Abstract
Objective
Tissue factor pathway inhibitor (TFPI) is produced in 2 isoforms: TFPIα, a soluble protein in plasma, platelets, and endothelial cells, and TFPIβ, a glycosylphosphatidylinositol-anchored protein on endothelium. Protein S (PS) functions as a cofactor for TFPIα, enhancing the inhibition of factor Xa. However, PS does not alter the inhibition of prothrombinase by TFPIα, and PS interactions with TFPIβ are undescribed. Thus, the physiological role and scope of the PS–TFPI system remain unclear.
Approach and Results
Here, the cofactor activity of PS toward platelet and endothelial TFPIα and endothelial TFPIβ was quantified. PS enhanced the inhibition of factor Xa by TFPIα from platelets and endothelial cells and stabilized the TFPIα/factor Xa inhibitory complex, delaying thrombin generation by prothrombinase. By contrast, PS did not enhance the inhibitory activity of TFPIβ or a membrane-anchored form of TFPI containing the PS-binding third Kunitz domain (K1K2K3) although PS did function as a cofactor for K1K2K3 enzymatically released from the cell surface.
Conclusions
The PS–TFPI anticoagulant system is limited to plasma TFPIα and TFPIα released from platelets and endothelial cells. PS likely functions to localize solution-phase TFPIα to the cell surface, where factor Xa is bound. PS does not alter the activity of membrane-associated TFPI. Because activated platelets release TFPIα and PS, the PS–TFPIα anticoagulant system may act physiologically to dampen thrombin generation at the platelet surface.
Keywords: blood coagulation, hemorrhage, thromboplastin, thrombosis
Protein S (PS)1 is a vitamin K–dependent anticoagulant protein that functions as a cofactor for activated protein C in the inactivation of factors Va (FVa) and VIIIa. Inherited deficiency of PS results in greatly increased risk for venous thrombosis.1 Aside from its role as a cofactor for activated protein C, PS also inhibits thrombin generation in an activated protein C–independent manner,2 an anticoagulant function attributed to either its enhancement of the inhibition of factor Xa (FXa) by tissue factor pathway inhibitor (TFPI)3,4 or direct inhibition of FXa and FVa, components of the thrombin-generating prothrombinase complex.5,6 The plasma concentration and anticoagulant activity of TFPI correlate with the plasma PS concentration, suggesting that these 2 proteins have a physiologically important interaction in vivo.7,8
TFPI is a reversible, slow, tight-binding inhibitor of FXa, which is produced in 2 isoforms: TFPIα and TFPIβ. TFPIα contains 3 Kunitz domains (K1, K2, and K3) and a highly basic C terminus,9 which interact for optimal inhibition of FXa. K1 is necessary for formation of the tight inhibitory complex; K2 directly binds the active site of FXa; and K3 and the C-terminal region enhance the initial binding.10–14 Circulating platelets store TFPIα and release it on activation.15,16 In addition, TFPIα circulates in plasma,17 but only ≈10% to 30% is fully active, full-length protein.14,18 Plasma TFPIα rapidly increases 2- to 4-fold after heparin infusion,19,20 suggesting that some TFPIα may be nonspecifically associated with glycosaminoglycans on the endothelium. However, studies of cultured endothelial cells suggest that the majority of endothelial TFPIα is stored within discrete intracellular granules and released after stimulation with heparin21,22 or thrombin.23 Surface-expressed TFPI on the endothelium seems to be primarily TFPIβ,24,25 in which the K3 domain and basic C terminus of TFPIα are replaced with a glycosylphosphatidylinositol (GPI)-anchor addition sequence, which localizes it to the cell surface.24–27
PS enhances the initial and the final (steady state) rates of inhibition of FXa by TFPIα.3,4,13 The interaction between PS and TFPIα is dependent on the presence of a membrane surface3,13 and K3,13,28 particularly residues Arg-19913 and Glu-226.28 The mechanism by which PS enhances the inhibitory activity of TFPI is not yet clear. The Gla domain of PS may localize TFPI to the membrane surface,29 thereby enhancing the interaction between TFPI and FXa, which also binds the membrane through a Gla domain. If so, one would predict that PS will serve as a cofactor only for soluble forms of TFPI. Alternatively, binding of PS may cause a conformational change in TFPI, which increases its affinity for FXa. If so, then PS may also serve as a cofactor for cell surface–expressed forms of TFPI, and membrane binding may promote the interaction between PS and TFPI.
It is currently unknown which pools of TFPI are involved in the PS–TFPI anticoagulant system. To date, all studies have used soluble TFPIα or plasma TFPI. However, the majority of intravascular TFPI is not circulating free in plasma30 and is instead either expressed on the surface of or released from the endothelium or released from platelets.15,16 We undertook studies to define the mechanism by which PS acts as a cofactor for TFPI and to define the physiological pools of TFPI involved in this anticoagulant system. The experimental results demonstrate that PS is a cofactor for TFPIα released from endothelial cells or platelets that acts by localizing TFPIα to a membrane surface. PS is not a cofactor for cell surface–associated forms of TFPI, regardless of the presence or absence of the K3 domain.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
PS Stabilizes the TFPIα–FXa Inhibitory Complex
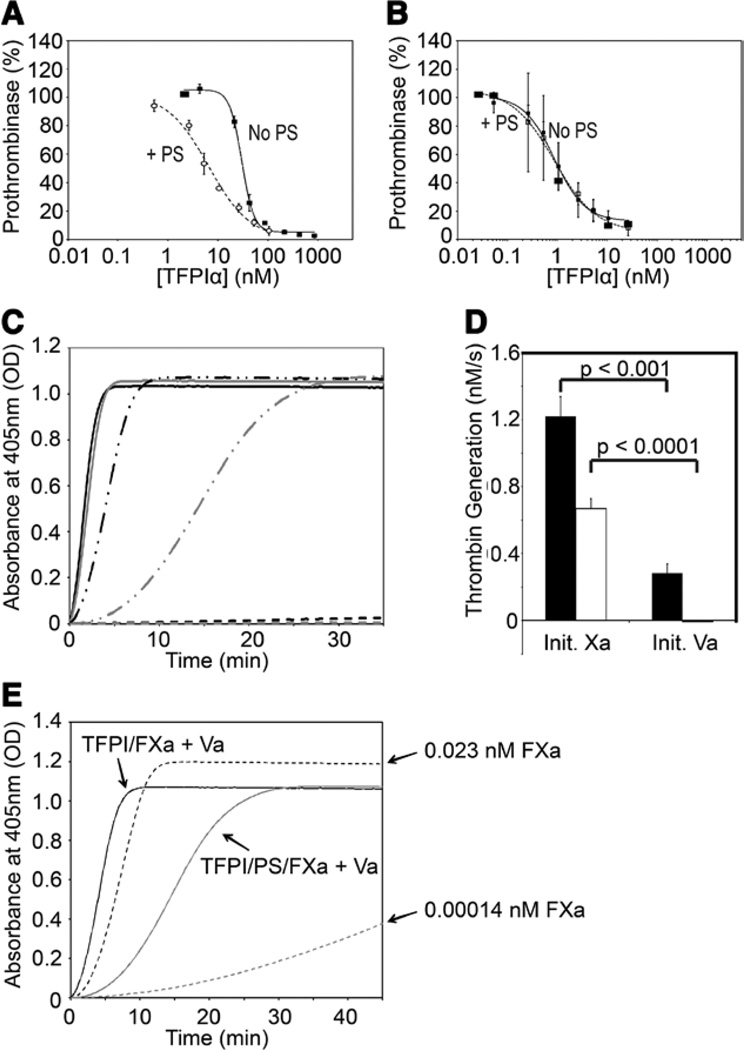
PS enhances both the initial and final rates of FXa inhibition by TFPIα but not TFPI-160, in a dose-dependent manner, as measured using a chromogenic FXa substrate, confirming previously published results3,4,13 (Figure I in the online-only Data Supplement). Physiologically, FXa acts in prothrombinase (FXa/FVa/Ca2+/phospholipids) to generate thrombin. TFPIα is an effective inhibitor of free FXa, but a poor inhibitor of thrombin generation by prothrombinase assembled with thrombin-activated FVa.31 Ndonwi et al13 reported that TFPIα remains a poor prothrombinase inhibitor in the presence of PS. We extended this observation by quantifying the TFPIα-mediated inhibition of prothrombinase in the presence or absence of 80 nmol/L PS (Figure 1A). PS enhanced the inhibitory activity of TFPIα (50% inhibition observed at 6.5 versus 31.4 nmol/L in the absence of PS), but the concentration necessary for 50% inhibition was still higher than the circulating concentration of TFPIα (<1 nmol/L), suggesting that this is not a physiological inhibitory function. In the absence of TFPIα, PS had little effect on prothrombinase activity, with ≈10% inhibition observed in the presence of 500 nmol/L PS (Figure II in the online-only Data Supplement).
Figure 1.
Protein S (PS) stabilizes the tissue factor pathway inhibitor-(TFPI)α/factor Xa (FXa) inhibitory complex. A and B, Reactions containing TFPIα (varying concentrations), thrombin-activated (A) or FXa-activated (B) factor Va (FVa; 0.5 nmol/L), the thrombin inhibitor dansylarginine-N-(3-ethyl-1,5-pentanediyl) amine (3 µmol/L), and phospholipid vesicles (20 µmol/L) were incubated either in the absence (●) or presence (○) of PS (80 nmol/L). Thrombin generation was initiated by the addition of prothrombin (1.4 µmol/L) and FXa (5 nmol/L). Initial rates of thrombin generation are shown as a percentage of the initial rate in the absence of TFPIα (mean±SEM; n=3). Experiments performed using FXa-activated FVa in the absence of PS were previously published32 and are reproduced in B. C, Reactions containing TFPIα (0.5 nmol/L), cephalin (1/500), FXa (0.1 nmol/L), FVa (5 nmol/L), prothrombin (1.4 µmol/L), and thrombin chromogenic substrate (0.5 mmol/L) were performed either in the presence (gray) or absence (black) of PS (80 nmol/L). Reactions were initiated either by the addition of FXa (solid lines) or FVa (dashed/dotted lines). In control experiments, FVa was omitted (dashed lines). The average of ≥3 progress curves is shown. D, Reactions were performed as in C, in the absence of TFPIα. E, Reactions were performed as in C, using the indicated FXa concentrations, in the absence of TFPIα or PS (dashed lines). Data from C (solid lines) are shown for reference.
We have demonstrated recently that TFPIα is an efficient inhibitor of prothrombinase assembled with FXa-activated FVa.32 Because this inhibition is dependent on both K2 and the C terminus, regions flanking the PS-binding K3 domain, we hypothesized that PS may enhance this function of TFPIα. However, the inhibition of prothrombinase containing FXa-activated FVa by TFPIα in the presence of 80 nmol/L PS was nearly identical to that reported in the absence of PS (IC50=0.9 nmol/L for each; Figure 1B),32 indicating that PS has no effect on the kinetics of this reaction.
These data suggest that PS does not have a physiologically relevant impact on prothrombinase inhibition by TFPIα. However, the TFPIα–PS system delays thrombin generation in plasma-based assays,3 an effect which may be explained if PS stabilizes the inhibitory TFPIα–FXa complex that forms before prothrombinase assembly. To test this hypothesis, assays were performed in 2 ways: (1) FVa and TFPIα were preincubated for 30 minutes with prothrombin, cephalin, and a thrombin chromogenic substrate, with or without PS, and reactions initiated by addition of FXa; and (2) FXa and TFPIα were preincubated for 30 minutes with prothrombin, cephalin, and substrate, with or without PS, and reactions initiated by addition of FVa (Figure 1C). This assay was absolutely dependent on prothrombinase assembly, as no significant thrombin generation was observed in the absence of FVa (Figure 1C), and inhibition was dependent on TFPIα as PS had minimal effect on thrombin generation in this system in the absence of TFPIα (Figure III in the online-only Data Supplement). When initiating with FXa, the inclusion of PS resulted in a slight delay in thrombin generation. When initiating with FVa, thrombin generation was substantially delayed, reflecting the need for TFPIα to dissociate from FXa before prothrombin may be cleaved. Inclusion of PS in these reactions further delayed thrombin generation, suggesting that PS further stabilizes the TFPIα–FXa inhibitory complex. The delays in prothrombinase function observed in Figure 1C were quantified by measuring initial rates of thrombin generation (Figure 1D). TFPIα reduced the initial rate of thrombin generation >4-fold in reactions initiated with FVa compared with reactions initiated with FXa (1.22±0.12 and 0.28±0.06 nmol/L per second, respectively). TFPIα in the presence of PS prevented any detectable thrombin generation during the first 2 minutes in reactions initiated with FVa, and also produced an ≈2-fold decrease in the initial rate of thrombin generation in reactions initiated with FXa (0.67±0.06 versus 1.22±0.12 nmol/L per second). The difference in thrombin generation observed when FXa was preincubated with TFPIα alone or TFPIα with PS (Figure 1C) may be because of a difference in the amount of FXa inhibited during this incubation step, a difference in the amount of FXa released from inhibition during the assay, or both. To assess this, the initial rates of thrombin generation (Figure 1D) were used to estimate the percentage of uninhibited FXa at time t=0, and reactions were performed in the presence of that amount of FXa (0.023 nmol/L to mimic TFPIα and 0.00014 nmol/L to mimic TFPIα–PS; Figure 1E). Although thrombin generation was dose dependently related to FXa concentration, as anticipated, thrombin generation in the presence of low FXa concentrations was substantially slower than in the presence of preinhibited FXa (particularly when comparing 0.00014 nmol/L FXa to 0.1 nmol/L FXa inhibited with TFPIα and PS). Thus, FXa must be released from inhibition during the assay to explain the thrombin generation kinetics observed.
PS Is a Cofactor for Platelet TFPIα
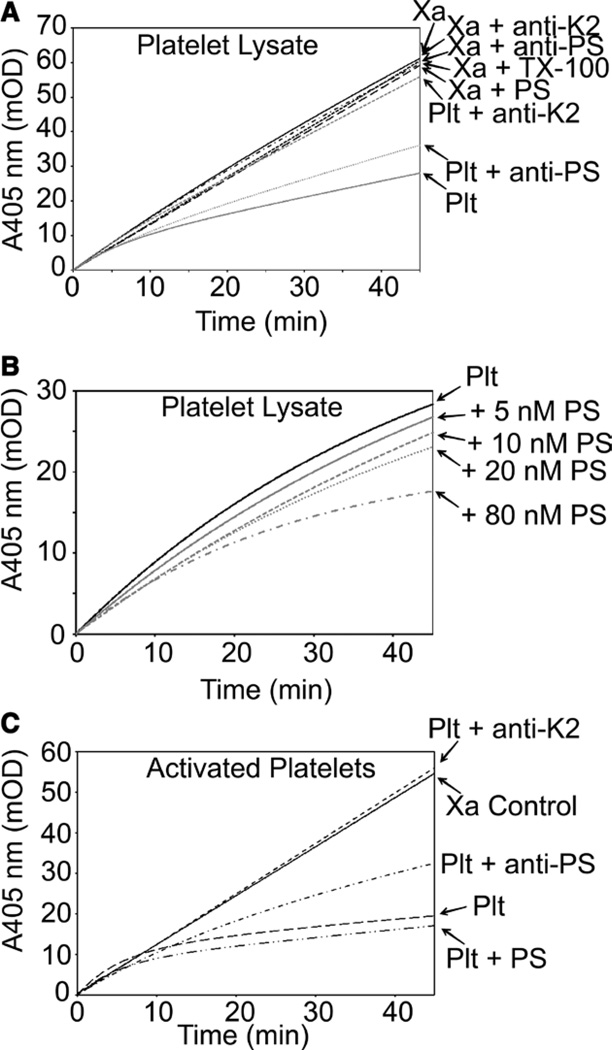
PS has been described previously as a cofactor for plasma TFPIα. However, much of intravascular TFPIα is within platelets and endothelial cells. Therefore, we undertook studies to determine the PS cofactor activity toward these pools of TFPIα. Platelets release TFPIα and PS on activation.15,16,33 The role of platelet PS as a cofactor for platelet TFPIα was assessed using lysed human platelets. The platelet lysate, containing a final concentration of 0.01% TX-100, inhibited FXa activity (Figure 2A). FXa activity was restored partially by addition of a polyclonal antibody against PS and almost completely restored by addition of a monoclonal antibody against the K2 domain of TFPI, demonstrating that the inhibitory activity is mediated by TFPIα and enhanced by PS (Figure 2A; Figure IV in the online-only Data Supplement). In the absence of platelet lysate, PS (80 nmol/L), the antibodies, and the TX-100 detergent had no effect on FXa activity (Figure 2A). The inhibition of FXa by platelet TFPIα was further enhanced by the addition of PS (5–80 nmol/L) to platelet lysate (Figure 2B), suggesting that platelet PS is a cofactor for platelet TFPIα under these conditions, but is not present at sufficient concentration to saturate the effect. This is perhaps expected, as a platelet lysate would contain an average of only 2.5 nmol/L PS under these conditions.33 The effect of PS was primarily on the initial rate of inhibition (Figure V in the online-only Data Supplement), but did not reach statistical significance because of large interindividual variability. The final inhibition of FXa was also enhanced by PS, but did not reach statistical significance because of large interindividual variability.
Figure 2.
Protein S (PS) is a cofactor for platelet tissue factor pathway inhibitor (TFPI)α. A and B, Washed human platelets were solubilized with TX-100 and assayed for factor Xa (FXa) inhibitory activity. A, Reactions performed in the presence and absence of antibodies against the TFPIα K2 domain and PS (100 nmol/L each). PS (80 nmol/L), the antibodies, and the TX-100 detergent had no effect on FXa activity in the absence of platelet lysate. B, Reactions performed with increasing concentrations of PS. C, Platelets were activated with calcium ionophore, and FXa inhibitory activity was measured in the presence of an antibody against the TFPIα K2 domain or against PS (100 nmol/L each) or in the presence of exogenous PS (80 nmol/L). For all panels, the average progress curves from 3 platelet donors are shown.
The inhibitory activity of platelet TFPIα and platelet PS may be different in the context of an intact activated platelet surface. Therefore, washed platelets were stimulated with calcium ionophore to induce activation and release of TFPIα and PS. These activated platelets exhibited FXa inhibitory activity, as expected, that was dependent on TFPIα and PS (Figure 2C). As in Figure 2A, addition of an antibody against PS resulted in partial restoration of FXa activity, whereas the anti-K2 antibody completely blocked the inhibitory activity. Further inhibition was observed with the addition of 80 nmol/L PS to the activated platelets although this enhancement was much less pronounced than that observed with platelet lysates.
PS Is a Cofactor for TFPIα Secreted From Cultured Endothelial Cells
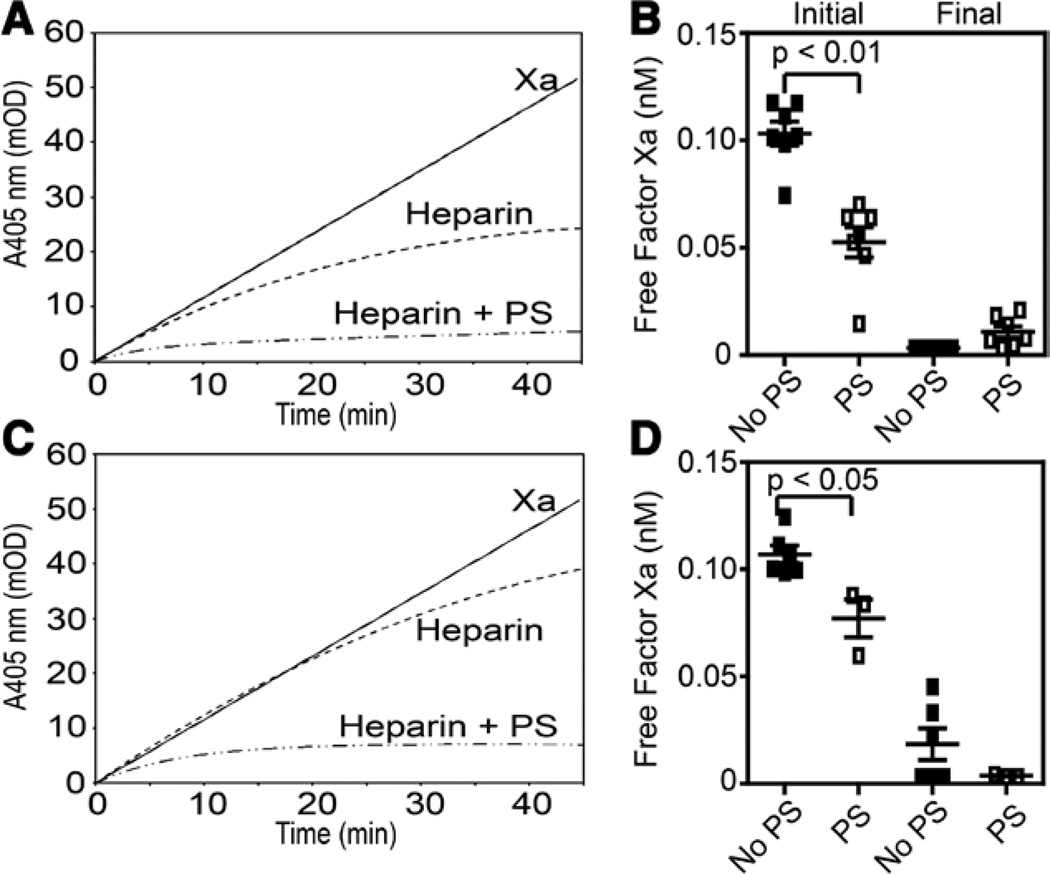
TFPIα is contained within cultured endothelial cells and released on treatment with heparin.20–22 The cofactor activity of PS toward endothelial TFPIα was examined by treating wild type and aerolysin-resistant EA.hy926 (AR-EA.hy926) cells with heparin, quantifying the amount of TFPIα released by ELISA (Table I in the only-only Data Supplement), and measuring FXa inhibition (Figure 3). The AR-EA.hy926 cells were studied because they do not express surface TFPI and, therefore, all of the heparin-releasable TFPI is TFPIα secreted from intracellular stores.34 Heparin increased the TFPIα secreted into the supernatants of EA.hy926 and AR-EA.hy926 cells 3.2- and 5.9-fold, respectively (Table I in the online-only Data Supplement). PS enhanced the initial rate of inhibition by the supernatants of both heparin-treated EA.hy926 cell lines (Figure 3). In control experiments, heparin did not alter PS cofactor activity when added to supernatants of buffer-treated cells (data not shown).
Figure 3.
Protein S (PS) enhances the inhibition of factor Xa (FXa) by tissue factor pathway inhibitor (TFPI)α released from EA.hy926 cells after heparin treatment. EA.hy926 (A and B) and AR-EA.hy926 (C and D) were treated with heparin for 1 hour, and the supernatants were tested in FXa amidolytic assays. A and C, The averages of ≥3 progress curves are shown for FXa alone (solid line), heparin treatment supernatant (dashed line), and heparin treatment supernatant with PS (80 nmol/L; dashed/dotted line). B and D, The amounts of initial and final free factor Xa were calculated from the individual progress curves and plotted (mean±SEM).
PS Is Not a Cofactor for TFPIβ
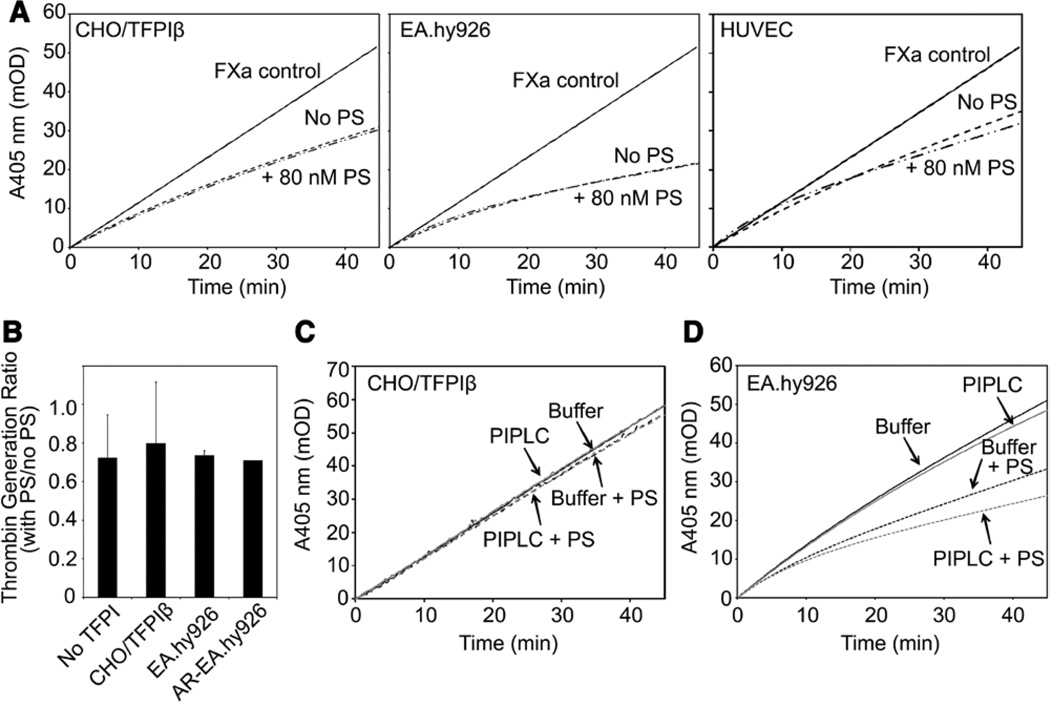
TFPIβ is the primary form of TFPI on the endothelial surface,24,25 but PS cofactor activity toward TFPIβ has not been studied previously. Although this activity would circumvent the interaction of PS with K3, it was hypothesized that PS may form a membrane-associated complex with TFPIβ and enhance its inhibitory activity. This hypothesis was tested using Chinese hamster ovary (CHO) cells expressing TFPIβ, EA.hy926 cells, and human umbilical vein endothelial cells. PS did not enhance the initial or final rates of inhibition of FXa by TFPI expressed on any cell line analyzed (Figure 4A). PS also did not alter the initial rate of thrombin generation by prothrombinase containing FVaIIa in assays performed using any of the cell lines tested (Figure 4B). For each cell line, an inhibitory polyclonal antibody against TFPI reversed the cellular inhibition of FXa, demonstrating that TFPI was responsible for the inhibitory activity observed. Further, CHO cells not expressing TFPIβ and AR-EA.hy926 cells did not inhibit FXa (data not shown). In addition, cells not expressing TFPI did not inhibit FXa in the presence of PS, suggesting that the cellular membrane conditions are sufficient to prevent the PS multimerization-induced FXa inhibition described by Seré et al.35
Figure 4.
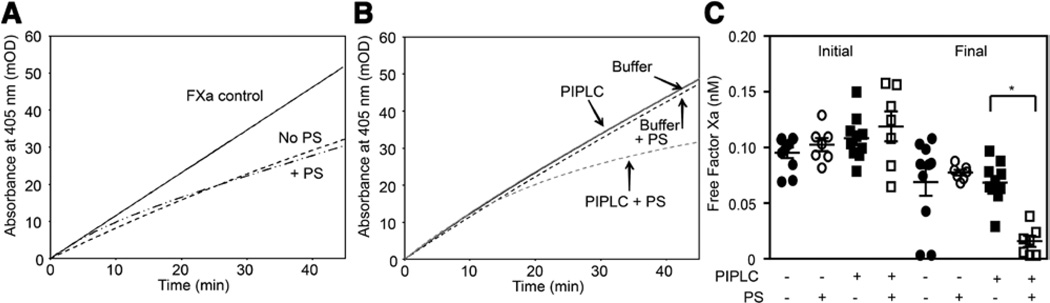
Protein S (PS) does not enhance the inhibition of factor Xa (FXa) by tissue factor pathway inhibitor (TFPI)β. A, FXa amidolytic assays were performed in the presence of Chinese hamster ovary (CHO) cells expressing TFPIβ (left), EA.hy926 cells (center), or human umbilical vein endothelial cells (HUVECs; right) in the presence or absence of PS (80 nmol/L). The averages of ≥4 progress curves are shown. B, Discontinuous thrombin generation assays were performed in the presence of CHO cells not expressing TFPI (No TFPI), CHO cells expressing TFPIβ, EA.hy926 cells, or AR-EA.hy926 cells, in the absence or presence of PS (80 nmol/L). Shown are the ratios of the thrombin generation rates in the presence and absence of PS (mean±SD; n=3). C and D, CHO/TFPIβ (C) or EA.hy926 (D) cells were treated with (gray) or without (black) phosphoinositol-specific phospholipase C (PIPLC; 1 U/mL) for 1 hour, and FXa amidolytic assays were performed in the presence of the supernatant and in the absence (lines) or presence (dashes) of PS (80 nmol/L). The averages of 6 progress curves are shown.
Because PS did not enhance the inhibitory activity of cell surface–associated TFPI, additional experiments were performed to determine whether cell-associated TFPI can interact with PS after release from the cell surface. Phosphoinositol-specific phospholipase C (PIPLC) was used to release TFPI from the surface of CHO/TFPIβ, EA.hy926, and AR-EA.hy926 cells. TFPIβ, released from PIPLC-treated CHO/TFPIβ cells, was not enhanced by PS (Figure 4C). PIPLC treatment produced a 3.1-fold increase in the amount of total TFPI released from EA.hy926 cells, compared with buffer-only treatment (Table I in the online-only Data Supplement). In contrast, only a small amount of TFPIα was released (1.2-fold over mock treatment), suggesting that the PIPLC-releasable TFPI on the EA.hy926 cells is TFPIβ. PIPLC did not release TFPI from the AR-EA.hy926 cells, consistent with the lack of TFPI on the surface of these cells.34 In the presence of 80 nmol/L PS, the supernatants of PIPLC-treated EA.hy926 cells showed slight, statistically significant increases in both the initial and final (steady state) inhibition of FXa, compared with buffer treatment samples (P<0.001; Figure 4D). PS cofactor activity was reversed by the addition of an antibody against the PS-binding K3 domain of TFPIα (Figure VI in the online-only Data Supplement), demonstrating that the observed cofactor activity is because of TFPIα secreted during the incubation period, and not PIPLC-released TFPIβ.
PS Is Not a Cofactor for Cell Surface–Associated TFPI Containing the K3 Domain
Because TFPIβ lacks the K3 domain, the absence of PS cofactor activity may be related to its inability to bind TFPIβ. PS cofactor activity toward cell surface–associated TFPI containing K3 was assessed using CHO cells expressing a GPI-anchored form of TFPI containing all 3 Kunitz domains (K1K2K3).36 PS did not enhance the initial or final rate of FXa inhibition by these cells (Figure 5A). To verify that PS is capable of interacting with this form of TFPI, the cells were treated with PIPLC, and the FXa inhibitory activity of the supernatants was assessed (Figure 5B and 5C). The released protein was a relatively poor FXa inhibitor in the absence of PS, as it lacks the TFPIα C terminus.12,13 However, the inhibition was enhanced by PS although this enhancement was less pronounced than with full-length TFPIα (Figure 5B and 5C), consistent with the data of Ndonwi et al.13 Thus, PS is a cofactor for K1K2K3 in solution, but is not a cofactor for K1K2K3 bound to the cell surface. Unlike with TFPIα, PS only enhanced the final rate of FXa inhibition by soluble K1K2K3. The reason for this is unclear although it may relate to the low K1K2K3 concentration in the supernatant samples.
Figure 5.
Protein S (PS) is a cofactor for soluble, but not membrane-anchored, K1K2K3. A, Factor Xa (FXa) amidolytic assays were performed in the presence of Chinese hamster ovary (CHO) cells expressing glycosylphosphatidylinositol (GPI)-anchored K1K2K3 and the absence or presence of 80 nmol/L PS. The averages of ≥7 progress curves are shown. B and C, CHO cells expressing GPI-anchored K1K2K3 were treated with (gray) or without (black) phosphoinositol-specific phospholipase C (PIPLC; 1 U/mL) for 1 hour, and FXa amidolytic assays were performed in the presence of the supernatant and in the absence (lines) or presence (dashes) of PS (80 nmol/L). The averages of ≥7 progress curves (B) and the calculated initial and final free FXa levels (C; mean±SEM) are shown. *P<0.001.
Discussion
The ability of PS to enhance the inhibition of FXa by plasma TFPI and by recombinant TFPIα produced in Escherichia coli was originally demonstrated by Hackeng et al.3 Subsequent studies have investigated the interaction of PS and TFPIα in plasma, finding that patients with PS deficiency have decreased plasma TFPIα, that immunodepletion of PS depletes plasma TFPIα as well, and that plasma TFPIα correlates with free PS, rather than with C4bp-bound PS.8 Thus, it seems that there is a physiologically relevant PS–TFPI anticoagulant system functioning in vivo, yet much remains unclear. The majority of intravascular TFPI is TFPIβ on the endothelium, which does not have the PS-binding K3 domain.24,25 In addition, the repercussions of FXa inhibition by PS–TFPIα are not apparent, as TFPIα is a poor inhibitor of thrombin production by the prothrombinase complex assembled with FXa and thrombin-activated FVa, and PS does not enhance this inhibitory function to physiologically relevant rates (Figure 1D).13,31
The current study was designed to further characterize the human PS–TFPI anticoagulant system by quantifying PS cofactor activity directed toward physiological pools of TFPI that have not been examined previously. Our results demonstrate that PS enhances the inhibition of FXa by solution-phase TFPIα, including platelet TFPIα and TFPIα released from cultured endothelial cells. In contrast, PS has no effect on the inhibition of FXa by surface-associated TFPIβ on transfected CHO cells or on cultured endothelial cells; or by solution-phase TFPIβ released from the cell surface by PIPLC. These findings suggest that PS exerts its cofactor activity by localizing TFPIα to the cell surface, where it can readily interact with membrane-associated FXa, and that cell surface TFPI is not affected by PS. This notion is supported by experiments using an altered form of TFPI expressed in CHO cells that contains the 3 Kunitz domains attached to the cell surface via a GPI-anchor. Inhibition of FXa by this form of TFPI was unaffected by PS when localized to the cell surface but was enhanced by PS after removal from the cell surface with PIPLC. However, these experimental results do not absolutely rule out a PS-induced conformational change in TFPIα that may contribute to the enhanced inhibitory activity because PS may not have been able to bind K3 when the GPI-anchored protein was bound to the cell surface. Regardless, the data presented in Figure 4 demonstrate that PS has no significant cofactor activity toward forms of TFPI endogenously expressed on the surface of endothelial cells.
Recent data from our laboratory have demonstrated that TFPIα is a potent inhibitor of prothrombinase assembled with FXa-activated FVa.32 This inhibition requires (1) an interaction between the K2 domain and the FXa active site and (2) an interaction between the basic TFPIα C terminus and an acidic region within the FV B-domain, which is retained after activation by FXa but removed after activation by thrombin. As such, this TFPIα inhibitory activity is relevant only during the initiation phase of thrombin generation. We hypothesized that PS would enhance this inhibitory activity. However, PS had no effect (Figure 1B) on the ability of TFPIα to inhibit prothrombinase assembled with FXa-activated FVa.
In contrast to the results with purified prothrombinase, the TFPIα–PS anticoagulant system inhibits thrombin generation in plasma-based assays.3 We assessed the impact of PS on the preformed TFPIα–FXa inhibitory complex in an effort to explain this observation, finding that PS stabilizes the inhibitory complex. This PS activity was observed in thrombin generation experiments in which the TFPIα–FXa or PS–TFPIα–FXa complexes were allowed to form before the addition of thrombin-activated FVa. A significant delay in thrombin generation occurred in reactions containing the PS–TFPIα–FXa complex compared with those lacking PS. If the inhibitory complex was not formed before initiation, then TFPIα was a poor inhibitor of prothrombinase, even in the presence of PS as previously described.13,31 These results suggest that the PS–TFPIα anticoagulant system may regulate prothrombinase function, inhibiting FXa and sequestering it from FVa or prothrombin. As TFPIα effectively inhibits cleavage of chromogenic substrates by prothrombinase,37 the more likely explanation is that dissociation of TFPIα from FXa allows prothrombinase to bind prothrombin.
Murine plasma TFPI contains only K1 and K2,25,38 and, consequently, PS does not serve as its cofactor.39 Because mice do not experience a hypercoagulable state, the key components of the PS–TFPIα anticoagulant system may be mediated through interactions of PS with TFPIα released from activated platelets, which secrete TFPIα15,16 and PS.33,40 In the studies presented here, using human platelets, platelet PS was a cofactor for platelet TFPIα inhibitory activity present either in platelet lysates or ionophore-activated platelets. Platelet PS was not saturating in the platelet lysates but was near saturating at the surface of activated platelets, suggesting that it may be released at sufficient localized concentration to saturate TFPIα within a platelet plug in vivo. The in vivo significance of the interaction among platelet PS, platelet TFPIα, and FXa will depend on the conditions required for PS and TFPIα release. If they are both released early in the hemostatic response, then they may be regulating initial prothrombinase. If they are only released after stimulation with thrombin, then they likely do not regulate initial prothrombinase but may serve a role in either spatially containing or downregulating the coagulant response after formation of the fibrin clot. In contrast to the work of Stavenuiter et al,41 we did not observe direct inhibition of FXa by platelet PS in the absence of TFPIα in our assay systems. The reason for these contrasting data is not apparent but may result from the different antibody reagents used in the 2 studies.
Our laboratory has demonstrated that hematopoietic cell TFPI, most likely from platelets, dampens thrombus growth in a murine model of large vessel vascular injury and directly modulates bleeding in a murine hemophilia model.42,43 In addition, it has been demonstrated that degradation of platelet TFPIα by neutrophil proteases increases platelet procoagulant activity both in vitro and in vivo.44,45 Although these actions of platelet TFPIα may result from inhibition of circulating forms of TF, platelet PS and platelet TFPIα may also assemble as a physiologically relevant anticoagulant complex on the surface of activated platelets where they act to prevent development of occlusive intravascular thrombi by slowing thrombin generation through the mechanism described above. It is important to note that the TFPIα–PS system is most effective at inhibiting prothrombinase when the inhibitory complex is allowed to form before introduction of FVa and prothrombin. If all components are present, then the IC50 for inhibition (6.5 nmol/L) suggests an unfavorable interaction. However, many factors may alter these kinetics, including (1) the potential for differential release of TFPIα, PS, and FVa from platelet storage granules, (2) the high local concentrations of these proteins within a platelet plug, and (3) the presence of Zn2+ bound to plasma PS, which is necessary for its direct inhibition of FXa.6
Platelets are thought to be the primary site of prothrombinase assembly and thrombin generation; however, other cell types may also support this function, including monocytes, lymphocytes, neutrophils, erythrocytes, and endothelial cells.2,46–48 Endothelial cells also release TFPIα from intracellular stores in response to agonists such as heparin and thrombin.20–23 Data presented here support this, demonstrating the release of TFPIα into the supernatants of cultured AR-EA. hy926 cells, which do not express surface TFPI. Thus, it is tempting to speculate that plasma PS may interact with plasma TFPIα or TFPIα released from activated endothelium to regulate thrombin production on vascular cells other than platelets.
Collectively, these data demonstrate that PS exerts anticoagulant cofactor activity with TFPIα from any physiological pool, likely by localizing TFPIα to membrane surfaces, stabilizing its interaction with membrane-bound FXa, and slowing thrombin generation. However, PS has no effect on TFPIβ or other forms of membrane-associated TFPI. We hypothesize that a physiological function of the PS–TFPIα anticoagulant system is to slow the generation of thrombin, on platelets and other vascular cells, by inhibiting FXa before FVa and prothrombin become available.
Supplementary Material
Significance.
Protein S (PS) is a cofactor for factor Xa inhibition by tissue factor pathway inhibitor (TFPI). Previous studies with PS have assessed its effect as a cofactor for TFPIα, using plasma or purified recombinant protein although the majority of vascular TFPI is TFPIβ. Here, we demonstrate that PS is a cofactor for TFPIα released from platelets or endothelial cells, and likely acts by localizing soluble TFPIα to a membrane surface, thereby stabilizing its interaction with membrane-bound factor Xa, sequestering it from prothrombin, and slowing thrombin generation. By contrast, PS has no cofactor activity toward TFPIβ. These data define the physiological scope of the TFPI/PS anticoagulant system and for the first time demonstrate that this system can slow prothrombinase assembly.
Acknowledgments
We thank C.E. Bonesho for technical assistance.
Sources of Funding
This work was supported by grants HL068835 (to A.E. Mast) and HL096419 and HL117702 (to S.A. Maroney). J.P. Wood was supported by training grant HL007209 (to G.C. White), from the National Heart, Lung, and Blood Institute. A.E. Mast received grant support from Novo Nordisk. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard Abbreviations and Acronyms
- CHO
Chinese hamster ovary
- FVa
factor Va
- FXa
factor Xa
- GPI
glycosylphosphatidylinositol
- PIPLC
phosphoinositol-specific phospholipase C
- PS
protein S
- TFPI
tissue factor pathway inhibitor
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.113.302655/-/DC1.
Disclosures
None.
References
- 1.Castoldi E, Hackeng TM. Regulation of coagulation by protein S. Curr Opin Hematol. 2008;15:529–536. doi: 10.1097/MOH.0b013e328309ec97. [DOI] [PubMed] [Google Scholar]
- 2.Hackeng TM, van ‘t Veer C, Meijers JC, Bouma BN. Human protein S inhibits prothrombinase complex activity on endothelial cells and platelets via direct interactions with factors Va and Xa. J Biol Chem. 1994;269:21051–21058. [PubMed] [Google Scholar]
- 3.Hackeng TM, Seré KM, Tans G, Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc Natl Acad Sci USA. 2006;103:3106–3111. doi: 10.1073/pnas.0504240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndonwi M, Broze G., Jr Protein S enhances the tissue factor pathway inhibitor inhibition of factor Xa but not its inhibition of factor VIIa-tissue factor. J Thromb Haemost. 2008;6:1044–1046. doi: 10.1111/j.1538-7836.2008.02980.x. [DOI] [PubMed] [Google Scholar]
- 5.Heeb MJ, Mesters RM, Tans G, Rosing J, Griffin JH. Binding of protein S to factor Va associated with inhibition of prothrombinase that is independent of activated protein C. J Biol Chem. 1993;268:2872–2877. [PubMed] [Google Scholar]
- 6.Heeb MJ, Prashun D, Griffin JH, Bouma BN. Plasma protein S contains zinc essential for efficient activated protein C-independent anticoagulant activity and binding to factor Xa, but not for efficient binding to tissue factor pathway inhibitor. FASEB J. 2009;23:2244–2253. doi: 10.1096/fj.08-123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castoldi E, Maurissen LF, Tormene D, Spiezia L, Gavasso S, Radu C, Hackeng TM, Rosing J, Simioni P. Similar hypercoagulable state and thrombosis risk in type I and type III protein S-deficient individuals from mixed type I/III families. Haematologica. 2010;95:1563–1571. doi: 10.3324/haematol.2010.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castoldi E, Simioni P, Tormene D, Rosing J, Hackeng TM. Hereditary and acquired protein S deficiencies are associated with low TFPI levels in plasma. J Thromb Haemost. 2010;8:294–300. doi: 10.1111/j.1538-7836.2009.03712.x. [DOI] [PubMed] [Google Scholar]
- 9.Wun TC, Kretzmer KK, Girard TJ, Miletich JP, Broze GJ., Jr Cloning and characterization of a cDNA coding for the lipoprotein-associated coagulation inhibitor shows that it consists of three tandem Kunitz-type inhibitory domains. J Biol Chem. 1988;263:6001–6004. [PubMed] [Google Scholar]
- 10.Cunningham AC, Hasty KA, Enghild JJ, Mast AE. Structural and functional characterization of tissue factor pathway inhibitor following degradation by matrix metalloproteinase-8. Biochem J. 2002;367(Pt 2):451–458. doi: 10.1042/BJ20020696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi DA, Wun TC, Likert KM, Broze GJ., Jr The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood. 1992;79:1712–1719. [PubMed] [Google Scholar]
- 12.Lockett JM, Mast AE. Contribution of regions distal to glycine-160 to the anticoagulant activity of tissue factor pathway inhibitor. Biochemistry. 2002;41:4989–4997. doi: 10.1021/bi016058n. [DOI] [PubMed] [Google Scholar]
- 13.Ndonwi M, Tuley EA, Broze GJ., Jr The Kunitz-3 domain of TFPI-alpha is required for protein S-dependent enhancement of factor Xa inhibition. Blood. 2010;116:1344–1351. doi: 10.1182/blood-2009-10-246686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesselschmidt R, Likert K, Girard T, Wun TC, Broze GJ., Jr Tissue factor pathway inhibitor: the carboxy-terminus is required for optimal inhibition of factor Xa. Blood. 1992;79:2004–2010. [PubMed] [Google Scholar]
- 15.Maroney SA, Haberichter SL, Friese P, Collins ML, Ferrel JP, Dale GL, Mast AE. Active tissue factor pathway inhibitor is expressed on the surface of coated platelets. Blood. 2007;109:1931–1937. doi: 10.1182/blood-2006-07-037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novotny WF, Girard TJ, Miletich JP, Broze GJ., Jr Platelets secrete a coagulation inhibitor functionally and antigenically similar to the lipoprotein associated coagulation inhibitor. Blood. 1988;72:2020–2025. [PubMed] [Google Scholar]
- 17.Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ., Jr Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–393. [PubMed] [Google Scholar]
- 18.Broze GJ, Jr, Lange GW, Duffin KL, MacPhail L. Heterogeneity of plasma tissue factor pathway inhibitor. Blood Coagul Fibrinolysis. 1994;5:551–559. [PubMed] [Google Scholar]
- 19.Novotny WF, Palmier M, Wun TC, Broze GJ, Jr, Miletich JP. Purification and properties of heparin-releasable lipoprotein-associated coagulation inhibitor. Blood. 1991;78:394–400. [PubMed] [Google Scholar]
- 20.Sandset PM, Abildgaard U, Larsen ML. Heparin induces release of extrinsic coagulation pathway inhibitor (EPI) Thromb Res. 1988;50:803–813. doi: 10.1016/0049-3848(88)90340-4. [DOI] [PubMed] [Google Scholar]
- 21.Hansen JB, Svensson B, Olsen R, Ezban M, Osterud B, Paulssen RH. Heparin induces synthesis and secretion of tissue factor pathway inhibitor from endothelial cells in vitro. Thromb Haemost. 2000;83:937–943. [PubMed] [Google Scholar]
- 22.Lupu C, Poulsen E, Roquefeuil S, Westmuckett AD, Kakkar VV, Lupu F. Cellular effects of heparin on the production and release of tissue factor pathway inhibitor in human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1999;19:2251–2262. doi: 10.1161/01.atv.19.9.2251. [DOI] [PubMed] [Google Scholar]
- 23.Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF. Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Arterioscler Thromb Vasc Biol. 1995;15:2055–2062. doi: 10.1161/01.atv.15.11.2055. [DOI] [PubMed] [Google Scholar]
- 24.Girard TJ, Tuley E, Broze GJ., Jr TFPIβ is the GPI-anchored TFPI isoform on human endothelial cells and placental microsomes. Blood. 2012;119:1256–1262. doi: 10.1182/blood-2011-10-388512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maroney SA, Ferrel JP, Pan S, White TA, Simari RD, McVey JH, Mast AE. Temporal expression of alternatively spliced forms of tissue factor pathway inhibitor in mice. J Thromb Haemost. 2009;7:1106–1113. doi: 10.1111/j.1538-7836.2009.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang JY, Monroe DM, Oliver JA, Roberts HR. TFPIbeta, a second product from the mouse tissue factor pathway inhibitor (TFPI) gene. Thromb Haemost. 1999;81:45–49. [PubMed] [Google Scholar]
- 27.Zhang J, Piro O, Lu L, Broze GJ., Jr Glycosyl phosphatidylinositol anchor- age of tissue factor pathway inhibitor. Circulation. 2003;108:623–627. doi: 10.1161/01.CIR.0000078642.45127.7B. [DOI] [PubMed] [Google Scholar]
- 28.Ahnström J, Andersson HM, Hockey V, Meng Y, McKinnon TA, Hamuro T, Crawley JT, Lane DA. Identification of functionally important residues in TFPI Kunitz domain 3 required for the enhancement of its activity by protein S. Blood. 2012;120:5059–5062. doi: 10.1182/blood-2012-05-432005. [DOI] [PubMed] [Google Scholar]
- 29.Dahlbäck B, Hildebrand B, Malm J. Characterization of functionally important domains in human vitamin K-dependent protein S using monoclonal antibodies. J Biol Chem. 1990;265:8127–8135. [PubMed] [Google Scholar]
- 30.Werling RW, Zacharski LR, Kisiel W, Bajaj SP, Memoli VA, Rousseau SM. Distribution of tissue factor pathway inhibitor in normal and malignant human tissues. Thromb Haemost. 1993;69:366–369. [PubMed] [Google Scholar]
- 31.Mast AE, Broze GJ., Jr Physiological concentrations of tissue factor pathway inhibitor do not inhibit prothrombinase. Blood. 1996;87:1845–1850. [PubMed] [Google Scholar]
- 32.Wood JP, Bunce MW, Maroney SA, Tracy PB, Camire RM, Mast AE. Tissue factor pathway inhibitor-alpha inhibits prothrombinase during the initiation of blood coagulation. Proc Natl Acad Sci USA. 2013;110:17838–17843. doi: 10.1073/pnas.1310444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz HP, Heeb MJ, Wencel-Drake JD, Griffin JH. Identification and quantitation of protein S in human platelets. Blood. 1985;66:1452–1455. [PubMed] [Google Scholar]
- 34.Maroney SA, Cunningham AC, Ferrel J, Hu R, Haberichter S, Mansbach CM, Brodsky RA, Dietzen DJ, Mast AE. A GPI-anchored co-receptor for tissue factor pathway inhibitor controls its intracellular trafficking and cell surface expression. J Thromb Haemost. 2006;4:1114–1124. doi: 10.1111/j.1538-7836.2006.01873.x. [DOI] [PubMed] [Google Scholar]
- 35.Seré KM, Willems GM, Rosing J, Hackeng TM. Protein S multimers are generated in vitro and affect protein S structure-function analyses. Semin Hematol. 2006;43(1 Suppl 1):S111–S120. doi: 10.1053/j.seminhematol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Maroney SA, Ellery PE, Wood JP, Ferrel JP, Bonesho CE, Mast AE. Caveolae optimize tissue factor-Factor VIIa inhibitory activity of cell-surface-associated tissue factor pathway inhibitor. Biochem J. 2012;443:259–266. doi: 10.1042/BJ20111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang ZF, Wun TC, Broze GJ., Jr Kinetics of factor Xa inhibition by tissue factor pathway inhibitor. J Biol Chem. 1993;268:26950–26955. [PubMed] [Google Scholar]
- 38.Maroney SA, Ferrel JP, Collins ML, Mast AE. Tissue factor pathway inhibitor-gamma is an active alternatively spliced form of tissue factor pathway inhibitor present in mice but not in humans. J Thromb Haemost. 2008;6:1344–1351. doi: 10.1111/j.1538-7836.2008.03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saller F, Brisset AC, Tchaikovski SN, Azevedo M, Chrast R, Fernández JA, Schapira M, Hackeng TM, Griffin JH, Angelillo-Scherrer A. Generation and phenotypic analysis of protein S-deficient mice. Blood. 2009;114:2307–2314. doi: 10.1182/blood-2009-03-209031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh PN. Platelet coagulant activities and hemostasis: a hypothesis. Blood. 1974;43:597–605. [PubMed] [Google Scholar]
- 41.Stavenuiter F, Davis NF, Duan E, Gale AJ, Heeb MJ. Platelet protein S directly inhibits procoagulant activity on platelets and microparticles. Thromb Haemost. 2013;109:229–237. doi: 10.1160/TH12-08-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Mast AE. Murine hematopoietic cell tissue factor pathway inhibitor limits thrombus growth. Arterioscler Thromb Vasc Biol. 2011;31:821–826. doi: 10.1161/ATVBAHA.110.220293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maroney SA, Cooley BC, Ferrel JP, Bonesho CE, Nielsen LV, Johansen PB, Hermit MB, Petersen LC, Mast AE. Absence of hematopoietic tissue factor pathway inhibitor mitigates bleeding in mice with hemophilia. Proc Natl Acad Sci USA. 2012;109:3927–3931. doi: 10.1073/pnas.1119858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 45.Zillmann A, Luther T, Müller I, Kotzsch M, Spannagl M, Kauke T, Oelschlägel U, Zahler S, Engelmann B. Platelet-associated tissue factor contributes to the collagen-triggered activation of blood coagulation. Biochem Biophys Res Commun. 2001;281:603–609. doi: 10.1006/bbrc.2001.4399. [DOI] [PubMed] [Google Scholar]
- 46.Tracy PB, Eide LL, Mann KG. Human prothrombinase complex assembly and function on isolated peripheral blood cell populations. J Biol Chem. 1985;260:2119–2124. [PubMed] [Google Scholar]
- 47.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–3845. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hackeng TM, Tans G, Koppelman SJ, de Groot PG, Rosing J, Bouma BN. Protein C activation on endothelial cells by prothrombin activation products generated in situ: meizothrombin is a better protein C activator than alpha-thrombin. Biochem J. 1996;319(Pt 2):399–405. doi: 10.1042/bj3190399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.