Abstract
Background
In congenital aortic stenosis (AS) chronic pressure load has detrimental effects on left ventricular (LV) systolic and diastolic function. Reduction in LV pressure load with balloon aortic valvuloplasty (BAVP) may improve diastolic function.
Methods and Results
Echocardiographic and catheterization data for 25 consecutive patients undergoing BAVP for congenital AS were retrospectively analyzed. Median age at BAVP was 11.5 years (3.2–40.1). LV end-diastolic pressure (EDP) pressure was elevated (≥ 15 mm Hg) in 72% of patients with a median of 17 mm Hg (range: 9–24). With BAVP, median AS gradient was reduced from 63 mm Hg (44–105) to 30 mm Hg (10–43). Aortic regurgitation (AR) increased from trivial (none to mild) to mild (trivial - moderate). Pre-BAVP early diastolic mitral inflow velocity/tissue Doppler early diastolic velocity (E/E') correlated with LVEDP (r= 0.52, p=0.007). On follow up echocardiogram (median 11 months post-BAVP), AS gradient was lower (p<0.001) and degree of AR was higher (P=0.01) compared to pre-BAVP echocardiograms. LV end-diastolic volume z-score increased (p=0.02), LV mass was unchanged, and LV mass: volume decreased (p=0.002). Mitral annular and septal E' (p<0.001) were higher and E/E' was lower post-dilation (10.8 vs. 14.2, p<0.001). Lower pre-BAVP E/E' and lower pre-BAVP LV mass z-score were associated with lower post-BAVP E/E'.
Conclusions
After BAVP, LV remodeling characterized by an increase in EDV and decrease in LV mass: volume occurs and echocardiographic measures of diastolic function and LVEDP improve in most patients. Risk factors for persistent diastolic dysfunction include higher pre-BAVP LV mass z-score and worse pre-BAVP diastolic function.
Keywords: congenital heart disease, aortic stenosis, balloon aortic valvuloplasty, diastolic function
Aortic valve stenosis (AS) imposes a chronic pressure load on the left ventricle (LV). The myocardial response to chronic pressure load has been well described in adult patients with calcific AS. In response to chronic pressure loading, LV remodels and develops concentric hypertrophy1–3. This initially adaptive response allows for peak systolic wall stress to remain normal and for systolic function to be preserved early in the course of the disease, but concentric remodeling is the substrate for later development of diastolic dysfunction1, 4–8. Later in the disease course the deleterious effects of concentric hypertrophy and associated myocardial fibrosis become apparent with the development of both systolic and diastolic dysfunction9–13. In adults with calcific AS, removal of LV pressure load with aortic valve replacement leads to partial, but not complete, halting or, in some cases, reversal of the maladaptive processes of concentric hypertrophy and myocardial fibrosis and to an improvement in diastolic function14–16.
The myocardial response to chronic pressure load in children is also characterized by concentric hypertrophy, myocardial fibrosis and impaired diastolic function17–20, although it has been less well described than in adults. The time course, pathophysiology and risk factors for progression of diastolic dysfunction may be different in younger AS patients21. The response of the LV myocardium in younger patients with congenital AS to removal of pressure load via balloon aortic valvuloplasty (BAVP) has only been described in the immediate post-BAVP period17. The aim of this study is to characterize the medium term effect BAVP on LV remodeling and diastolic function in patients with congenital AS.
Methods
Patients
We retrospectively reviewed the records all patients ≥ 3 years of age undergoing BAVP for isolated congenital AS at our institution from January 2006 through June 2011. Patients with complete echocardiographic assessment of diastolic function, including mitral inflow pulsed-Doppler, tissue Doppler imaging (TDI), pulmonary vein Doppler, and left atrial volume measurement, both within one month prior to catheterization and 6 to 24 months post catheterization were included. Forty-two patients underwent BAVP in this time period and met inclusion criteria, 25 of whom had both pre- and post-BAVP echo data available and were included in the analysis. Patients with structural heart disease apart from patent foramen ovale, bicommissural aortic valve, and aortic coarctation were excluded. Additionally, patients who underwent any cardiac surgery requiring cardiopulmonary bypass (prior to catheterization or after catheterization and before follow up echocardiogram) were excluded in order to avoid the confounding effect of cardiopulmonary bypass on myocardial function.
Catheterizations
Hemodynamic data were collected retrospectively from reports produced at the time of the catheterization. Hemodynamic data, including ordinal AR grade (0=none, 1=trivial, 2=mild, 3=moderate, 4=severe) and peak-to-peak AS gradient, before and after intervention were included in the analysis. Baseline (pre-intervention) hemodynamic data was used for evaluation of left ventricular end-diastolic pressure (LVEDP), left atrial pressure, pulmonary vascular resistance (PVR) and pulmonary artery pressure.
Echocardiograms
All patients had a pre-catheterization (within one month of catheterization) and at least one post-catheterization echocardiogram (between 6 and 24 months post catheterization) included in the analysis. For patients with multiple post-BAVP echocardiograms, the most recent echocardiogram up to 24 months post-BAVP was used in the analysis.
The following LV variables and z-scores for each variable were recorded from reports produced at the time of the study: end-diastolic volume (EDV), mass, mass: volume, ejection fraction (EF), end systolic stress, velocity of circumferential fiber shortening, and sphericity index. LV EDV was calculated using the 5/6 area-length formula and LV mass using volumetric 2D measurements22 Maximum instantaneous AS gradient from the apical imaging window or mean AS gradient form the suprasternal notch imaging window (whichever was higher) and qualitative aortic regurgitation grade (0–4) on an ordinal scale were collected.
Diastolic function assessment included pulsed-Doppler of the mitral inflow, TDI, and left atrial volume for both the pre- and post-BAVP echocardiograms. All measurements of diastolic variables were retrospectively re-measured by a single echocardiographer (KF) from images obtained at the time of the study. Conventional pulsed-Doppler indices of diastolic function, including peak early (E) and late (A) diastolic transmitral velocities, E:A ratio, A-wave duration, and E-wave deceleration time were measured from the spectral Doppler signal of the mitral valve inflow. Pulsed-wave TDI velocities were obtained from the lateral mitral annulus and the interventricular septum from the apical 4-chamber view. TDI measurements for each of the myocardial segments included peak early diastolic velocity (E') and peak late diastolic velocity (A'). Only tracings that demonstrated a clear E' were used. Each TDI velocity was measured on 3 consecutive cardiac cycles and the average of these values was used for the analysis. Peak early diastolic mitral inflow velocity/early septal TDI velocity (E/E') was calculated. Left atrial volumes were calculated using the prolate-ellipse formula23.
Statistical Analysis
Mean and standard deviation were used to express measures of central tendency and dispersion for echocardiographic data and median and range were used for catheterization data. Associations between pre-dilation echocardiographic and pre-dilation hemodynamic data were evaluated using Pearson correlation coefficients. Receiver-operator curves were constructed to asses the ability of pre-BAVP E/E' to predict LVEDP measured at catheterization. Paired t-tests were used to compare pre-and post-BAVP echocardiographic variables. Factors associated with elevated post-dilation E/E' (≥12) were sought using Fischer’s exact test or Mann-Whitney test as appropriate. All statistical analysis were 2-sided and type I error was controlled at a level of .05. Analyses were performed with SPSS (version 16.0, SPSS Inc, Chicago, Ill).
Results
Demographic and clinical data for the 25 patients meeting inclusion criteria are shown in Table 1. Median age at catheterization was 11.5 years. All patients had either a bicommissural (60%) or unicommissural (40%) aortic valve. Nine patients (39%) underwent prior BAVP with 4 patients having undergone prior BAVP twice and 5 patients having a single prior BAVP. Median residual AS gradient after BAVP in these 9 patients was 20 mm Hg (range: 20–43). Six patients (24%) previously underwent coarctation repair none of whom had significant residual arch obstruction. Blood pressure at most recent evaluation was within normal range in all patients.
Table 1.
Patient Characteristics
| All patients (n=25) | |
|---|---|
| Male (n, %) | 20 (80%) |
| Previous catheterization | 10 (40%) |
| Age at Catheterization (years) (median, range) | 11.5 (3.2–40.1) |
| Bicommissural Aortic Valve | 15 (60%) |
| Unicomissural Aortic Valve | 10 (40%) |
| Critical Aortic Stenosis | 3 (12%) |
| Aortic Valve Intervention within 1st year of life | 7 (28%) |
| Previous Coarctation Repair | 6 (24%) |
| Weight (kg) | 37.1 (17.9–79.8) |
| Weight % | 66 (5–98) |
| Systolic blood pressure (mm Hg) | 114 (85–126) |
| Diastolic blood pressure (mm Hg) | 66 (50–86) |
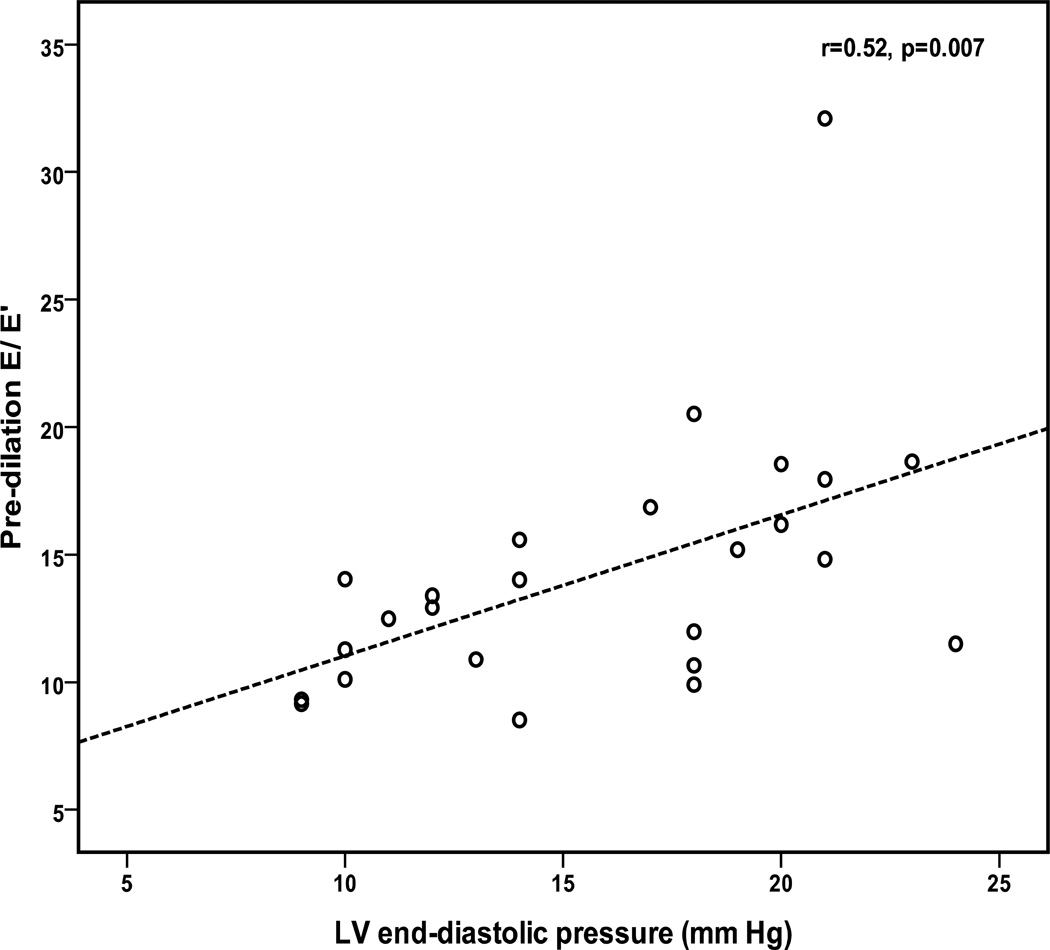
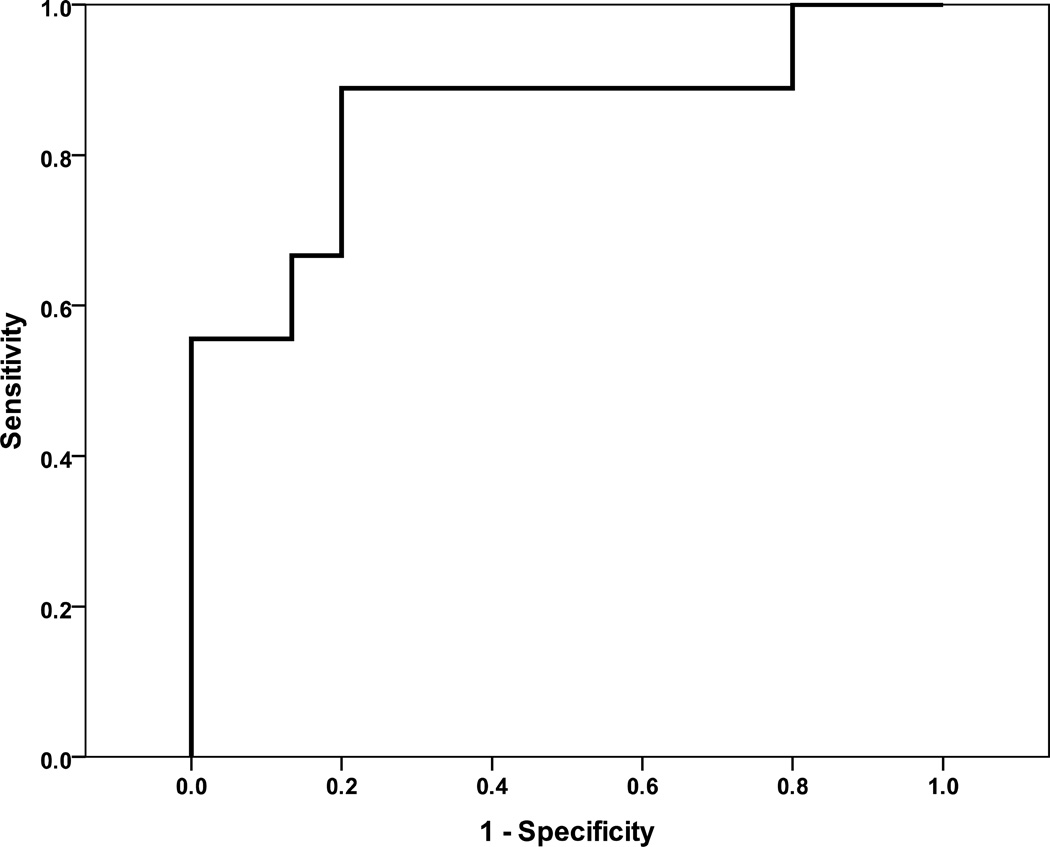
Median pre-BAVP LVEDP was 17 mm Hg with the majority of patients (18 patients, 72%) having a LVEDP ≥ 15 mm Hg (Table 2). Pre-BAVP E/E' correlated with LVEDP (r= 0.57, p=0.007) (Figure 1). E/E'> 12 predicted LVEDP ≥ 18 mm Hg with 88% sensitivity and 78% specificity (Figure 2). Six patients (24%) had PVR > 2 Wood units (Wu) with 5 having mildly elevated PVR between 2 and 3 Wu and one patient having significantly elevated PVR at 6.9 Wu. Significant AR before BAVP was rare with 14 patients (56%) having trivial or less AR and only one patient having more than mild AR.
Table 2.
Catheterization Data
| All patients (n=25) | |
|---|---|
| Pulmonary capillary wedge pressure (mmHg) | 17 (10–27) |
| LV end-diastolic pressure (mm Hg) | 17 (9–24) |
| LV end-diastolic pressure ≥ 15 mm Hg (n,%) | 18 (72%) |
| Mean PA pressure (mm Hg) | 24 (14–35) |
| Pulmonary Vascular Resistance (Wood units) | 1.5 (0.2–6.9) |
| Pre-BAVP AR grade† | 1 (0–2.5) |
| Post-BAVP AR grade† | 2 (1–3) |
| Pre-BAVP AS gradient (mm Hg) | 63 (44–105) |
| Post-BAVP AS gradient (mm Hg) | 30 (10–43) |
| AS Gradient reduction (mm Hg) | 30 (15–75) |
| AS Gradient reduction (%) | 54 (27–83) |
LV, left ventricle; PA, pulmonary artery; BAVP, balloon aortic valvuloplasty; AR, aortic regurgitation; AS, aortic stenosis.
Values are expressed as median (range)
AR ordinal grading system: 0=none, 1=trivial, 2=mild, 3=moderate, 4=severe
Figure 1.
Scatter-plot showing positive correlation between left ventricular (LV) end-diastolic pressure and pre-balloon aortic valvuloplasty (BAVP) early diastolic pulsed-Doppler mitral inflow (E)/early diastolic tissue Doppler velocity (E'). Dashed line represents line of best fit.
Figure 2.
Receiver-operator curve for E/E' predicting left ventricular end diastolic pressure ≥18 mm Hg. E/E’>12 is 86% sensitive and 78% specific for left ventricular end diastolic pressure ≥18 mm Hg. Area under the curve=0.85.
BAVP resulted in a median AS gradient reduction of 54% and a reduction in peak to peak gradient to ≤ 45 mm Hg in all patients (Table 2). On post-BAVP angiography, 18 patients (72%) had mild or less AR, 5 (20%) had mild to moderate AR, and 2 (8%) had moderate AR.
At a median follow-up time of 11 months post-BAVP (range 6–23 months), echocardiography showed lower AS gradient (p<0.001) and higher AR grade (p=0.01) than pre-BAVP (Table 3). Post-BAVP, LV EDV (p=0.004) and LV EDV (p=0.02) z-score were higher (p=0.02), LV mass was unchanged, and LV mass: volume was lower (p=0.002) than pre-BAVP. Most patients (72%) had an increase in LVEDV z-score with 9 patients (36%) having a marked increase (more than 2 z-score units). Greater than mild AR on follow-up echo was associated with both higher LVEDV z-score at follow up (p=0.002) and larger increase in LVEDV z-score between catheterization and follow-up (p=0.009). The majority of patients (80%) had a decrease in LV mass: volume z-score. Greater than mild AR on follow up echo was associated with a higher percentage decrease in LV mass: volume (−33 % vs. −7 %, p=0.027). Residual AS gradient was not associated with the magnitude of change in LV mass: volume.
Table 3.
Echocardiographic Data
| Pre-BAVP | Post-BAVP | p value | |
|---|---|---|---|
| Anatomic variables | |||
| LV end-diastolic volume (ml) | 89 (+/−42) | 113 (+/−44) | 0.004 |
| LV end-diastolic volume z-score | −0.1 (+/−1.6) | 0.9 (+/−1.9) | 0.02 |
| LV mass (grams) | 106 (+/−48) | 113 (+/−51) | 0.24 |
| LV mass z-score | 1.7 (+/−1.4) | 1.8 (+/−1.9) | 0.82 |
| LV mass: volume | 1.3 (+/−0.3) | 1.0 (+/−0.2) | 0.002 |
| LV mass: volume z-score | 2.4 (+/−2.2) | 1.0 (+/−1.7) | 0.006 |
| LV ejection fraction | 70 (+/−6) | 65 (+/−5) | <0.001 |
| Peak aortic stenosis gradient (mm Hg) | 64 (+/−15) | 37 (+/8) | <0.001 |
| Aortic regurgitation grade‡ | 2 (0−2.5) | 2.5 (1−4) | 0.01 |
| Diastolic Parameters | |||
| Mitral Inflow E:A | 2.2 (+/−0.7) | 2.1 (+/0.9) | 0.53 |
| Mitral Inflow E:A z-score | 0.1 (+/−1.1) | −0.2 (+/1.4) | 0.47 |
| Abnormal Mitral inflow E:A | 2 (8%) | 2 (8%) | 0.99 |
| Mitral Inflow E-wave decel time (msecs) | 136 (+/−26) | 141(+/−44) | 0.68 |
| Mitral Inflow E-wave decel time z-score | −0.3 (+/−0.8) | −0.3 (+/−1.2) | 0.90 |
| Mitral Annular E' (cm/s) | 12.1 (+/−2.4) | 14.3 (+/−2.6) | 0.004 |
| Mitral Annular E' z-score | −2.1 (+/−0.8) | −1.3 (+/−1.0) | 0.006 |
| Septal E' (cm/s) | 8.4 (+/−1.6) | 10.0 (+/−1.9) | <0.001 |
| Septal E' z-score | −2.4 (+/−0.8) | −1.6 (+/−1.0) | <0.001 |
| E/E' | 14.2 (+/−4.9) | 10.8 (+/−3.4) | <0.001 |
| Left atrial volume (ml/m2) | 19 (+/−7) | 20 (+/−6) | 0.37 |
| LV sphericity index | 0.66 (+/−0.09) | 0.69 (+/−0.07) | 0.17 |
| VCFc (circ s−1) | 1.25 (+/−0.17) | 1.12 (+/−0.11) | 0.008 |
| End-systolic stress (g/cm2) | 31 (+/−13.3) | 40 (+/−8) | 0.001 |
LV, left ventricle; E, early diastolic pulsed-Doppler mitral inflow velocity; A, late diastolic pulsed-Doppler mitral inflow velocity; decel, deceleration; E', early diastolic tissue Doppler velocity; VCFc, heart rate-corrected velocity of circumferential fiber shortening
Values are expressed as mean +/− standard deviation
Aortic regurgitation ordinal grading system: 0=none, 1=trivial, 2=mild, 3=moderate, 4=severe
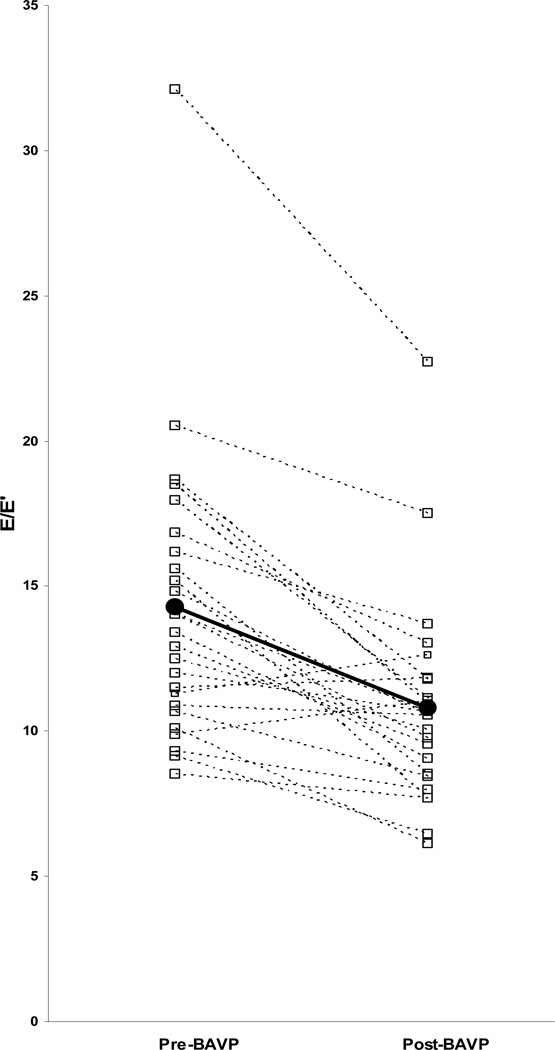
Comparison of pre- and post-BAVP diastolic function parameters showed higher mitral annular E´ (p=0.004) and septal E´(p<0.001) and lower E/E´ after BAVP (p<0.001) (Table 3). Prior to BAVP, septal E´ and mitral annular E´ z-scores were abnormally low (z-score ≤−2) in 18 (72%) and 19 (76%) patients, respectively. Post-BAVP, the majority of patients had normalization of septal E´ and mitral annular E´ with only 6 patients (24%) having z-score ≤−2.0 for each value. Pre-BAVP E/E´ was elevated (≥ 12) in 16 patients (64%), while after BAVP only 5 patients (20%) continued to have elevated E/ E´ (Figure 3). No differences were found in mitral inflow spectral Doppler derived variables, including E:A and mitral E-wave deceleration time, or in left atrial volume before and after BAVP.
Figure 3.
Early diastolic pulsed-Doppler mitral inflow (E)/early diastolic tissue Doppler velocity (E') pre- and post-balloon aortic valvuloplasty (BAVP). Median values represented by black circles and bold line.
In univariate analysis, factors associated with E/E' ≥ 12 at follow up included higher pre-BAVP LV mass (163 vs. 77 grams vs. p=0.006), pre-BAVP LV mass z-score (2.7 vs. 1.7 p=0.03), higher pre-BAVP E/E' (16.9 vs. 12.7, p=0.035) and higher LV mass z-score at follow-up (2.6 vs.1.7, p=0.05). There was a trend towards higher residual peak to peak AS gradient immediately post-BAVP being associated with E/E' ≥ 12 at follow-up (36 vs. 29 mm Hg, p=0.07). Age at intervention, need for previous BAVP, pre-BAVP AS gradient, duration of post BAVP at follow up echocardiogram, and AR grade post-BAVP were not associated with post-BAVP E/E'.
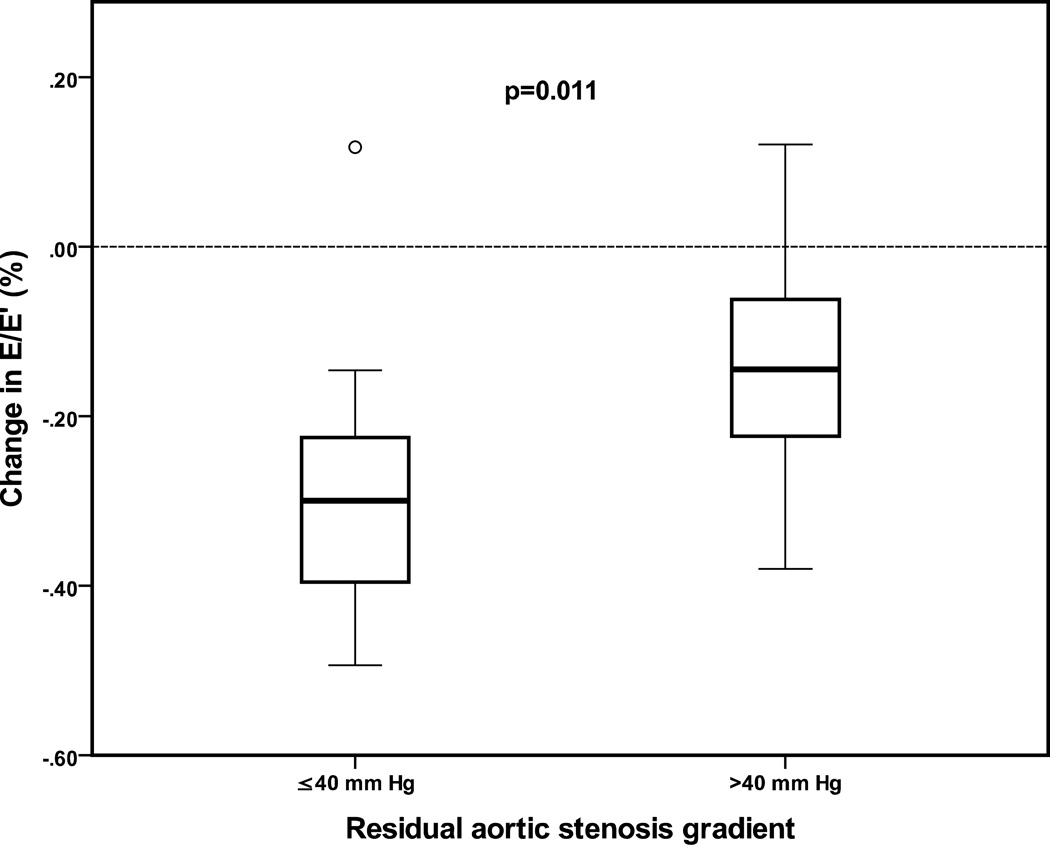
Residual AS gradient at follow up was associated with magnitude of decrease in E/E'. Patients with AS gradient ≤ 40 mm Hg on follow up echocardiography had a median decrease in E/E' of 30% compared to 14% in patients with AS gradient > 40 mm Hg (p=0.011) (Figure 4).
Figure 4.
Box plot showing percentage change in early diastolic pulsed-Doppler mitral inflow (E)/early diastolic tissue Doppler velocity (E') from pre-to post-balloon aortic valvuloplasty echocardiograms comparing patients with residual aortic stenosis gradient on follow up echocardiogram ≤ 40 mm Hg to patients with residual AS gradient > 40 mm Hg. Boxes represent interquartile range, dark line represents median value, and whiskers represent range.
Discussion
In this study we show that diastolic dysfunction and elevated left heart filling pressures are common in patients with congenital AS and echocardiographic measures of diastolic function and LVEDP improve after BAVP. In the majority of patients, diastolic function and echocardiographic estimate of LVEDP (E/E') improve after removal of pressure load, but a minority have significant ongoing elevation of E/E' and evidence of persistent diastolic dysfunction. Factors associated with ongoing elevation of E/E' are higher pre-and post-BAVP LV mass and higher E/E' prior to BAVP. Additionally, this study describes LV remodeling after BAVP, which is characterized primarily by an increase in LV EDV and decrease in LV mass: volume without a change in LV mass.
Our results agree with previous studies showing diastolic dysfunction in children with left heart obstructive lesions. Lam et al reported on 23 patients with congenital AS and showed that they have more echocardiographic evidence of diastolic dysfunction and elevated LVEDP than patients with aortic coarctation or controls20. de Kort et al. reported echocardiographic diastolic function data on 9 children before and shortly after BAVP (1–4 days after catheterization) using TDI and strain imaging and showed that values improved post-BAVP in most cases17. Unique from previous studies, this study examines diastolic function over a longer follow up period (6–24 months) which allows for LV remodeling to occur post-BAVP. Additionally, we are able to identify factors associated with persistent diastolic dysfunction after BAVP.
Despite low residual AS gradients in the vast majority of patients, LV mass did not change after BAV. Yet, diastolic function and E/E' improve, suggesting the hypertrophy alone is not entirely responsible for diastolic dysfunction in this population. In addition to ventricular hypertrophy, possible mechanisms for diastolic dysfunction in this population include impaired active relaxation due to alterations in calcium handling24–26 and myocardial fibrosis6, 7, 12, 27, 28 secondary to chronic pressure load.
Despite substantial relief of LV pressure load in all patients, 20% of patients continued to have echocardiographic evidence of impaired diastolic function and elevated left atrial pressure. The association between higher pre-and post-BAVP LV mass z-score and higher post-BAVP E/E' suggests that there may be a subset of patients in whom longer duration of LV pressure load has lead to myocardial changes, including hypertrophy and myocardial fibrosis13, 29, 30, as well as alterations in active relaxation24–26 that are less reversible. Future studies are needed to identify mechanisms of impaired diastolic function in this population more precisely and to determine their reversibility.31 Severe, and in some cases, irreversible diastolic heart failure has been described in a small number of patients with congenital AS who underwent BAVP as infants21. As patients who have undergone BAVP as children are now increasingly reaching their 3rd and 4th decades of life, long-term clinical follow-up will establish how many of these patients have persistent diastolic dysfunction and if these abnormalities will lead to development of symptoms of diastolic heart failure or decreased exercise capacity. Current approaches to the timing of BAVP and effectiveness of therapy have focused primarily systolic function and on the degree of AS and AR31. A future approach using not only the degree of aortic valve disease and systolic function but also diastolic function, particularly signs of less reversible changes and evidence of myocardial fibrosis, in timing of intervention may improve management.
Limitations of this study include the retrospective design and small cohort size which may limit ability to identify all factors associated with changes in diastolic function after BAVP. The retrospective nature of the study resulted in variable post-BAVP follow up periods with range of 6–24 months. Patients under 3 years of age were excluded due to the wide variability in the quality and reproducibility of diastolic function measures in this age range and lack of normative data in infants and young children. Follow up LVEDP was estimated as elevated or normal using E/ E'≥12 rather than measured invasively as few patients in this cohort had repeat catheterizations within this time-frame. Lastly, although degree of post-BAVP AR grade did not have an influence on diastolic function in this cohort, the study design and small cohort patients did not allow us to definitively determine the effect of AR and mixed aortic valve disease on diastolic function.
Conclusion
After BAVP, LV remodeling characterized by an increase in LV EDV and decrease in LV mass: volume occurs and echocardiographic measures of diastolic function and LVEDP improve in the majority of patients. Risk factors for persistently elevated E/E' after BAVP include higher LV mass z-score and higher pre-BAVP E/E'.
What is Known
Chronic pressure load on the left ventricle due to aortic stenosis results in ventricular hypertrophy, myocardial fibrosis, and abnormal myocardial mechanics.
These processes can result in both systolic and diastolic dysfunction.
What This Study Adds
Diastolic function and non-invasive measures of left atrial pressure improve in the majority of patients after effective relief of aortic stenosis with balloon aortic valvuloplasty.
Acknowledgments
Sources of Funding
Hinden Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol. 1960;5:370–382. doi: 10.1016/0002-9149(60)90084-9. [DOI] [PubMed] [Google Scholar]
- 3.Savage DD, Garrison RJ, Kannel WB, Levy D, Anderson SJ, Stokes J, III, Feinleib M, Castelli WP. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham Study. Circulation. 1987;75:I26–I33. [PubMed] [Google Scholar]
- 4.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 5.Krayenbuehl HP, Hess OM, Schneider J, Turina M. Left ventricular function and myocardial structure in aortic valve disease before and after surgery. Herz. 1984;9:270–278. [PubMed] [Google Scholar]
- 6.Peterson KL, Ricci D, Tsuji J, Sasayama S, Ross J., Jr Evaluation of chamber and myocardial compliance in pressure overload hypertrophy. Eur J Cardiol. 1978;7(Suppl):195–211. [PubMed] [Google Scholar]
- 7.Piper C, Schultheiss HP, Akdemir D, Rudolf J, Horstkotte D, Pauschinger M. Remodeling of the cardiac extracellular matrix differs between volume- and pressure-overloaded ventricles and is specific for each heart valve lesion. J Heart Valve Dis. 2003;12:592–600. [PubMed] [Google Scholar]
- 8.Tsang W, Lang RM. Myocardial fibrosis in severe aortic stenosis. Curr Cardiol Rep. 2010;12:196–198. doi: 10.1007/s11886-010-0097-6. [DOI] [PubMed] [Google Scholar]
- 9.Hess OM, Villari B, Krayenbuehl HP. Diastolic dysfunction in aortic stenosis. Circulation. 1993;87:IV73–IV76. [PubMed] [Google Scholar]
- 10.Peterson KL, Tsuji J, Johnson A, DiDonna J, LeWinter M. Diastolic left ventricular pressure-volume and stress-strain relations in patients with valvular aortic stenosis and left ventricular hypertrophy. Circulation. 1978;58:77–89. doi: 10.1161/01.cir.58.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Stewart RA, Kerr AJ, Whalley GA, Legget ME, Zeng I, Williams MJ, Lainchbury J, Hamer A, Doughty R, Richards MA, White HD. Left ventricular systolic and diastolic function assessed by tissue Doppler imaging and outcome in asymptomatic aortic stenosis. Eur Heart J. 2001;31:2216–2222. doi: 10.1093/eurheartj/ehq159. [DOI] [PubMed] [Google Scholar]
- 12.Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, Krayenbuehl HP. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477–1484. doi: 10.1016/0735-1097(93)90560-n. [DOI] [PubMed] [Google Scholar]
- 13.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 14.Gjertsson P, Caidahl K, Bech-Hanssen O. Left ventricular diastolic dysfunction late after aortic valve replacement in patients with aortic stenosis. Am J Cardiol. 2005;96:722–727. doi: 10.1016/j.amjcard.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 15.Villari B, Vassalli G, Monrad ES, Chiariello M, Turina M, Hess OM. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation. 1995;91:2353–2358. doi: 10.1161/01.cir.91.9.2353. [DOI] [PubMed] [Google Scholar]
- 16.Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG. Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications. J Thorac Cardiovasc Surg. 2012;143:656–664. doi: 10.1016/j.jtcvs.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Kort E, Thijssen JM, Daniels O, de Korte CL, Kapusta L. Improvement of heart function after balloon dilation of congenital valvar aortic stenosis: a pilot study with ultrasound tissue Doppler and strain rate imaging. Ultrasound Med Biol. 2006;32:1123–1128. doi: 10.1016/j.ultrasmedbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Fifer MA, Borow KM, Colan SD, Lorell BH. Early diastolic left ventricular function in children and adults with aortic stenosis. J Am Coll Cardiol. 1985;5:1147–1154. doi: 10.1016/s0735-1097(85)80017-6. [DOI] [PubMed] [Google Scholar]
- 19.Kiraly P, Kapusta L, Thijssen JM, Daniels O. Left ventricular myocardial function in congenital valvar aortic stenosis assessed by ultrasound tissue-velocity and strain-rate techniques. Ultrasound Med Biol. 2003;29:615–620. doi: 10.1016/s0301-5629(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 20.Lam YY, Kaya MG, Li W, Gatzoulis MA, Henein MY. Effect of chronic afterload increase on left ventricular myocardial function in patients with congenital left-sided obstructive lesions. Am J Cardiol. 2007;99:1582–1587. doi: 10.1016/j.amjcard.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Robinson JD, del Nido PJ, Geggel RL, Perez-Atayde AR, Lock JE, Powell AJ. Left ventricular diastolic heart failure in teenagers who underwent balloon aortic valvuloplasty in early infancy. Am J Cardiol. 2010;106:426–429. doi: 10.1016/j.amjcard.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 22.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Jiamsripong P, Honda T, Reuss CS, Hurst RT, Chaliki HP, Grill DE, Schneck SL, Tyler R, Khandheria BK, Lester SJ. Three methods for evaluation of left atrial volume. Eur J Echocardiogr. 2008;9:351–355. doi: 10.1016/j.euje.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Borbely A, Papp Z, Edes I, Paulus WJ. Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep. 2009;61:139–145. doi: 10.1016/s1734-1140(09)70016-7. [DOI] [PubMed] [Google Scholar]
- 25.Selby DE, Palmer BM, Lewinter MM, Meyer M. Tachycardia-induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol. 2011;58:147–154. doi: 10.1016/j.jacc.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zile MR, Gaasch WH. Abnormal calcium homeostasis: one mechanism in diastolic heart failure. J Am Coll Cardiol. 2011;58:155–157. doi: 10.1016/j.jacc.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 27.Cheitlin MD, Robinowitz M, McAllister H, Hoffman JI, Bharati S, Lev M. The distribution of fibrosis in the left ventricle in congenital aortic stenosis and coarctation of the aorta. Circulation. 1980;62:823–830. doi: 10.1161/01.cir.62.4.823. [DOI] [PubMed] [Google Scholar]
- 28.Oldershaw PJ, Brooksby IA, Davies MJ, Coltart DJ, Jenkins BS, Webb-Peploe MM. Correlations of fibrosis in endomyocardial biopsies from patients with aortic valve disease. Br Heart J. 1980;44:609–611. doi: 10.1136/hrt.44.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heymans S, Schroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, Van de WF, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph A, bdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–291. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 31.Brown DW, Dipilato AE, Chong EC, Lock JE, McElhinney DB. Aortic valve reinterventions after balloon aortic valvuloplasty for congenital aortic stenosis intermediate and late follow-up. J Am Coll Cardiol. 2010;56:1740–1749. doi: 10.1016/j.jacc.2010.06.040. [DOI] [PubMed] [Google Scholar]