Abstract
High glucose inhibits mitochondrial respiration, known as the “Crabtree effect”, in cancer cells and possibly other cell types. The upstream pathways regulating this phenomenon are poorly understood. In diabetes, where glucose levels are elevated, the p90 ribosomal S6 kinase (p90RSK) has received much attention as a potential upstream mediator of the effects of high glucose. Evidence is also emerging that p90RSK may play a role in cancer cell signaling, although the role of p90RSK in regulating cancer cell metabolism is unclear. Herein we provide an overview of the Crabtree effect and its relationship to mitochondrial metabolism. Furthermore, preliminary data are presented suggesting a role for p90RSK and its upstream components, the ERK family of mitogen-activated protein kinases (MAPKs), in the Crabtree effect.
Keywords: Cancer, Crabtree, Warburg, ERK5, Mitochondria, p90RSK
Introduction
The exposure of cells to high concentrations of glucose results in a number of physiological and pathological responses. In the field of diabetes research, the effects of glucose on mitochondrial reactive oxygen species (ROS) generation have been well-studied, and are believed to underlie much of the secondary pathology of diabetes, particularly vascular complications [1]. In the field of cancer research, the effects of glucose on cancer cell metabolism have also been studied extensively, but the majority of attention has been given to the Warburg effect – the phenomenon in which cancer cells appear to prefer anaerobic glycolysis as a source of ATP even in the presence of adequate O2 for mitochondrial oxidative phosphorylation (Ox-Phos) to occur [2,3].
In contrast the Crabtree effect, postulated in 1929, has languished in the shadow of the Warburg effect in terms of research attention [4]. The Crabtree effect is simply defined as the observation that high concentrations of glucose result in a decrease in mitochondrial respiration in cancer cells [4]. The role of inhibited mitochondrial function in the Warburg effect is still unclear [5], and as such it is possible that the Crabtree effect may be an underlying cause of the Warburg effect. Recently, it has become apparent that the Crabtree effect is present in many types of dividing cells (e.g. endothelial cells), and not just in cancer cells [6]. Furthermore, it may be related to the phenomenon of glucose repression which is observed in yeast [7]. Although it was discovered eight decades ago, the molecular mechanisms underpinning the Crabtree effect have yet to be elucidated.
Recent evidence has implicated the p90 ribosomal S6 kinase (p90RSK) in glucose sensing and diabetes [8]. Thus, it is possible that p90RSK may play a role in the Crabtree effect. The current paper presents evidence favoring a role for p90RSK in the Crabtree effect, and discusses this in the context of cancer signaling.
ERK5/MEK5/p90RSK pathway in cancer
Mitogen-activated protein kinases (MAPKs) are essential components many cell signaling events including proliferation, differentiation and cell death. The biological actions of ERK5 (Big MAP Kinase 1) are well described [9,10] in several cell proliferative and developmental pathways, as well as cancer. MEK5 [10,11] has been closely linked to ERK5, and nearly all cellular processes in which MEK5 has been implicated, are attributed to regulation by ERK5.
Within the field of cancer research, the MEK5-ERK5 pathway has been implicated in cell growth control [12], and a role for ERK5 has been postulated in EGF-induced proliferation and cell-cycle progression [13]. Furthermore, studies in ERK5 knock-out mice show a crucial role for ERK5 in angiogenesis and vascular cell growth [14], especially as a modulator of other antigenic signals.
In patients suffering from multiple myeloma, ERK5 is expressed in B-cells [15] and it is constitutively activated in breast cancer cells over-expressing the Erb B2 receptor [16]. ERK5 activation is required for the proliferation of myeloma and breast cancer cell lines in response to IL-6 and neuregulins respectively. Cyclin D1, whose deregulation is implicated in a wide variety of cancers, was identified as a target of the MEK5/ERK5 pathway [17]. The authors showed that the kinase activity of ERK5 is required for induction of cyclin D1 in response to serum in some breast cancer cell lines [17]. In prostate and breast cancers, MEK5 over-expression is associated with poor survival and resistance to chemotherapy respectively [18,19]. Furthermore, prostate cancer cell growth can be suppressed by inhibition of MEK5 signaling, through the enhanced degradation of the protein [20]. ERK5 and p90RSK have some overlapping functions in cancer: p90RSK, like ERK5, is thought to stimulate cell cycle progression [21]. ERK5 also responds to cAMP [22] and plays a role in signaling to the pro-apoptotic protein Bad [23], which is a direct substrate of p90RSK [24].
Overall, there are several lines of evidence linking p90RSK to cancer and to glucose sensing, but surprisingly the role of this serine/threonine kinase in mediating the Warburg or Crabtree effects has not been investigated.
Experimental
The metabolic function of the H9c2 cardiomyocyte cell line was measured using an XF24 extracellular flux analyzer (Seahorse Bioscience, Billerica MA) [25,26]. Cells were incubated in a glucose free Krebs Ringer solution (pH 7.3 w. HEPES) for 30 min., with or without 20 mM glucose, with the addition of kinase inhibitors and other pharmacologic agents, as follows: (i) BIX02188 (MEK5 inhibitor, 20 μM); (ii) UO126 (MEK1/2 inhibitor, 10 μM); (iii) FMK (fluoromethylketone pyrrolopyrimidine, p90RSK inhibitor [21], 10 μM); (iv) Splitomicin (SIRT1 inhibitor, 10 μM).
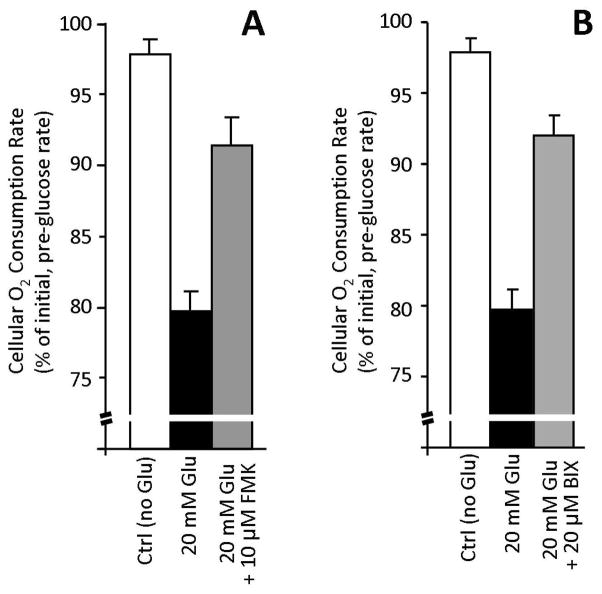
Figure 1 shows that H9c2 cells exhibited a robust Crabtree effect, with 30 min. exposure to 20 mM glucose resulting in a 20% drop in cellular oxygen consumption rate. This data is in broad agreement with those of Sweet et al. [6], who reported a robust Crabtree effect in endothelial cells. Thus, the Crabtree effect is not unique to cancer cells, and may apply to many types of dividing cells. Notably, this effect was blocked by pre-incubation with the p90RSK inhibitor FMK (Fig. 1A) or the MEK5 inhibitor BIX (Fig. 1B). Other pharmacophores tested included the MEK1/2 (ERK1/2) inhibitor UO126, or the SIRT1 inhibitor splitomicin, but neither had a significant effect on the inhibition of respiration by glucose (data not shown).
Figure 1. Crabtree Effect in H9c2 Cells, Effect of Inhibitors.
Oxygen consumption of H9c2 cells was measured in glucose free or replete (20 mM) media, in the presence or absence of the p90RSK inhibitor FMK (panel A), and the MEK5 inhibitor BIX (panel B). Data are means ± SEM, N >3. See text for further details.
Discussion
The role of MAP kinases in the response to glucose in general has been well-studied, and it was shown that some mitochondrial responses to high glucose could be blocked by high concentrations of the non-specific MEK1/2 (ERK1/2) inhibitor PD98059 [27], although this effect may have been due to off-target effects of PD98059 [28].
More recently, attention within the MAPK field has focused on another member of the ERK family, ERK5 (Big MAP Kinase), which is an upstream mediator of p90RSK activity [29,30]. This has been facilitated by the commercial availability of BIX02188, an inhibitor of MEK5, which is the kinase immediately upstream of ERK5. Our data in Fig. 1B suggest that indeed MEK5, through ERK5, is an upstream kinase involved in the activation of p90RSK in response to high glucose. This signaling pathway is shown in Figure 2, suggesting key roles for both MEK5 and p90RSK in the Crabtree effect.
Figure 2.
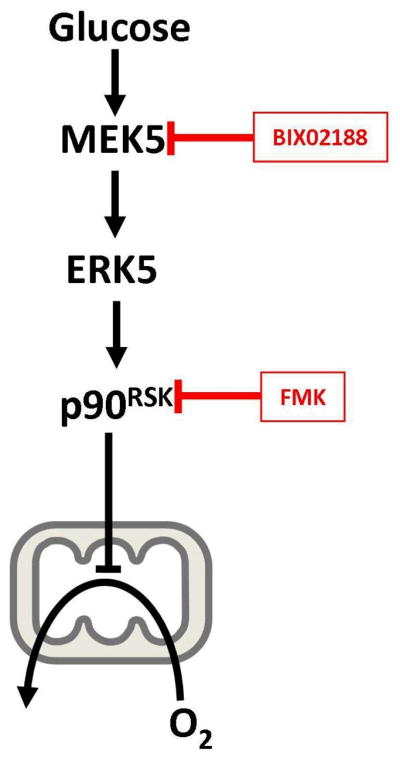
Proposed Signaling Pathway Linking High Glucose to Respiratory Inhibition.
To the best of our knowledge, this is the first identification of any signaling pathway involved in mediating the Crabtree effect. Notably, p90RSK has been linked to signaling by the tumor suppressor protein p53 [31]. In addition, p53 is emerging as an important regulator of mitochondrial function [32–34]. Thus, p90RSK may play additional roles in cancer signaling beyond the Crabtree effect, as reviewed above.
The relationship between the Crabtree effect and mitochondrial morphology is also worthy of further consideration. Specifically, a link has been established between mitochondrial fragmentation and ROS generation in response to high glucose [27,35]. Thus it is interesting to speculate that mitochondrial fragmentation may be an intermediate step between p90RSK and the inhibition of respiration. Clearly, the elucidation of this complex signaling pathway which drives the response of cancer and other dividing cell types to high glucose, may lead to the development of novel therapeutic approaches, based on the inhibition of p90RSK and its upstream signals.
Acknowledgments
Work in the laboratory of PSB is funded by a grant from the US National Institutes of Health (HL-071158).
Literature Cited
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 4.Crabtree HG. Observations of the carbohydrate metabolism of tumours. Biochem J. 1929;23:536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Ruiz R, Rigoulet M, Devin A. The Warburg and Crabtree effects: On the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys Acta. 2011;1807(6):568–76. doi: 10.1016/j.bbabio.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Sweet IR, Gilbert M, Maloney E, Hockenberry DM, Schwartz MW, Kim F. Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phospate but not increased mitochondrial respiration. Diabetologia. 2009;52:921–931. doi: 10.1007/s00125-009-1272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz-Ruiz R, Uribe-Carvajal S, Devin A, Rigoulet M. Tumor cell energy metabolism and its common features with yeast metabolism. Biochim Biophys Acta. 2009;1796:252–265. doi: 10.1016/j.bbcan.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Itoh S, Ding B, Bains CP, Wang N, Takeishi Y, Jalili T, King GL, Walsh RA, Yan C, Abe J. Role of p90 ribosomal S6 kinase (p90RSK) in reactive oxygen species and protein kinase C β (PKC-β)-mediated cardiac troponin I phosphorylation. J Biol Chem. 2005;280:24135–24142. doi: 10.1074/jbc.M413015200. [DOI] [PubMed] [Google Scholar]
- 9.Lee JD, Ulevitch RJ, Han J. Primary structure of BMK1: a new mammalian map kinase. Biochem Biophys Res Commun. 1995;213(2):715–724. doi: 10.1006/bbrc.1995.2189. [DOI] [PubMed] [Google Scholar]
- 10.Zhou GF, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270(21):12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 11.English JM, Vanderbilt CA, Xu S, Marcus S, Cobb MH. Isolation of MEK5 and differential expression of alternatively spliced forms. J Biol Chem. 1995;270(48):28897–28902. doi: 10.1074/jbc.270.48.28897. [DOI] [PubMed] [Google Scholar]
- 12.English JM, Pearson GF, Hockenberry TF, Shivakumar LF, White MA, Cobb MH. Contribution of the ERK5/MEK5 pathway to Ras/Raf signaling and growth control. J Biol Chem. 1999;274(44):31588–31592. doi: 10.1074/jbc.274.44.31588. [DOI] [PubMed] [Google Scholar]
- 13.Kato YF, Kravchenko VV, Tapping RI, Han JF, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16(23):7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn SJ, Sarvis BK, Cado D, Winoto A. ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J Biol Chem. 2002;277(45):43344–43351. doi: 10.1074/jbc.M207573200. [DOI] [PubMed] [Google Scholar]
- 15.Carvajal-Vergara X, Tabera S, Montero JC, Esparís-Ogando A, López-Pérez R, Mateo G, Gutiérrez N, Parmo-Cabañas M, Teixidó J, San Miguel JF, Pandiella A. Multifunctional role of Erk5 in multiple myeloma. Blood. 2005;105(11):4492–4499. doi: 10.1182/blood-2004-08-2985. [DOI] [PubMed] [Google Scholar]
- 16.Esparís-Ogando A, Díaz-Rodríguez E, Montero JC, Yuste L, Crespo P, Pandiella A. Erk5 participates in neuregulin signal transduction and is constitutively active in breast cancer cells overexpressing ErbB2. Mol Cell Biol. 2002;22(1):270–285. doi: 10.1128/MCB.22.1.270-285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulloy R, Salinas S, Philips A, Hipskind RA. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene. 2003;22(35):5387–5398. doi: 10.1038/sj.onc.1206839. [DOI] [PubMed] [Google Scholar]
- 18.Mehta PB, Jenkins BL, McCarthy L, Thilak L, Robson CN, Neal DE, Leung HY. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene. 2003;22(9):1381–1389. doi: 10.1038/sj.onc.1206154. [DOI] [PubMed] [Google Scholar]
- 19.Weldon CB, Scandurro AB, Rolfe KW, Clayton JL, Elliott S, Butler NN, Melnik LI, Alam J, McLachlan JA, Jaffe BM, Beckman BS, Burow ME. Identification of mitogen-activated protein kinase kinase as a chemoresistant pathway in MCF-7 cells by using gene expression microarray. Surgery. 2002;132(2):293–301. doi: 10.1067/msy.2002.125389. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh AK, Steele R, Ray RB. c-myc Promoter-binding protein 1 (MBP-1) regulates prostate cancer cell growth by inhibiting MAPK Pathway. J Biol Chem. 2005;280(14):14325–14330. doi: 10.1074/jbc.M413313200. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson GW, Cobb MH. Cell condition-dependent regulation of ERK5 by cAMP. J Biol Chem. 2002;277(50):48094–48098. doi: 10.1074/jbc.M208535200. [DOI] [PubMed] [Google Scholar]
- 23.Pi XF, Yan CF, Berk BC. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ Res. 2003;94(3):362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- 24.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and –independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 25.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Guo S, Olm-Shipman A, Walters A, Urciuoli WR, Devito S, Nadtochiy SM, Wojtovich AP, Brookes PS. A cell-based phenotypic assay to identify cardioprotective agents. Circ Res. 2012;110(7):948–57. doi: 10.1161/CIRCRESAHA.111.263715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu T, Jhun BS, Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal. 2011;14(3):425–437. doi: 10.1089/ars.2010.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The MAP kinase ERK5 binds to and phosphorylates p90 RSK. Arch Biochem Biophys. 2006;449:8–16. doi: 10.1016/j.abb.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi M, Fearns C, Eliceiri B, Yang Y, Lee JD. Big mitogen-activated protein kinase 1/extracellular signal-regulated kinase 5 signaling pathway is essential for tumor-associated angiogenesis. Cancer Res. 2005;65:7699–7706. doi: 10.1158/0008-5472.CAN-04-4540. [DOI] [PubMed] [Google Scholar]
- 31.Jackson MW, Linnea EP, LaRusch GA, Donner DB, Stark GR, Mayo LD. Hdm2 nuclear export, regulated by insulin-like growth factor-I/MAPK/p90RSK signaling, mediates the transformation of human cells. J Biol Chem. 2006;281:16814–16820. doi: 10.1074/jbc.M511617200. [DOI] [PubMed] [Google Scholar]
- 32.Feng Z, Levine AJ. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010;20:427–434. doi: 10.1016/j.tcb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 34.Murphy ME, Leu JI, George DL. p53 moves to mitochondria: a turn on the path to apoptosis. Cell Cycle. 2004;3:836–839. [PubMed] [Google Scholar]
- 35.Yu T, Sheu S, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated levels of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]