Abstract
Hepatocellular carcinoma (HCC) is one of the most common highly aggressive malignant tumors worldwide. AKR1B10 was first isolated from HCC and further identified to be over-expressed in many cancers from various organs. AKR1B10 contributes to detoxification of xenobiotics by lipid peroxidation and metabolizes physiological substrates such as farnesal, retinal and carbonyls. Metabolizing these lipid substrates plays a crucial role in promoting carcinogenesis. In the present study, immunohistochemical analysis was performed to determine the prevalence/pattern of AKR1B10 expression in HCC, and its usefulness to differentiate benign liver lesions from HCC. Oncogenic function of AKR1B10 was examined in hepatocellular carcinoma cells in vitro using western blotting and shRNA knockdown approaches, with emphasis on cell apoptosis and response to chemotherapy. Immunohistochemistry analysis revealed AKR1B10 was over-expressed in 97% (86/89) of hepatocellular carcinomas, with minimal to no expression in adjacent hepatic tissue, while hepatic adenomas and focal nodular hyperplasia did not exhibit expression of AKR1B10. shRNA-mediated silencing of AKR1B10 expression in hepatocellular carcinoma cells resulted in 1) increased cell apoptosis, 2) decreased colony formation and size, and 3) enhanced cytoreductive response following exposure to doxorubicin chemotherapy. Our findings provide first time evidence that AKR1B10 is a unique biomarker involved in hepatocellular carcinogenesis via modulation of proliferation, cell apoptosis and chemoresistance, and is a potential promising biomarker to differentiate HCCs from benign hepatic lesions.
Keywords: Hepatocellular carcinoma, hepatic adenoma, focal nodular hyperplasia, AKR1B10
Introduction
Hepatocellular carcinoma (HCC) is the most common malignant primary tumor in the liver and the 2nd leading cause of cancer deaths worldwide 1. HCC represents the fastest growing cause of cancer mortality 2–3 with the incidence increasing due to viral hepatitis B and C, obesity and the synergystic effects of alcohol 4–5. The median survival is less than 2 years 2 and surgical resection is only potentially curative. Due to tumor burden or liver dysfunction, additional therapeutic modalities such as transarterial chemoembolization (TACE), radiofrequency ablation (RFA) 6–8 or transplantation 9–10 have been employed. Tremendous opportunity to improve patient outcomes exists and can be achieved by enhancing screening, detection, treatment approaches and drug development.
Proteomic and genomic studies of HCC have identified aldoketoreductase 1B10 (AKR1B10) as a possible clinical biomarker 11–13. Cao et al. isolated AKR1B10 (ARL1, aldose reductase-like 1) in 1998 as a gene upregulated during hepatocarcinogenesis 14, which has subsequently been identified in non-small cell lung, esophageal, uterine and pancreatic carcinomas 15–18. Aldoketoreductases (AKRs) are a gene superfamily involved in elimination reactions and have an (α/β)8-barrel structural motif which contains a cofactor binding site, catalytic domain and loops at the back of the structure that dictate substrate specificity. These enzymes can utilize sugar and lipid aldehydes, steroid hormones, prostaglandins and xenobiotics as substrates; their broad substrate specificity parallels the cytochrome P450 superfamily.
AKR1B10 has restricted specificity with activity to the carbonyls, farnesal and geranylgeranial, and retinal as its substrates 19–20. AKR1B10 may promote carcinogenesis through conversion of highly reactive aldehyde and ketone groups into hydroxyl groups. Studies have shown AKR1B10 contributes to the detoxification of xenobiotics by lipid peroxidation, including the chemotherapy drugs doxorubicin and mitomycin 21–25. The expression of AKR1B10 in neoplastic cells protects against carbonyl-induced apoptosis and resistance to several anti-cancer drugs. The carbonyl groups have been shown to be metabolized by AKR1B10 and converted to their corresponding alcohols, rendering cells resistant to these agents 21–25. AKR1B10 may also promote carcinogenesis by the conversion of retinal to retinol resulting in suppression of the final conversion of retinal to retinoic acid - the major active anti-neoplastic metabolite 26. Finally, the metabolization of farnesyl and geranylgeranyl by AKR1B10 appears to be an important process for protein prenylation, which is involved in activating several key proteins including the RAS oncogene 27
Recent proteomic, transcriptional and immunohistochemical studies have examined HCC in relation to tumor differentiation, proliferation, and staging 12,13,28,29. However, there have been no studies examining the utility of this enzyme to differentiate benign from malignant tumors of the liver. In the present study, we set out to determine the prevalence and pattern of AKR1B10 protein expression in HCC along with its usefulness to differentiate benign mass-forming from malignant liver lesions. To investigate the oncogenic role of AKR1B10 in HCC, we further analyzed the effect of AKR1B10 dynamic silencing using an shRNA knockdown approach on apoptosis, proliferation and chemosensitivity.
Materials and Methods
Clinical Material
Formalin-fixed, paraffin-embedded blocks from 89 patients with HCC between 2005 and 2011 were retrieved from the surgical pathology archives of Northwestern Memorial Hospital following IRB approval. Patients with hepatic adenoma (HA, n=24) and focal nodular hyperplasia (FNH, n=9) were included. Hematoxylin and eosin stained slides and AKR1B10 immunohistochemical stains were reviewed by 2 pathologists (G-YY and KAM) to confirm the tumor classification and grade. Clinical notes, pathology reports and slides were reviewed to establish the etiology of HCC.
Immunohistochemistry
Sections (5 µm) were deparaffinized, rehydrated in alcohols, and subjected to heat-induced epitope retrieval in 0.1 M citrate buffer at pH 6.0 in a microwave for 20 minutes. Slides were then incubated with a primary mouse monoclonal antibody specific for AKR1B10 (Abnova; dilution of 1:300) for 1 hour at room temperature. After incubation with rabbit anti-mouse secondary antibody, a subsequent reaction was performed with biotin-free horseradish peroxidase enzyme-labeled polymer of ImmPRESS Polymer Detection Kit (Vector Laboratories, Burlingame, CA). 3,3'-diaminobenzidine was used as the chromogen (Sigma-Aldrich, St. Louis, MO) and the sections were counterstained with hematoxylin. Immunoreactivity was semi-quantitatively evaluated as negative (0; 0–5% of cells stained), minimal positive (1+; 5% to 10% of cells stained), positive (2+; 10% to 50% of cells stained), or diffusely positive (3+; >50% of cells stained). Staining intensity was graded from 0 to 3+ with a mean intensity calculated. Adjacent benign hepatic tissue was present in sections from HCC and was also assessed for percent of cells stained. Colonic tissue was used as a positive control.
shRNA AKR1B10 Transfection
Small-hairpin RNA inhibition was performed using GIPZ Lentiviral shRNA targeting human AKR1B10 from Open Biosystems (Waltham, MA). Lentivirus expression of AKR1B10 shRNA particles was produced in HEK293T cells. For stable transfection, 8×104 Hu7 human hepatocellular carcinoma cells were plated per well in a 6-well plate with 2 ml of medium without antibiotics. Following overnight incubation, the media was removed and replaced with 1 ml of medium containing lentiviral produced particles in 10 μg/ml polybrene. Twenty-four hours later, the media was removed and 2ml of fresh media added to each well. Forty-eight hours later, media was removed and media containing 2mg/ml puromycin was added to each well and replaced every 3 to 4 days for colony selection. Single colonies were obtained after 2 weeks of puromycin selection.
Protein Extraction and Immunoblot Analysis
Cultured cells were washed with 1X DPBS buffer, scraped with sterile cell lifters and pelleted at 1,000 rpm for 5 min. Cells were then lysed on ice by vortexing every 5 min for 30 min using RIPA buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.004% sodium azide, 10 mg/ml leupeptin, 10mg/ml aprotinin, 1% phosphatase inhibitor cocktail 1 and 2 (Sigma, St. Louis, MO), 2% protease inhibitor cocktail and 1% PMSF (Sigma, St. Louis, MO). The extracts were collected tion at 10,000 rpm for 3 min at 4°C, followed by transfer of the supernatant to a new tube. All protein concentrations were determined using Bradford reagent (Bio-Rad).
35µg of protein was loaded on a 10% SDS-polyacrylamide gel and transferred to a Immun-Blot PVDF Membrane (BioRad, Hercules, CA), blocked using 1X TBST containing 0.05% Tween-20 and 5% non-fat powdered milk, followed by membrane incubation with polyclonal rabbit anti-human AKR1B10 primary antibody (1:300; LifeSpan BioSciences, Inc., Seattle, WA) solution overnight at 4°C. The membrane was then washed with 1X TBST and incubated with HRP-linked anti-rabbit (1:2000) IgG and HRP-linked anti-biotin antibodies (1:1000; Cell Signaling Technology, Inc., Danvers, MA) for 1 hour at room temperature. The protein-antibody complex was detected using the 1X LumiGLO chemiluminescent substrate (Cell Signaling Technology) according to the manufacturer's instructions and the emitted light captured on X-ray film. Mouse anti-human β-actin (1:5000: Sigma-Aldrich) used with HRP-linked anti-mouse (1:2000) IgG, served as an internal loading control.
Cell Apoptosis Assay
Apoptosis was evaluated by flow cytometry using cellular Annexin V and 7-Aminoactinomycin D (7-AAD) binding (BD Pharmingen, San Jose, CA). In brief, cells were stained with Annexin V and 7-AAD according to the manufacturer’s instructions and then analyzed using a BD Accuri 6 flow cytometer (BD BioSciences, San Jose, CA). Cells in early apoptosis are Annexin V positive and 7-AAD negative, while cells in late apoptosis are both Annexin V and 7-AAD positive. Annexin V and 7-AAD double-negative cells were considered viable.
Carbonyl- and Chemotherapy-induced Apoptosis
Hu7 cells were treated with either 50µM of the carboxyl free radical trans-4-hydroxy-2-hexenal (HHE), or 3µM of the chemotherapeutic agent doxorubicin for 24 hours followed by cell apoptosis assays as described above. HHE is a major alpha, beta-unsaturated aldehyde product of omega-3 polyunsaturated fatty acid oxidation and is an active biochemical mediator resulting from lipid peroxidation. HHE in these experiments serves as a positive control whereby its aldehyde group with a conjugated double bond and a hydroxyl next to the unsaturation will form an adduct with AKR1B10 in order to induce cytotoxicity and apoptosis.
Statistical Analysis
The Biostatistics Core facility of The Robert H. Lurie Comprehensive Cancer Center provided the statistical support and analyses for these studies. For continuous measures, analysis of variance (ANOVA) methods have been used. If the data exhibited an extreme deviation from normality, then log-transformed data was used to obtain p-values. Comparison between patients with hepatocellular carcinoma and patients with hepatic adenoma or focal nodular hyperplasia was performed using Fisher's exact test. A p-value less than 0.05 are considered statistically significant.
Results
Patient Characteristics
For the 89 patients with HCC, the age of patients ranged from 43 to 74 years (mean=61.16 years), with a strong male predominance (73.04% male vs 26.96% female). The majority of patients had a history Hepatitis C viral infection (53.9%), while other etiologies included Hepatitis B (7.9%), hemochromatosis (1.1%), alcohol (19.1%), primary sclerosing cholangitis (2.2%), primary biliary cirrhosis (2.2%) among several others. Additional patient characteristics are summarized in Table 1.
Table 1.
Clinical Parameters of Patient Groups.
| Hepatocellular Carcinoma (n=89) |
Hepatic Adenoma (n=24) |
Focal Nodular Hyperplasia (n=9) |
|
|---|---|---|---|
| Age, mean (range) y | 61.16 (43-79) | 42.42 (27-74) | 36.22 (23-53) |
| Male, n (%) | 65 (73.04) | 11 (45.8) | 0 (0) |
| Female, n (%) | 24 (26.96) | 13 (54.2) | 9 (100) |
| Etiology, n | |||
| Hepatitis C | 48 | - | - |
| Hepatitis B | 7 | - | - |
| Alcohol | 17 | - | - |
| NASH | 8 | - | - |
| Autoimmune | 1 | - | - |
| PBC | 2 | - | - |
| PSC | 2 | - | - |
| Hemochromatosis | 1 | - | - |
| Cryptogenic | 9 | - | - |
| HIV | 2 | - | - |
| 2 or more from above | 9 | - | - |
Expression of AKR1B10 in HCC and adjacent liver parenchyma
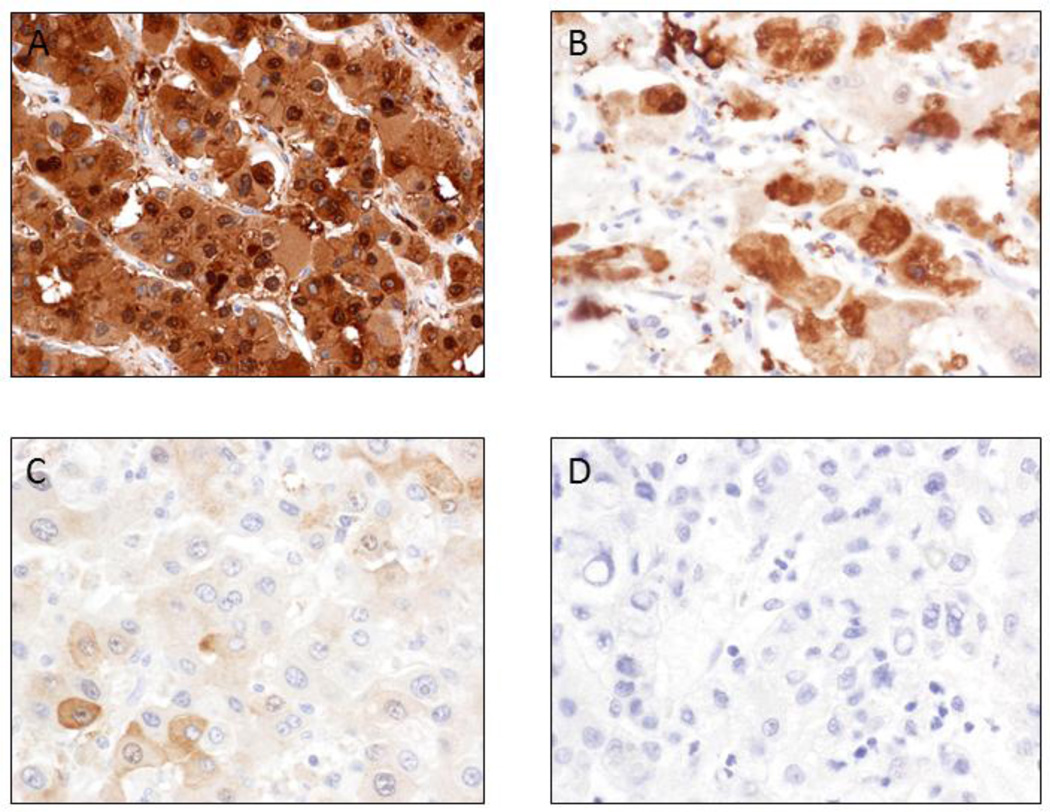
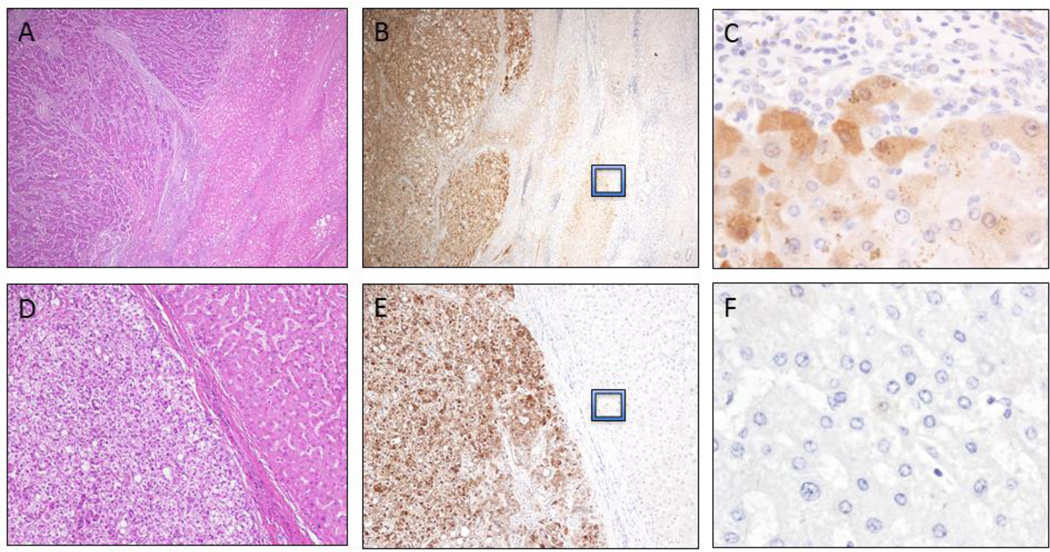
Strong and diffuse cytoplasmic and focal nuclear AKR1B10 staining was identified in HCC cells (Figure 1) with 97% (86/89) of HCC exhibiting AKR1B10 positive staining. Semi-quantitative analysis of AKR1B10 staining intensity was performed based on the established criteria (as set out in Figure 1) and showed that 75 cases (84.2%) had strong and diffuse positivity (including 69 cases of HCC with 3+ staining, 6 cases with 2+ staining); while 11 cases displayed focal positivity (1+ staining, 12.4%), and 3 cases exhibited no staining (0, 3.4%), as summarized in Tables 2 and 3. Of the tumors exhibiting strong (3+) staining intensity (Figure 1A), 30 (33.7%) were well-differentiated, 35 (39.3%) were moderately-differentiated and 4 (4.5%) were poorly-differentiated. Three (3.3%) well-differentiated, 2 (2.2%) moderately-differentiated and 1 (1.1%) poorly-differentiated tumor displayed moderate staining intensity (2+, as seen Figure 1B). Of the focal positive-staining tumors (1+, as seen in Figure 1C), 6 (6.7%) were well-differentiated, 4 (4.5%) were moderately-differentiated and 1 (1.1%) was poorly-differentiated. One (1.1%) well-differentiated and 2 (2.2%) moderately-differentiated tumors had no or less than 5% of cells staining for AKR1B10 (as seen in Figure 1D). No statistically significant association with grade (p = 0.72) or stage (p = 0.69) of HCC was observed and the data are shown in Table 3. Furthermore, in 69 HCC arising in the background of cirrhosis, there was no association identified between AKR1B10 expression and the etiology of cirrhosis. In cirrhotic livers with HCC (Figure 2A-2C), the hepatocytes at the interface between the HCC and adjacent uninvolved liver parenchyma exhibited faint AKR1B10 expression (Figure 2B and 2C), but liver parenchyma away from HCC showed no expression (Figure 2B). In 62 of the 69 cases with cirrhosis (89.8%), the nodules immediately adjacent to the carcinoma exhibited scattered hepatocytes at the periphery with weak cytoplasmic staining. In non-cirrhotic HCC cases (n=20), the adjacent hepatic parenchyma had no AKR1B10 expression (Figure 2E-F).
Figure 1.
Semi-quantitative analysis of AKR1B10 staining intensity in hepatocellular carcinoma. Staining intensity for each slide was graded from 0 to 3+ and a mean intensity was calculated. Immunoreactivity was semi-quantitatively evaluated as strong and diffuse cytoplasmic and nuclear positivity (3+) with >50% of cells staining for AKR1B10 (A), positive (2+) with 10%–50% of cells staining (B), minimally positive (1+) with 5%–10% of cells staining (C) and negative (0) with <5% of cells staining for AKR1B10 (D).
Table 2.
Expression of AKR1B10 Protein in Liver Lesions.
| Percentage of cell staining |
Hepatocellular carcinoma |
Hepatic adenoma | Focal nodular hyperplasia |
|---|---|---|---|
| 3+ (>50%) | 69/89(77.5%) | 0/24 | 0/9 |
| 2+ (11-50% | 6/89 (6.7% | 0/24 | 0/9 |
| 1+ (5-10%) | 11/89 (12.4%) | 1/24 (4.5%) | 0/9 |
| 0 (0-5%) | 3/89 (3.4%) | 23/24 (95.5%) | 9/9 (100%) |
| Intensity (mean) | 2.58 | 0.05 | 0 |
The vast majority of hepatocellular carcinomas exhibited a high level of protein expression, while negligible expression was seen in benign lesions such as hepatic adenomas and focal nodular hyperplasia.
Table 3.
Breakdown of AKR1B10 Expression in Liver Lesions 476 and Based on Tumor Grade and 477 Stage.
| AKR1B10 Expression | |||||
|---|---|---|---|---|---|
| 0 n (%) |
1 n (%) |
2 n (%) |
3 n (%) |
Total | |
| Lesion | |||||
| HA | 23 (65.71) | 1 (8.33) | 0 (0) | 0 (0) | 24 |
| FNH | 9 (25.71) | 0 (0) | 0 (0) | 0 (0) | 9 |
| HCC | 3 (8.57) | 11 (91.67) | 6 (100) | 69 (100) | 89 |
| Total | 35 | 12 | 6 | 69 | 122 |
| Fisher’s exact p-value <0.0001 | |||||
| HCC Grade | |||||
| 1 | 1 (33.33) | 6 (54.55) | 3 (50) | (30 (43.48) | 40 |
| 2 | 2 (66.67) | 4 (36.36) | 2 (33.33) | 35 (50.72) | 43 |
| 3 | 0 (0) | 1 (9.09) | 1 (16.67) | 4 (5.80) | 6 |
| Total | 3 | 11 | 6 | 69 | 89 |
| Fisher’s exact p-value = 0.72 | |||||
| HCC Stage | |||||
| pT1 | 3 (100) | 7 (63.64) | 3 (50) | 30 (43.48) | 43 |
| pT2 | 0 (0) | 4 (36.36) | 3 (50) | 34 (49.28) | 41 |
| pT3 | 0 (0) | 0 (0) | 0 (0) | 3 (4.35) | 3 |
| pT4 | 0 (0) | 0 (0) | 0 (0) | 2 (2.90) | 2 |
| Total | 3 | 11 | 6 | 69 | 89 |
| Fisher’s exact p-value = 0.69 | |||||
Figure 2.
AKR1B10 expression in hepatocellular carcinomas. Hepatocellular carcinoma (HCC) arising in a cirrhotic liver (A; H&E stain, 100X). Strong and diffuse AKR1B10 protein expression in HCC with faint cytoplasmic staining observed in the proliferating cells at the periphery of regenerative nodules (B, 100X and C, 600X). HCC in a non-cirrhotic liver (D; H&E stain, 200X) highlights strong and diffuse cytoplasmic and nuclear staining of AKR1B10 (E, 200X) and negligible AKR1B10 expression in the adjacent hepatic parenchyma (F, 600X).
Expression of AKR1B10 in benign liver lesions
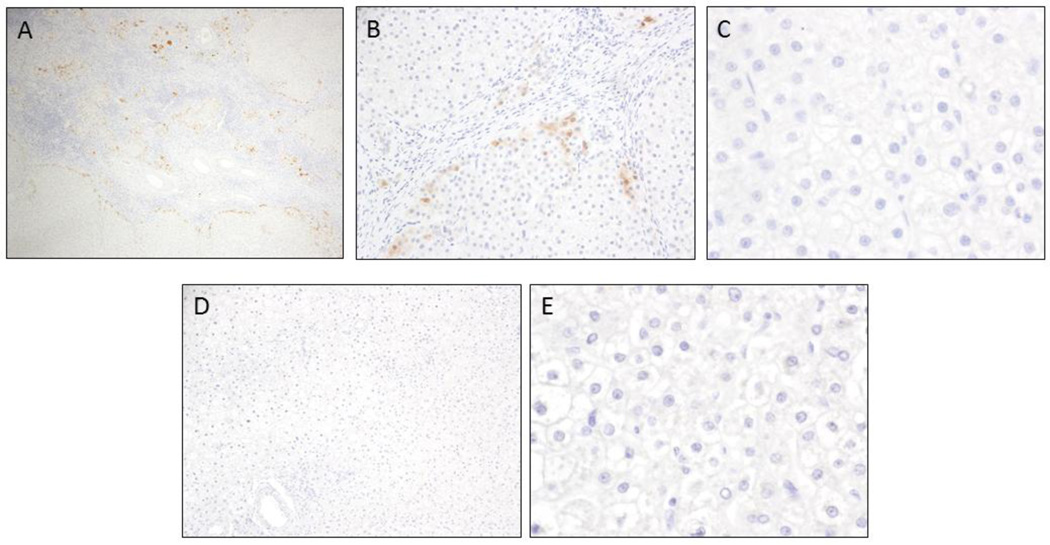
To determine if AKR1B10 is a unique biomarker to differentiated HCC from benign liver lesions, AKR1B10 immunostaining was performed on focal nodular hyperplasia (FNH) and hepatic adenoma (HA). For FNH (n=9), there was focal scattered AKR1B10 positivity in hepatocytes located near the central scar (proliferating cells) (Figure 3A-B), but the remainder of the hepatic parenchyma in all 9 (100%) cases was negative (Figure 3C). For HA (n=24, including 21 hepatocellular adenoma and 3 inflammatory hepatocellular adenoma), only one HA shows 1+ staining and all remaining 23 cases shows no AKR1B10 expression (Figure 3D-E). The difference of AKR1B10 expression between HCC and HA or FNH was statistically significant (p<0.0001), as summarized in Table 3.
Figure 3.
AKR1B10 expression in benign mass-forming liver lesions. Faint cytoplasmic staining of AKR1B10 can be observed in the proliferating cells adjacent to the central scar in focal nodular hyperplasia (A, 100X; B, 400X; C, 600X). Negligible AKR1B10 expression was identified in hepatic adenomas (D, 200X; E, 600X).
Small-hairpin RNA (shRNA)-mediated AKR1B10 silencing in human Hu7 hepatocellular carcinoma cells
To further demonstrate the biologic role of AKR1B10 in HCC, silencing of AKR1B10 dynamic expression in the human Hu7 hepatocellular carcinoma cell line was achieved using a shRNA approach. Two separate clones (labeled as #1 and #2, respectively) of Hu7 cells transfected with AKR1B10-shRNA showed a significant down-regulation of AKR1B10 expression when compared to vector controls as detected by western blot (Figure 4, Panel A). Cell proliferation and tumorigenesis were further determined based on the extent of colony formation and size and revealed a significant decrease in the overall number of colonies which was parallel with the levels of down-regulated expression of AKR1B10 in two separate clones (Figure 4, Panel B and C). The number of cells comprising each colony in Hu7 cells with AKR1B10 silencing was markedly reduced when compared to the vector control and correlated with the extent of AKR1B10 inhibition (Figure 4B). Thus, the involvement of AKR1B10 in the proliferative potential is evidenced by significant suppression in Hu7 cells with dynamic silencing of AKR1B10.
Figure 4.
Silencing of AKR1B10 dynamic expression using a shRNA approach. Western blot demonstrates markedly decreased expression of AKR1B10 in 2 separate Hu7 knockdown clones (#1 and #2, respectively) (A) with concomitant decrease in colony size and formation that parallels AKR1B10 expression (B and C; *p<0.05).
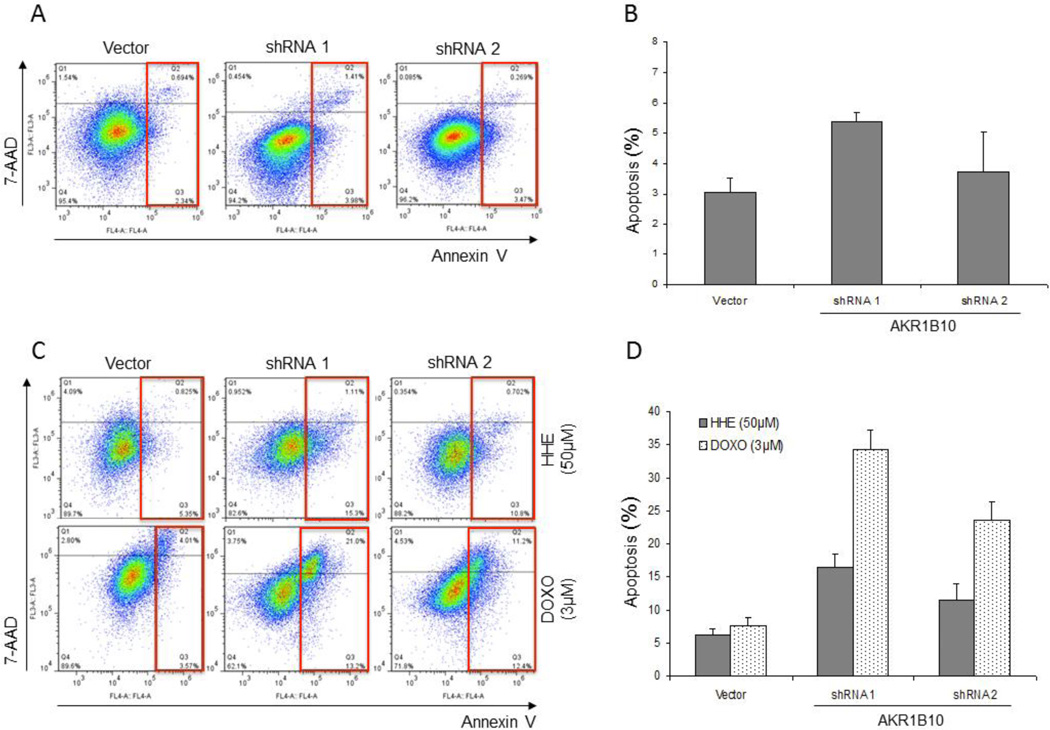
Silencing of AKR1B10 dynamic expression induces apoptotic cell death and increases chemosensitivity in hepatocellular carcinoma cell line
Since active carbonyls are one of specific substrates of AKR1B10, over-expression may represent a possible mechanism of cell resistance to the carbonyl-induced cell death and apoptosis. Flow cytometry for Annexin V and 7-Aminoactinomycin D (7-AAD) revealed that silencing of AKR1B10 expression resulted in increased apoptosis as seen by Annexin V positivity in the scatter plots (red boxes) (Figure 5A). Vector control Hu7 cells exhibited 3.03±0.5% apoptosis, in contrast to 5.39±0.3% and 3.74±1.3% percent in the shRNA clones #1 and #2, respectively; with clone #1 Hu7 cells reaching statistical significance (p<0.05, Figure 5B).
Figure 5.
Effect of shRNA-mediated silencing of AKR1B10 expression on apoptosis and chemosensitivity based on flow cytometry. Inhibition of AKR1B10 expression results in increased cell apoptosis based on Annexin 5 expression. Compared to vector, Hu7 cells silenced using shRNA to AKR1B10 had increased levels of apoptosis (A; red squares), which was a function of AKR1B10 expression levels. Hu7 cells treated with vector control exhibited 3.03±0.5% apoptosis, in contrast to 5.39±0.3% and 3.74±1.3% percent in the shRNA clones #1 and #2, respectively (B). Dynamically silenced clones were treated with 50μM HHE as control or with the chemotherapeutic agent doxorubicin (3μM) for 24 hrs and analyzed for apoptosis by flow cytometry. Silencing of AKR1B10 expression resulted in increased apoptosis following treatment with HHE as control as seen by Annexin V positivity in the scatter plots (red boxes) (C; red boxes). Exposure of shRNA treated cells to doxorubicin resulted in a marked increase in apoptosis compared to vector as well as HHE treated cells. Hu7 cells treated with vector control had similar levels of apoptosis following treatment with HHE and doxorubicin (6.17±1.0% vs. 7.58±1.2%). In contrast, Hu7 cells treated with shRNA to AKR1B10 had marked apoptosis following treatment with doxorubicin for shRNA#1 and #2 (34.2±3.0% and 23.6±2.8%, respectively) (D).
In order to study the role of AKR1B10 in chemoresistance, vector and AKR1B10-shRNA transfected Hu7 cells were treated with either HHE as control or with the chemotherapeutic agent doxorubicin for 24 hrs. Flow cytometry for Annexin V and 7-Aminoactinomycin D (7-AAD) revealed that silencing of AKR1B10 expression resulted in increased apoptosis following treatment with HHE (positive control) as demonstrated by Annexin V positivity in the scatter plots (red boxes) (Figure 5C). Treatment of AKR1B10 silenced cells with doxorubicin resulted in a marked increase in apoptosis compared to both vector control and HHE-treated cells. Vector control Hu7 cells had similar levels of apoptosis following treatment with HHE and doxorubicin (6.17±1.0% vs. 7.58±1.2%, respectively; Figure 5D). In contrast, AKR1B10-shRNA transfected Hu7 cells had a significant increase of cell apoptosis following treatment with doxorubicin for both clones #1 and #2 (34.2±3.0% and 23.6±2.8%, respectively; Figure 5D; p<0.001).
Discussion
The differential diagnosis of a distinct nodule in cirrhotic livers includes HCC as well as benign mimickers like hepatic adenoma, focal nodular hyperplasia or large regeneration nodules are challenge clinically. A variety of tumor markers have been studied in HCC. Recent studies have reported the use of Glypican-3 immunostaining to be useful in the differentiation of well-differentiated hepatocellular carcinoma from adenomas in non-cirrhotic livers 31, while others report a higher sensitivity in poorly-differentiated HCC 32. In addition, nuclear staining of β-catenin has been reported to aid in the differentiation between well-differentiated HCC from high-risk/atypical adenoma 33. A combination of several markers has been suggested to increase the specificity for HCC, however this can be difficult in a limited biopsy specimen. In the present study, AKR1B10 is extensively expressed in HCC and minimally in the benign liver lesions including cirrhotic liver, HA and FNH, indicating that AKR1B10 is a potential promising biomarker for HCC.
Our study implies the usefulness of AKR1B10 in the differential diagnosis of HCC from the benign liver lesions, including hepatic adenoma (HA) and focal nodular hyperplasia (FNH). Our data showed AKR1B10 was a sensitive IHC marker with 96.6% of HCC cases expressing AKR1B10 expression. Sixty-nine (77.5%) of the 89 HCC cases studies expressed strong and diffuse staining of AKR1B10, while six additional cases showed positive staining in 10–50% of tumor cells. No or minimal expression of AKR1B10 was observed in case of HA, and FNH. It is worth mentioning that AKR1B10 expression in HCC was detected in well, moderately and poorly differentiated tumors, and AKR1B10 expression levels in HCC were high irrespective of tumor etiology, implying a high sensitivity for detecting HCC.
There is increasing evidence supporting the AKR superfamily as important players involved in chemoresistance. Previous studies have demonstrated that AKRs converts doxorubicin into a less toxic form by reducing ketone groups 34. AKR1B10, AKR1B1 and several AKR1C isoforms have also been shown to be up-regulated in several cancers exposed to anti-cancer drugs. As of early 2012, there have only been two reported clinical studies examining AKR overexpression and chemoresistance in cancer patients, specifically in ovarian and cervical carcinomas 35,36. Our AKR1B10-shRNA knockdown experiment demonstrated an increased sensitivity to doxorubicin. These findings suggest that AKR1B10 up-regulation maybe responsible for the resistance to anthracycline chemotherapy and may account for why HCC is often unresponsive to current chemotherapeutic approaches. Based on these findings, AKR1B10 appears to be a promising biomarker of and a potential new therapeutic target in hepatocellular carcinoma.
AKR1B10 may play a crucial role in tumorigenesis based on its metabolic fates. AKR1B10 is involved in metabolizing lipids/isoprenoids, active carbonyl and in regulating retinal homeostasis. Intracellular farnesal/geranylgeranial can be reduced by AKR1B10 and further phosphorylated into farnesyl and geranylgeranyl pyrophosphates, which would serve as another source of substrates for protein prenylation. Prenylation is a post-translational modification of proteins and has been shown to have a significant role in cell development, proliferation and differentiation, such as oncogenic RAS gene. The conversion of highly reactive aldehyde and ketone groups into hydroxyl groups by AKR1B10 prevents the cell from undergoing carbonyl-induced apoptosis. AKR1B10 is also an efficient retinal reductase; the conversion of retinal to retinol by AKR1B10 results in the suppression of retinal to retinoic acid conversion, which is important because retinoic acid is a major active antineoplastic metabolite. Dynamically silencing AKR1B10 in Hu7 HCC cells by shRNA resulted in significantly decreased colony formation and cell growth as well as induction of cell apoptosis, further supporting the role of AKR1B10 in liver carcinogenesis. Additional studies are needed to further address the role of these metabolic fates in carcinogenesis.
In conclusion, immunohistochemical analysis revealed that AKR1B10 was over-expressed in hepatocellular carcinomas, and minimal to no expression was identified in adjacent hepatic tissue, hepatic adenomas and focal nodular hyperplasia. Our results indicate that AKR1B10 is a promising and highly potential biomarker to differentiate between benign and malignant primary lesions of the liver. shRNA-mediated silencing of AKR1B10 expression in hepatocellular carcinoma cells resulted in 1) increased cell apoptosis, 2) decreased colony formation and size, and 3) enhanced cytoreductive response following exposure to chemotherapeutic agent. These findings provide first time evidence that AKR1B10 is a unique biomarker involved in hepatocellular carcinogenesis via modulation of proliferation, cell apoptosis and chemoresistance.
Acknowledgments
The authors would like to thank Alfred W. Rademaker, PhD and Mrs. Irene B Helenowski of the Biostatistics Core Facility at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University for their assistance with the statistical analysis of the data.
Sources of Support: This study was supported by National Institutes of Health (NIH) R01 grant CA164041 to G-YY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong R, Corley DA. Racial and ethnic variations in hepatocellular carcinoma incidence within the United States. Am J Med. 2008;121(6):525–531. doi: 10.1016/j.amjmed.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 6.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 7.Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW, Burroughs AK. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30(1):6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 8.Choi DM, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17(3):684–692. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, Byrne T, Vargas HE, Mulligan D, Rakela J, Wiesner RH. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10(1):36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 10.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249(5):799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 11.Teramoto R, Minagawa H, Honda M, Miyazaki K, Tabuse Y, Kamijo K, Ueda T, Kaneko S. Protein expression profile characteristic to hepatocellular carcinoma revealed by 2D-DIGE with supervised learning. Biochim Biophys Acta. 2008;1784(5):764–772. doi: 10.1016/j.bbapap.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Heringlake S, Hofdmann M, Fiebeler A, Manns MP, Schmiegel W, Tannapfel A. Identification and expression analysis of the aldo-ketoreductase1-B10 gene in primary malignant liver tumours. J Hepatol. 2010;52(2):220–227. doi: 10.1016/j.jhep.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Satow R, Shitashige M, Kanai Y, Takeshita F, Ojima H, Jigami T, Honda K, Kosuge T, Ochiya T, Hirohashi S, Yamada T. Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res. 2010;16(9):2518–2528. doi: 10.1158/1078-0432.CCR-09-2214. [DOI] [PubMed] [Google Scholar]
- 14.Cao D, Fan ST, Chung SS. Identification and characterization of a novel human aldose reductase-like gene. J Biol Chem. 1998;273(19):11429–11435. doi: 10.1074/jbc.273.19.11429. [DOI] [PubMed] [Google Scholar]
- 15.Penning TM. AKR1B10: a new diagnostic marker of non-small cell lung carcinoma in smokers. Clin Cancer Res. 2005;11(5):1687–1690. doi: 10.1158/1078-0432.CCR-05-0071. [DOI] [PubMed] [Google Scholar]
- 16.Breton J, Gage MC, Hay AW, Keen JN, Wild CP, Donnellan C, Findlay JB, Hardie LJ. Proteomic screening of a cell line model of esophageal carcinogenesis identifies cathepsin D and aldo-keto reductase 1C2 and 1B10 dysregulation in Barrett's esophagus and esophageal adenocarcinoma. J Proteome Res. 2008;7(5):1953–1962. doi: 10.1021/pr7007835. [DOI] [PubMed] [Google Scholar]
- 17.Yoshitake H, Takahashi M, Ishikawa H, Nojima M, Iwanari H, Watanabe A, Aburatani H, Yoshida K, Ishi K, Takamori K, Ogawa H, Hamakubo T, Kodama T, Araki Y. Aldo-keto reductase family 1, member B10 in uterine carcinomas: a potential risk factor of recurrence after surgical therapy in cervical cancer. Int J Gynecol Cancer. 2007;17(6):1300–1306. doi: 10.1111/j.1525-1438.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 18.Chung YT, Matkowskyj K, Li H, Bai H, Zhang W, Tsao MS, Liao J, Yang G-Y. Overexpression and oncogenic function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic carcinoma. Mod Pathol. 2012;25(5):758–766. doi: 10.1038/modpathol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallego O, Ruiz FX, Ardevol A, Dominguez M, Alvarez R, de Lera AR, et al. Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proc Natl Acad Sci USA. 2007;104:20764–20769. doi: 10.1073/pnas.0705659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo S, Matsunaga T, Mamiya H, Ohta C, Soda M, Kitade Y, et al. Kinetic studies of AKR1B10, human aldose reductase-like protein: endogenous substrates and inhibition by steroids. Arch Biochem Biophys. 2009;487:1–9. doi: 10.1016/j.abb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Balendiran GK. Fibrates in the chemical action of daunorubicin. Curr Cancer Drug Targets. 2009;9(3):366–369. doi: 10.2174/156800909788166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong LSH, Huang C, Jing H, Cao D. AKR1B10 induces cell resistance to daunorubicin and idarubicin by reducing C13 ketonic group. Toxicol Appl Pharmacol. 2011;255:40–47. doi: 10.1016/j.taap.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsunaga T, Wada Y, Endo S, Soda M, El-Kabbani O, Hara A. Aldo-Keto Reductase 1B10 and Its Role in Proliferation Capacity of Drug-Resistant Cancers. Front Pharmacol. 2012;3(5):1–11. doi: 10.3389/fphar.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang XD, Tang DN, Wang J, Cao DL. Screening of the drug resistance-associated gene in HepG2 cell line transfected with aldose reductase like gene-1 (ARL-1) Ai Zheng. 2003;22(12):1289–1295. [PubMed] [Google Scholar]
- 25.Martin HJ, Breyer-Pfaff U, Wsol V, Venz S, Block S, Maser E. Purification and characterization of AKR1B10 from human liver: role in carbonyl reduction of xenobiotics. Drug Metab Dispos. 2006;34(3):464–470. doi: 10.1124/dmd.105.007971. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz FX, Gallego O, Ardevol A, Moro A, Dominguez M, Alvarez S, et al. Aldo-keto reductases from the AKR1B subfamily: retinoid specificity and control of cellular retinoic acid levels. Chem Biol Interact. 2009;178:171–177. doi: 10.1016/j.cbi.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Liao J, Yang GY. CAAX-box protein, prenylation process and carcinogenesis. Am J Transl Res. 2009:312–325. [PMC free article] [PubMed] [Google Scholar]
- 28.Wei W, Liang HJ, Cui JF, Guo K, Kang XN, Cao J, Su JJ, Li Y, Liu YK. Effects of AKR1B10 gene silence on the growth and gene expression of HCC cell line MHCC97H. Zhonghua Gan Zang Bing Za Zhi. 2010;18(9):666–671. doi: 10.3760/cma.j.issn.1007-3418.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KJ, Sotiropoulos GC, Baba HA, Schmid KW, Müller D, Paul A, Auer T, Gamerith G, Loeffler-Ragg J. AKR1B10 expression is associated with less aggressive hepatocellular carcinoma: a clinicopathological study of 168 cases. Liver Int. 2011;31(6):810–816. doi: 10.1111/j.1478-3231.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- 30.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coston WM, Loera S, Lau SK, Ishizawa S, Jiang Z, Wu CL, Yen Y, Weiss LM, Chu PG. Distinction of hepatocellular carcinoma from benign hepatic mimickers using Glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol. 2008;32(3):433–444. doi: 10.1097/PAS.0b013e318158142f. [DOI] [PubMed] [Google Scholar]
- 32.Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Modern Pathology. 2008;21:1011–1018. doi: 10.1038/modpathol.2008.85. [DOI] [PubMed] [Google Scholar]
- 33.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43(3):515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 34.Zhong L, Shen H, Huang C, Jing H, Cao D. AKR1B10 induces cell resistance to daunorubicin and idarubicin by reducing C13 ketonic group. Toxicol Appl Pharmacol. 2011;255(1):40–47. doi: 10.1016/j.taap.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YJ, Yuan CC, Chow KC, Wang PH, Lai CR, Yen MS, Wang LS. Overexpression of dihydrodiol dehydrogenase is associated with cisplatin-based chemotherapy resistance in ovarian cancer patients. Gynecol Oncol. 2005;97(1):110–117. doi: 10.1016/j.ygyno.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 36.Ueda M, Hung YC, Chen JT, Chiou SH, Huang HH, Lin TY, Terai Y, Chow KC. Infection of human papillomavirus and overexpression of dihydrodiol dehydrogenase in uterine cervical cancer. Gynecol Oncol. 2006;102(2):173–181. doi: 10.1016/j.ygyno.2005.12.009. [DOI] [PubMed] [Google Scholar]