Abstract
Rationale
Vascular calcification is a serious cardiovascular complication that contributes to the increased morbidity and mortality of patients with diabetes. Hyperglycemia, a hallmark of diabetes, is associated with increased vascular calcification as well as increased modification of proteins by O-linked N-acetylglucosamine (O-GlcNAcylation).
Objective
We sought to determine the role of protein O-GlcNAcylation in regulating vascular calcification and the underlying mechanisms.
Methods and Results
Low-dose streptozotocin-induced diabetic mice exhibited increased aortic O-GlcNAcylation and vascular calcification, which also was associated with impaired aortic compliance in mice. Elevation of O-GlcNAcylation by administration of Thiamet-G, a potent inhibitor for O-GlcNAcase (OGA) that removes O-GlcNAcylation, further accelerated vascular calcification and worsened aortic compliance of diabetic mice in vivo. Increased O-GlcNAcylation, either by Thiamet-G or OGA knockdown, promoted calcification of primary mouse vascular smooth muscle cells (VSMC). Increased O-GlcNAcylation in diabetic arteries or in the OGA knockdown VSMC upregulated expression of the osteogenic transcription factor Runx2 and enhanced activation of AKT. O-GlcNAcylation of AKT at two new O-sites, T430 and T479, promoted AKT phosphorylation, which in turn enhanced VSMC calcification. Site-directed mutation of AKT at T430 and T479 decreased O-GlcNAcylation, inhibited phosphorylation of AKT at S473 and binding of mTOR complex 2 to AKT, and subsequently blocked Runx2 transactivity and VSMC calcification.
Conclusions
O-GlcNAcylation of AKT at two new sites enhanced AKT phosphorylation and activation, thus promoting vascular calcification. Our studies have identified a novel causative effect of O-GlcNAcylation in regulating vascular calcification in diabetes and uncovered a key molecular mechanism underlying O-GlcNAcylation-mediated activation of AKT.
Keywords: Diabetes mellitus, O-GlcNAcylation, vascular calcification, smooth muscle cells, AKT activation
INTRODUCTION
Diabetes has been strongly associated with chronic cardiovascular and renal complications, which leads to an increased morbidity and mortality in affected patients1, 2. Increased vascular calcification is commonly observed in diabetic arteries, in the intimal and medial layers of the vessel walls3-6, which increases arterial stiffness, reduces compliance of the blood vessels7, 8, and increases the risk of cardiovascular events and mortality9. Therefore, understanding of the molecular mechanisms underlying diabetic vascular calcification should provide important insights into overcoming these adverse clinical outcomes.
Hyperglycemia, or elevated blood glucose, is a characteristic feature of diabetes10. In addition to producing bioenergetic substrates via the tricarboxylic acid cycle, glucose metabolism through the hexosamine biosynthesis pathway generates UDP-GlcNAc, a substrate for protein O-linked β-N-acetylglucosamine modification (O-GlcNAcylation)11. Hyperglycemia has also been associated with vascular calcification in vitro5, 12; however, the mechanistic function of hyperglycemia and O-GlcNAcylation in regulating diabetic vascular calcification is unknown. O-GlcNAcylation is a dynamic and reversible modification that regulates the activity and function of numerous cytoplasmic and nuclear proteins13, 14. Like protein phosphorylation, O-GlcNAcylation occurs on serine and threonine residues, and thus these two modifications on proteins crosstalk to regulate cellular signaling and function. Unlike phosphorylation, however, O-GlcNAcylation is tightly regulated by two specific enzymes: β-N-acetylglucosaminyl-transferase (OGT) adds O-GlcNAc onto target proteins, whereas β-N-acetylglucosaminidase (OGA) removes O-GlcNAc modification.
Protein O-GlcNAcylation regulates a variety of cellular functions in different tissues, including the cardiovascular system, related to diabetes and vascular injury. O-GlcNAcylation was found to serve as a cellular nutrient and stress sensor by modulating the function of specific proteins in response to glucose levels15. In cardiomyocytes, O-GlcNAcylation is associated with cell survival in response to oxidative stress, and preserves heart function in models of heart failure16-18. In contrast, increased O-GlcNAcylation has been observed to negatively influence contractility in left ventricular tissue from humans with heart failure19. In human diabetic carotid plaques, the overall O-GlcNAcylation level is increased20. Additionally, diabetic patients have a higher incidence of calcified plaque3. However, the contribution of elevated O-GlcNAcylation in the diabetic vasculature to vascular calcification is unknown.
The present studies investigate the function of O-GlcNAcylation in regulating vascular calcification and the underlying molecular mechanisms. We have demonstrated elevated O-GlcNAcylation and increased vascular calcification in arteries from diabetic mice, which was associated with impaired aortic compliance. Elevation of O-GlcNAcylation by Thiamet-G treatment, a potent inhibitor for OGA, further accelerated vascular calcification and worsened aortic compliance of diabetic mice in vivo. Using primary cultured mouse vascular smooth muscle cells (VSMC), we have determined that O-GlcNAcylation of AKT at two new sites increases phosphorylation and activation of AKT, which promoted VSMC calcification. These studies have demonstrated a novel and causative link between protein O-GlcNAcylation and vascular calcification in diabetes. Understanding the molecular mechanisms underlying hyperglycemia in regulating diabetic vascular calcification should provide important insights into identification of new targets and strategies for prevention and therapy of diabetic calcification.
METHODS
Experimental animals
To induce hyperglycemia and diabetes, low-dose streptozotocin (STZ) injection was performed as previously described21-23. Briefly, C57BL/6 mice were intraperitoneally injected with STZ (50 mg/kg) for 5 consecutive days, and blood glucose was monitored weekly for 4 months using the AlphaTrak glucose meter and strips (Abbott, Abbott Park, IL). For Thiamet-G treatment, mice were injected intravenously with Thiamet-G24 (20 mg/kg) one week after STZ treatment and weekly for 2 months. Both food and fluid intake were given ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Tissue harvest and processing
At the experimental end points, mice were sacrificed, the aortic arch and descending aorta were dissected under a microscope and used for characterization of calcium content, RNA and protein expression and immunostaining as we previously described25.
Aortic calcium measurement
Aortic calcium content was measured by Arsenazo III assay as we previously described25. Descending aortas were homogenized and digested by collagenase. Protein amount was determined by BCA assay26, and calcium was extracted with 0.6 mmol/L HCl and quantified colorimetrically by Arsenazo III calcium measurement kit (StanBio)25. The amount of vascular calcium was normalized to the total protein amount in the tissues and expressed as fold change compared to control.
Echocardiography and measurement of pulse wave velocity
Pulse wave velocity (PWV) was analyzed by echocardiography with the high resolution imaging system VEVO 770 (Visual Sonics, Toronto, Canada). Detailed methods are available in online supplemental materials.
In vitro calcification of VSMC
Primary VSMC were isolated from the aortas of C57BL/6 mice as we described26. VSMC calcification was induced in osteogenic medium containing DMEM, supplemented with 20% fetal bovine serum, L-ascorbic acid (0.25 mM), β-glycerophosphate (10 mM), and dexamethasone (10−8 M, Sigma-Aldrich) for 3 weeks. Calcification was determined by Alizarin red staining as we described25. In parallel sets of dishes, cells were lysed with 0.5 N HCl and total calcium content was quantified with Arsenazo III calcium measurement kit (StanBio) and normalized to the amount of total proteins25.
Induction of O-GlcNAcylation in VSMC
O-GlcNAcylation was induced by inhibition of OGA, using a pharmacological inhibitor, Thiamet-G24, or OGA knockdown by lentivirus-mediated short hairpin RNA specific targeting OGA (GenBank NC_000085.6, shRNA, Thermo Scientific, Waltham, MA) as we have described previously26.
OGA, OGT and O-GlcNAcylation were determined by Western blot analysis using specific antibodies for OGA (Santa Cruz Biotechnology, Santa Cruz, CA), OGT (Sigma Aldrich, St. Louis, MO), and O-GlcNAcylation (RL-2, Abcam)27.
O-GlcNAcylation of AKT
To determine AKT O-GlcNAcylation and its impact on phosphorylation, immunoprecipitation was performed with AKT antibody (Cell Signaling). In brief, cell extracts were incubated with AKT antibody or isotope-matched IgG (Santa Cruz, as negative control) at 4°C overnight and then mixed with protein G agarose beads (Sigma Aldrich) for 3 hours. Beads were washed, and proteins pulled down were analyzed by Western blotting using specific antibodies to detect O-GlcNAcylation (RL-2) and AKT phosphorylation (Cell Signaling, listed above).
Dual-Luciferase reporter assay
Runx2 transactivity was determined as we described by Dual-Luciferase Reporter assay (Promega, Madison, WI) with the use of a luciferase reporter construct containing six Runx binding elements (p6xRunx-Luc)28.
Generation of AKT mutants
Constructs carrying cDNA encoding wild type (wt-AKT) and constitutively active AKT (CA-AKT) were originally provided by Dr. Hongju Wu (Tulane University)29. Point mutations in the sequence of the lentiviral CA-AKT were made at serine 122, threonine 430 and threonine 479 to replace the residues with alanine using the QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies, Santa Clara, CA) and confirmed by sequencing analysis. VSMC stably infected with lentivirus expressing wt-AKT, CA-AKT, and mutant AKT, CA-AKT-S122A, Ca-AKT-T430A and CA-AKT-T479A, were characterized for AKT O-GlcNAcylation and their effects on AKT phosphorylation, Runx2 activity and VSMC calcification. The effects of AKT mutants on the binding of AKT to its kinases and phosphatase were determined by immunoprecipitation followed by Western blot analysis of Rictor, mTOR, PDK1, PHLPP with specific antibodies (Cell Signaling).
Statistical analysis
Results are presented as the mean ± SD. Differences between groups were determined with the use of Student t tests or 1-way ANOVA where appropriate. Significance was defined as p<0.05.
RESULTS
Increased vascular O-GlcNAcylation and calcification in diabetic mice
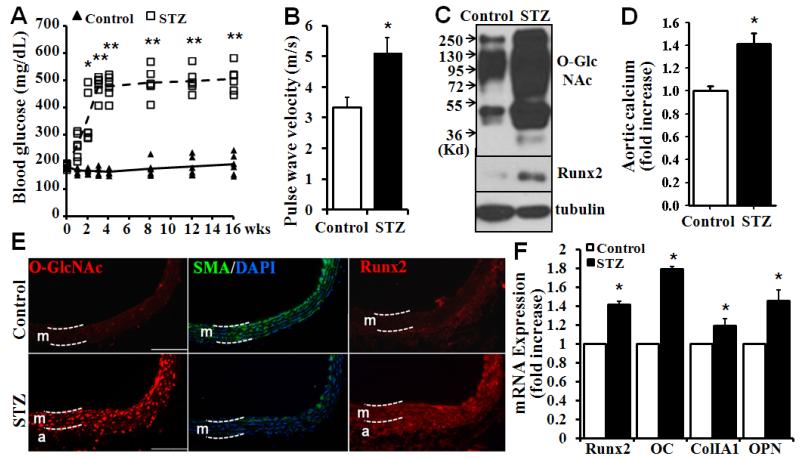
Using low-dose STZ injection-induced diabetic mouse model, we characterized O-GlcNAcylation and vascular calcification in mice. Blood glucose levels were monitored in the STZ-injected mice and compared with those in the control mice (Fig 1A). Elevation of blood glucose levels was observed at one week in the STZ-injected mice (Fig 1A). Severe hyperglycemia was observed after three weeks, which was sustained until the end of the experiments at 16 weeks after administration of STZ. Echocardiography analysis demonstrated a significant increase in pulse wave velocity (PWV), an indicator for aortic stiffness30, in the diabetic mice 16 weeks after administration of STZ (Fig 1B), suggesting impaired aortic compliance in the diabetic mice. Therefore, STZ-induced hyperglycemia was linked to impaired aortic function in the diabetic mice.
Figure 1. Increased O-GlcNAcylation and vascular calcification in diabetic mice.
A) STZ injection increased blood glucose. Mice were injected intraperitoneally with sodium citrate buffer (Control) or 50 mg/kg STZ for 5 consecutive days. Blood glucose was measured using the AlphaTrak glucose meter for 16 weeks following the STZ injection (n=6 mice for each group, *p<0.05, **p<0.01 compared to control). B) Increased aortic stiffness in STZ-injected diabetic mice. Echocardiography was performed in mice 16 week after the STZ injection, to determine pulse wave velocity, an indicator for aortic stiffness (n=4, *p=0.02). C) Increased vascular O-GlcNAcylation and Runx2 expression in diabetic mice. Descending aortas from control and STZ-injected mice were explanted, and protein extracts were analyzed for O-GlcNAcylation and Runx2 by Western blot. D) Increased vascular calcification in diabetic mice. Calcium content was determined in descending aortas from control and STZ-injected mice. The calcium content in aortas from control mice was defined as 1 (n=4 mice for each group, *p=0.002). E) Increased O-GlcNAcylation and Runx2 in aortic media of diabetic mice. Aortic sections from control and STZ-injected mice were stained with specific antibodies as labeled. White dashed lines delineate aortic media (m) and adventitia (a). Scale bar=100 μm. F) Increased expression of Runx2 and osteogenic markers in aortas from diabetic mice. Real-time PCR analysis was performed to determine the expression of Runx2 and osteogenic marker genes, including OC, ColIA1 and OPN, in aortas from control and STZ-injected diabetic mice (n=4, *p<0.001).
As hyperglycemia has been linked to increased protein O-GlcNAc modification, we determined protein O-GlcNAcylation profile in the diabetic vasculature. Dramatic increases in O-GlcNAcylation were demonstrated in the aortas from STZ-injected mice (Fig 1C, top panel). Increased O-GlcNAcylation in the diabetic arteries was associated with increased expression of the osteogenic transcription factor Runx2 (Fig 1C, middle panel). We have previously demonstrated that increased Runx2 determines vascular calcification25,26. Consistently, increased calcification was also observed in the aortas from STZ-injected mice (Fig 1D). Additionally, increased O-GlcNAcylation was observed in the vasculature of mice 4 weeks after STZ administration, but calcification was not significantly increased at this time point (Suppl. Fig I), indicating that increased vascular O-GlcNAcylation may contribute to calcification in diabetes.
Immunofluorescent staining further demonstrated increased vascular O-GlcNAcylation in the media of arteries from STZ-injected diabetic mice compared to those from controls (Fig 1E, O-GlcNAc). Increased O-GlcNAcylation was correlated with decreased smooth muscle specific α-actin (α-SMA) and increased Runx2 expression (Fig 1E). In addition, increased expression of Runx2 and osteogenic marker genes, including osteocalcin (OC), collagen IA1 (ColIA1), and osteopontin (OPN) was demonstrated in aortas from the diabetic mice (Fig 1F), further confirming an association of O-GlcNAcylation with SMC dedifferentiation and calcification.
Increased O-GlcNAcylation in VSMC induces vascular calcification
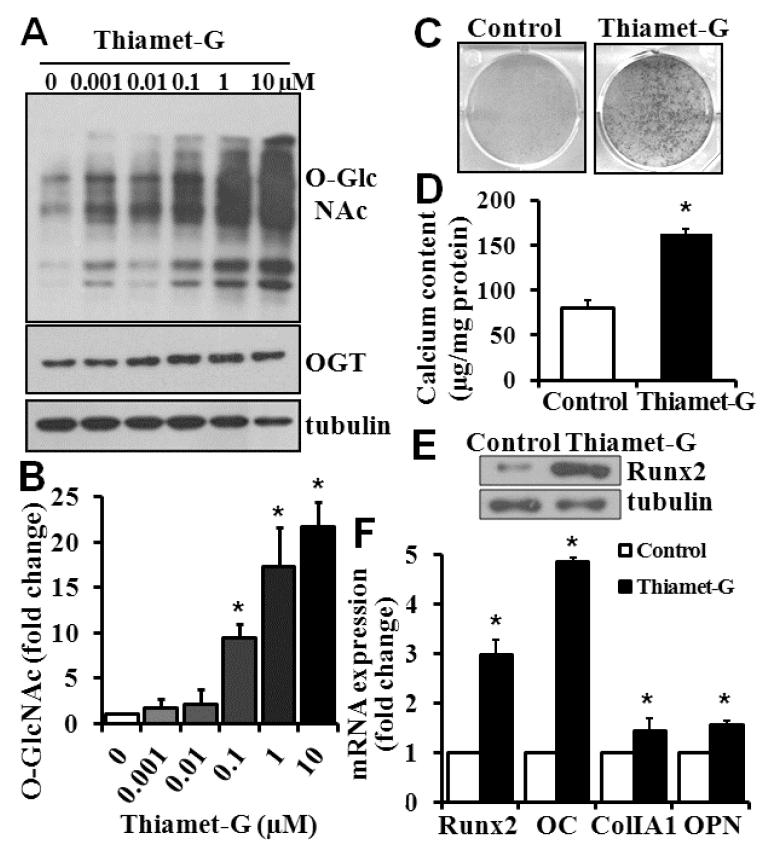
To determine a direct effect of increased O-GlcNAcylation on vascular calcification, we induced O-GlcNAcylation in cultured VSMC using Thiamet-G, a highly potent and selective inhibitor of OGA that has been shown to increase O-GlcNAc modification24. We found that Thiamet-G dose-dependently increased O-GlcNAcylation, independent of OGT (Fig 2A, and 2B). Thiamet-G at the concentration used did not affect cell viability and proliferation (data not shown). Importantly, increased O-GlcNAcylation by Thiamet-G was found to induce VSMC calcification, as shown by Alizarin red staining (Fig 2C), calcium content quantification by Arsenazo III assay (Fig. 2D), and increase expression of Runx2 (Fig 2E). Similar to the observation with diabetic arteries in vivo (Fig 1F), increased O-GlcNAcylation in VSMC by the Thiamet-G treatment was found to upregulate expression of Runx2 as well as other osteogenic marker genes (Fig 2E).
Figure 2. Inhibition of OGA increases O-GlcNAcylation and calcification in VSMC.
A) and B) Thiamet-G increased O-GlcNAcylation in VSMC. VSMC were treated with Thiamet-G for 6 hours at 0-10 μM, and Western blot was performed to determine O-GlcNAcylation. Representative blots from 4 independent experiments are shown in A. B) The intensity of the bands in each condition in A was quantified by NIH ImageJ and compared with that in the control (Thiamet-G, 0), defined as 1. C) and D) Thiamet-G induced VSMC calcification. VSMC were treated with osteogenic medium alone (Control) or osteogenic medium with 10 μM Thiamet-G for 3 weeks. Calcification was determined by Alizarin red staining (C) or quantified by Arsenazo III assay (D, n=3, *p<0.001). E) and F) Thiamet-G increased the expression of Runx2 and osteogenic marker genes, as determined by Western blot (E) and Real-time PCR analysis (F) in parallel experiments as in C and D (n=3, *p<0.01 compared to control).
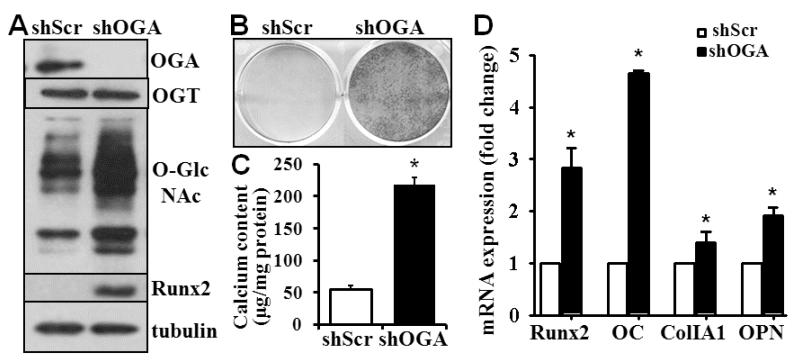
To confirm that the effects of Thiamet-G were mediated through OGA, we selectively knocked down OGA in VSMC using lentivirus containing shRNA for OGA. Western blot analysis demonstrated effective knockdown of OGA in VSMC, without affecting the expression of OGT (Fig 3A). The viability and proliferation of VSMC was not affected by the OGA knockdown (data not shown). Consistent with the results from Thiamet-G treatment (Fig 2A), the OGA knockdown in VSMC increased O-GlcNAcylation (Fig 3A). Importantly, the OGA knockdown was sufficient to induce expression of Runx2 and other osteogenic marker genes, as well as VSMC calcification (Fig 3B-D). Therefore, these studies have demonstrated a direct effect of increased O-GlcNAcylation on osteogenic differentiation and calcification of VSMC in vitro.
Figure 3. Knockdown of OGA increases VSMC calcification.
A) Knockdown of OGA in VSMC by shRNA increased O-GlcNAcylation and Runx2. VSMC were infected with lentivirus containing scrambled shRNA (shScr) or shRNA for OGA (shOGA). Infected cells were selected using puromycin. The amount of OGA, OGT, Runx2 and O-GlcNAc was determined by Western blot analysis. Representative blots from 4 independent experiments are shown. B) and C) OGA knockdown induced VSMC calcification. Control and OGA knockdown cells were cultured in osteogenic medium for 3 weeks. Calcification was determined by Alizarin red staining (B) or quantified by Arsenazo assay (C) in separate dishes (n=3, *p<0.001). D) OGA knockdown increased expression of Runx2 and osteogenic marker genes. Real-time PCR analysis was performed to determine the expression of Runx2 and osteogenic marker genes in parallel experiments as in B and C (n=3, *p<0.005 compared to shScr).
Increased O-GlcNAcylation enhances diabetic vascular calcification in vivo
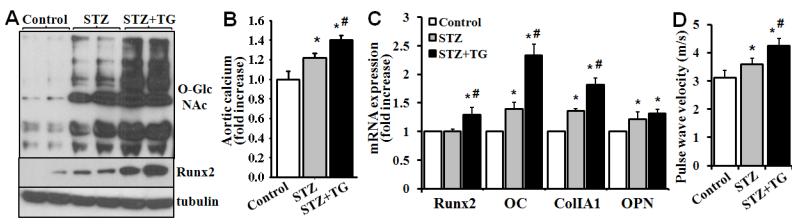
The effects of increased O-GlcNAcylation on vascular calcification was further determined in diabetic mice in vivo. STZ-induced vascular O-GlcNAcylation was dramatically enhanced by administration of Thiamet-G (Fig 4A). At 8 weeks after the STZ injection, STZ alone induced a significant increase in vascular calcification. Strikingly, administration of Thiamet-G further enhanced vascular calcification (Fig 4B). The effect of Thiamet-G on diabetic vascular calcification (Fig. 4B) was well associated with increased expression of Runx2 and the osteogenic marker genes (Fig 4C). Consistently, administration of Thiamet-G further increased aortic stiffness and worsened aortic compliance, indicated by increased pulse wave velocity, in the diabetic mice (Fig 4D). Taken together, these data demonstrated a causative link between increased O-GlcNAcylation and vascular calcification in diabetic mice in vivo.
Figure 4. Thiamet-G treatment accelerates vascular calcification in diabetic mice.
Mice were treated with control, STZ and STZ plus Thiamet G for 8 weeks as described in material and methods. A) Thiamet-G increased vascular O-GlcNAcylation and Runx2 in diabetic mice. Descending aortas were explanted, and protein extracts were analyzed for aortic O-GlcNAcylation and Runx2 by Western blot. B) Thiamet-G accelerated vascular calcification in diabetic mice. Calcium content was determined in mouse descending aortas and compared with that in the vehicle-treated control group, which is defined as 1 (n=4, *p<0.05 compared with the control, #p<0.05 compared with STZ). C) Thiamet-G increased the expression of Runx2 and osteogenic marker genes in diabetic mice. Real-time PCR analysis was performed to determine the expression of Runx2 and osteogenic marker genes in descending aortas from mice treated with vehicle control, STZ and STZ plus Thiamet-G (n=4, *p<0.05 compared with control, #p<0.05 compared with STZ alone). D) Thiamet-G increased aortic stiffness of diabetic mice. Echocardiography was performed to determine pulse wave velocity (n=4, *p<0.05 compared with the control, #p<0.05 compared with STZ).
Increased O-GlcNAcylation enhances activation of AKT
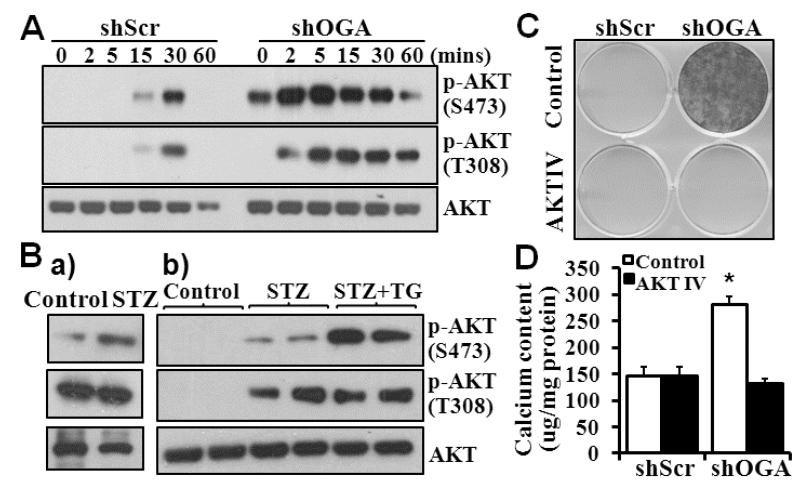
To determine the molecular mechanisms underlying O-GlcNAcylation on VSMC calcification, we characterized the activation of protein kinase AKT, a critical upstream kinase that we have previously determined to regulate Runx2 activity and VSMC calcification26. Glucose-induced phosphorylation/activation of AKT at serine 473 (S473) and threonine 308 (T308) was demonstrated in the control VSMC (Fig 5A, shScr). Increased O-GlcNAcylation in the OGA knockdown VSMC (shOGA) resulted in basal activation of AKT by phosphorylation at S473, but not T308. Furthermore, increased and sustained phosphorylation/activation at both S473 and T308 was demonstrated in OGA knockdown VSMC after stimulation with glucose (Fig 5A), suggesting a direct effect of O-GlcNAc modification on activation of AKT.
Figure 5. Increased O-GlcNAcylation enhances activation of AKT.
A) OGA knockdown enhanced AKT activation. Control or OGA knockdown VSMC were exposed to glucose (25 mM) for 0 to 60 minutes. Western blot analysis was performed to determine phosphorylation/activation of AKT at S473 and T308. Representative blots from 3 experiments are shown. B) Increased vascular O-GlcNAcylation is associated with increased AKT activation in diabetic mouse aortas. Western blot analysis was performed to determine AKT phosphorylation at S473 and T308, in a) aortas from control or STZ-injected mice after 16 weeks of STZ injection; and b) aortas from control, STZ and STZ plus Thiamet G-treated mice 8 weeks after STZ injection. Representative blots of 4 mice in each group are shown. C) and D) Inhibition of AKT blocks increased O-GlcNAcylation-induced VSMC calcification. Control and OGA knockdown VSMC were cultured in osteogenic medium with or without AKT inhibitor IV (5 μM) for 3 weeks. Calcification was determined by Alizarin red staining (C) or quantified by Arsenazo III assay (D) in separate dishes (n=3, *p<0.001)
Consistently, increased phosphorylation of AKT at both S473 and T308 was observed in the vasculature of diabetic mice injected with STZ (Fig 5Ba). Furthermore, administration of Thiamet-G enhanced AKT phosphorylation at S473 (Fig 5Bb), suggesting the role of increased activation of AKT in mediating the effect of increased O-GlcNAcylation on vascular calcification in diabetes. Using the AKT IV inhibitor, we demonstrated that inhibition of AKT activation blocked increased O-GlcNAcylation-induced VSMC calcification in the OGA knockdown VSMC (Fig 5C, D), supporting a critical role of the AKT activation in mediating O-GlcNAcylation-induced VSMC calcification.
O-GlcNAcylation of AKT directly regulates activation/phosphorylation of AKT and vascular calcification
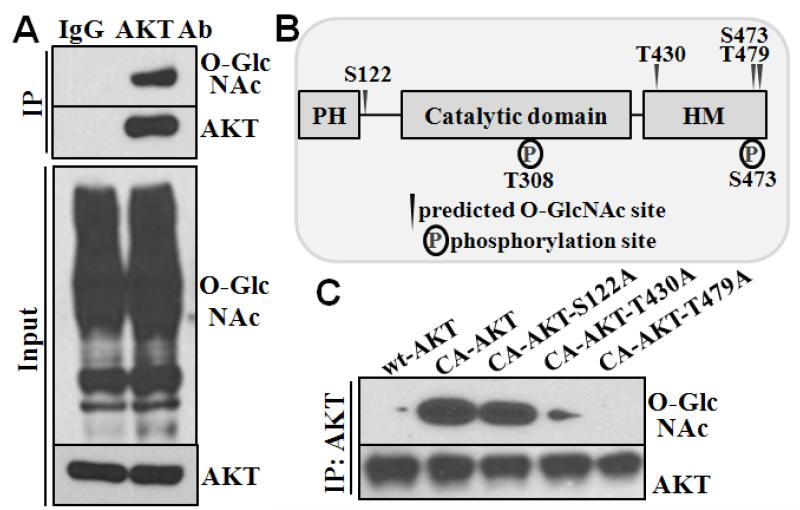
We further characterized whether AKT was directly modified by O-GlcNAcylation, and how the modification alters the AKT activation. O-GlcNAcylation of AKT was detected by Western blot analysis of the AKT immunoprecipitated complex using O-GlcNAc specific antibody (Fig 6A). Four putative O-GlcNAc modification sites were predicted on AKT with the YinOYang 1.2 software (http://www.cbs.dtu.dk/services/YinOYang/): serine 122 (S122), threonine 430 (T430), serine 473 (S473), and threonine 479 (T479, Fig 6B). To determine the effect of O-GlcNAcylation on activation of AKT, a lentiviral constitutively-active AKT vector (CA-AKT) was used to mutate the putative glycosylation sites to encode an alanine residue (A). Because S473 is a known site that determines AKT phosphorylation and activation, it was not targeted for mutagenesis. Mutations at both T430 and T479 inhibited O-GlcNAcylation of AKT, whereas mutation at S122 did not affect O-GlcNAcylation of AKT (Fig 6C).
Figure 6. O-GlcNAc modification of AKT at T430 and T479.
A) AKT was O-GlcNAcylated in VSMC. Immunoprecipitation was performed with antibody for AKT in cell lysate from VSMC cultured in growth media with high glucose (25 mM), and subsequently analyzed by Western blotting. Immunoprecipitation with control IgG antibody was used as a negative control. B) Putative glycosylation sites on AKT. YinOYang software was used to predict putative O-GlcNAcylation sites of AKT at serine and theronine residues. C) T430 and T479 are required for O-GlcNAcylation of AKT. Site-directed mutagenesis was performed on the constitutively active AKT to replace the putative O-GlcNAcylation sites with alanine. Lentivirus carrying wild-type (wt-AKT), constitutively active (CA-AKT), and the AKT point mutants (CA-AKT-S122A, CA-AKT-T430A, CA-AKT-T479A) were stably transfected into VSMC. Immunoprecipitation was performed with AKT antibody, followed by Western blot analysis to determine O-GlcNAcylation of AKT with RL-2 antibody.
Since phosphorylation of AKT is known to occur at S473 and T308, we characterized the effects of altered AKT O-GlcNAcylation on its activation with each of the AKT mutants. Decreased O-GlcNAcylation of AKT in either T430A or T479A mutant resulted in decreased phosphorylation of AKT at S473, but not at T308 (Figure 7A). These data support a positive correlation of AKT O-GlcNAcylation at T430 or T479 and its phosphorylation at S473.
Figure 7. O-GlcNAcylation of AKT at T430 and T479 is required for AKT activation and vascular calcification.
A) Effect of O-GlcNAcylation of AKT on its phosphorylation/activation. Western blot analysis was performed with cell lysate from VSMC stably expressing wild type (wt), constitutively active (CA) and mutant AKT to determine activation of AKT by phosphorylation at S473 and T308. B) AKT O-GlcNAc mutants exhibit decreased binding to Rictor. Immunoprecipitation was performed in cell lysates from A with AKT antibody, followed by Western blot analysis to determine the binding of AKT to PDK1, Rictor, mTOR and PHLPP. Representative blots form 3 independent experiments are shown. C) Effect of O-GlcNAcylation of AKT on VSMC calcification. VSMC stably expressing wt-AKT, CA-AKT and AKT mutants were cultured in osteogenic medium for 3 weeks. Calcification was quantified by Arsenazo III assay. D) Effect of O-GlcNAcylation of AKT on Runx2 transactivity. VSMC stably expressing wt-AKT, CA-AKT and AKT mutants were transfected with a luciferase reporter for Runx2 transactivity and a Renilla luciferase reporter plasmid. Luciferase activity was measured and normalized to Renilla activity (n=4 for each experiment; *p<0.05 compared to wt-AKT, #p<0.05 compared to CA-AKT).
To explore the mechanisms underlying the regulation of AKT phosphorylation by AKT O-GlcNAcylation, we determined the effects of these AKT mutations on AKT binding to kinases and phosphatase that are known to regulate AKT phosphorylation. All AKT mutants were found to bind to PDK1 and PHLPP similarly to wt-AKT or CA-AKT (Fig 7B). Decreased O-GlcNAcylation of AKT in either T430A or T479A mutant, but not in S122A, inhibited AKT binding to mTOR and Rictor (Fig 7B), a component of the mTOR complex 2, known to phosphorylate AKT at S47331. Inhibition of mTOR signals with rapamycin inhibited vascular calcification induced by increased-O-GlcNAcylation in the OGA knockdown VSMC, which was associated with inhibition of AKT phosphorylation (Suppl. Fig II). Therefore, the reduced binding of the mTOR complex 2 to AKT may contribute to inhibited AKT phosphorylation at S473 by impaired O-GlcNAcylation at T430/479, which led to the decrease in vascular calcification.
The contribution of AKT O-GlcNAcylation to VSMC calcification was further demonstrated in VSMC stably expressing different AKT variants. Importantly, expression of CA-AKT in VSMC is sufficient to promote VSMC calcification, as shown by increased calcium content (Fig 7C), suggesting AKT is a key regulator in the VSMC calcification. Mutation at AKT S122A, which did not affect O-GlcNAcylation and phosphorylation of AKT, had no impact on VSMC calcification. In sharp contrast, T430A and T479A, which are the two mutants that markedly inhibited O-GlcNAcylation and phosphorylation of AKT, significantly reduced VSMC calcification (Fig 7C). Consistently, CA-AKT induced Runx2 transactivity, which was inhibited by AKT mutations of T430A and T479A, but not S122A (Fig 7D). Taken together, these data support a critical role of O-GlcNAcylation of AKT at T430/479 in regulating its phosphorylation/activation at S473, thus inducing Runx2 transactivity and promoting VSMC calcification.
DISCUSSION
Vascular calcification is prevalent in diabetes and is correlated with adverse cardiovascular outcome32, 33; however, the molecular mechanisms underlying increased vascular calcification in diabetes are largely unknown. Elevation of O-GlcNAcylation is found in human diabetic carotid plaques20 and diabetic mouse vasculature34. Coincidently, increased vascular calcification has been identified in both type I and type II diabetic patients 35 and diabetic mouse models6. Nevertheless, the role of O-GlcNAcylation in vascular calcification has not been previously determined. The present study has demonstrated a causative effect of O-GlcNAcylation on diabetic vascular calcification. Our studies revealed that activation of AKT by O-GlcNAcylation in vasculature is key to diabetic vascular calcification. Two novel O-GlcNAcylation sites on AKT play a crucial role in enhancing AKT phosphorylation at S473 to increase vascular calcification. Because O-GlcNAcylation is tightly regulated by two specific enzymes, the new findings have exciting implications for prevention and treatment of diabetic vascular calcification through therapies targeting O-GlcNAcylation and signaling.
We found that increased O-GlcNAcylation in response to chronic hyperglycemia induced vascular calcification in the low-dose STZ-induced diabetic mouse model (Fig 1). Previous studies have demonstrated that acute increases in O-GlcNAcylation (less than 24 hours) protect cardiomyocytes from oxidative stress-induced calcium overload and structural damage in ischemia/reperfusion models of heart failure18,36,37. However, few studies have examined the function of chronic increases in O-GlcNAcylation. This study and others38 indicate chronic O-GlcNAcylation over an extended period of time, as observed in the later stages of diabetes, may cause adverse complications in the cardiovascular system. The distinct function of O-GlcNAcylation in chronic and acute disease model may be related to differential activation of unknown signaling cascades. While the STZ model has its limitation due to its toxicity in vitro and its inhibitory effect on OGA also, its diabetogenic mechanism of action has been shown to be independent of these side effects since STZ has a very short half-life24. Using Thiamet-G, a potent and selective OGA inhibitor, our studies have provided the first evidence that increased vascular O-GlcNAcylation enhanced vascular calcification in diabetic mice in vivo (Fig 4). Consistent with the clinical observations demonstrating an association between increased vascular calcification35 and other vascular complications33,34 in diabetes, our findings have demonstrated reduced aortic compliance in the STZ-induced diabetic mice, which was further worsened by increased O-GlcNAcylation achieved by the Thiamet-G treatment (Fig 1, 4). Together, our studies have revealed a causative link between increased O-GlcNAcylation and diabetic vascular calcification and impaired vascular compliance in vivo.
Using VSMC in culture, we further demonstrated that increased O-GlcNAcylation by OGA inhibition or knockdown induced VSMC calcification. Previously considered a passive process by deposition of calcium, vascular calcification has now been recognized as a regulated dynamic process involving osteochondrogenic differentiation of vascular cells26,39. Increased O-GlcNAcylation has been associated with osteogenesis38 and chondrogenesis40; however, the underlying mechanisms are unknown. Our studies have revealed a direct effect of O-GlcNAcylation in regulating osteogenic differentiation of VSMC, which may also provide new insights into the function of O-GlcNAcylation in regulating differentiation of osteoblasts and chondrocytes. We have previously reported that oxidative stress induces AKT activation and VSMC calcification26. Both oxidative stress and high glucose induce vascular calcification, and have been found to increase O-GlcNAcylation41,42. Accordingly, it is also likely that hyperglycemia induces oxidative stress that contributes to increased vascular calcification.
We found that increased activation of AKT was associated with increased vascular calcification in the STZ-induced diabetic arteries (Fig 5B). These observations are consistent with previous studies showing that VSMC in diabetic models exhibit sustained activation of AKT after chronic hyperglycemia43, although other studies reported blunted AKT activation in diabetic cardiomyocytes and myotubes44,45. Apparently, AKT may be differentially regulated depending upon cell types, cellular environment and disease status. Using Thiamet-G to induce O-GlcNAcylation in vivo, we demonstrated a direct effect of O-GlcNAcylation on AKT activation in the diabetic vasculature. Consistent with these findings, blockade of OGA in cultured VSMC increased O-GlcNAcylation and simultaneously increased and sustained activation of AKT (Fig 5). Furthermore, inhibition of AKT activation attenuated VSMC calcification, demonstrating an essential role of AKT activation in mediating O-GlcNAcylation-induced vascular calcification. Remarkably, constitutively active AKT was sufficient to induce VSMC calcification of VSMC (Fig 7), demonstrating that AKT activation is a key regulator of vascular calcification.
Importantly, the present studies have provided paradigm-shifting novel mechanisms underlying the regulation of AKT phosphorylation and activation by it O-GlcNAcylation. Although activation of AKT has been associated with altered O-GlcNAcylation in different cells, whether the AKT activation is regulated directly by O-GlcNAcylation is not clear14, 46. Our studies have identified that O-GlcNAcylation at T430 and T479 plays an important role in AKT phosphorylation at S473, which promotes vascular calcification. A recent study suggested that O-GlcNAcylation of AKT at T305 and T312 inhibits AKT phosphorylation at T30847, in COS-7 cells, which is in agreement with the prevailing belief that protein O-GlcNAcylation and phosphorylation reciprocally regulate protein activity48. However, mutation at T305 and T312 did not affect AKT phosphorylation at S473 or VSMC calcification (Suppl. Fig III). In addition, increased O-GlcNAcylation by the OGA knockdown in VSMC did not affect basal AKT phosphorylation at T308 (Fig 5). Furthermore, we found that inhibition of O-GlcNAcylation at T430 and T479 did not affect activation of AKT at T308, but only inhibited activation of AKT at S473, suggesting the selective effect of O-GlcNAcylation on the key residues. Therefore, we have identified unique O-GlcNAc modification at two novel sites, T430/479 that are critical for AKT phosphorylation and its function to promote VSMC calcification.
Moreover, mechanistic studies further revealed that AKT O-GlcNAcylation at T430/479 is important for the binding of AKT to Rictor, a component of the mTOR complex 2 (Fig 7B), Because mTOR complex 2 is known to phosphorylate AKT at of S47331, disruption of AKT binding to Rictor by the T430A and T479A mutations may contribute to the inhibited phosphorylation of AKT at S473. Inhibition of O-GlcNAcylation-induced VSMC calcification by rapamycin (Suppl. Fig II) further support a role of the mTOR signals in mediating O-GlcNAcylation-induced AKT activation and VSMC calcification. The precise mechanism of how O-GlcNAcylation at T430/479 affects its binding to Rictor remains to be determined. The T430, T479 and S473 residues lie in the hydrophobic motif of AKT49, which plays a major role in AKT protein stability50. It is possible that O-GlcNAc modifications at T430/479 may change the conformation of the hydrophobic motif so that facilitates its binding to mTOR complex 2 and thus leading to its phosphorylation at S473 site. Although S473 does not reside in the catalytic domain of AKT, phosphorylation of S473 may lead to a conformational change that modulates complete kinase activity and phosphorylation of downstream targets50. This study revealed an essential role of AKT phosphorylation at S473 in regulating osteogenic transcription factor Runx2 and VSMC calcification.
The function of O-GlcNAcylation-induced phosphorylation/activation of AKT in promoting VSMC calcification appear to be independent of its regulation of cell proliferation and apoptosis, as we found that increased O-GlcNAcylation, by the OGA knockdown, did not affect VSMC proliferation/viability. Consistently, we have reported that AKT inhibition does not induce apoptosis of VSMC26. Further studies are warranted to dissect the precise signaling cascades that are responsible for AKT activation-induced Runx2 upregulation. Nevertheless, the novel regulation of AKT activation by O-GlcNAcylation uncovered in this study may have significant impact not only on the biological function of AKT activation, but also provide novel mechanistic insights into pathogenesis of vascular disease featuring increased activation of AKT.
In summary, the present studies have demonstrated a novel causative link between chronic increases in vascular O-GlcNAcylation and vascular calcification in diabetes, and uncovered a novel mechanism underlying the regulation of AKT activation by its O-GlcNAcylation, which induces Runx2 upregulation and promotes VSMC calcification (Suppl. Fig IV). These findings have determined O-GlcNAcylation as a novel contributor to the process of vascular calcification and identified O-GlcNAcylation of AKT as a possible target for the development of therapies for vascular calcification in diabetes.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Patients with diabetes have increased prevalence of vascular calcification, which correlates with higher risk for adverse cardiovascular events.
Hyperglycemia, a characteristic feature of diabetes, is associated with increased vascular calcification as well as increased protein O-GlcNAcylation.
Protein O-GlcNAcylation is tightly regulated by two enzymes, OGT and OGA.
Activation of AKT is important for oxidative stress-induced calcification of vascular smooth muscle cells (VSMC).
What New Information Does This Article Contribute?
Streptozotocin-induced diabetes in mice t increased O-GlcNAcylation and vascular calcification, which were associated with impaired aortic compliance.
Inhibition of OGA in VSMC, either by Thiamet-G or the OGA knockdown, increases O-GlcNAcylation in VSMC, which promotes VSMC calcification.
Administration of Thiamet-G in diabetic mice further enhances vascular O-GlcNAcylation, accelerated vascular calcification and worsened aortic compliance.
Increased O-GlcNAcylation in diabetic arteries or in the OGA knockdown VSMC enhances activation of AKT that upregulates expression of Runx2.
AKT activation increased O-GlcNAcylation-induced VSMC calcification.
Site-directed mutation of AKT at T430 and T479 decreases O-GlcNAcylation that inhibits phosphorylation of AKT at S473 and binding of the mTOR complex 2 to AKT, which leads to inhibition of the Runx2 transactivity and subsequent VSMC calcification.
O-GlcNAcylation of AKT at T430 and T479 promotes phosphorylation, which represents a novel mechanism underlying AKT activation and vascular calcification in diabetes.
Vascular calcification is often observed in diabetic arteries, and is associated with increased frequency of cardiovascular events and mortality in diabetes patients. O-GlcNAcylation is increased in response to stressors, such as hyperglycemia and oxidative stress. The present studies demonstrate a novel and causative link between protein O-GlcNAcylation and vascular calcification in diabetes, and reveal that O-GlcNAcylation of AKT at two new sites enhances AKT phosphorylation and subsequently induces VSMC calcification. These findings suggest that O-GlcNAcylation is a novel regulator of vascular calcification and uncover a novel mechanism underlying AKT activation by O-GlcNAcylation. Thus, O-GlcNAcylation of AKT may be a potential new target for the development of therapies for vascular calcification in diabetes.
ACKNOWLEDGMENT
We thank Dr. Jay McDonald, University of Alabama at Birmingham for critical review and helpful discussion.
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health (NIH) HL092215 and Veterans Administration BX000369 and BX001591 (project 2) to YC. JH was supported by American Heart Association Grant 12PRE11840009. JCC was supported by grants from the NIH HL101192 and HL110366.
Nonstandard Abbreviations and Acronyms
- α-SMA
smooth muscle specific α-actin
- CA-AKT
constitutively active AKT
- ColIA1
type I collagen A1
- mTOR
mammalian target of rapamycin
- OC
osteocalcin
- OGA
β-N-acetylglucosaminidase
- OGT
β-N-acetylglucosaminyltransferase
- O-GlcNAcylation
O-linked β-N-acetylglucosamine modification
- OPN
osteopontin
- PWV
pulse wave velocity
- Runx2
runt-related transcription factor 2
- VSMC
vascular smooth muscle cells
- wt-AKT
wild type AKT
Footnotes
DISCLOSURES
None.
Subject codes:
[191] Other diabetes
[162] Smooth muscle proliferation and differentiation
[97] Other vascular biology
REFERENCES
- 1.Chiha M, Njeim M, Chedrawy EG. Diabetes and coronary heart disease: A risk factor for the global epidemic. Int J Hypertens. 2012;2012:697240. doi: 10.1155/2012/697240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: An integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 3.Carr JJ, Register TC, Hsu FC, Lohman K, Lenchik L, Bowden DW, Langefeld CD, Xu J, Rich SS, Wagenknecht LE, Freedman BI. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: The diabetes heart study. Bone. 2008;42:43–52. doi: 10.1016/j.bone.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification – calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis – calcific uremic arteriolopathy: The emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4:4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen NX, Moe SM. Arterial calcification in diabetes. Curr Diab Rep. 2003;3:28–32. doi: 10.1007/s11892-003-0049-2. [DOI] [PubMed] [Google Scholar]
- 6.Boström KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekikawa A, Shin C, Curb JD, Barinas-Mitchell E, Masaki K, El-Saed A, Seto TB, Mackey RH, Choo J, Fujiyoshi A, Miura K, Edmundowicz D, Kuller LH, Ueshima H, Sutton-Tyrrell K. Aortic stiffness and calcification in men in a population-based international study. Atherosclerosis. 2012;222:473–477. doi: 10.1016/j.atherosclerosis.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara Y, Kawasaki M, Hattori A, Imai H, Takahashi S, Sato H, Kubota T, Okubo M, Ojio S, Nishigaki K, Takemura G, Fujiwara H, Minatoguchi S. Relationship among coronary plaque compliance, coronary risk factors and tissue characteristics evaluated by integrated backscatter intravascular ultrasound. Cardiovasc Ultrasound. 2012;10:32. doi: 10.1186/1476-7120-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 10.Ruderman NB, Williamson JR, Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992;6:2905–2914. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Zhong H, Liang JY, Fu P, Luo ZJ, Zhou L, Gou R, Huang J. Effect of high glucose levels on the calcification of vascular smooth muscle cells by inducing osteoblastic differentiation and intracellular calcium deposition via BMP-2/Cbfα-1 pathway. J Zhejiang Univ Sci B. 2010;11:905–911. doi: 10.1631/jzus.B1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing D, Gong K, Feng W, Nozell S, Chen Y, Chatham J, Oparil S. O-GlcNAc modification of NFκB p65 inhibits TNF-α-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS One. 2011;6:e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg H, Whiteside C, Fantus IG. O-linked β-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am. J. Physiol. Endocrinol Metab. 2011;301:E713–E726. doi: 10.1152/ajpendo.00108.2011. [DOI] [PubMed] [Google Scholar]
- 15.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 16.Hilgers RH, Xing D, Gong K, Chen YF, Chatham JC, Oparil S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am J Physiol Heart Circ Physiol. 2012;303:H513–H522. doi: 10.1152/ajpheart.01175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh SA, Powell PC, Dell’italia LJ, Chatham JC. Cardiac O-GlcNAcylation blunts autophagic signaling in the diabetic heart. Life Sci. 2012 doi: 10.1016/j.lfs.2012.06.011. DOI:10.1016/j.lfs.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunde IG, Aronsen JM, Kvaløy H, Qvigstad E, Sjaastad I, Tønnessen T, Christensen G, Grønning-Wang LM, Carlson CR. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol Genomics. 2012;44:162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 20.Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 21.Rossini AA, Like AA, Chick WL, Appel MC, Cahill GF., Jr. Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci USA. 1977;74:2485–2489. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leiter EH. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: Influence of inbred background, sex, and thymus. Proc Natl Acad Sci USA. 1982;79:630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiter EH. Differential susceptibility of BALB/c sublines to diabetes induction by multi-dose streptozotocin treatment. Curr Top Microbiol Immunol. 1985;122:78–85. doi: 10.1007/978-3-642-70740-7_12. [DOI] [PubMed] [Google Scholar]
- 24.Bone RN, Icyuz M, Zhang Y, Zhang Y, Cui W, Wang H, Peng JB, Matthews QL, Siegal GP, Wu H. Gene transfer of active Akt1 by an infectivity-enhanced adenovirus impacts beta-cell survival and proliferation differentially in vitro and in vivo. Islets. 2012:4. doi: 10.4161/isl.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific Runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byon C, Javed A, Dai Q, Kappes J, Clemens T, Darley-Usmar V, McDonald J, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;30:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys. 2003;415:155–163. doi: 10.1016/s0003-9861(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 28.Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y. Runx2-upregulated receptor activator of nuclear factor κb ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1387–1396. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuzwa S, Macauley M, Heinonen J, Shan X, Dennis R, He Y, Whitworth G, Stubbs K, McEachern E, Davies G, Vocadlo D. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 30.Hart JR, Vogt PK. Phosphorylation of Akt: A mutational analysis. Oncotarget. 2011;2:467–476. doi: 10.18632/oncotarget.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen N, Naik V, Speer MY. Diabetes mellitus accelerates cartilaginous metaplasia and calcification in atherosclerotic vessels of LDLr mutant mice. Cardiovasc Pathol. 2012;22:167–175. doi: 10.1016/j.carpath.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erbel R, Budoff M. Improvement of cardiovascular risk prediction using coronary imaging: Subclinical atherosclerosis: The memory of lifetime risk factor exposure. Eur Heart J. 2012;33:1201–1213. doi: 10.1093/eurheartj/ehs076. [DOI] [PubMed] [Google Scholar]
- 34.Bennett CE, Johnsen VL, Shearer J, Belke DD. Exercise training mitigates aberrant cardiac protein O-GlcNAcylation in streptozotocin-induced diabetic mice. Life Sci. 2012 doi: 10.1016/j.lfs.2012.09.007. DOI:10.1016/j.lfs.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Snell-Bergeon JK, Hokanson JE, Jensen L, MacKenzie T, Kinney G, Dabelea D, Eckel RH, Ehrlich J, Garg S, Rewers M. Progression of coronary artery calcification in type 1 diabetes: The importance of glycemic control. Diabetes Care. 2003;26:2923–2928. doi: 10.2337/diacare.26.10.2923. [DOI] [PubMed] [Google Scholar]
- 36.Laczy B, Marsh SA, Brocks CA, Wittmann I, Chatham JC. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am J Physiol Heart Circ Physiol. 2010;299:H1715–H1727. doi: 10.1152/ajpheart.00337.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2010;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medford HM, Chatham JC, Marsh SA. Chronic ingestion of a western diet increases O-linked-β-N-acetylglucosamine (O-GlcNAc) protein modification in the rat heart. Life Sci. 2012;90:883–888. doi: 10.1016/j.lfs.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrés-Bergós J, Tardio L, Larranaga-Vera A, Gómez R, Herrero-Beaumont G, Largo R. The increase in O-GlcNAc protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem. 2012;287:33615–33628. doi: 10.1074/jbc.M112.354241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abhijit S, Bhaskaran R, Narayanasamy A, Chakroborty A, Manickam N, Dixit M, Mohan V, Balasubramanyam M. Hyperinsulinemia-induced vascular smooth muscle cell (VSMC) migration and proliferation is mediated by converging mechanisms of mitochondrial dysfunction and oxidative stress. Mol Cell Biochem. 2012;373:95–105. doi: 10.1007/s11010-012-1478-5. [DOI] [PubMed] [Google Scholar]
- 44.Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, Heinrichs J, Blumensatt M, Cuvelier C, Akhyari P, Ruige JB, Ouwens DM, Eckel J. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation. 2012;126:2324–2334. doi: 10.1161/CIRCULATIONAHA.111.039586. [DOI] [PubMed] [Google Scholar]
- 45.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2012;62:401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krześlak A, Jóźwiak P, Lipinska A. Down-regulation of β-N-acetyl-D-glucosaminidase increases Akt1 activity in thyroid anaplastic cancer cells. Oncol. Rep. 2011;26:743–749. doi: 10.3892/or.2011.1333. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Huang X, Sun D, Xin X, Pan Q, Peng S, Liang Z, Luo C, Yang Y, Jiang H, Huang M, Chai W, Ding J, Geng M. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates Akt signaling. PLoS One. 2012;7:e37427. doi: 10.1371/journal.pone.0037427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Cron P, Good VM, Thompson V, Hemmings BA, Barford D. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9:940–944. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 50.Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.