a decrease in CRP and improved forearm blood flow in response to acetylcholine or sodium nitroprusside after treatment with p38 MAPK inhibitor losmapipod [21]. A study on COPD patients demonstrated that the p38 MAPK inhibitor SB-681323 decreased levels of activated serum heat shock protein 27, a marker of p38 activity, and decreased LPS-stimulated TNFα release into serum. Interestingly, prednisolone decreased LPS-stimulated TNFα release in the serum with little decrease in HSP 27 activation, suggesting the involvement of multiple inflammatory pathways in COPD [22]. Barnes et al reported that patients with moderate stable COPD receiving SB681323 for 28 days had a reduced sputum neutrophils and plasma fibrinogen with improvement in forced vital capacity as compared with placebo. A 6 week trial of p38 MAPK inhibitor PH797804 in patients with moderate to severe COPD decreased serum CRP levels as well as improved trough forced expiratory volume in 1 second (FEV1) and dyspnea index scores when compared to placebo. While these results are promising, there are some potential problems that make the p38 MAPK pathway a less desirable target for controlling inflammation. As seen in Figure 1, airway inflammation involves multiple kinases and signaling pathways, and blocking one kinase may lead to increased activity of others. Additionally, the p38α MAPK modulates activity of upstream MAPK kinase kinases such as TAK1 [23], and inhibition of p38α MAPK may alter these feedback loops and increase activation of kinases such as TAK1 and JNK2. Importantly, many p38 inhibitors have failed in clinical trials due to unacceptable safety profiles. Multiple side effects have been reported with p38 MAPK inhibitors including elevated liver enzymes, skin rash, cardiotoxicity, infections, and CNS and GI toxicity [24]. Inhaled p38 MAPK therapy is being explored for COPD, and p38 MAPK inhibitors ARRY371797 and PF03715455 show promise as p38 MAPK inhibitors that can be administered via inhalation [25].
Figure 1.
Role of TNFα, IKK2 and p38MAPK in modulating gene expression. Multiple stimuli induce p38MAPK phosphorylation, including inflammatory cytokines and oxidative stress. Once activated, p38 can activate multiple transcription factors including AP-1, ATF2, and ELK 1 to modulate gene transcription. TNFα binds its receptor and causes activation of NFκB by activating the IKK complex. IKK2 phosphorylates and inactivates IκBα, exposing the nuclear localization of NFκB and activating it. This figure is a simplification of the pathways involved with these mediators; multiple NFκB inducers have been identified including IL-1β and LPS, and there are interactions among kinases and transcription factors that are not elaborated here. ATF2 – activating transcription factor 2; CREB – cAMP response element binding; ELK1 – extracellular signal regulated-like kinase 1; IKK- IκB kinase; JNK – c-Jun N-terminal Kinase ; MAPK – mitogen-activated protein kinase, MKK- mitogen-activated protein kinase kinase; NEMO - NFκB essential modulator ; R – prototype receptor (e.g. cytokine, eicosanoid, prostaglandin), STAT1 – signal transducers and activators of transcription 1; TNF- Tumor necrosis factor alpha, TNFR – TNF receptor.
TNFα Inhibitors
Tumor necrosis factor alpha (TNFα) is a pleiotropic cytokine and a member of the TNF superfamily, a group of membrane-bound and soluble proteins implicated in inflammation. TNFα is produced as an intregral membrane protein that is translocated to the cell surface, and is released in soluble form by TNFα converting enzyme (TACE); TNFα may also play a central role in COPD pathogenesis (Figure 1). Induced sputum from patients with COPD has higher levels of TNFα than sputum obtained from smokers without COPD or nonsmoking controls [26]; levels of TNFα in induced sputum correlate directly with pack-years of smoking and inversely with forced exipiratory volume in one second (FEV1) [27]. Sputum TNFα levels were also increased in COPD patients with exacerbations associated with bacterial infections [28]. Interestingly, in animal models instillation of TNFα in the lungs of mice induces inflammatory and pathological features that are similar to that observed in COPD patients and TNF receptor knockout mice exposed to cigarette smoke had less airspace destruction and decreased levels of inflammatory mediators in BAL after cigarette smoke exposure. Further, TNFα over-expression downregulates oxidative stress enzymes and proteins, thereby decreasing the protective capacity of the lungs in response to oxidative damage [29]. Blocking TNFα has gained interest as an agent that attenuates airway inflammation. Two strategies have been employed to neutralize TNFα effects: etanercept is a monoclonal antibody that is a fusion of type II TNF receptor and the Fc portion of IgG to block unbound TNFα; infliximab and adalimumab are antibodies with a human Fc portion and mouse variable portions that directly bind TNFα. Infliximab and adalimumab also have the capability to activate complement mediated cellular lysis whereas ethanercept lacks this function [30]. Infliximab was used in 16 mild to severe COPD patients for a period of six weeks and found to have little effect on levels of inflammatory mediators in BAL or serum [31]. A study of 14 smokers with mild to moderate COPD given infliximab noted no change in spirometry measurements, eNO, or AHR, as compared to patients given placebo [32]. Rennard et al. studied 157 patients with mild to severe COPD, measuring exacerbations, FEV1, and transition dyspnea index, and found that little difference between patients treated with infliximab or placebo, however infliximab increased the incidence of pneumonia and malignancy, and a higher percentage of patients receiving infliximab needed to discontinue therapy due to adverse events [33]. An observational nested cohort study of 15,771 patients with both rheumatoid arthritis and COPD showed an association between patients given etanercept and a reduction in the rate of COPD hospitalizations, but no such association for patients given infliximab [34]. The lack of efficacy of infliximab may be due to a multiple factors. Although TNFα levels are elevated in COPD patients, none of the current studies measured serum or sputum TNFα levels. A subgroup of COPD patients may exist with increased TNFα levels who may benefit, but this remains to be seen. Additionally, the clinical trials were conducted for different time courses, varying from 6 weeks to 24 weeks, and longer studies may be needed to address efficacy. The side effects of blocking TNFα systemically include increased risk of infection, recurrence of tuberculosis and reactivation of hepatitis B, as well as worsening of congestive heart failure [30]. These side effects may overshadow the therapeutic benefits for COPD. Accordingly, anti-TNFα therapy may be more beneficial for exacerbations rather than as maintenance therapy. A study found that levels of TNFα in bronchial secretions could distinguish patients with COPD exacerbations and Pseudomonas aeruginosa infections from COPD exacerbation patients with common bacterial or viral infections [35], further suggesting that a subtype of patients may benefit from anti-TNFα therapy. Additionally, TNFα expression is increased by NFκB activation, and increases in TNFα levels seen in patients with COPD and/or COPD exacerbations may be due to NFκB activation. Further studies are needed to determine whether alternate delivery routes for TNFα inhibitors or identification of subgroups of COPD patients who may benefit from TNFα inhibitors. Alternatively, targeting release of soluble TNFα by TACE may be effective in limiting chronic inflammation, and TACE inhibitors PKF242-484 and PKF241-466 inhibited TNFα release from human macrophages in vitro and decreased LPS-induced and increases of cellular infiltration, TNFα levels, and myeloperoxidase and elastase activities in an animal model [36]. Other members of the TNF superfamily may also be targets for COPD treatment. Blockade of TNF superfamily ligand LIGHT decreases lung fibrosis, airway smooth muscle hyperplasia and airway hyperresponsiveness in a mouse model of chronic asthma [37].
IKK2 inhibitors
NFκB is a family of transcription factors that is activated in the inflammatory response of COPD. Both TNFα and cigarette smoke extract increase NFκB activation [38], and NFκB regulates the expression of multiple pro-inflammatory mediators (e.g TNFα, interleukins, vascular cell adhesion molecules, matrix metalloproteinases and cyclooxyenases [39], many of which are involved in COPD pathogenesis [40]. Patients with COPD have increased NFκB activation in BAL macrophages and epithelial cells, and NFκB activation further increases during exacerbations [3]. NFκB normally resides in the cytoplasm bound to IκBα, a chaperone protein that inhibits NFκB activation. As shown in Figure 1, NFκB is activated when an IκB kinase containing catalytic subunits IκB kinase 1 and IκB kinase 2 (IKK2) phosphorylate IκBα, causing its ubiquitination and proteolysis, releasing NFκB to translocate to the nucleus and initiate gene transcription [39]. IKK2 inhibition abrogates NFκB activation and nuclear translocation and decreases the inflammatory response seen in COPD. While IKK2 knockout mice were not viable, B-cells obtained from conditional IKK2 knockout mice demonstrate severely decreased proliferation in response to stimulation with anti-IgM, LPS, or anti-CD40, mediators that activate NFκB in normal conditions [41]. Numerous IKK2 inhibitors are being evaluated as potential anti-inflammatory therapies, including IMD-0354, IMD-0650, BMS-345541, PS-1145, SC-514, ACHP, Bay 65-1942, and AS602868 [42], and while there have been no clinical trials using IKK2 inhibitors, in vitro IKK2 inhibitors decrease NFκB activation induced by both TNFα and viral exposure as well as expression of NFκB dependent genes ICAM-1, IL-8, RANTES, IP-10, I-TAC and COX-2 [43–45]. The IKK2 inhibitor PHA-408 fed to rats exposed to aerosolized LPS or cigarette smoke decreased NFκB –DNA binding as well as neutrophils and pro-inflammatory mediators TNFα, IL-6, GM-CSF and IL-1β BAL when compared to controls [46]. Additonally, rats given the inhaled IKK2 inhibitor PF-184 and exposed to LPS had decreased NFκB activation as well as decreased levels of TNFα and PGE2 in the BAL. BAL cells challenged with LPS ex vivo demonstrated decreased secretion of nitric oxide, IL-1β, TNF-α, and Gro-α [47]. There are multiple alternatives to IKK2 inhibition to decrease NFκB activation, including overexpression or inhibiting degradation of IκBα, such as targeting of IκB ubiquitin ligase, inhibition of other kinases besides IKKs, or inhibition of non-canonical NFκB activation by inhibiting NFκB inducing kinase. Inhibition of NFκB-induced gene transcription with ‘decoy’ oligonucleotides [48] or using small interfering RNAs (siRNAs) to target expression of specific genes regulated by NFκB is another therapeutic strategy.
Animal studies suggest that inhibiting NFκB activation may have multiple side effects, including increased susceptibility to infections and liver apoptosis, and these potential adverse effects will need to be evaluated prior to initiating human studies.
To summarize, COPD is a disease defined by chronic airflow limitation and airway inflammation, and is a cause of significant morbidity and mortality. Research into the molecular mechanisms of inflammation underlying COPD pathogenesis have yielded multiple targets for new therapies, including inhibitors of p38 MAPK, TNFα, and IKK2. While these treatments have all demonstrated promise in vitro as well as in animal models, they all have significant side effects and human studies are currently lacking in evidence of efficacy. Further studies are necessary focusing on delivery methods to minimize adverse side effects as well as identification of subpopulations of COPD patients who may benefit most from these new therapies.
Highlights.
COPD causes significant morbidity and mortality throughout the world.
Drugs that target inflammation have promise as therapies for COPD
TNF alpha, NF kappa B, and p38 MAP kinase may all contribute to COPD pathogenesis
Inhibitors of TNF alpha, IKK2 and p38 MAPK may be useful as COPD therapies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 3:CD008532. doi: 10.1002/14651858.CD008532.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer S, et al. Inhaled corticosteroids versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 12:CD007033. doi: 10.1002/14651858.CD007033.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Emerging pharmacotherapies for COPD. Chest. 2008;134(6):1278–86. doi: 10.1378/chest.08-1385. [DOI] [PubMed] [Google Scholar]
- 4.Williams AS, et al. Role of p38 mitogen-activated protein kinase in ozone-induced airway hyperresponsiveness and inflammation. Eur J Pharmacol. 2008;600(1–3):117–22. doi: 10.1016/j.ejphar.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Renda T, et al. Increased activation of p38 MAPK in COPD. Eur Respir J. 2008;31(1):62–9. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- 6.Rahman I, I, Adcock M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28(1):219–42. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 7.Volpi G, et al. Cigarette smoke and alpha, beta-unsaturated aldehydes elicit VEGF release through the p38 MAPK pathway in human airway smooth muscle cells and lung fibroblasts. Br J Pharmacol. 163(3):649–61. doi: 10.1111/j.1476-5381.2011.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patenaude J, et al. LPS response and endotoxin tolerance in Flt-3L-induced bone marrow-derived dendritic cells. Cell Immunol. 271(1):184–91. doi: 10.1016/j.cellimm.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, et al. Lipopolysaccharide upregulates the expression of corticotropin-releasing hormone via MAP kinase pathway in rat peritoneal macrophages. Mol Cell Biochem. doi: 10.1007/s11010-011-1080-2. [DOI] [PubMed] [Google Scholar]
- 10.Szatmary Z, Garabedian MJ, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem. 2004;279(42):43708–15. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry. 2004;9(1):65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa T, et al. Involvement of the p38 MAPK pathway in IL-13-induced mucous cell metaplasia in mouse tracheal epithelial cells. Respirology. 2008;13(2):191–202. doi: 10.1111/j.1440-1843.2008.01237.x. [DOI] [PubMed] [Google Scholar]
- 13.Spahn JD, et al. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol. 1996;157(6):2654–9. [PubMed] [Google Scholar]
- 14.Adams JL, et al. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog Med Chem. 2001;38:1–60. doi: 10.1016/s0079-6468(08)70091-2. [DOI] [PubMed] [Google Scholar]
- 15.Kent LM, et al. Inhibition of lipopolysaccharide-stimulated chronic obstructive pulmonary disease macrophage inflammatory gene expression by dexamethasone and the p38 mitogenactivated protein kinase inhibitor N-cyano-N′-(2-{[8-(2,6-difluorophenyl)-4-(4-fluoro-2-methylphenyl)-7-oxo-7,8-dihydropyrido[2,3-d] pyrimidin-2-yl]amino}ethyl)guanidine (SB706504) J Pharmacol Exp Ther. 2009;328(2):458–68. doi: 10.1124/jpet.108.142950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medicherla S, et al. p38alpha-selective mitogen-activated protein kinase inhibitor SD-282 reduces inflammation in a subchronic model of tobacco smoke-induced airway inflammation. J Pharmacol Exp Ther. 2008;324(3):921–9. doi: 10.1124/jpet.107.127092. [DOI] [PubMed] [Google Scholar]
- 17.Underwood DC, et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L895–902. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- 18.Hope HR, et al. Anti-inflammatory properties of a novel N-phenyl pyridinone inhibitor of p38 mitogen-activated protein kinase: preclinical-to-clinical translation. J Pharmacol Exp Ther. 2009;331(3):882–95. doi: 10.1124/jpet.109.158329. [DOI] [PubMed] [Google Scholar]
- 19.Genovese MC, et al. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J Rheumatol. 38(5):846–54. doi: 10.3899/jrheum.100602. [DOI] [PubMed] [Google Scholar]
- 20.Sarov-Blat L, et al. Inhibition of p38 mitogen-activated protein kinase reduces inflammation after coronary vascular injury in humans. Arterioscler Thromb Vasc Biol. 30(11):2256–63. doi: 10.1161/ATVBAHA.110.209205. [DOI] [PubMed] [Google Scholar]
- 21.Cheriyan J, et al. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 123(5):515–23. doi: 10.1161/CIRCULATIONAHA.110.971986. [DOI] [PubMed] [Google Scholar]
- *22.Singh D, et al. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 50(1):94–100. doi: 10.1177/0091270009347873. In this double blind, double dummy randomized crossover study, patients were given p38 MAPK inhibitor SB-681323, prednisolone, or placebo, and blood was evaluated for inflammatory mediatiors. The investigators found that the p38 MAPK inhibitor decreased heat shock protein levels as well as TNF alpha levels, while prednisolone decreased TNF alpha levels but had little effect on TNF alpha levels. This trial demonstrated that p38 MAPK inhibitors can decrease serum levels of inflammatory mediators in COPD patients. [DOI] [PubMed] [Google Scholar]
- 23.Shin MS, et al. Cross interference with TNF-alpha-induced TAK1 activation via EGFR-mediated p38 phosphorylation of TAK1-binding protein 1. Biochim Biophys Acta. 2009;1793(7):1156–64. doi: 10.1016/j.bbamcr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Pettus LH, Wurz RP. Small molecule p38 MAP kinase inhibitors for the treatment of inflammatory diseases: novel structures and developments during 2006–2008. Curr Top Med Chem. 2008;8(16):1452–67. doi: 10.2174/156802608786264245. [DOI] [PubMed] [Google Scholar]
- 25.Millan DS, et al. Design and synthesis of inhaled p38 inhibitors for the treatment of chronic obstructive pulmonary disease. J Med Chem. 54(22):7797–814. doi: 10.1021/jm200677b. [DOI] [PubMed] [Google Scholar]
- 26.Keatings VM, et al. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–4. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 27.Hacievliyagil SS, et al. Association between cytokines in induced sputum and severity of chronic obstructive pulmonary disease. Respir Med. 2006;100(5):846–54. doi: 10.1016/j.rmed.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Bathoorn E, et al. Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis. 2009;4:101–9. doi: 10.2147/copd.s4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFalpha in pulmonary pathophysiology. Respir Res. 2006;7:125. doi: 10.1186/1465-9921-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matera MG, Calzetta L, Cazzola M. TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm Pharmacol Ther. 23(2):121–8. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Dentener MA, et al. Effect of infliximab on local and systemic inflammation in chronic obstructive pulmonary disease: a pilot study. Respiration. 2008;76(3):275–82. doi: 10.1159/000117386. [DOI] [PubMed] [Google Scholar]
- 32.van der Vaart H, et al. First study of infliximab treatment in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(4):465–9. doi: 10.1164/rccm.200501-147OC. [DOI] [PubMed] [Google Scholar]
- *33.Rennard SI, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(9):926–34. doi: 10.1164/rccm.200607-995OC. In this double blind, placebo-controlled, parallell group study patients with moderate to severe COPD were given infliximab vs placebo. The investigators noted no change in either chronic respiratory questionnaire total score or FEV1, six minute walk distance, dyspnea index, or COPD exacerbations. This and other studies have not shown benefit to infliximab therapy in COPD. [DOI] [PubMed] [Google Scholar]
- *34.Suissa S, Ernst P, Hudson M. TNF-alpha antagonists and the prevention of hospitalisation for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2008;21(1):234–8. doi: 10.1016/j.pupt.2007.03.003. This was an observational study of patients with both RA and COPD. The investigators found that ethanercept, but not infliximab, was associated with a lower rate of hospitalization with COPD exacerbation and suggests that further study of TNF inhibitors in COPD should be done. [DOI] [PubMed] [Google Scholar]
- 35.Dal Negro RW, et al. A two-stage logistic model based on the measurement of pro-inflammatory cytokines in bronchial secretions for assessing bacterial, viral, and non-infectious origin of COPD exacerbations. Copd. 2005;2(1):7–16. doi: 10.1081/copd-200050680. [DOI] [PubMed] [Google Scholar]
- 36.Trifilieff A, et al. Pharmacological profile of PKF242-484 and PKF241-466, novel dual inhibitors of TNF-alpha converting enzyme and matrix metalloproteinases, in models of airway inflammation. Br J Pharmacol. 2002;135(7):1655–64. doi: 10.1038/sj.bjp.0704616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doherty TA, et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 17(5):596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, et al. NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respir Res. 2008;9:66. doi: 10.1186/1465-9921-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 40.Adcock IM, et al. Kinase inhibitors and airway inflammation. Eur J Pharmacol. 2006;533(1–3):118–32. doi: 10.1016/j.ejphar.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 41.Li ZW, et al. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170(9):4630–7. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- 42.Xie J, et al. Aminopyridinecarboxamide-based inhaled IKK-2 inhibitors for asthma and COPD: Structure-activity relationship. Bioorg Med Chem. 19(3):1242–55. doi: 10.1016/j.bmc.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Caposio P, et al. Targeting the NF-kappaB pathway through pharmacological inhibition of IKK2 prevents human cytomegalovirus replication and virus-induced inflammatory response in infected endothelial cells. Antiviral Res. 2007;73(3):175–84. doi: 10.1016/j.antiviral.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Frelin C, et al. AS602868, a pharmacological inhibitor of IKK2, reveals the apoptotic potential of TNF-alpha in Jurkat leukemic cells. Oncogene. 2003;22(50):8187–94. doi: 10.1038/sj.onc.1206963. [DOI] [PubMed] [Google Scholar]
- 45.Hideshima T, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277(19):16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- *46.Rajendrasozhan S, et al. Anti-inflammatory effect of a selective IkappaB kinase-beta inhibitor in rat lung in response to LPS and cigarette smoke. Pulm Pharmacol Ther. 23(3):172–81. doi: 10.1016/j.pupt.2010.01.002. In this study, investigators administered oral IKK2 inhibitor PHA-408 and exposed pt to LPS aerosol or cigarette smoke and observed decreased levels of neutrophils and inflammatory cytokines in BAL, demonstrating that IKK2 inhibitors decrease inflammation in vivo in an animal model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **47.Sommers CD, et al. Novel tight-binding inhibitory factor-kappaB kinase (IKK-2) inhibitors demonstrate target-specific anti-inflammatory activities in cellular assays and following oral and local delivery in an in vivo model of airway inflammation. J Pharmacol Exp Ther. 2009;330(2):377–88. doi: 10.1124/jpet.108.147538. In this study, investigators compared inhaled administration of IKK2 inhibitor PF-184 to oral PHA-408 and demonstrted that inhaled PF-184 had similar effects as oral PHA-408 on markers of pulmonary inflammation without changing serum TNF levels, suggesting that inhaled IKK2 inhibitors may be inhibit pulmonary inflammation without systemic side effects. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura H, Morishita R, Kaneda Y. Molecular therapy via transcriptional regulation with double-stranded oligodeoxynucleotides as decoys. In Vivo. 2002;16(1):45–8. [PubMed] [Google Scholar]