Summary
We conducted a phase 1/2 study of bortezomib administered in combination with high-dose melphalan followed by tandem autologous transplants in patients with primary resistant multiple myeloma. Thirty patients received 2 cycles of salvage bortezomib followed by stem cell mobilization with G-CSF and harvest. Melphalan 100 mg/m2/day on two consecutive days was administered, immediately followed by one dose of bortezomib (dose escalation) and stem cell infusion. The median beta 2-microglobulin was 4.35 mg/L (range: 1.8 - 11.4); albumin was 3.7 g/dL (range: 3.1 - 4.9); high-risk karyotypes were noted in 45% of patients. The maximum planned dose of bortezomib at 1.3 mg/m2 was well tolerated and a formal maximum tolerated dose was not determined. The peak of best overall response (≥PR) and complete response rates after tandem transplants were 84% and 36%, respectively. With a median follow-up of 48 months, the median progression-free survival was 15 (95% CI: 11 - 21) months and the median overall survival was 35 (95% CI: 22 - 43) months. Correlative studies demonstrated decreased expression of FANCD1 (P=0.0072) and FANCF (P=0.0458) mRNA following bortezomib treatment. Bortezomib combined with high-dose melphalan is a well tolerated conditioning with some activity in patients with resistant myeloma.
Keywords: Bortezomib, autologous transplantation, multiple myeloma, Fanconi anemia, DNA repair pathway
Introduction
Multiple myeloma (MM) represents approximately 10% of all hematologic malignancies and the median overall survival (OS) of those diagnosed in the last decade has approached 4 years (Jemal, et al 2011, Kumar, et al 2008). The role of high-dose chemotherapy followed by autologous hematopoietic cell transplantation (HCT) has been established (Attal, et al 1996, Child, et al 2003, Koreth, et al 2007, Kumar, et al 2009). High-dose melphalan at 200 mg/m2 remains an integral modality of myeloma treatment and tandem transplants continue to be offered to a fraction of patients (Attal, et al 1996).
Historically, approximately 20-30% of patients with newly diagnosed MM fail to respond to induction treatment regimens such as vincristine, adriamycin and dexamethasone (VAD) or thalidomide and dexamethasone (Kumar, et al 2004), hence these patients were thought to have poorly responsive disease. Their median progression free survival ranges from 4 to 8 months with the median OS was approximately 12 months (Alexanian and Dimopoulos 1994). In the recent years, these primary refractory patients were shown to benefit from high-dose chemotherapy with CR rates of 16-40% (Alexanian, et al 2004, Kumar, et al 2004, Singhal, et al 2002). However, the results are sill inferior to patients with responsive disease prior to transplant. With the introduction of novel agents including lenalidomide and bortezomib the overall response rates (ORR) with newer induction regimens have increased dramatically in the range of 80% to 90% (Harousseau, et al 2010, Rajkumar, et al 2010, Richardson, et al 2010). Plasma cell leukemia (PCL) represents, perhaps, the most aggressive form of MM (Sher, et al 2010). The European Group for Blood and Marrow Transplantation (EBMT) reported an OS of 25.7 months in 272 patients with PCL who underwent autologous HCT in a retrospective analysis (Drake, et al 2010), albeit limited by a selection bias. Currently there are no standard therapeutic strategies for PCL. To this end, there is an unmet need to improve and develop better treatment regimens for patients with primary resistant MM and PCL.
Bortezomib has demonstrated significant clinical anti-myeloma activity when used as single agent (Richardson, et al 2003, Richardson, et al 2005) and also in combination with melphalan (Berenson, et al 2006, San Miguel, et al 2008). Bortezomib targets both acquired and de novo mechanisms of drug resistance and sensitizes melphalan-sensitive and melphalan-resistant MM cell lines to melphalan via the down regulation of cellular responses to genotoxic stress (Ma, et al 2003, Mitsiades, et al 2003). Inhibition of NF-κB by bortezomib significantly reduces Fanconi anemia (FA)/BRCA gene expression and FANCD2 protein expression in MM cells with a resultant reduction in DNA damage repair and enhanced melphalan sensitivity (Yarde, et al 2009). These studies suggested the utilization of bortezomib in combination with high-dose melphalan may be synergistic and provide significant clinical benefit.
Based on both preclinical and clinical data, we hypothesized that bortezomib would sensitize patients with primary resistant MM (defined in the method section) to high-dose melphalan, overcome both acquired and de novo drug resistance, and ultimately result in increased efficacy of autologous HCTs, and that these effects may result from the down regulation of the FA/BRCA gene expression. We designed this study to (a) determine the maximum tolerated dose (MTD) and to (b) investigate the safety, tolerability, and response rates of bortezomib given in combination with high-dose melphalan, as a conditioning regimen for tandem autologous HCTs in patients with primary resistant MM, and to (c) examine the effects of bortezomib on the FA/BRCA gene expression in vivo.
Methods
Patient eligibility
Patients with primary resistant MM or PCL who are 18 years or older were eligible. Primary resistant disease was defined as failure to achieve at least a partial response, based on International Uniform Response Criteria (Durie, et al 2006), after induction therapy in bortezomib naïve myeloma population, as bortezomib was only available for patients who failed two prior therapies at the time of study initiation (Bross, et al 2004). All patients were required to have histologically confirmed diagnosis by pathology review at the H. Lee Moffitt Cancer Center and Research Institute (MCC). Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki on the protocol approved by the Institutional Review Board of University of South Florida. The trial was registered at ClinicalTrials.gov as NCT 00307086. From June 2005 to February 2009, 34 patients were enrolled onto this study. Three patients were excluded due to insurance denial for the participation in the study and one patient withdrew the informed consent.
Patients with inadequate hematologic (a platelet count of <30 × 109/L and an absolute neutrophil count of <1.0 × 109/L), renal (serum creatinine >2.0 mg/dL or creatinine clearance <40 mL/min, requiring hemodialysis or peritoneal dialysis), endocrine, cardiopulmonary and hepatic functions were ineligible. Other notable criteria for exclusion at the time of enrollment were >grade 2 peripheral neuropathy, hypersensitivity to bortezomib, boron, or mannitol, active central nervous system (CNS) involvement, previous malignancy other than non-melanoma skin cancer, radiation therapy within 14 days prior to enrollment, previous bortezomib therapy, and prior stem cell transplantation.
Initial diagnostic and staging evaluations, baseline cytogenetics and fluorescent in situ hybridization (FISH), initial therapies and myeloma status at diagnosis were documented for all patients. High risk group by cytogenetic changes was defined as the presence of hypodiploidy, t(4;14), t(14;16), partial or whole deletion of chromosome 13q, or loss of 17p13 by conventional cytogenetics and 17p deletion (p53), t(4;14) (IgH, FGFR3), or deletion 13q14 (Rb-1) by FISH. Standard risk group by cytogenetic changes was defined as hyperdiploidy, or normal karyotype by conventional cytogenetics and other abnormalities not included in the high risk group by FISH. Patients were staged by both the Durie-Salmon staging system and the International Staging System (ISS) (Greipp, et al 2005, Woodruff, et al 1979).
Study design
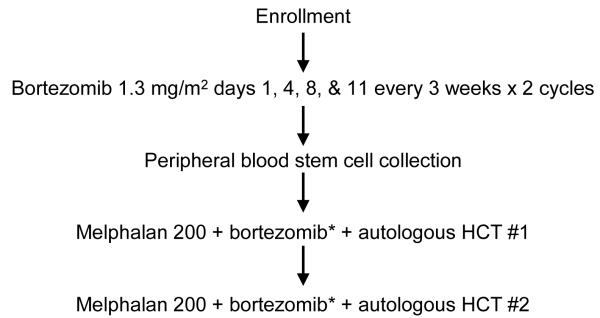
Patients were initially treated with bortezomib at a dose of 1.3 mg/m2 intravenously on days 1, 4, 8, and 11, every three weeks for two cycles. After the completion of bortezomib therapy, patients underwent peripheral-blood stem cell mobilization with granulocyte colony-stimulating factor (G-CSF) mobilization at a dose of 20 μg/kg/day for 4 consecutive days, followed by stem cell collection. The goal of peripheral blood stem cell collection was to achieve CD34+ cell dose of 4-10 × 106/kg of body weight (BW) to perform tandem transplantation. Study schema is depicted in Figure 1.
Figure 1. Study schema.
HCT: Hematopoietic cell transplantation
Melphalan 200: Melphalan 200 mg/m2
*Bortezomib given on day -3 (dose escalation: 0.7 mg/m2, 1.0 mg/m2, and 1.3 mg/m2)
For the first transplant, Melphalan (100 mg/m2) was administered intravenously via central catheter over 30 minutes once daily on day -4 and day -3, with bortezomib dosed according to a phase I dose escalation schema and infused by intravenous push over 3 to 5 seconds on day -3, immediately after the melphalan. A minimum of 2 × 106 autologous CD34+ cells/kg of BW was infused 72 hours after the conditioning regimen was completed. The day of peripheral blood stem cell infusion was designated as day 0. Ninety (±15) days after the first transplant, patients underwent a second transplant using the same regimen and schedule as in the first transplant (Figure 1).
For bortezomib in combination with high-dose melphalan, a phase I dose-escalation scheme with 3 patients in each cohort (standard 3+3 design) was employed. Escalated doses of bortezomib were administered in sequential cohorts of patients: 0.7 mg/m2 (cohort 1), 1.0 mg/m2 (cohort 2) and 1.3 mg/m2 (cohort 3). As specified in the protocol, cohort 2 was expanded as the last patient on cohort 3 was less than 30 days after first HCT and the entire cohort has not completed the toxicity evaluation at the time of enrollment. Cohort 3 was subsequently expanded as a phase 2 component of the study. We estimated that with 30 evaluable patients the width of a 95% confidence interval for the response rate would not exceed 37% using the exact Binomial distribution.
Posttransplantation supportive care
Patients received supportive care according to institutional clinical guidelines for autologous stem cell transplantation. G-CSF was administered at a dose of 5 μg/kg per day from day +7 until a self-sustained absolute neutrophil count (ANC) recovery of ≥ 500/μL was achieved. No maintenance therapy post tandem autologous HCTs were allowed in this protocol. Herpes simplex and herpes zoster virus prophylaxis consisted of acyclovir 400 - 800 mg orally twice daily for one year posttransplant.
Assessment of response and toxicity
Treatment response and relapse definitions were based on International Uniform Response Criteria (Durie, et al 2006). Responses were scored separately after 2 cycles of bortezomib and 3 months post tandem autologous HCT. After the 90 day evaluation, myeloma responses were recorded approximately every 3 months until progression or relapse was documented. Toxicity assessment was performed using the Common Terminology Criteria for Adverse Events 3.0 (CTCAE version 3.0). Dose-limiting toxicities (DLTs) were defined as delayed engraftment and/or the development of grade 3 or 4 toxicities. The toxicities expected during stem cell transplantation including leukopenia, neutropenia, thrombocytopenia, mucositis, nausea, vomiting, and alopecia were not considered dose limiting.
Isolation of multiple myeloma cells
Serial bone marrow aspirations were collected (1) at baseline, (2) after 1 dose of bortezomib, and (3) after 2 cycles of bortezomib. Plasma cells were isolated via negative selection protocol (RosetteSep, Stem Cell Technology), aiming for >85% purity. First, 1 mL of bone marrow aspirate was centrifuged in a Ficoll-Paque PLUS gradient (Amersham Biosciences), and a cytospin of the isolated cells was used to determine the initial purity of the plasma cell population. In parallel, 10 mL of the bone marrow sample was incubated with the Millennium Rosette Antibody Cocktail (Millennium Pharmaceuticals Inc.) for 20 minutes followed by Ficoll extraction. A new cytospin was prepared from these cells to assess the efficiency of selection (Yarde, et al 2009).
FA/BRCA pathway gene expression
RNA was extracted from CD 138-enriched fresh myeloma cells using RNeasy Micro kit (Qiagen) and cDNA was synthesized using SuperScript First Stand Synthesis kit (Invitrogen). FA/BRCA DNA repair pathway mRNA expression was assessed by quantitative-PCR (q-PRC) with customized microfluidic cards (Applied Biosystems) designed to simultaneously analyze the expression of 11 FA/BRCA related genes, brca1, brca2, fanca, fancc, fancd2, fance, fancf, fancg, fancl, rad51, and rad51c as previously described (Yarde, et al 2009). Gene expression was normalized to the internal standard of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an endogenous control, and externally standardized against the screening sample. For longitudinal intra-patient analysis of FA/BRCA pathway mRNA expression was reported as fold-change from screening bone marrow. The inter-patient comparison of FA/BRCA pathway expression involved an additional step. To control for comparison of values from different microfluidic cards, an additional myeloma cell line control sample was used on each card. All FA/BRCA pathway values from initial bone marrow were normalized to this, allowing for an accurate inter-patient comparison of pretreatment FA/BRCA pathway expression taking into account the card to card variability.
Study endpoints and statistical analysis
The primary objectives of this study were (a) to determine the maximum tolerated dose (MTD) and (b) to investigate the safety, tolerability, and response rates of bortezomib given in combination with high-dose melphalan in primary resistant multiple myeloma or plasma cell leukemia. Primary efficacy endpoints were analyzed on those patients who received at least one transplant. Following treatment with two cycles of standard dose bortezomib, sequential cohorts of patients were given escalating doses of bortezomib in a standard 3+3 design combined with high dose melphalan. Neutrophil engraftment was defined as the first day the ANC was greater than or equal to 500/μL for 3 consecutive days. Platelet engraftment was defined as the first day the platelet count was greater than or equal to 20,000/μL for 7 consecutive days independent of transfusions. Engraftment was considered successful (not delayed) if the time to achieve the predefined ANC occurred at or prior to 14 days, and the time to achieve the pre-defined platelet count occurred at or prior to 30 days.
Those patients who received at least one transplant at the MTD or maximum planned dose (MPD) were deemed as evaluable patients for efficacy analysis. The 95% confidence interval of the response rate was computed using the exact Binominal distribution. OS and progression-free survival (PFS) were calculated using the methods of Kaplan and Meier with standard errors computed using Greenwood’s formula with log-log transformation, and were compared using log-rank test.
Statistical methods, including t-test, Spearman correlation, and Cox Proportional-Hazards model, were used to evaluate clinical association of FA/BRCA gene expression. Specifically, t-test was used to examine if the gene expression changed from baseline to after 1 dose of bortezomib (and after 2 cycles). Spearman correlation was used to compare the FA/BRCA gene expression to the monoclonal spike ratios and disease response categories. Cox Proportional-Hazards model was used to test the relationship between the FA/BRCA gene expression to OS and time to progression. Prior to data analysis, the FA/BRCA gene expression was first normalized to baseline using the ratio and then transformed by logarithm to make data less skew.
Results
Patient characteristics and response to salvage bortezomib
A total of 30 patients received at least one dose of salvage bortezomib. Clinical characteristics of these patients are summarized in the Table 1. Fifteen were males (50%) and the median age was 54.5 years (range, 36-70 years). Information on conventional cytogenetics was available in 28 patients and FISH analysis was available in 29 patients. Thirteen patients (45%) had high risk chromosomal abnormality. Induction therapy had consisted of combination chemotherapy including dexamethasone, thalidomide, lenalidomide, or doxorubicin formulation. Twenty three patients received one line of therapy and 7 received two or more lines of therapy. Of those 29 patients with MM, 16 (55%) had disease progression either while on induction therapy or within 60 days of discontinuing the therapy prior to enrollment to the study.
Table 1.
Patient characteristics
| N = 30 | No. | Percent |
|---|---|---|
| Age at transplant, median, year (range) | 54.5 (36-70) | - |
| Male | 15 | 50 |
| Multiple myeloma subtype | ||
| IgG | 15 | 50 |
| IgA | 9 | 30 |
| Light chain | 5 | 17 |
| Plasma cell leukemia | 1 | 3 |
| Chromosomal abnormality*1 | ||
| High risk | 13 | 45 |
| 13q deletion | 11 | 38 |
| 17p deletion | 2 | 7 |
| t(4;14) | 2 | 7 |
| Standard risk | 16 | 55 |
| β2 microglobulin, median, mg/L (range) | 4.35 (1.8-11.4) | - |
| Refractory disease*2 | 16 | 55 |
| Albumin, median, g/dL (range) | 3.7 (3.1-4.9) | - |
| Time from diagnosis to enrollment, median, month (range) |
7 (3 – 80)*3 | - |
| Prior chemotherapy | ||
| Thalidomide/dexamethasone | 14 | 46 |
| Lenalidomide-based regimen*4 | 11 | 37 |
| Vincristine/Adriamycin/dexamethasone (VAD) | 5 | 17 |
| Liposomal doxorubicin-based regimen | 3 | 10 |
| Number of prior regimens | ||
| 1 | 23 | 77 |
| ≥2 | 7 | 23 |
| Durie-Salmon Staging System*5 | ||
| 1 | 3 | 10 |
| 2 | 6 | 21 |
| 3 | 20 | 69 |
| International Staging System (ISS) | ||
| I | 8 | 27 |
| II | 16 | 53 |
| III | 6 | 20 |
One patient with plasma cell leukemia was not included in the chromosomal risk classification.
Disease progression during induction therapy or within 60 days of discontinuing induction therapy (prior to enrollment)
One patient was diagnosed as asymptomatic myeloma 6 years prior to study enrollment and only required systemic therapy 4 months before the enrollment.
Three patients received liposomal doxorubicin containing regimen.
One patient with plasma cell leukemia was excluded.
After 2 cycles of bortezomib (n=30), the overall response (CR+VGPR+PR) and CR rates were 37% (95% confidence interval (CI): 19.9-56.1%) and 3%, respectively. Two patients had progressive disease after the first cycle of bortezomib. One patient with obesity hypoventilation syndrome was not a candidate for high-dose chemotherapy. Adverse events included 10 patients (33%) with grade 1-2 sensory peripheral neuropathy and 1 patient (3%) with grade 1-2 cranial neuropathy. Grade 3 adverse events include anemia (10%), thrombocytopenia (13%), neutropenia (10%), and pneumonia (3%). One patient developed significant cytokine release syndrome and pulmonary dysfunction after G-CSF mobilization and was taken off study. One patient experienced diffuse alveolar hemorrhage after the first cycle of bortezomib, which resolved, but prompted removal from the study. Thus, 25 patients proceeded to transplant conditioning with melphalan plus bortezomib.
Stem cell collections and engraftments
All 29 patients receiving mobilization therapy collected sufficient autologous stem cells for transplantation. Patients received a median of 2.59 × 106 (range: 2.07-11.2) CD34+ cell/kg for the autologous HCT #1 and a median of 2.71 × 106 (range: 2.0-11.2) CD34+ cell/kg for the autologous HCT #2. Neutrophils (ANC ≥ 500/μL for 3 days) recovered in medians of 12 (range: 10-23) days after autologous HCT #1 and 12 (range: 11-16) days after autologous HCT #2. Platelets (≥ 20,000/μL for 7 days without transfusions) recovered in medians of 13 (range: 11-20) days after HCT #1 and 12 (range: 9-14) days after HCT #2, respectively.
Bortezomib dose escalation and conditioning regimen safety
Bortezomib was administered at 0.7 mg/m2 to 6 patients in cohort 1, at 1.0 mg/m2 to 6 patients in cohort 2, and successfully escalated to 1.3 mg/m2 and administered to a total of 18 patients in cohort 3 which was the maximal planned dose (MPD). One patient in cohort 1 experienced delayed neutrophil engraftment and this patient did not receive G-CSF support post-transplant. The cohort 1 was expanded and there were no further DLTs. Cohort 2 was expanded while 3 initial patients on cohort 3 were still less than 30 days post tandem transplants as specified in the protocol. No DLTs were observed on either cohort 2 or 3. Thus, no formal MTD was determined. Three patients did not proceed with second HCT because of disease progression (n=1) and delayed recovery from the first HCT (n=2), hence a total of 22 patients received tandem transplants. Observed grade 3-4 adverse events are summarized in Table 2. There was no treatment-related mortality.
Table 2.
Grades 3-4 adverse events after autologous HCTs (n=25 and 22)*
| Grade 3, n (%) |
Grade 4, n (%) |
|||
|---|---|---|---|---|
| HCT #1 | HCT #2 | HCT #1 | HCT #2 | |
| Febrile neutropenia | 12 (48) | 13 (59) | 0 (0) | 1 (5) |
| Hypokalemia | 7 (28) | 5 (23) | 0 (0) | 0 (0) |
| Bacteremia | 6 (24) | 2 (9) | 0 (0) | 1 (5) |
| Mucositis | 3 (12) | 4 (18) | 1 (4) | 0 (0) |
| Hypophosphatemia | 2 (8) | 3 (14) | 0 (0) | 0 (0) |
| Pneumonia | 2 (8) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 1 (4) | 1 (5) | 0 (0) | 0 (0) |
| Nausea | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Clostridium difficile infection | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Systolic dysfunction | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Diastolic dysfunction | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Hypoxia | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Dyspnea | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Hypotension | 0 (0) | 0 (0) | 0 (0) | 1 (5) |
There were no grade 5 toxicities.
HCT: hematopoietic cell transplantation
Myeloma responses after transplants
The responses at 3 months after completing at least one transplant (n=25) were as follows: CR 20%, VGPR 24%, PR 40%, SD 4%, PD 4%, and 8% were not evaluable with an ORR of 84% (95% CI: 63.9-95.5%). The CR rate increased to 36% at best response (median of 8.1 months: range 4.3 – 12.3) and the overall response was at 84% (95% CI: 70.8-97.1%). Figure 2 shows the distribution of disease responses before and after the 2 cycles of bortezomib, at 3 months after the completion of transplant, and at their best responses.
Figure 2. Responses after treatment.
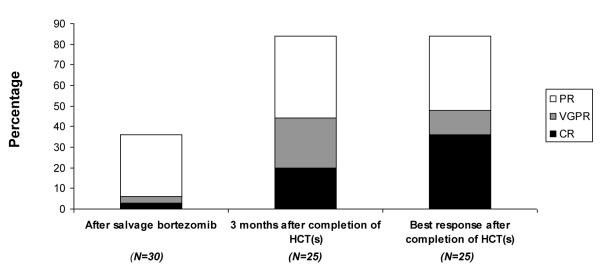
The overall response and complete response rates increased after autologous hematopoiectic cell transplantation.
Abbreviations:
CR, complete response; HCT, hematopoietic cell transplantation; PR, partial response; VGPR, very good partial response
Progression-free and overall survivals
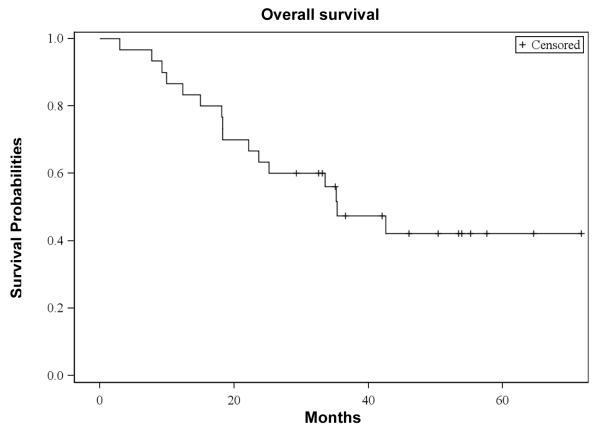
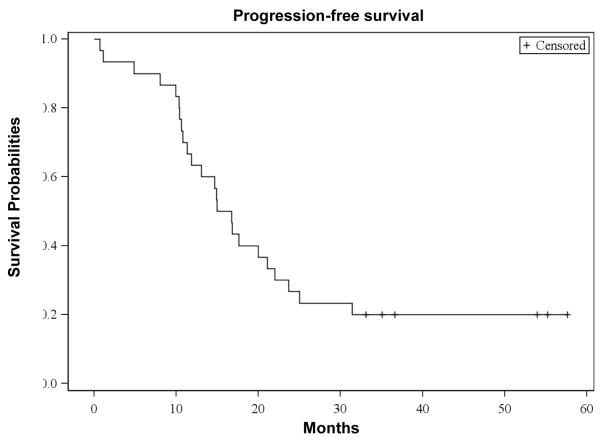
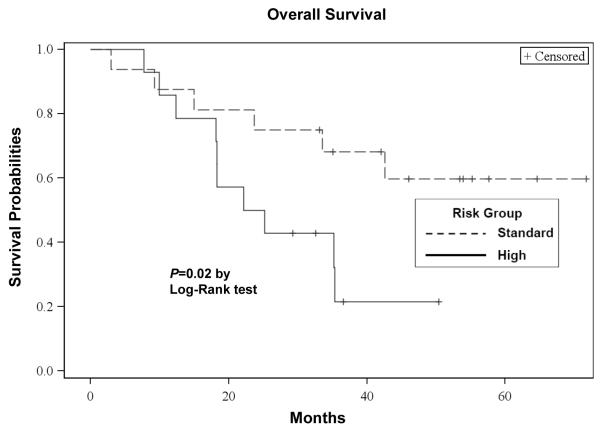
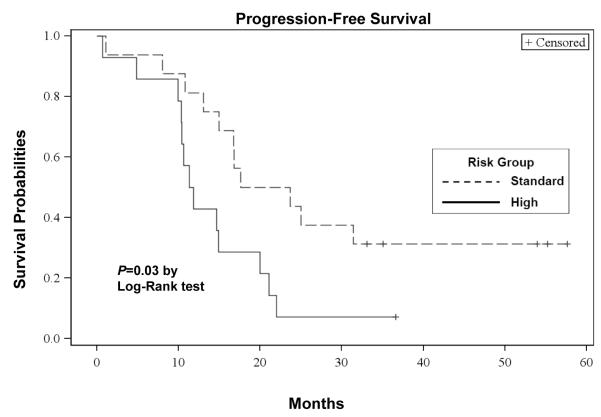
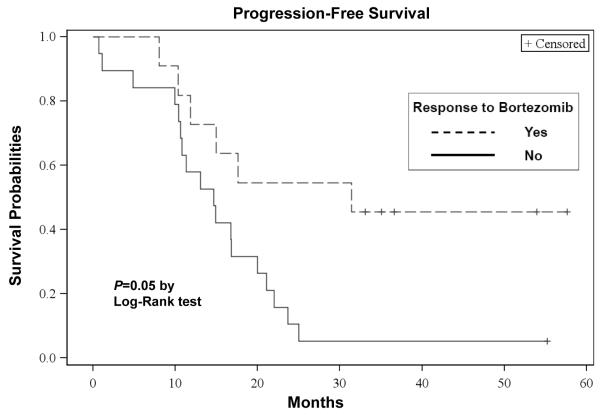
The median OS from the first dose of bortezomib was 35 (95% CI: 22 - 43) months, with a median follow-up of 48 (range, 3 - 62) months. The median progression-free survival (PFS) from the first dose of bortezomib was 15 (95% CI: 11 - 21) months. The OS and PFS curves are depicted in Figures 3a and 3b, respectively. For patients receiving transplant, the median OS from the time of first transplant was 40 (95% CI: 32 - 40) months and the median PFS from the first transplant was 15 (95% CI: 10 - 20) months, respectively. Of those 25 transplanted patients, 19 had disease progression. Patients with standard risk by cytogenetics had a significantly higher OS (P=0.02: Figure 3c) and PFS (P=0.03: Figure 3d) than high risk patients. There were no significant differences in the OS between the group with initial response (≥ PR) to salvage bortezomib and those without the response (P=0.60). However, there was a suggestion of longer PFS in patients responsive to salvage bortezomib compared to non-responders (P=0.05: Figure 3e).
Figure 3a. Overall Survival (OS).
Kaplan-Meier probability of overall survival. Vertical tick marks indicate censored patients.
Figure 3b. Progression-Free Survival (PFS).
Kaplan-Meier probability of progression-free survival. Vertical tick marks indicate censored patients.
Figure 3c. Overall Survival According to Risk Groups.
Kaplan-Meier probability of overall survival based on risk groups. Vertical tick marks indicate censored patients.
Figure 3d. PFS According to Risk Groups.
Kaplan-Meier probability of progression-free survival based on risk groups. Vertical tick marks indicate censored patients.
Figure 3e. PFS According to Response to Salvage Bortezomib.
Kaplan-Meier probability of progression-free survival based on response to salvage bortezomib. Vertical tick marks indicate censored patients.
FA/BRCA pathway gene expression after bortezomib and correlation with time to progression and survival
A total of 12 patients had both baseline bone marrow aspirates and samples after first dose of bortezomib for FA/BRCA pathway gene expression analysis. Eleven patients had samples for both baseline and after 2 cycles of salvage bortezomib. There was a statistically significant lower FANCD1 (=BRCA2) and FANCF mRNA expression after first dose of bortezomib (P=0.007 and P=0.046, respectively; Figure 4a) and there was a trend toward lower expression of FANCC, FANCD2, RAD51, and BRCA1 after first dose of bortezomib (Figure 4a). There was also a statistically significant lower FANCD1 mRNA expression after 2 cycles of bortezomib (P = 0.0055; Figure 4b). There was no statistically significant association between baseline expression of the FA/BRCA pathway genes and bortezomib induced down regulation of gene expression with either time to progression or OS when examined individually or when combined as a pathway.
Figure 4a. FA/BRCA gene expression by RT-PCR after first dose of bortezomib.
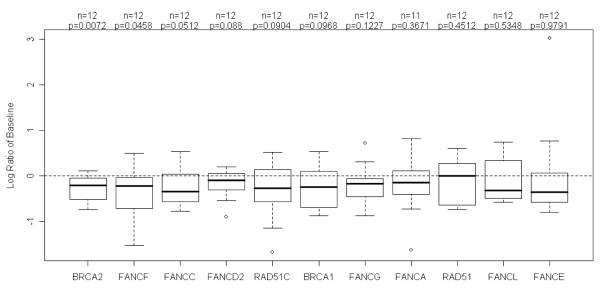
FANCD1, also known as BRCA2, and FANCF expressions were significantly lower after first dose of bortezomib (P=0.0072 and P=0.0458, respectively). There was a trend toward decreased expression of 4 other genes including FANCC, FANCD2, RAD51 and BRCA1.
Figure 4b. FA/BRCA gene expression by RT-PCR after 2 cycles of bortezomib.

FANCD1 (=BRCA2) expression remained significant lower after 2 cycles of bortezomib (P = 0.0055).
Discussion
Our trial offers several distinctive features in comparison to previously reported studies. First, the patient population studied was specifically primary resistant MM defined as failure to achieve at least a partial response after induction therapy and were bortezomib naïve. Second, we evaluated the ability of single agent bortezomib to salvage this population. Third, bortezomib was added to a standard high-dose melphalan conditioning regimen in tandem transplants and demonstrated its safety and unaffected engraftment kinetics. Additionally, we explored the effect on FA/BRCA DNA damage repair pathway and showed significantly decreased mRNA expression after bortezomib.
The use of high-dose chemotherapy in patients with refractory MM has been proven to be feasible and efficacious, as was demonstrated by the Intergroup Trial (Vesole, et al 1994). Acknowledging the methodological differences in documenting disease response utilizing more sensitive free light chain assay or immunofixation, previous high-dose therapy studies have shown ORR of 70-92% and CR rates of 16-40% with single autologous transplant in patients with primary refractory MM (Alexanian, et al 2004, Kumar, et al 2004, Singhal, et al 2002). However, median PFS and OS were 18-30 months and less than 5 years, respectively, therefore supporting the need for better treatment modalities for this patient population.
The patients enrolled to our trial had primary resistant MM or PCL. More specifically, 55% of MM patients had disease progression either while on induction therapy or within 60 days of discontinuing the therapy prior to enrollment to the study albeit they were bortezomib naïve. This poor response to initial therapy occurred despite the use of lenalidomide in more than one third of our patients. OS from first transplant was longer than OS from the time of first bortezomib, reflecting the aggressive disease biology and progression prior to transplant. ORR of 84% was seen after tandem transplants with novel combination of bortezomib plus melphalan. CR rate of 20% at 3 months after the transplants increased to 36% at the peak of best response. This suggests that the addition of bortezomib to high-dose therapy may enhance the CR rate for a group of bortezomib naïve patients with primary resistant disease. However, OS of 35 months with PFS of 15 months is in line with the results reported by Gertz et al. (OS of 30.4 months and PFS of 13.1 months) for patients who did not achieve PR after thalidomide and/or lenalidomide (Gertz, et al 2010). Taken together, the evidence suggests the durable role of autologous HCT as salvage therapy in refractory patient population, albeit with a short response duration. The combination of bortezomib plus high-dose melphalan would need to be examined in a larger controlled trial before more definitive conclusions are drawn.
In a similar concept, others have combined bortezomib with high-dose melphalan in different cohorts. In a multicenter phase II trial, Roussel et al. reported at least 70% VGPR rate including CR rate of 32% after autologous HCT in newly diagnosed MM patients with melphalan plus bortezomib conditioning (Roussel, et al 2010). A matched cohort comparison with Intergroupe Francophone du Myélome (IFM) 2005-01 showed higher CR rate with combination of bortezomib and high-dose melphalan than standard regimen (35% vs 11%, P=0.001). Bortezomib was administered intravenously at 1 mg/m2 on days -6, -3, +1, and +4. Lonial et al. performed a dose and schedule finding randomized phase I/II study by administering bortezomib either before or after high-dose melphalan in patients who did not achieve VGPR after induction (Lonial, et al 2010). Pharmacodynamic studies showed greater plasma cell apoptosis among patients who received bortezomib following melphalan (Lonial, et al 2010). The data were only available only after we completed accrual to our current study, however, the result was in line with our trial and it underscores the importance of administration sequence for the synergism enhancement (Mitsiades, et al 2003).
Although the pathogenesis of primary resistance is poorly understood, it likely involves factors intrinsic to the myeloma cells, as well as those posed by the bone marrow microenvironment (Dalton, et al 2004, Damiano, et al 1999, Damiano, et al 2001, Mudry, et al 2000). Increasing activity of NF-κB in multiple myeloma correlates with an increase in tumor cell survival (Andela, et al 2000, Huang, et al 2000, Kim, et al 2000, Palombella, et al 1994). Regulation of anti-apoptotic products via NF-κB activation is one example of cell-adhesion mediated drug resistance (CAM-DR) (Meads, et al 2009). Bortezomib has been shown to reduce binding interactions between myeloma cells and bone marrow stromal cells (Hideshima, et al 2001), and sensitize highly resistant myeloma cell lines to chemotherapy (Ma, et al 2003). Sensitization of myeloma cells to melphalan by bortezomib has been showed in vitro which may overcome chemoresistance (Hideshima, et al 2001, Mitsiades, et al 2003).
The mechanisms by which bortezomib enhances melphalan response in myeloma cells are still not fully understood. The FA/BRCA DNA damage repair pathway has been reported to be a critical determinant of melphalan response and resistance (Chen, et al 2005, Hazlehurst, et al 2003). Measurement of FA/BRCA gene expression was incorporated to functionally evaluate the effect of bortezomib on FA/BRCA DNA damage repair pathway expression. Despite the small sample size, we have demonstrated statistically significant lower FANCD1 (BRCA2) and FANCF expression as well as a trend toward lower expression of 4 other FA/BRCA genes after the dose of bortezomib. Reduced FAND1 and FANCF expression following bortezomib treatment would be sufficient to afford a drug sensitive phenotype, as in Fanconi anemia where loss of function mutations in individual FA/BRCA pathway complementation groups results in decreased DNA repair capacity and predisposition to cancer.(Howlett, et al 2002) FAND1 is a critically important downstream effector of FA complex in homologous recombination, unlike other FA genes’ products where the altered function may result in only mildly reduced DNA repair capacity (Nakanishi, et al 2005, Sung and Klein 2006). These findings suggest the significant impact of decreased FANCD1 and/or FANCF expression in FA/BRCA DNA repair pathway which would result in melphalan sensitization with bortezomib. No statistically significant associations among the FA/BRCA expression, disease response, and time to progression were observed which may be in part due to limited sample size and the timing of assessment. The effect of bortezomib on FA/BRCA pathway expression appears transient and decreases within 24-48 hours (Yarde, et al 2009). To this end, it may be more telling to examine FA/BRCA expression within 24 hours of the combined dosing and compare with clinical endpoints. Nonetheless, our data suggest that targeting the FA/BRCA DNA damage repair pathway may potentially enhance chemotherapeutic response in myeloma patients and may provide important insights into the mechanisms associated with the observed synergy between bortezomib and high-dose melphalan.
This report demonstrates the feasibility and safety of adding sequential treatment with bortezomib followed by a combination of bortezomib and high-dose melphalan as a conditioning regimen for tandem autologous HCTs in patients with primary resistant MM. As bortezomib in combination with high-dose melphalan was safely administered in these resistant MM patients and FA/BRCA gene expression was decreased after bortezomib, further exploration to discern true efficacy of this novel combination is warranted in the context of randomized controlled trials with chemosensitive MM. Further evaluation of FA/BRCA DNA damage repair pathway gene analyses is also required to dissect the mechanisms of bortezomib synergy with high-dose melphalan.
Acknowledgements
We thank the patients, physicians, nurses and research staffs at MCC. We thank Mrs. Beth Maddox and Christine M. Simonelli for their excellent work in data collection. This work was supported in part by the NIH/NCI grant number P30 CA 76292-11 (W.S.D.) and by Research Support from Millennium Pharmaceuticals Inc. Correlative studies were performed at the Translational Core at MCC (PI: Christopher Cubitt, PhD).
Footnotes
D.S., R.B., L.P., M.A.KD., J.L.OB., H.F.F., J.R., and M.A. performed the research, T.J.A., C.A., and M.A. designed the research study, K.S., D.N.Y., and V.O. contributed to the DNA repair pathway analysis, T.N., K.S., W.F. G.H., J.K., DT.C., C.A., and M.A. analysed the data and T.N., J.P., C.A., and M.A. wrote the paper.
Disclosure of Conflicts of Interest Declared conflicts of interest include: MA Consultant and Research support from Millennium Pharmaceuticals Inc.
References
- Alexanian R, Dimopoulos M. The treatment of multiple myeloma. N Engl J Med. 1994;330:484–489. doi: 10.1056/NEJM199402173300709. [DOI] [PubMed] [Google Scholar]
- Alexanian R, Weber D, Delasalle K, Handy B, Champlin R, Giralt S. Clinical outcomes with intensive therapy for patients with primary resistant multiple myeloma. Bone Marrow Transplant. 2004;34:229–234. doi: 10.1038/sj.bmt.1704562. [DOI] [PubMed] [Google Scholar]
- Andela VB, Schwarz EM, Puzas JE, O’Keefe RJ, Rosier RN. Tumor metastasis and the reciprocal regulation of prometastatic and antimetastatic factors by nuclear factor kappaB. Cancer Res. 2000;60:6557–6562. [PubMed] [Google Scholar]
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- Berenson J, Yang H, Sadler K, Jarutirasarn S, Vescio R, Mapes R, Purner M, Lee S, Wilson J, Morrison B, Adams J, Schenkein D, Swift R. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24:937–944. doi: 10.1200/JCO.2005.03.2383. [DOI] [PubMed] [Google Scholar]
- Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, Bradley S, Gobburu JV, Goheer A, Lee SL, Leighton J, Liang CY, Lostritto RT, McGuinn WD, Morse DE, Rahman A, Rosario LA, Verbois SL, Williams G, Wang YC, Pazdur R. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- Chen Q, Van der Sluis P, Boulware D, Hazlehurst L, Dalton W. The FA/BRCA pathway is involved in melphalan-induced DNA interstrand cross-link repair and accounts for melphalan resistance in multiple myeloma cells. Blood. 2005;106:698–705. doi: 10.1182/blood-2004-11-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- Dalton WS, Hazlehurst L, Shain K, Landowski T, Alsina M. Targeting the bone marrow microenvironment in hematologic malignancies. Semin Hematol. 2004;41:1–5. doi: 10.1053/j.seminhematol.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- Damiano JS, Hazlehurst LA, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia. 2001;15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- Drake M, Iacobelli S, van Biezen A, Morris C, Apperley J, Niederwieser D, Björkstrand B, Gahrton G, Net E.G.f.B.a.M.T.a.t.E.L. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 2010;95:804–809. doi: 10.3324/haematol.2009.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie B, Harousseau J, Miguel J, Bladé J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar S. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR, Buadi FK, Hogan WJ. Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood. 2010;115:2348–2353. doi: 10.1182/blood-2009-07-235531. quiz 2560. [DOI] [PubMed] [Google Scholar]
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, Lenain P, Hulin C, Facon T, Casassus P, Michallet M, Maisonneuve H, Benboubker L, Maloisel F, Petillon MO, Webb I, Mathiot C, Moreau P. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- Hazlehurst L, Enkemann S, Beam C, Argilagos R, Painter J, Shain K, Saporta S, Boulware D, Moscinski L, Alsina M, Dalton W. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Res. 2003;63:7900–7906. [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, Ikeda H, Fox EA, D’Andrea AD. Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002;297:606–609. doi: 10.1126/science.1073834. [DOI] [PubMed] [Google Scholar]
- Huang Y, Johnson KR, Norris JS, Fan W. Nuclear factor-kappaB/IkappaB signaling pathway may contribute to the mediation of paclitaxel-induced apoptosis in solid tumor cells. Cancer Res. 2000;60:4426–4432. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee S, Hwangbo B, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. NF-kappaB activation is related to the resistance of lung cancer cells to TNF-alpha-induced apoptosis. Biochem Biophys Res Commun. 2000;273:140–146. doi: 10.1006/bbrc.2000.2909. [DOI] [PubMed] [Google Scholar]
- Koreth J, Cutler C, Djulbegovic B, Behl R, Schlossman R, Munshi N, Richardson P, Anderson K, Soiffer R, Alyea E.r. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13:183–196. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kharfan-Dabaja M, Glasmacher A, Djulbegovic B. Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: a systematic review and meta-analysis. J Natl Cancer Inst. 2009;101:100–106. doi: 10.1093/jnci/djn439. [DOI] [PubMed] [Google Scholar]
- Kumar S, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Geyer S, Allmer C, Witzig TE, Lust JA, Greipp PR, Kyle RA, Litzow MR, Gertz MA. High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant. 2004;34:161–167. doi: 10.1038/sj.bmt.1704545. [DOI] [PubMed] [Google Scholar]
- Kumar S, Rajkumar S, Dispenzieri A, Lacy M, Hayman S, Buadi F, Zeldenrust S, Dingli D, Russell S, Lust J, Greipp P, Kyle R, Gertz M. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonial S, Kaufman J, Tighiouart M, Nooka A, Langston AA, Heffner LT, Torre C, McMillan S, Renfroe H, Harvey RD, Lechowicz MJ, Khoury HJ, Flowers CR, Waller EK. A phase I/II trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res. 2010;16:5079–5086. doi: 10.1158/1078-0432.CCR-10-1662. [DOI] [PubMed] [Google Scholar]
- Ma MH, Yang HH, Parker K, Manyak S, Friedman JM, Altamirano C, Wu ZQ, Borad MJ, Frantzen M, Roussos E, Neeser J, Mikail A, Adams J, Sjak-Shie N, Vescio RA, Berenson JR. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–1144. [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades C, Richardson P, Poulaki V, Tai Y, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann T, Schlossman R, Munshi N, Hideshima T, Anderson K. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- Mudry RE, Fortney JE, York T, Hall BM, Gibson LF. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96:1926–1932. [PubMed] [Google Scholar]
- Nakanishi K, Yang YG, Pierce AJ, Taniguchi T, Digweed M, D’Andrea AD, Wang ZQ, Jasin M. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc Natl Acad Sci U S A. 2005;102:1110–1115. doi: 10.1073/pnas.0407796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Rajkumar S, Jacobus S, Callander N, Fonseca R, Vesole D, Williams M, Abonour R, Siegel D, Katz M, Greipp P, Group ECO. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar S, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski R, Kuter D, Limentani S, Lee S, Hideshima T, Esseltine D, Kauffman M, Adams J, Schenkein D, Anderson K. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson P, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, Harousseau J, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel J, Bladé J, Boccadoro M, Cavenagh J, Dalton W, Boral A, Esseltine D, Porter J, Schenkein D, Anderson K. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- Richardson P, Weller E, Lonial S, Jakubowiak A, Jagannath S, Raje N, Avigan D, Xie W, Ghobrial I, Schlossman R, Mazumder A, Munshi N, Vesole D, Joyce R, Kaufman J, Doss D, Warren D, Lunde L, Kaster S, Delaney C, Hideshima T, Mitsiades C, Knight R, Esseltine D, Anderson K. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M, Moreau P, Huynh A, Mary J, Danho C, Caillot D, Hulin C, Fruchart C, Marit G, Pégourié B, Lenain P, Araujo C, Kolb B, Randriamalala E, Royer B, Stoppa A, Dib M, Dorvaux V, Garderet L, Mathiot C, Avet-Loiseau H, Harousseau J, Attal M, (IFM) I.F.d.M. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM) Blood. 2010;115:32–37. doi: 10.1182/blood-2009-06-229658. [DOI] [PubMed] [Google Scholar]
- San Miguel J, Schlag R, Khuageva N, Dimopoulos M, Shpilberg O, Kropff M, Spicka I, Petrucci M, Palumbo A, Samoilova O, Dmoszynska A, Abdulkadyrov K, Schots R, Jiang B, Mateos M, Anderson K, Esseltine D, Liu K, Cakana A, van de Velde H, Richardson P. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Sher T, Miller K, Deeb G, Lee K, Chanan-Khan A. Plasma cell leukaemia and other aggressive plasma cell malignancies. Br J Haematol. 2010;150:418–427. doi: 10.1111/j.1365-2141.2010.08157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal S, Powles R, Sirohi B, Treleaven J, Kulkarni S, Mehta J. Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and autotransplantation in myeloma. Bone Marrow Transplant. 2002;30:673–679. doi: 10.1038/sj.bmt.1703717. [DOI] [PubMed] [Google Scholar]
- Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol. 2006;7:739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- Vesole D, Barlogie B, Jagannath S, Cheson B, Tricot G, Alexanian R, Crowley J. High-dose therapy for refractory multiple myeloma: improved prognosis with better supportive care and double transplants. Blood. 1994;84:950–956. [PubMed] [Google Scholar]
- Woodruff RK, Wadsworth J, Malpas JS, Tobias JS. Clinical staging in multiple myeloma. Br J Haematol. 1979;42:199–205. doi: 10.1111/j.1365-2141.1979.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Yarde DN, Oliveira V, Mathews L, Wang X, Villagra A, Boulware D, Shain KH, Hazlehurst LA, Alsina M, Chen DT, Beg AA, Dalton WS. Targeting the Fanconi anemia/BRCA pathway circumvents drug resistance in multiple myeloma. Cancer Res. 2009;69:9367–9375. doi: 10.1158/0008-5472.CAN-09-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]