Abstract
A decrease in dopamine D2 receptor (D2R) binding in the striatum is one of the most common findings in disorders that involve a dysregulation of motivation, including obesity, addiction, and attention deficit hyperactivity disorder. Since disruption of D2R signaling in the ventral striatum – including the Nucleus Accumbens (NAc) - impairs motivation, we sought to determine whether potentiating postsynaptic D2R-dependent signaling in the NAc would improve motivation. In this study, we used a viral vector strategy to overexpress postsynaptic D2Rs in either the NAc or the dorsal striatum. We investigated the effects of D2R overexpression on instrumental learning, willingness to work, use of reward value representations and modulation of motivation by reward associated cues. Overexpression of postsynaptic D2R in the NAc selectively increased motivation without altering consummatory behavior, the representation of the value of the reinforcer, or the capacity to use reward associated cues in flexible ways. In contrast, D2R overexpression in the dorsal striatum did not alter performance on any of the tasks. Thus, consistent with numerous studies showing that reduced D2R signaling impairs motivated behavior, our data show that post-synaptic D2R overexpression in the NAc specifically increases an animal’s willingness to expend effort to obtain a goal. Taken together, these results provide insight into the potential impact of future therapeutic strategies that enhance D2R signaling in the NAc.
Keywords: D2 Receptor, Nucleus Accumbens, motivation, reward, dopamine, incentive salience, viral vector
INTRODUCTION
The mesoaccumbens dopamine (DA) pathway modulates motivation1–3. In the nucleus accumbens (NAc), DA mediates these effects through D1-like and D2-like receptors. A decrease in D2 receptor (D2R) availability in the striatum, including the NAc, is a common imaging phenotype in disorders that involve the dysregulation of motivation, including obesity4, addiction5 and attention deficit hyperactivity disorder (ADHD)6. Moreover, imaging studies in human subjects have shown that striatal D2R levels correlate with characteristics such as sensation seeking and motivation7–9. These studies clearly establish the relevance of D2R function to human disorders of motivation, and highlight the importance of determining causal relationships between D2R expression and motivation. One powerful approach for revealing such relationships is to combine in rodents region-specific manipulations of D2R expression with validated methods for measuring specific aspects of motivation.
Changes in motivated behavior can be driven by a number of underlying mechanisms, including effort the animal is willing to expend for the reward - i.e. primary motivational stimuli or goals10 - as well as the organism’s valuation of a reward11–13. Both of these aspects of motivation have been shown to depend on dopaminergic transmission in the NAc. In rodents, willingness to work for a reward is modified by altering D2R signaling in the NAc. Blocking D2Rs in the NAc shifts the animals’ choice away from more effortful toward less effortful behavior11 and the genetic deletion of the D2R also impairs motivated, reward-seeking behavior14. Similarly, selective lesioning of the NAc in rats impairs their willingness to work for a reward15,16. On the other hand, DA release in the NAc is associated with the animal’s valuation of an appetitive stimulus even in the absence of a work requirement to obtain the stimulus13.
The goal of the present study was to investigate whether increasing postsynaptic NAc D2R levels can enhance motivation, and if so, whether distinct aspects of motivated behavior might be selectively modulated. The D2R was overexpressed in the striatum using viral gene transfer in adult mice. In order to explore the effect of D2R overexpression on motivation, overexpression in the NAc was compared to that in the caudate/putamen in different groups of mice. Behavior was studied using operant tasks that assess instrumental learning, willingness to expend effort to obtain a goal, the ability to use representations of reward to guide responding, and the capacity of reward associated cues to modulate motivation.
MATERIAL AND METHODS
Viruses
As described previously17, adeno-associated viruses 1/2 expressing either 1) D2LR fused to mVenus or 2) GFP were used (see supplemental information).
Aequorin assay
A functional assay based on luminescence of mitochondrial aequorin following intracellular Ca2+ release was performed as previously described18.
Animals
All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Columbia University and the New York State Psychiatric Institute. Wild-type female congenic C57Bl/6j mice were used for behavioral experiments (postnatal ages 90 days). D2R knockout and their C57Bl/6j WT littermates (KO)19 were used for immunohistochemical experiments (See Supplemental Information).
Surgeries/viral injections
Viral injections were performed as previously described17. CPu was targeted with two bilateral injections, (4 sites total, 0.75ul Virus injected into each): A-P 1.5 mm and 0, M-L +/−1.5 mm and +/−2.5 mm, all 3 mm ventral to brain surface. NAc was targeted with a single injection site bilaterally (2 sites total, 0.5 μl virus injected in each site), A-P 1.7 mm, M-L +/− 1.7 mm, both 3.8 mm ventral to brain surface. All co-ordinates given are relative to Bregma. Behavioral testing began one month after surgery.
[3H] N-methylspiperone (NMSP) binding assay
Binding assay was performed to investigate the degree of D2R overexpression (See supplemental Information).
Behavioral Testing
Apparatus
The apparatus has been described in detail in Simpson et al. (2011) (See supplemental information) 20.
Procedures
Seven animals in each group were used in the current study, except for the PIT experiment where there were only 6 mice in the CPu-GFP group due to the death of one animal. The same animals performed each of the behavioral procedures. In order to asses instrumental learning and willingness to work in face of increasing effort, dipper training, lever pressing training, fixed-interval (FI) training and progressive ratio (PR) testing were performed as described in Simpson et al.20 with some modifications (see supplemental information).
Random ratio (RR) testing
RR consists of a constant probability of reinforcement for each lever press. The mice were first trained for 5 days in 1-hour RR5 sessions. Animals were then tested in concurrent RR/choice procedure that has been extensively shown to assess effort in instrumental responding11. This task consisted of having 8–12 g of lab chow available in a dish in the operant chamber while the mouse worked on the RR schedule. Increasing ratios were used (RR5, RR10, RR20). A previous study using a preference test showed that evaporated milk serves as a reinforcer for mice using a preference task21. For each RR, both concurrent choice and simple RR sessions were repeated in a pseudo-random manner.
Devaluation Procedure
This task was used to assess alteration of the outcome value. Mice had free access to either chow or the evaporated milk reward for 1 h in the home cage (single-housed) prior to a lever press test conducted in a 15 minute extinction session. Following this session, the animals received 2 days of retraining using an RR20 procedure prior to the second test. This test was identical to the first except that the mice were pre-fed with the other outcome. The order of pre-feeding was counterbalanced across subjects within each group.
Pavlovian to instrumental transfer (PIT) test
PIT was performed 2 weeks after the final devaluation test. In the Pavlovian training an auditory stimulus (85 dB, 2000 Hz) served as conditioned stimulus (CS). Pavlovian training consisted in 5 presentations of 2 minutes CS with a variable ITI (mean 8 minutes) for 5 days. The evaporated milk reward was made available for 30 seconds with a variable delay after the beginning of the tone-CS (mean 1 minute). We limited the Pavlovian training to 5 sessions since previous studies have shown that overtraining impairs PIT22. The PIT test was conducted on the 6th day and consisted of a 10 minute extinction followed by 5 cycles of 2 minutes CS presentation with 2 minutes ITI, without reward delivery.
Histology/immunohistochemistry
Brain tissue preparation, immunohistochemistry, confocal microscopy and image acquisition were performed as described previously17. The following primary antibodies were used in this study: home-made rabbit polyclonal anti-D217 (1/500) and rabbit polyclonal anti-MAP2 (Abcam ab32454; 1/2000).
Statistical analysis
Data were analyzed using mixed ANOVAs with appropriate terms, followed by post-hoc Bonferroni comparisons (when appropriate) or t-tests. For all the tasks analyzed, the number of lever presses was used as the dependent measure (see also supplemental information).
RESULTS
Characterization of D2R-mVenus overexpression
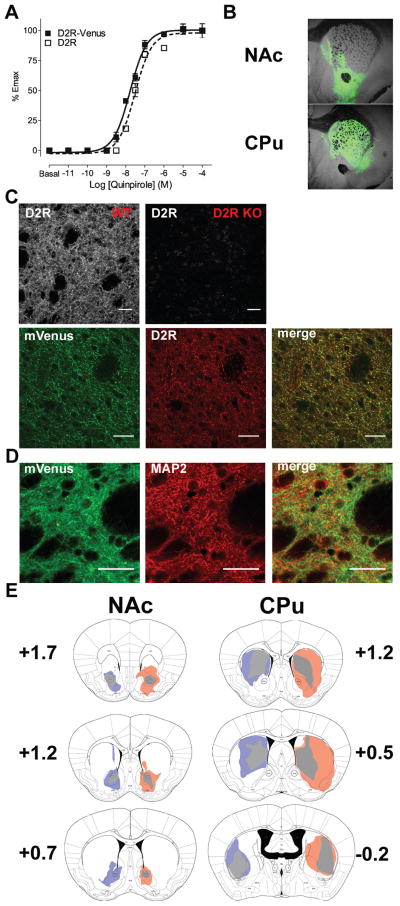
To facilitate visualization of the exogenous D2Rs and discriminate between exogenous and endogenous receptors, we generated a fusion construct of D2R tagged with mVenus at its C-terminus. To verify that the fusion construct was functional we expressed D2R-mVenus in HEK cells and assessed G protein activation by the agonist quinpirole using an aequorin-based functional assay (see supplemental information). As shown in Figure 1A, fusion of mVenus to the D2R receptor did not alter the ability of the receptor to activate G protein.
Figure 1. Characterization of the D2R-mVenus overexpression.
(A) Fusion of mVenus to the D2R receptor did not alter the ability of the agonist quinpirole to activate G protein as demonstrated in an aequorin-based assay. Quinpirole-induced luminescence was determined as described in Material and Methods and expressed as a percentage of the maximal response for each construct. Results of 3 independent experiments are represented as mean ± S.E.M fit to a sigmoidal dose response non-linear regression. (B) Representative examples of D2R-mVenus expression by AAV injection in NAc (top) and CPu (bottom). (C) Specificity of the anti-D2R antibody was determined by comparison of a wildtype and D2R KO stained sections (top row). D2R-mVenus expression highly overlaps with endogenous D2R expression (bottom row). (D) D2R-mVenus co-localized with MAP2, consistent with a dendritic/postsynaptic localization of the receptor. Scale bars: 30 μm. (E) Diagrammatic representation of the maximal (colors) and minimal (gray) extent of spread of D2R-mVenus (orange) and GFP (blue) for CPu (right diagram) and NAc (left diagram); numbers indicate distance from Bregma.
We overexpressed D2R-mVenus or GFP alone as a control in either the nucleus accumbens (NAc) or the dorsal striatum (CPu) of adult mice using AAV-mediated gene transfer (Figure 1B) and quantified the increase in D2R expression by performing a [3H]N-methylspiperone (NMSP) binding assay. Expression of D2R-mVenus resulted in an approximately 10-fold increase in D2R binding in both NAc (D2R-mVenus: Bmax=17.8±1.2 pmol/mg protein, Kd=36.3±10.4 pM; GFP: Bmax=1.65±0.15 pmol/mg protein, Kd=28.9±4.8 pM) and CPu (D2R-mVenus: Bmax=17.3±0.5 pmol/mg protein, Kd=36.6±10.1 pM; GFP: Bmax=1.64±0.03 pmol/mg protein, Kd=42.5±9.8 pM).Immunofluorescence experiments revealed that D2R-mVenus had very low somatic expression (Figure 1C) and partially colocalized with the dendritic marker MAP2 (Figure 1D), consistent with the known predominant post-synaptic localization of the receptor23. Together these data suggest that our viral overexpression system leads to a large increase in functional D2R with the expected subcellular localization.
The minimal and maximal extents of D2R-mVenus and GFP expression are depicted in the left and right hemispheres, respectively, of coronal sections in Fig. 1E. D2R-Venus expression in the NAc largely targeted the core in all animals and partially the shell, with very low spread to the dorsal striatum. D2R-Venus expression in the CPu did not reach the NAc except in one animal (see Fig. 1E) and spread throughout roughly 40–70% of the entire CPu. We did not observe significant expression in extra-striatal areas in any of the animals.
D2R overexpression does not alter simple instrumental conditioning
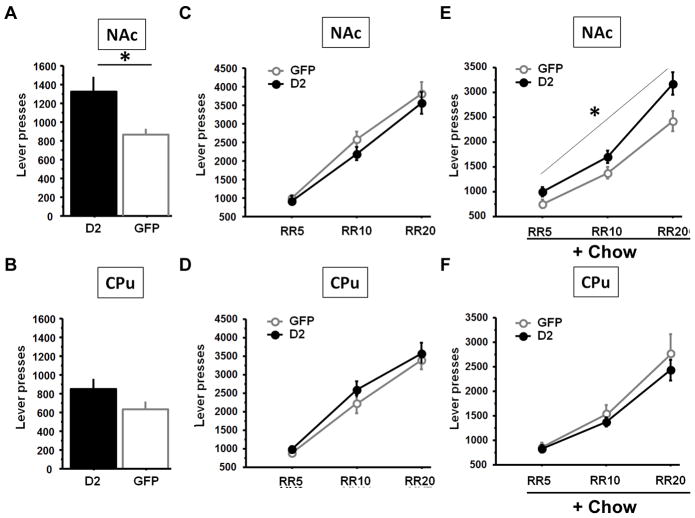
D2R overexpression in the NAc or CPu did not alter learning of instrumental conditioning in the continuous reinforcement (CRF) schedule compared to GFP overexpression as shown by the number of lever presses (NAc: F(1, 12)=0.30; p=0.59, CPu F(1, 12)=0.02; p=0.89) (Fig. 2 A, B). Previous studies show that fixed interval (FI) training before progressive ratio (PR) testing creates a sensitive assay of motivation24. We analyzed the number of lever presses during FI schedules with increasing intervals (Fig. 2 A, B). D2R overexpression had no effect at any interval tested (all p’s>0.25). All mice learned the operant response procedures within three days of training, showing that D2R overexpression did not impact learning or any phase of the initial training.
Figure 2. D2R overexpression in the NAc or the CPu does not alter operant learning.
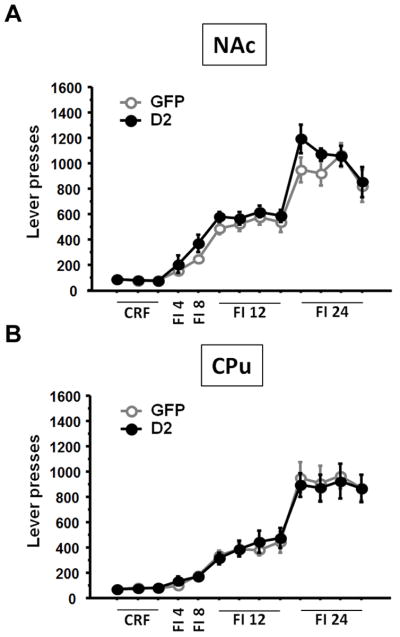
D2R overexpression in the NAc (A) or CPu (B) did not alter learning of the operant procedure in a continuous reinforcement schedule (CRF) or performance in fixed interval (FI) schedules.
D2R overexpression in the NAc, but not the CPu, increases effort for a reward
To test the effect on motivation, we used the progressive ratio (PR) schedule, which assesses the amount of effort a subject is willing to expend to obtain a reward. We analyzed only two consecutive PR sessions because repeated exposure to PR sessions lowers operant responding over time. Measure of the breakpoint showed a trend toward an increase in the animals overexpressing D2R in the NAc (t=−1.68; p<0.08) but not in the CPu (t=−1.36; p=0.20) (suppl. Figure 1). However, since in the current PR schedule the criterion doubled for each successive trial (from 512 to 1024 to 2048 lever presses to obtain the next reward), the number of lever presses was used as a more sensitive continuous primary outcome measure. Independent group comparison revealed that D2R overexpression led to ~50% increase in the number of lever presses in the NAc group (t=2.51; p=0.03) but did not alter total lever presses in the CPu group (t=1.66; p=0.12) (Fig. 3A, B).
Figure 3. D2R overexpression in the NAc, but not the CPu, increases effort in a progressive ratio task and a choice paradigm.
D2R overexpression in the NAc (A) but not in the CPu (B) enhanced the average number of lever presses in a progressive ratio task. When tested in random ratio (RR) paradigms, D2R overexpression in the NAc (C) or the CPu (D) had no effect on operant response in any of the random ratios tested (number of sessions pooled for each ratios: RR5=13; RR10=4; RR20=7). However, when tested in a choice lever pressing/chow feeding procedure (number of sessions pooled for each ratios: RR5=9; RR10=8; RR20=12), mice overexpressing D2R in the NAc (E) showed an enhancement in their rate of lever press, whereas overexpression of D2R in the CPu (F) had no effect. (* statistically significant).
We next examined the performance of mice in the simple random-ratio (RR) tasks and in a lever-pressing/chow-feeding choice procedure in which the mice can lever press for preferred food (evaporated milk) or can consume a less preferred food (home cage chow) that is freely available in the chamber11. Unlike the PR schedule, which measures willingness to continue working to obtain a reward in the face of an increasing effort requirement, this procedure allows an assessment of the animals’ choice to expend effort to obtain a preferred food when a less preferred food is available for little effort. Overall, D2R-overexpression had no effect on the number of lever presses emitted on the RR schedule when home cage chow was not available (non-concurrent task), either in the NAc (virus effect: F(1, 12)= 1.25; p=0.28) (Fig. 3C) or the CPu (virus effect: F(1, 11)=0.65; p=0.44) (Fig. 3D). However, when tested in the choice phase (chow freely available), mice with D2R overexpression in the NAc (Fig. 3E) showed enhanced lever presses for the reward in response to the increasing ratio requirements (virus effect: F(1, 12)=5.51; p=0.04) and a virus x ratio interaction (F(2, 24)=4.490; p=0.02) compared to controls. In contrast D2R overexpression in the CPu had no effect (virus effect: F(1, 11)=0.55; p=0.47. virus x ratio interaction: F(2, 22)= 0.67; p=0.52) (Fig. 3F). The difference in lever presses in the NAc group was directly due to an increase in lever presses in mice overexpressing D2R since the two GFP-expressing control groups were similar (F(1, 11)= 0.68; p=0.43).
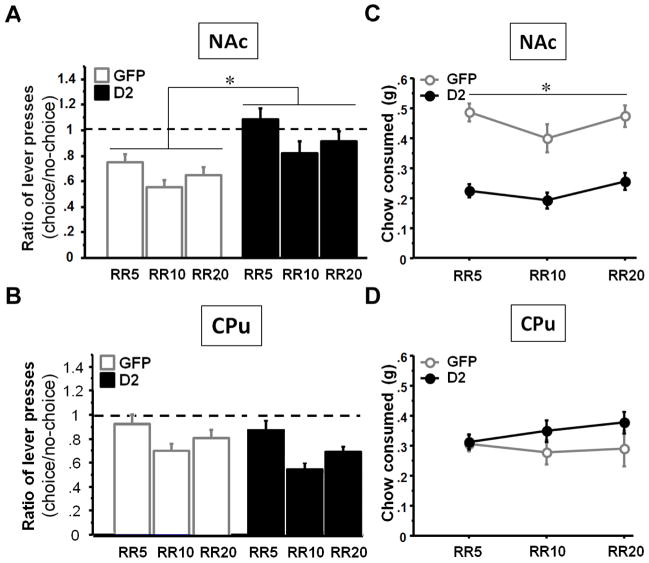
Interestingly, analyses of the ratio (lever presses in choice)/(lever presses in no choice) in the NAc group showed that the D2R-overexpressing animals maintained their rate of lever pressing, whereas GFP-expressing control animals significantly decreased their lever presses when free chow was available in the cage, (Fig. 4A). ANOVA revealed a virus effect (F(1, 12)=11.09; p<0.01), and one-sample analysis revealed that the ratio (lever presses in choice)/(lever presses in no choice) was significantly lower than 1 in the NAc GFP group for each RR (overall analysis: t=−9.85; p<0.01; one-sample analysis for each RR: all p’s<0.01) but not in the NAc D2R group (overall analysis: t=−1.14; p=0.27; one-sample analysis for each RR: all p’s>0.1). In contrast, in the CPu group (Fig. 4B) there was no virus effect (F(1,11)=2.81; p>0.12) and the ratios (lever presses in choice)/(lever presses in no choice) were significantly lower than 1 in both groups (overall analysis: CPu GFP: t=−4.65; p<0.01; CPuD2: t=−6.76; p<0.01) confirming that the animals decreased their rate of lever presses when chow was available in the cage similar to the NAc GFP group. Mice overexpressing D2R in the NAc (Fig. 4C) consumed significantly less free chow than controls at all three ratios (ANOVA: F(1, 12)=31.39, p<0.01; post-hoc: all p’s<0.01), demonstrating that the increase in willingness to work for the reward was accompanied by a reduction in consumption of freely available, less preferred food25. In contrast, D2R overexpression in the CPu group (Fig. 4D) had no effect on the amount of chow consumed in the choice phase (F(1, 11)=1.65; p=0.22). Altogether, these data demonstrate that D2R overexpression in the NAc enhances the willingness to work for the reward.
Figure 4. Animals that overexpress D2R in the NAc - but not the CPu – maintain their rate of lever presses in the choice procedure and eat less of the free chow.
(A) Ratio (lever presses in choice)/(lever presses in no-choice) in the NAc and CPu (B) groups. Amount of chow consumed in the choice paradigm in the NAc group (C) and CPu (D) groups. (* statistically significant).
D2R overexpression does not affect the reactivity to reward, the capacity to represent reward value or the ability of reward-associated cues to alter the motivation to respond
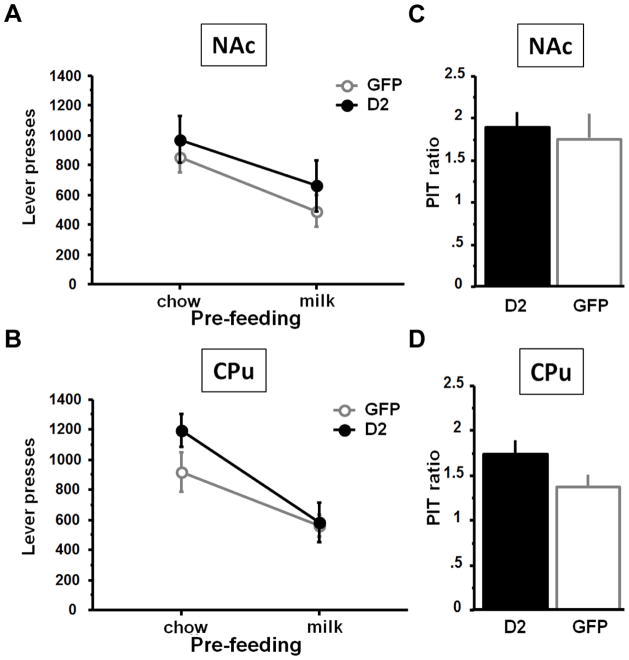
We tested whether the increase in lever pressing in the NAc D2R overexpressing mice in the reinforcement schedules with high work requirement was related to differences in the capacity to form and update representations of reward value with an outcome devaluation procedure. After subjects have learned that bar-pressing leads to a specific outcome (milk) they are allowed to satiate on the reward and then given the opportunity to make the response that previously led to that outcome22, 26, 27.
In the devaluation procedure, free consumption of the evaporated milk reward prior to testing reduced response rates in all groups significantly more than free consumption of an alternative food, indicating a significant degree of devaluation specific to the reinforcer obtained by bar pressing (Fig. 5A, B). ANOVA revealed a significant type of pre-feeding effect on lever-pressing in both NAc and CPu groups (both p’s<0.01) but no effect of D2R overexpression in either the NAc (F(1, 12)=0.67; p=0.43) or the CPu (F(1, 12)=1.42; p=0.26), indicating a similar sensitivity to the current value of outcomes. Post-hoc comparisons confirmed that all groups experienced outcome devaluation when pre-fed with evaporated milk compared to chow (all p values <0.03) independent of the overexpression (all p values >0.13). Similarly, ANOVA revealed a pre-feeding effect on the number of head entries in the food magazine (both p’s<0.01) but no effect of D2R overexpression in either NAc (F(1, 12)=0.32; p=0.58) or the CPu (F(1, 12)=0.92; p=0.36) (data not shown). There was a significant virus x pre-feeding effect (F(1, 12)=5.54; p=0.04) in the NAc group but unpaired comparisons for each pre-feeding did not show any difference (both p’s>0.18) confirming a similar sensitivity to the value of outcomes. There was also no difference in the amount of free evaporated milk consumed by each group during pre-feeding (Mean (in grams) ± SEM: NAc-GFP: 4.3 ± 0.2; NAc-D2R: 4.7 ± 0.2; CPu-GFP: 4.2 ± 0.4; CPu-D2R: 4.7 ± 0.2. t-tests: p’s>0.3), indicating that reactivity to the reward itself was not affected by D2R overexpression.
Figure 5. D2R overexpression does not affect representation of outcome or incentive value.
D2R overexpression in the NAc (A, C) or the CPu (B, D) had no effect on satiety devaluation (A, B) by pre-feeding with either chow or evaporated milk, or on Pavlovian-to-instrumental transfer (C, D).
The ability to learn about cues associated with reward and their capacity to enhance motivation was tested in the Pavlovian to instrumental Transfer (PIT) – a paradigm in which a neutral stimulus is paired with an unconditioned reward and its ability to modulate the motivation to make an instrumental response is tested22, 26, 27. Specifically, PIT was measured by first training the mice to associate a tone-conditioned stimulus (CS) with the occurrence of the milk reward. ANOVAs revealed that overall both NAc and CPu groups increased their rate of head-entries during the tone compared to the inter-trial interval (ITI) (NAc group: F(1, 12)=13.6; p<0.01, CPu group: F(1, 11)=14.6; p=0.01) and there were no differences based on GFP or D2R overexpression (virus x session effect: NAc group: F(1, 12)=0.04; p=0.84, CPu group: F(1, 11)=3.32; p=0.10) (suppl. Figure 2)). Thus, the overexpression had no impact on the capacity to learn a CS-US association. The level of PIT was tested in a session in which no primary reward was delivered, but the tone CS was presented intermittently. During this PIT transfer test mice from both NAc (PIT ratio: t=−3.43; p<0.01) and CPu (PIT ratio: t=−3.31; p<0.01) groups pressed significantly more during the tone CS than during the ITI (Fig. 5C, D). Moreover, the level of PIT transfer (increase in responding during the CS) did not differ between D2R overexpression and control groups in the NAc (t=−0.41; p=0.69) or the CPu (t=−1.92; p=0.09) (Fig. 5C, D) suggesting that the tone was equally effective at modulating motivation in both groups. Similarly, the number of head entries in the food magazine was significantly increased in both groups during the tone presentation compared to ITI (both F’s<13,60; both p’s<0.01) but there was no effect of D2R overexpression (both F’s<0.65; both p’s>0.44) (data not shown). The similar sensitivities of the mice to reward devaluation and PIT support the idea that the increased motivation in NAc D2R overexpressing animals is not related to an alteration in reactivity to the reward itself, the representation of reward value or the capacity of stimuli associated with reward to modulate motivation; but rather to a specific enhancement of willingness to work for the reward.
DISCUSSION
Our data show that postsynaptic D2R overexpression in the NAc - but not in the CPu – of adult mice increases motivation. This was shown in two ways: 1) higher levels of operant responding as work requirements increased; and 2) higher levels of responding for the earned reward in the presence of a less preferred but freely consumable reinforcer. The increased motivation was not due to a change in sensitivity to the value of the reinforcer, since the NAc D2R-overexpressing and control animals consumed similar amounts of the food reward when freely available and all groups were similarly sensitive to devaluation of the reward. D2R overexpression also had no effect on acquisition of Pavlovian goal tracking or enhancement of instrumental responding in the presence of an appetitive cue, indicating no change in the Pavlovian components of the instrumental response. Thus, augmenting postsynaptic expression of D2Rs in the NAc in adulthood selectively enhances the ability to sustain the instrumental response – or willingness to work - without changing the animal’s representation of the value of the reward stimulus per se. Rather, the effect specifically involves an alteration of the representation of how effortful the response is and/or the computation of the difference between cost and benefit.
Imaging studies in humans suggest that optimal goal-directed behaviors and motivation seem to correlate with higher D2R levels in the striatum7–9, 28. Similarly, high D2R availability in the striatum is associated with a resilience against the development of addiction29. Miscalculation of cost-benefit30, 31, 32 as well as decreased D2R availability in the striatum6, 33–35 are common feature among human pathologies that involve a dysregulation of motivation. Notably, increasing dopamine transmission at the D2R in the ventral striatum correlates with improvement in symptoms in ADHD6, and in cocaine abusers, the successful response to a motivation-based treatment is associated with enhanced dopamine transmission at the D2R in the ventral striatum36. Our current findings are consistent with these studies in implicating an important role for ventral striatal D2R signaling in motivation. This may be particularly true for disorders in which a loss of willingness to work is a prominent phenotype.
Our results are in agreement with previous studies showing a critical role for mesolimbic D2R-mediated dopamine transmission in the regulation of decisions based upon effort expenditure11. Salamone and colleagues have shown that dopamine signaling at the D2R has a powerful effect on an animal’s willingness to work for a reward, based on the work-related response costs and the value of the reinforcer itself11, 37–41. Indeed, intra-NAc infusions of low doses of D2R antagonists or DA depletion impair motivation and shift behavior away from food-reinforced tasks that have a high response requirement and toward low cost options with less reinforcement11. Conversely, amphetamine administration in humans42 or into the NAc in animals facilitates motivation43–45, as do manipulations that increase endogenous levels of extracellular dopamine. Indeed, Cagniard et al.46 showed that knock down of the dopamine transporter in mice, which results in elevated extracellular dopamine as a result of decreased clearance, enhanced the tendency to work for a food reward, without effects on Pavlovian and operant learning. Similarly, Bello et al.47 showed that mice lacking dopamine D2 autoreceptors and thus with elevated striatal dopamine synthesis and release due to a loss of feedback inhibition, display enhanced motivation to obtain a food reward. It is notable that manipulations that increase extracellular dopamine levels enhance motivation, but also lead to other behaviors such as impulsivity and/or hyperlocomotion that can interfere with learned appetitive behaviors43, 48, 49, whereas the mice in this study did not display generalized increases in unconditioned behavior (suppl. Figure 3). The present findings are consistent with the above studies showing that blocking D2R signaling impairs motivation and that increasing extracellular dopamine can enhance motivation, but the current study shows that potentiating postsynaptic DA signaling at D2R in the NAc selectively enhances the organism’s willingness to work for reward.
The present results highlight a potential dissociation between D2Rs in the NAc and CPu in modulating motivation. Recognition that the NAc is involved in goal-directed behavior has led to the hypothesis that mesoaccumbens dopamine signaling encodes information regarding the motivational significance of a stimulus50. Early work showed that neurotoxic lesions of the nucleus accumbens specifically impair an animal’s response to conditioned and unconditioned reinforcers, whereas lesions of the dorsal striatum affect behaviors such as response initiation and reaction time to acquire the reinforcers 45, 51–53. Thus, it has been suggested that instrumental responding for natural rewards is dependent on the NAc whereas the dorsal striatal pathways recruit stimulus-response processes54. Given evidence that the dorsal striatum plays a role in instrumental learning, with activity of the nigrostriatal DA pathway acting as a reinforcement signal3, 55, it is somewhat surprising that D2R-overexpression in the CPu did not have a significant effect on any of the tasks performed in the current study. Indeed, previous studies demonstrated that restoring DA signaling selectively in the dorsal striatum of DA-depleted mice is sufficient to restore reward-based learning56. Anatomical and functional studies suggest that the dorsal striatum is divided into a medial system that supports action-goal associations and a lateral system involved in stimulus-response association57. The lack of effect of D2R overexpression in the CPu in the current study could result from the concomitant alteration of both instrumental systems. However this seems unlikely since action of DA in either one of these subregions of the dorsal striatum seems to be able to support instrumental processes independently of dopamine signaling in the other area58. Further studies using overexpression of D2R in specific subregions of the CPu and operant schedules that discriminate between stimulus-response and action-goal associations will be required to understand the role of the D2R in the dorsal striatum for instrumental behavior.
Previous studies have shown that genetically-driven conditional overexpression of D2R in the striatum of mice results in a reversible decrease in motivation20, 21, 24. In those studies, the D2R was overexpressed in mice throughout their development and was associated with a reduction in the operant responding required to earn a reward, opposite to the current findings. However, in that transgenic mouse model the decrease in motivation likely reflects an effect of D2R overexpression across development, which results in compensatory alterations in the dopamine system59. Moreover, in that developmental mouse model, D2R overexpression is of a smaller magnitude and is not selective for ventral or dorsal striatum, but is restricted to medium spiny projection neurons throughout the striatum. In the viral model presented here, we specifically targeted the NAc or CPU and produced a large increase in D2R expression in all cells within these regions.
In the striatum, expression of D1R and D2R is largely segregated in neurons of the striatonigral and striatopallidal pathways, respectively60, although a subpopulation of MSNs in the NAc coexpress D1R and D2R and may represent a third neuronal pathway61. It is possible that NAc D2R overexpression in our study enhances the role of this atypical projection pathway. Additionally, cholinergic interneurons in the striatum express D2R62 and are strongly modulated by reward probability63. Thus, the effect on motivation could also be directly related to overexpression of D2Rs in cholinergic interneurons. Ultimately, further studies using targeted manipulation that allows the specific overexpression of D2Rs in discrete neuronal subpopulations in the NAc will be required to determine which neurons are involved in the modulation of motivation. While it is not yet known which neuronal population mediates this effect, our results provide an important proof of concept by showing that increasing postsynaptic D2R density in the NAc selectively enhances motivation by increasing the willingness to expend effort to obtain a goal without major changes in other reward-related processes.
This raises the prospect that therapies that enhance postsynaptic D2R signaling in the NAc could be successful in psychiatric disorders that involve dysregulation of motivation. Along this line, determination of the molecular mechanisms mediating the effect of ventral striatal postsynaptic DA D2Rs on motivation, from the level of gene regulation to specific D2R signaling pathways in neuronal subpopulations, can reveal novel pharmacological targets for motivation enhancement. While a challenging hurdle for pharmacological intervention, development of a functionally-selective agonist for postsynaptic D2Rs or specific signaling pathways coupled to these receptors is quite feasible64. Another approach that could selectively enhance post-synaptic D2R expression in vivo in the NAc is viral-mediated expression. Of note, adeno-associated viral gene delivery in preclinical studies, in both rodents and non-human primates, has led to promising results in the context of neurodegenerative diseases65. Our data support the idea that treatments that increase D2R in specific brain regions might allow the selective modulation of motivation.
Supplementary Material
Acknowledgments
We thank Claudia Schmauss for the generous gift of D2R KO mice and Christoph Kellendonk for discussion and comments on the manuscript. This work was supported in part by US National Institutes of Health grants DA022413, MH054137 (J.A.J.), MH068073 (PDB), MH068073 (PDB), MH086404 (J.A.J., H.M., E.H.S., P.D.B.), by The Sidney R. Baer, Jr. Foundation (H.M.), by a Research Associate Award from the Research Foundation for Mental Hygiene (P.T.), an EMBO Long-Term fellowship and the Basque Country Government (E.U.), National Institute of Mental Health grant F32MH090750-01 (RDW) and by the Lieber Center for Schizophrenia Research and Treatment. PT, JAJ, EHS, PDB, HM designed research. PT, BF, EU, VW performed research. PT, RDW, KMT, PDB, and HM analyzed data. PT, EHS, PDB, HM, DM and JAJ wrote the paper. All the authors participated in interpreting the data and edited the manuscript.
Footnotes
CONFLICT OF INTEREST: The authors declare no competing financial interest.
References
- 1.Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1(3):186–189. [PubMed] [Google Scholar]
- 2.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14(2–3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 4.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med. 2009;3(1):8–18. doi: 10.1097/ADM.0b013e31819a86f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16(11):1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci U S A. 2010;107(8):3870–3875. doi: 10.1073/pnas.0912319107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CL, Yang YK, Chu CL, Lee IH, Yeh TL, Chen PS, et al. The association between the Lie scale of the Maudsley personality inventory and striatal dopamine D2/D3 receptor availability of healthy Chinese community subjects. Eur Psychiatry. 2006;21(1):62–65. doi: 10.1016/j.eurpsy.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biol Psychol. 2008;77(1):98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191(3):461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 12.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 14.Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Matsumoto G, et al. Altered accumbens neural response to prediction of reward associated with place in dopamine D2 receptor knockout mice. Proc Natl Acad Sci U S A. 2002;99(13):8986–8991. doi: 10.1073/pnas.132284599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292(5526):2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 16.Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex. 2009;19(10):2240–2247. doi: 10.1093/cercor/bhn241. [DOI] [PubMed] [Google Scholar]
- 17.Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, et al. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques. 2011;51(2):111–118. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5(9):688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, et al. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91(3):911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- 20.Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, et al. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry. 2011;69(10):928–935. doi: 10.1016/j.biopsych.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, et al. Dissociation of Hedonic Reaction to Reward and Incentive Motivation in an Animal Model of the Negative Symptoms of Schizophrenia. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes NM, Marchand AR, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34(8):1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Pickel VM, Garzon M, Mengual E. Electron microscopic immunolabeling of transporters and receptors identifies transmitter-specific functional sites envisioned in Cajal’s neuron. Prog Brain Res. 2002;136:145–155. doi: 10.1016/s0079-6123(02)36014-x. [DOI] [PubMed] [Google Scholar]
- 24.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology. 2012;37(7):1699–1707. doi: 10.1038/npp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanich ML, Fowler H. Transfer from Pavlovian appetitive to instrumental appetitive conditioning: signaling versus discrepancy interpretations. J Exp Psychol Anim Behav Process. 1978;4(1):37–49. doi: 10.1037//0097-7403.4.1.37. [DOI] [PubMed] [Google Scholar]
- 27.Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Ann N Y Acad Sci. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- 28.Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci. 2010;30(42):14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46(2):79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 30.Sonuga-Barke EJ, Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol Psychiatry. 2012;72(2):126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Monterosso J, Piray P, Luo S. Neuroeconomics and the study of addiction. Biol Psychiatry. 2012;72(2):107–112. doi: 10.1016/j.biopsych.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Rowland NE, Vaughan CH, Mathes CM, Mitra A. Feeding behavior, obesity, and neuroeconomics. Physiol Behav. 2008;93(1–2):97–109. doi: 10.1016/j.physbeh.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaus S, Antke C, Beu M, Muller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Reviews in the neurosciences. 2010;21(2):119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168(6):634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, et al. The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology (Berl) 2009;204(1):103–112. doi: 10.1007/s00213-008-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69(3–4):373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- 39.Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Muller CE, et al. Adenosine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-based decision making in mice. Neuropharmacology. 2012;62(5–6):2068–2077. doi: 10.1016/j.neuropharm.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 40.Pereira M, Farrar AM, Hockemeyer J, Muller CE, Salamone JD, Morrell JI. Effect of the adenosine A2A receptor antagonist MSX-3 on motivational disruptions of maternal behavior induced by dopamine antagonism in the early postpartum rat. Psychopharmacology (Berl) 2011;213(1):69–79. doi: 10.1007/s00213-010-2015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology (Berl) 2008;196(4):565–574. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32(18):6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117(2):202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 44.Wirtshafter D, Stratford TR. Evidence for motivational effects elicited by activation of GABA-A or dopamine receptors in the nucleus accumbens shell. Pharmacol Biochem Behav. 2010;96(3):342–346. doi: 10.1016/j.pbb.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology (Berl) 1991;103(2):187–196. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- 46.Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31(7):1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- 47.Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14(8):1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin HH, Zhuang X, Balleine BW. Instrumental learning in hyperdopaminergic mice. Neurobiol Learn Mem. 2006;85(3):283–288. doi: 10.1016/j.nlm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23(28):9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30(5):188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Robbins TW, Roberts DC, Koob GF. Effects of d-amphetamine and apomorphine upon operant behavior and schedule-induced licking in rats with 6-hydroxydopamine-induced lesions of the nucleus accumbens. J Pharmacol Exp Ther. 1983;224(3):662–673. [PubMed] [Google Scholar]
- 52.Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30(1):77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 53.Amalric M, Koob GF. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog Brain Res. 1993;99:209–226. doi: 10.1016/s0079-6123(08)61348-5. [DOI] [PubMed] [Google Scholar]
- 54.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15(4–6):507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 56.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199(1):43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 58.Darvas M, Palmiter RD. Restricting dopaminergic signaling to either dorsolateral or medial striatum facilitates cognition. J Neurosci. 2010;30(3):1158–1165. doi: 10.1523/JNEUROSCI.4576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49(4):603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 60.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perreault ML, Hasbi A, O’Dowd BF, George SR. The dopamine d1–d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in Basal Ganglia. Front Neuroanat. 2011;5:31. doi: 10.3389/fnana.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alcantara AA, Chen V, Herring BE, Mendenhall JM, Berlanga ML. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003;986(1–2):22–29. doi: 10.1016/s0006-8993(03)03165-2. [DOI] [PubMed] [Google Scholar]
- 63.Apicella P, Ravel S, Deffains M, Legallet E. The role of striatal tonically active neurons in reward prediction error signaling during instrumental task performance. J Neurosci. 2011;31(4):1507–1515. doi: 10.1523/JNEUROSCI.4880-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, et al. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther. 2002;301(3):1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- 65.Morgenstern PF, Marongiu R, Musatov SA, Kaplitt MG. Adeno-associated viral gene delivery in neurodegenerative disease. Methods Mol Biol. 2011;793:443–455. doi: 10.1007/978-1-61779-328-8_29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.