Abstract
Objectives
To determine the relationships between conventional and segmentation-derived optical coherence tomography (OCT) retinal layer thickness measures with intracranial volume (a surrogate of head size), and brain-substructure volumes in multiple sclerosis.
Design
Cross-sectional study
Setting
Johns Hopkins, Baltimore, Maryland
Participants
84 multiple sclerosis patients and 24 healthy controls
Main outcome measures
High-definition spectral-domain OCT conventional and automated segmentation-derived discrete retinal layer thicknesses, and 3-Tesla MRI brain-substructure volumes
Results
Peripapillary retinal nerve fiber layer and composite ganglion cell layer + inner plexiform layer (GCIP) thicknesses in multiple sclerosis eyes without a history of optic neuritis were associated with cortical-gray matter (p=0.01 and p=0.04 respectively) and caudate volumes (p=0.04 and p=0.03 respectively). Inner nuclear layer thickness, also in eyes without a prior history of optic neuritis, was associated with FLAIR-lesion volume (p=0.007), and inversely associated with normal appearing white matter volume (p=0.005) in relapsing-remitting multiple sclerosis. As intracranial volume was found to be related with several of the OCT measures in multiple sclerosis patients and healthy controls, and is already known to be associated with brain-substructure volumes, all OCT-brain-substructure relationships were adjusted for intracranial volume.
Conclusions
Retinal measures reflect global central nervous system pathology in multiple sclerosis, with thicknesses of discrete retinal layers each appearing to be associated with distinct central nervous system processes. Moreover, OCT measures appear to correlate with intracranial volume in multiple sclerosis and healthy controls, an important unexpected factor unaccounted for in prior studies examining the relationships between peripapillary retinal nerve fiber layer thickness and brain-substructure volumes.
Keywords: Optical coherence tomography, retinal segmentation, multiple sclerosis, brain-substructure volumes, gray matter pathology
Introduction
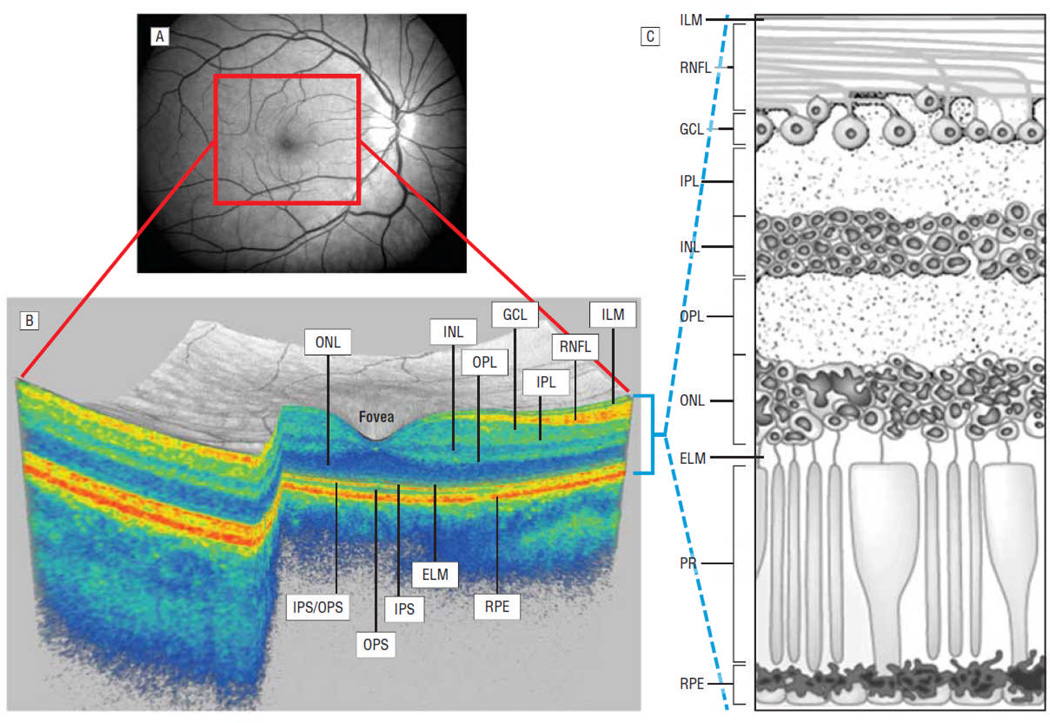
Optical coherence tomography (OCT) is a high-resolution imaging technique enabling the quantitative estimation of peripapillary-retinal nerve fiber layer (p-RNFL) thickness. In addition, modern high-definition spectral-domain OCT renders high resolution images, from which the individual retinal layers can also be objectively and precisely quantified. These include the macular-RNFL (m-RNFL), ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL) (Figure1).1–4 The RNFL is the innermost layer of the retina and is composed of unmyelinated axons. These axons, which are derived from the ganglion cell neurons located in the GCL below the RNFL (Figure1), coalesce at the optic discs to form the optic nerves, and subsequently exit through the lamina cribrosa to become myelinated.5, 6
Figure 1.
Panel A represents a fundus photograph from a healthy control. Panel B is a 3-D macular volume cube generated by Cirrus HD-OCT from the macular region denoted by the red box in Panel A from the same healthy control. Note the individual layers of the retina are readily discernible, except for the ganglion cell layer (GCL) and inner plexform layer (IPL) which are difficult to distinguish. During the segmentation process (performed in 3-D), the segmentation software identifies the outer boundaries of the macular retinal nerve fiber layer (RNFL), inner plexiform layer (IPL) and outer plexiform layer (OPL), as well as the inner boundary of the retinal pigment epithelium (RPE) which is identified by the conventional Cirrus HD-OCT algorithm. The identification of these boundaries facilitates OCT-segmentation, enabling determination of the thicknesses of the macular-RNFL, GCL+IPL (GCIP), the inner nuclear layer (INL)+OPL, and the outer nuclear layer (ONL) including the inner and outer photoreceptor segments. Panel C illustrates the cellular composition of the retinal layers depicted in panel B.
Abbreviations; IS: inner photoreceptor segments, OS: outer photoreceptor segments, IS/OS: IS/OS junction, PR: photoreceptors, ILM: inner limiting membrane, ELM: external limiting membrane
Multiple sclerosis (MS) has a predilection to affect the optic nerves clinically (e.g. from optic neuritis; ON) and sub-clinically, such that 94–99% of MS patients demonstrate demyelinating optic nerve lesions on post-mortem examination.7, 8 Optic nerve demyelination is thought to result in retrograde degeneration of the constituent axons of the optic nerve, leading to RNFL and GCL atrophy. 9–11 Consistent with post-mortem findings,9, 10 OCT demonstrates lower p-RNFL and GCL thicknesses in MS eyes, as compared to healthy controls, irrespective of a history of ON.2, 3, 12, 13 Although reduced RNFL and GCL thicknesses in MS are thought to derive from the same pathologic process (optic neuropathy), GCL thickness appears to correlate better than p-RNFL thickness with visual function and disability in MS.2 This observation may represent, at least in part, the superior reproducibility of GCL over RNFL thickness measurements.1, 2
In addition to inner retinal (RNFL and GCL) pathology, deeper retinal (INL and ONL) pathology also occurs in MS. Consistent with electroretinographic and post-mortem findings,9, 14–17 OCT-segmentation demonstrates quantitative INL and ONL abnormalities in MS.1, 2, 4 Although their etiology remains to be elucidated, INL and ONL pathology in MS may be the result of primary retinal mechanisms of pathology, rather than optic nerve mediated retrograde transynaptic degeneration,1–4 since atrophy or dysfunction of the INL or ONL has not been demonstrated following optic nerve transection in animal and electrophysiologic studies.18–23 Interestingly, the presence of INL and/or ONL abnormalities may be associated with more rapid accumulation of disability in MS.1
The relationships of conventional and segmentation-derived OCT measures with brain-substructure volumes in MS are unclear. Although time-domain OCT-derived p-RNFL thickness has been shown to correlate with brain parenchymal fraction (BPF) in MS,24–27 potentially reflecting global CNS pathology, p-RNFL associations with brain-substructure volumes remain inconclusive.24–26, 28 Specifically, it is unclear whether p-RNFL thickness correlates with normal appearing cerebral white matter (NAWM), white matter (WM)-lesion, or cortical-gray matter (GM) volumes, making it difficult to ascertain the aspects of global CNS pathology reflected by p-RNFL thickness. As these prior studies utilized time-domain OCT, which has lower reproducibility and resolution than newer spectral-domain devices,29, 30 the association between spectral-domain OCT-derived p-RNFL thickness and brain-substructure volumes in MS remains largely unexplored. Moreover, since OCT-segmentation was unavailable at the time of these studies, the associations between GCL, INL and ONL thicknesses with brain-substructure volumes in MS were not examined. Finally, brain-substructure volumes correlate with ICV, a surrogate of head size. Consequently, individual brain-substructure volumes are conventionally normalized or adjusted for ICV to account for these relationships.31 Since the retina is part of the central nervous system, it is plausible that retinal layer thicknesses may also correlate with ICV, although this has not been previously assessed. The identification of a relationship between OCT measures and ICV, would suggest the need to adjust examined OCT-brain-substructure relationships for ICV.
Since RNFL and GCL pathology in MS are related, it is plausible that RNFL and GCL thicknesses may reflect similar global CNS processes in MS. Conversely, since INL and ONL pathology in MS may be unrelated to optic neuropathy, it is plausible that INL and ONL thicknesses in MS may reflect global CNS processes distinct from those reflected by RNFL and GCL thicknesses. It is also unclear if INL and ONL pathology in MS may reflect the same or different global CNS processes as one another.
The principal objectives of this cross-sectional study were:
To determine if OCT-derived thicknesses and intracranial volume are related, so that these associations, in addition to the known associations between brain-substructure volumes and ICV,31 may be accounted for when examining OCT-brain-substructure relationships.
To determine the relationships of OCT-derived RNFL and GCL thicknesses with NAWM, WM-lesion, and cortical-GM volumes in MS, and whether these relationships are similar or different.
To determine the relationships of OCT-derived INL and ONL thicknesses with NAWM, WM-lesion, and cortical-GM volumes in MS, and whether these relationships are similar or different.
An exploratory objective was to ascertain whether OCT-derived retinal thicknesses were related with other brain-substructure volumes (e.g. deep GM structures, cerebellum, and brainstem), perhaps reflecting other aspects of MS-related neurodegeneration.
Methods
Patients
Johns Hopkins University Institutional Review Board approval was obtained for all study protocols, and written informed consent was obtained from recruited participants. MS subjects, recruited by convenience sampling from the Johns Hopkins Multiple Sclerosis Center, had their diagnosis confirmed by the treating neurologist (PAC) based on 2010 revised McDonald criteria.32 MS disease subtype was classified as relapsing-remitting (RRMS), secondary-progressive (SPMS), or primary-progressive MS (PPMS).33 Expanded Disability Status Scale (EDSS) scores were determined by a Neurostatus-certified EDSS examiner within 30-days of OCT and MRI examinations. Patient medical records were reviewed to determine MS disease-duration and history of ON, including date and side. Subjects with refractive errors of +/− six-diopters, other neurologic disorders, known ocular pathology, and/or diabetes were excluded from the study. To minimize confounding of OCT measurements and MRI-derived brain-substructure volumes, patients within three-months of ON, and one-month of steroid therapy were excluded. Healthy controls (HCs) were recruited from amongst Johns Hopkins University staff.
Optical coherence tomography (OCT)
Retinal imaging was performed with spectral-domain Cirrus HD-OCT (model 4000, software version 5.0; Carl Zeiss Meditec, Dublin, California), as described in detail elsewhere.29, 30 Briefly, peri-papillary and macular data were obtained with the Optic Disc Cube 200×200 protocol and Macular Cube 512x128 protocol respectively. OCT scanning was performed by experienced technicians, and scans were monitored to ensure fixation was reliable.29 Scans with signal strength less than 7/10, or with artifact, were excluded from analyses.
Macular cube scans were analyzed in a blinded fashion utilizing segmentation software, as described in detail elsewhere.2, 3 Briefly, segmentation performed in 3-D identifies the ILM, the outer boundaries of the m-RNFL, inner plexiform layer (IPL), outer plexiform layer (OPL), and the inner boundary of the retinal pigment epithelium (Figure1). Following the identification of these boundaries, thicknesses of the m-RNFL, GCL+IPL (GCIP), INL+OPL, and ONL (including inner and outer photoreceptor segments) were calculated, in an annulus of inner radius 0.54mm, and outer radius 2.4mm, centered on the fovea. This segmentation protocol has been shown to be reproducible in MS and HCs (inter-rater intra-class correlation coefficients: 0.91–0.99 for all segmentation measurements described).1
Magnetic Resonance Imaging
Brain MRI was performed with a 3-tesla Philips Intera scanner (Philips Medical System, Best, The Netherlands). Two-axial whole-brain sequences without gaps were used: multi-slice fluid-attenuated inversion recovery (FLAIR; acquired resolution: 0.8x0.8x2.2 or 0.8x0.8x4.4mm; TE: 68ms; TR: 11s; TI: 2.8s; SENSE factor: 2; averages: 1); and 3-D magnetization-prepared rapid acquisition of gradient echoes (MPRAGE; acquired resolution: 1.1x1.1x1.1mm; TE: 6ms; TR: ~10ms; TI: 835ms; flip angle: 8deg; SENSE factor: 2; averages: 1).
Acquired images were analyzed with Lesion-TOADS (Topology-preserving Anatomy-Driven Segmentation), a validated, automated, segmentation method, described in detail elsewhere.34, 35 This technique computes ICV and parcellates the brain into its component structures, yielding the following brain-substructure volumes – FLAIR-WM-lesions, ventricular-CSF, sulcal-CSF, cortical-GM, total cerebral-WM (including lesions), cerebellar-GM, cerebellar-WM, caudate, putamen, thalamus and brainstem. NAWM volumes were calculated by subtracting FLAIR-lesion volumes from total cerebral-WM volumes. Cerebral volume fraction (CVF), analogous to BPF, was calculated by dividing (normalizing) the summed volume of brain substructures (excluding ventricular and sulcal CSF) by ICV. All segmentations were verified by a trained observer.
Statistical analyses
Statistical analyses were completed on STATA Version 11 (StataCorp, College Station, TX). As OCT measures from both eyes of participants were included in analyses, mixed-effects, multivariable linear regression (MER) models were used to account for within-subject, inter-eye correlations. Relationships between OCT measures and ICV were assessed using MER, adjusting for age and sex (and disease-duration in MS). Partial correlations adjusting for age and sex (and disease-duration in MS), but not accounting for within-subject inter-eye correlations, were also performed to illustrate the relationships between OCT measures and ICV. Because we found significant relationships between OCT measures and ICV in MS and HCs (see results), MER models were adjusted for ICV, in addition to age, sex and disease-duration, when assessing the relationships between OCT measures and MRI-derived brain-substructure volumes in MS. This means that the relationships between OCT measures and brain sub-structure volumes were also adjusted for ICV.
In comparisons of MRI-derived brain-substructure volumes between MS and HCs, and in the assessment of MRI-derived brain-substructure volume relationships with EDSS, individual brain-substructure volumes were normalized to ICV.31 For comparative analyses of OCT measures and normalized brain-substructure volumes between MS and HCs, MER adjusting for age and sex was utilized. To assess associations between OCT measures and normalized brain-substructure volumes with EDSS, MER adjusting for age, sex, disease-duration, and ON history was used. Statistical significance was defined as p < 0.05. As this was an exploratory study, reported p-values should be interpreted as descriptive. Accordingly, correction for multiple comparisons was not performed.36
Results
Eighty-four MS patients (161-eyes; 7-eyes were excluded as their OCT scans had inadequate signal strength) and 24 HCs (48-eyes) were included in the study (Table1). The mean age of the MS cohort was 37-years, and the majority of the cohort had RRMS (62%). The mean age of the HC cohort was 36-years. 26% of RRMS eyes (n=32) and 28% of SPMS eyes (n=5) had a prior history of ON.
Table 1.
Summary of demographics & clinical characteristics
| All MS | RRMS | SPMS | PPMS | HCs | |
|---|---|---|---|---|---|
| n (eyes) | 84 (161) | 58 (114) | 18 (33) | 8 (14) | 24 (48) |
| Age, years (SD) | 43.6 (13.0) | 37.4 (10.2) | 58.3 (4.9) | 55.0 (9.0) | 36.0 (11.1) |
| Females (%) | 61 (73%) | 44 (76%) | 14 (78%) | 3 (38%) | 18 (75%) |
| Eyes with ON history (%) | 37 (23%) | 32 (26%) | 5 (15%) | - | - |
| Disease-duration, years (SD) | 11.5 (9.7) | 7.5 (6.4) | 21.7 (8.4) | 15.8 (13.1) | - |
| pRNFL, µm (SD) | 84.5 (13.0) | 84.9 (13.4) | 81.0 (10.9) | 89.6 (12.1) | 91.9 (9.4) |
| GCIP, µm (SD) | 70.6 (9.6) | 70.8 (10.0) | 68.8 (9.1) | 73.3 (5.9) | 81.9 (4.9) |
| mRNFL, µm (SD) | 28.2 (5.3) | 28.3 (5.7) | 27.6 (4.2) | 29.0 (2.8) | 33.7 (3.4) |
| INL+OPL, µm (SD) | 64.8 (4.6) | 65.2 (4.7) | 63.0 (4.2) | 66.0 (3.6) | 65.0 (4.8) |
| ONL, µm (SD) | 118.8 (8.1) | 119 (7.9) | 117 (7.5) | 126.3 (7.7) | 119.8 (5.9) |
| CVF (SD) | 0.655 (0.04) | 0.662 (0.03) | 0.640 (0.02) | 0.633 (0.06) | 0.682 (0.03) |
| n-Total WM (SD) | 0.267 (0.02) | 0.269 (0.02) | 0.263 (0.02) | 0.258 (0.04) | 0.275 (0.02) |
| n-Total GM (SD) | 0.377 (0.02) | 0.382 (0.02) | 0.366 (0.02) | 0.365 (0.03) | 0.393 (0.02) |
| Median EDSS score (IQR) | 3 (2–6) | 2.5 (1.5–3.25) | 6 (6–6.5) | 6.25 (6–6.75) | - |
Data presented above represent means, with their corresponding standard deviation (SD), unless otherwise noted.
MS: Multiple Sclerosis, RRMS: Relapsing-Remitting MS, PPMS: Primary Progressive MS, HCs: Healthy Controls, ON: Optic Neuritis, pRNLF: peripapillary Retinal Nerve Fiber Layer, GCIP: Ganglion Cell Layer + Inner Plexiform Layer, mRNFL: macular RNFL, INL + OPL: Inner Nuclear Layer + Outer Plexiform Layer, ONL: Outer Nuclear Layer including the inner & outer photo-receptor segments, CVF: Cerebral Volume Fraction, n-Total WM: normalized Total White Matter volume, n-Total GM: normalized Total Gray Matter volume, EDSS: Expanded Disability Status Scale, IQR: Inter-Quartile Range
Associations between OCT measures and ICV
ICV was associated with GCIP (p=0.008), m-RNFL (p=0.004) and ONL (p=0.005) thicknesses, but not p-RNFL (p=0.27) or INL thicknesses (p=0.36) in MS (Table2, Figure2A). Partial correlation coefficients between ICV and OCT thickness measures in MS were as follows – p-RNFL:r=0.11;GCIP:r=0.25;m-RNFL:r=0.28;INL:r=0.10; ONL:r=0.28. In HCs, p-RNFL and GCIP thicknesses were also related with ICV (p=0.04 for both) (Table2, Figure2B). Moreover, partial correlation coefficients between ICV and OCT thickness measures were higher in HCs than MS, supporting biological relationships between OCT thickness measures and ICV – p-RNFL:r=0.39;GCIP:r=0.40; m-RNFL:r=0.38; INL:r=0.19; ONL:r=0.30.
Table 2.
Relationships between OCT measures and ICV in MS and HCs
| r-values (p-values) | ||
|---|---|---|
| OCT measure | MS | HCs |
| p-RNFL | 0.11 (0.27) | 0.39 (0.04) |
| GCIP | 0.25 (0.008) | 0.40 (0.04) |
| m-RNFL | 0.28 (0.004) | 0.38 (0.05) |
| INL+OPL | 0.10 (0.36) | 0.19 (0.34) |
| ONL | 0.28 (0.005) | 0.30 (0.13) |
MS: eyes = 161, HCs: eyes = 48
r-value entries represent partial correlation coefficients adjusted for age and sex (and disease-duration in MS). p-value entries generated with mixed effects linear regression accounting for within-subject inter-eye correlations, adjusting for age and sex (and disease-duration in MS). Significant results are indicated in boldface.
OCT: Optical Coherence Tomography, ICV: Intracranial Volume. Other abbreviations as per Table 1
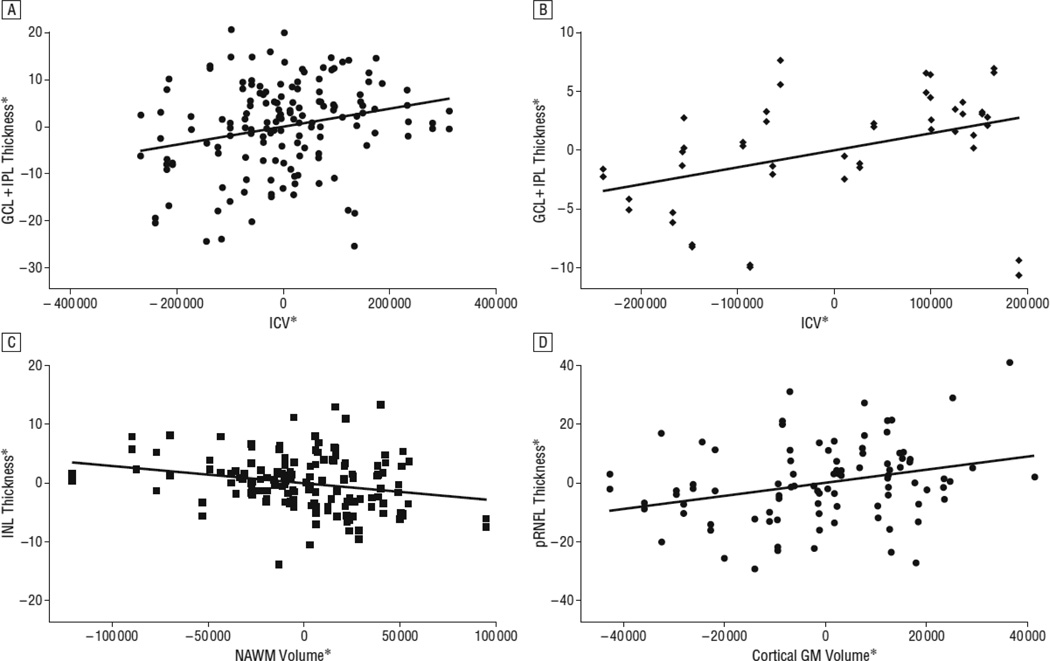
Figure 2.
*residual values from multivariate regression models
Panel A represents an adjusted variables plot of ganglion cell layer+inner plexiform layer (GCIP) thickness and intracranial volume (ICV) in multiple sclerosis (MS), adjusted for age, sex and disease duration. The solid red line graphically illustrates the independent relationship between GCIP thickness and ICV in MS. Note that as ICV increases, GCIP thickness similarly increases, consistent with the detection of significant associations between GCIP thickness and ICV in MS (p=0.008). Panel B represents an adjusted variables plot of ganglion cell layer+inner plexiform layer (GCIP) thickness and intracranial volume (ICV) in healthy controls (HCs), adjusted for age and sex. The solid red line graphically illustrates the independent relationship between GCIP thickness and ICV in HCs. Note that as ICV increases, GCIP thickness similarly increases, consistent with the detection of significant associations between GCIP thickness and ICV in HCs (p=0.04). Panel C represents an adjusted variables plot of inner nuclear layer (INL) thickness and normal appearing white matter (NAWM) volume in MS, adjusted for age, sex, disease duration and ICV. The solid red line graphically illustrates the independent relationship between INL thickness and NAWM volume in MS. Note that as INL thickness increases, NAWM volume decreases, consistent with the detection of significant inverse associations between INL thickness and NAWM volume in MS (p=0.01). Moreover, although not depicted in this figure, higher INL thickness was also associated with higher FLAIR-lesion volume in relapsing-remitting MS (RRMS) (p=0.02). Since INL pathology in MS is thought to result from primary retinal mechanisms of pathology, rather than being related to optic neuropathy, these findings raise the possibility that the potential mechanism underlying the proposed occurrence of primary retinal pathology affecting the INL in MS may be inflammatory, such as related to retinal periphlebitis. Panel D represents an adjusted variables plot of peri-papillary retinal nerve fiber layer (RNFL) thickness and cortical-gray matter (GM) volume in RRMS, adjusted for age, sex, disease duration and ICV. The solid red line graphically illustrates the independent relationship between RNFL thickness and cortical-GM volume in RRMS. Note that as RNFL thickness decreases, cortical-GM volume similarly decreases, consistent with the detection of significant associations between RNFL thickness and cortical-GM volume in RRMS (p=0.01).
Comparisons of OCT measures and brain-substructure volumes between MS and HCs
In accordance with published data,2, 4 p-RNFL and GCIP thicknesses were lower in MS than HCs (p=0.01 and p<0.001 respectively). Likewise, m-RNFL thickness was lower in all MS subtypes relative to HCs (p<0.001), consistent with the lower p-RNFL and GCIP thicknesses (Table1). Eyes with a history of ON had lower p-RNFL, GCIP, and m-RNFL thicknesses than eyes without a history of ON (p<0.001 for all).
CVF and normalized-total GM volume were lower in MS than HCs (p=0.02 and p=0.01 respectively), whereas normalized-total WM volume was not different between the two groups (p=0.19). Compared to HCs, MS patients demonstrated lower normalized-thalamic (−0.001+/−0.0003;p=0.003) and normalized-caudate volumes (−0.0007+/−0.0001;p<0.001). Normalized-cerebellar WM (−0.001+/−0.0004;p=0.008), normalized-cerebellar GM (−0.003+/−0.001;p=0.006), as well as normalized-brainstem (−0.001+/−0.003;p=0.001) volumes were also lower in MS than HCs.
GCIP (p=0.04) and m-RNFL (p=0.01) thicknesses, and normalized-NAWM volume (p=0.03), were inversely associated with EDSS in RRMS. Of OCT and MRI measures, only INL thickness was associated (inversely) with EDSS in SPMS (p=0.003) and PPMS (p=0.003).
Relationships between OCT measures and brain-substructure volumes in MS
Peripapillary-RNFL and GCIP thicknesses were associated with cortical-GM volume in the entire MS cohort (p=0.005 and p=0.04 respectively) (Table3). These associations were predominantly driven by relationships in eyes without a history of ON. Similarly, p-RNFL (p=0.04) and GCIP (p=0.03) thicknesses in non-ON eyes were also associated with caudate volume. INL thickness and NAWM volume were inversely associated in the entire MS cohort (p=0.01) (Figure2C), and again this association was predominantly driven by relationships in eyes without a history of ON.
Table 3.
Relationships between OCT measures and brain substructure volumes in MS
|
All Eyes (n=161): p-values | ||||||
| Cortical GM | NAWM | Caudate | Thalamus | Lesions | Brainstem | |
| P-RNFL | 0.005 | 0.98* | 0.05 | 0.18 | 0.78 | 0.10 |
| GCIP | 0.04 | 0.65 | 0.005 | 0.29 | 0.91 | 0.04 |
| m-RNFL | 0.44 | 0.73 | 0.007 | 0.09 | 0.73* | 0.28 |
| INL | 0.93* | 0.01* | 0.52* | 0.67* | 0.18 | 0.99 |
| ONL | 0.32 | 0.38* | 0.03 | 0.18 | 0.29* | 0.62 |
|
Non-ON Eyes (n=124): p-values | ||||||
| Cortical GM | NAWM | Caudate | Thalamus | Lesions | Brainstem | |
| P-RNFL | 0.01 | 0.84* | 0.04 | 0.12 | 0.88 | 0.18 |
| GCIP | 0.04 | 0.78* | 0.03 | 0.13 | 0.73* | 0.22 |
| m-RNFL | 0.53 | 0.68* | 0.04 | 0.03 | 0.50* | 0.72 |
| INL | 0.90* | 0.02* | 0.65* | 0.62* | 0.16 | 0.81 |
| ONL | 0.46 | 0.20* | 0.11 | 0.25 | 0.28* | 0.89* |
|
ON Eyes (n=37): p-values | ||||||
| Cortical GM | NAWM | Caudate | Thalamus | Lesions | Brainstem | |
| P-RNFL | 0.43 | 0.61* | 0.49 | 0.56 | 0.32 | 0.10 |
| GCIP | 0.94 | 0.58 | 0.24 | 0.95 | 0.69 | 0.16 |
| m-RNFL | 0.60* | 0.38 | 0.11 | 0.38 | 0.78* | 0.10 |
| INL | 0.42 | 0.83* | 0.78 | 0.89* | 0.69 | 0.67* |
| ONL | 0.19 | 0.80 | 0.09 | 0.27 | 0.27* | 0.97 |
p-value entries generated with mixed effects linear regression accounting for within-subject inter-eye correlations, adjusting for age, sex, disease-duration and intracranial volume (ICV).
In subgroup analyses, the majority of the above relationships were observed in RRMS (Figure2D, Table 4), with p-RNFL and GCIP thicknesses also associated with brainstem and cerebellar-WM volumes, and INL thickness in eyes without a history of ON also associated with FLAIR-lesion volume (p=0.007). Of OCT measurements, ONL thickness alone was only significantly associated with cerebellar-WM volume in RRMS eyes with a history of prior ON (p=0.03). However, ONL thickness in RRMS eyes with a history of ON also trended toward significant associations with cortical-GM (p=0.05), caudate (p=0.08), thalamus (p=0.08) and brainstem volumes (p=0.10), as well as an inverse association with FLAIR-lesion volume (p=0.08).
Table 4.
Relationships between OCT measures and brain substructure volumes in RRMS
|
All Eyes (n=114): p-values | |||||||
| Cortical GM | NAWM | Cerebellar WM | Caudate | Thalamus | Lesions | Brainstem | |
| p-RNFL | 0.01 | 0.76 | 0.03 | 0.008 | 0.24 | 0.20* | 0.003 |
| GCIP | 0.07 | 0.56 | 0.04 | 0.01 | 0.26 | 0.32* | 0.01 |
| m-RNFL | 0.57 | 0.50 | 0.13 | 0.003 | 0.07 | 0.08* | 0.23 |
| INL | 0.60 | 0.004* | 0.60* | 0.46* | 0.67* | 0.02 | 0.48 |
| ONL | 0.17 | 0.44* | 0.22 | 0.09 | 0.26 | 0.43* | 0.23 |
|
Non-ON Eyes (n=82): p-values | |||||||
| Cortical GM | NAWM | Cerebellar WM | Caudate | Thalamus | Lesions | Brainstem | |
| p-RNFL | 0.06 | 0.81* | 0.34 | 0.009 | 0.16 | 0.09* | 0.06 |
| GCIP | 0.06 | 0.93* | 0.24 | 0.04 | 0.13 | 0.07* | 0.20 |
| m-RNFL | 0.54 | 0.97* | 0.65 | 0.02 | 0.04 | 0.01* | 0.97* |
| INL | 0.56 | 0.005* | 0.43* | 0.64* | 0.61* | 0.007 | 0.43 |
| ONL | 0.30 | 0.21* | 0.57 | 0.39 | 0.50 | 0.45* | 0.52 |
|
ON Eyes (n=32): p-values | |||||||
| Cortical GM | NAWM | Cerebellar WM | Caudate | Thalamus | Lesions | Brainstem | |
| p-RNFL | 0.93* | 0.71* | 0.30 | 0.86 | 0.60* | 0.16 | 0.04 |
| GCIP | 0.56* | 0.73 | 0.79 | 0.50 | 0.70* | 0.56 | 0.15 |
| m-RNFL | 0.15* | 0.60 | 0.78 | 0.25 | 0.77 | 0.97 | 0.14 |
| INL | 0.41 | 0.79* | 0.94 | 0.78* | 0.84* | 0.74 | 0.99 |
| ONL | 0.05 | 0.42 | 0.03 | 0.07 | 0.08 | 0.08* | 0.10 |
p-value entries generated with mixed effects linear regression accounting for within-subject inter-eye correlations, adjusting for age, sex, disease-duration and intracranial volume (ICV).
Asterisk (*) indicates inverse relationships. Significant results are indicated in boldface.
In further subgroup analyses, ONL thickness was associated with cortical-GM volume in SPMS (p=0.04) and brainstem volume in PPMS (p=0.01). Although OCT measures and brain-substructure volumes were generally not correlated in SPMS, several associations, consistent with those demonstrated in RRMS, were also observed in PPMS (Supplementary Table). In addition to cortical-GM volume (p=0.002), p-RNFL thickness was associated with NAWM volume in PPMS (p=0.001).
Discussion
Results of this study suggest inner and outer retinal layer thicknesses, measured with spectral-domain OCT in MS, may reflect global and potentially distinct CNS processes, and that OCT may be a useful complementary in-vivo technique to MRI in evaluating MS. In particular, lower p-RNFL and GCIP thicknesses appear to reflect lower cortical-GM volume in MS, suggesting that measurements obtained with OCT, a relatively inexpensive, non-invasive, reproducible, rapid and well-tolerated investigation may partially represent clinically relevant processes known to affect the cortical-GM compartment in MS.6, 37–39 Furthermore, p-RNFL and GCIP thicknesses may also reflect caudate volume in MS, as well as brainstem and cerebellar-WM volumes in RRMS. Moreover, INL and ONL thicknesses, particularly in RRMS, may reflect global, although somewhat different CNS processes than those reflected by RNFL and GCIP thicknesses. Interestingly, higher INL thickness may be associated with higher FLAIR-lesion volume and lower NAWM volume. These findings raise the possibility that the potential mechanism underlying the proposed occurrence of primary retinal pathology affecting the INL in MS may be inflammatory,1, 2 such as related to retinal periphlebitis. Retinal periphlebitis is known to occur in up to 20% of MS patients,10, 40 and it has previously been shown that active retinal periphlebitis and disruption of the blood-brain-barrier tend to occur simultaneously in MS.41 Moreover, the presence of retinal periphlebitis in MS patients has been shown to be a risk factor for the subsequent development of relapses and gadolinium-enhancing lesions.28 ONL thickness also appears to have relationships with brain-substructure volumes. Lower ONL thickness appears to reflect lower cerebellar-WM volume, and moreover several additional trends suggesting potential associations between lower ONL thickness and lower cortical-GM, caudate, thalamic and brainstem volumes, as well as higher FLAIR-lesion volume, were observed, raising the possibility that ONL thickness may reflect the global nature of neurodegeneration in MS.
A conspicuously novel finding in this study, unaccounted for in prior studies assessing retinal-brain relationships, was the apparent relationship between OCT thickness measures with ICV. Since brain-substructure volumes correlate with ICV,31 individual brain-substructure volumes are conventionally normalized or adjusted for ICV to account for these associations.31 Our finding that OCT measures and ICV may be related, suggest that OCT measures should also be adjusted for ICV when assessing OCT-brain-substructure relationships. Of note, the majority of OCT measures examined in this study were associated with ICV in MS, and the correlation coefficients between OCT measures and ICV in HCs were higher than in MS, supporting the plausibility of a biological relationship. These findings merit further investigation in future studies.
As the optic nerve is frequently affected both clinically and subclinically in MS, the anterior visual system has been proposed as a model within which to study MS-related neurodegeneration.42 Demyelinated, yet intact, retrobulbar axons may be potentially remyelinated by viable oligodendrocytes, protecting against axonal (RNFL) and neuronal (GCL) degeneration. OCT has several compelling characteristics, including significant structure-function correlations (with vision and disability), which make it useful for detecting and monitoring neurodegeneration in MS, and for documenting potentially neuroprotective and/or neurorestorative effects of therapeutic agents.5, 6 Since our results suggest retinal layer thicknesses in MS may reflect global CNS pathology, it is possible that neuroprotective and/or neurorestorative effects detected in the anterior visual pathway with OCT might partially represent more global CNS effects, making OCT a potentially useful adjunct to MRI outcome measures in clinical trials.
Associations between p-RNFL, m-RNFL and GCIP thickness measures and brain-substructure volumes were similar, and most pronounced in eyes without a history of ON, suggesting that subclinical optic neuropathy may be an ideal surrogate of global neurodegeneration in MS. Although similar subclinical processes also likely occur in eyes with a history of ON, disproportionate local tissue degeneration following ON may potentially obscure these relationships. Higher INL thickness in non-ON eyes was associated with higher FLAIR-lesion volume and lower NAWM volume, raising the possibility that an inflammatory process, such as retinal periphlebitis, may be operative in the INL in MS eyes. However, determination of the underlying pathobiology of either INL or ONL changes was beyond the scope of this study. Nonetheless, our findings highlight that INL and ONL thicknesses in MS may reflect global CNS processes that differ from those reflected by RNFL and GCIP thicknesses, which is consistent with our previous work.1 Interestingly, INL and ONL thicknesses did not appear to reflect the same global CNS processes, raising the possibility that INL and ONL abnormalities in MS may be pathophysiologically distinct.
ONL thickness in eyes with a history of ON was associated with cerebellar-WM volume, and several trends were detected between ONL thickness and brain-substructure volumes in RRMS eyes with ON history. The lack of significance for some of these results is likely in part attributable to under-powering of eyes with a history of ON. Since selective photoreceptor pathology of undetermined etiology may occur in optic neuropathies,43 our observations raise the possibility that this process may likewise occur following MS related ON, and its occurrence may be a harbinger of increased susceptibility to neurodegeneration. INL and ONL pathology in MS warrants more focused and detailed study in the future.
This preliminary cross-sectional study has several limitations. First, the majority of patients included had RRMS. Thus, more accurate characterization of the relationships between OCT measures and brain-substructure volumes by MS subtype, which will require enrollment of greater numbers of SPMS and PPMS, as well as RRMS patients, is imperative. Furthermore, limited MS and HC enrollment, in part related to the cost of MRI, and under-powering may have contributed to the lack of significance for some of our analyses, particularly in the progressive cohorts.
Third, as this was an exploratory study, we did not adjust for multiple comparisons,36 thus it is conceivable that the identified relationships could have occurred by chance alone. However, since our results are consistent with and expand upon prior studies demonstrating relationships between p-RNFL thickness and whole brain volume (BPF), it is highly unlikely that all of our findings were spurious, and most of the observed associations likely reflect true biological relationships. Nonetheless, our findings require further confirmation with other OCT devices and segmentation techniques, as well as in other MS cohorts, and also need to be examined longitudinally.
Finally, this preliminary study does not explain the mechanisms underlying the relationships observed between OCT measures and brain-substructure volumes. For example, it is unclear why p-RNFL and GCIP thicknesses may reflect caudate, brainstem or cerebellar-WM volumes. While this could simply represent a reflection of global neurodegeneration, these potential relationships warrant further study, and may be illuminating to study longitudinally.
In summary, retinal axonal and neuronal layer thicknesses in MS may reflect global CNS processes. Lower p-RNFL and GCIP thicknesses related to subclinical optic neuropathy predominantly reflect lower cortical-GM and caudate volumes. INL and ONL thicknesses in MS may reflect global CNS processes somewhat distinct from one another, and those reflected by RNFL and GCIP thicknesses. Higher INL thickness in non-ON eyes (potentially reflecting inflammation) may reflect higher FLAIR-lesion and lower NAWM volumes, while ONL thickness in eyes with a history of ON may reflect global neurodegeneration. OCT measures may also correlate with ICV, a finding with potential implications for future studies examining retinal-brain relationships. Although our observations require verification, they provide support for the potential utility of OCT as an adjunctive outcome measure in MS clinical trials.
Supplementary Material
Acknowledgements
Drs Shiv Saidha & Peter Calabresi take full responsibility for the data, the analyses and their interpretation, and the conduct of the research, had full access to all of the data and have the right to publish any and all data separate and apart from any sponsor.
Study Funding: National Multiple Sclerosis Society (TR 3760-A-3 to P.A.C and RG 4212-A-4 to Laura J. Balcer subcontracted to P.A.C), National Eye Institute (R01 EY 014993 and R01 EY 019473 to Laura J. Balcer subcontracted to P.A.C), and Braxton Debbie Angela Dillon and Skip (DADS) Donor Advisor Fund (to P.A.C, E.M.F)
Funding
National Multiple Sclerosis Society (TR 3760-A-3 to P.A.C and RG 4212-A-4 to L.J.B subcontracted to P.A.C), National Eye Institute (R01 EY 014993 to L.J.B subcontracted to P.A.C), and Braxton Debbie Angela Dillon and Skip (DADS) Donor Advisor Fund (to P.A.C, E.M.F & L.J.B). The study was partially supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (to D.S.R).
Footnotes
Disclosures:
Dr. Shiv Saidha has received consulting fees from MedicalLogix for the development of continuing medical education programs in neurology, & educational grant support from Teva Neurosciences.
Dr. Jiwon Oh has received educational grant support from Teva Neurosciences
Mary Durbin, Jonathan Oakley & Scott Meyer are employed by Carl Zeiss Meditec Inc.
Dr. Scott Newsome has received speaker honoraria and consulting fees from Biogen Idec.
Dr. John Ratchford has consulted for Sun Pharmaceuticals.
Dr. Laura Balcer has received speaking and consulting honoraria from Biogen-Idec, Bayer, and Novartis.
Dr. Elliot Frohman has received speaker honoraria and consulting fees from Biogen Idec, TEVA, and Athena. He has also received consulting fees from Abbott Laboratories.
Dr. Ciprian Crainiceanu has received consulting fees from Merck and On-X.
Dr. Daniel Reich receives research support from the Intramural Research Program of the National Institute of Neurological Disorders and Stroke.
Dr. Peter Calabresi has provided consultation services to Novartis, EMD-Serono, Teva, Biogen-IDEC, Vertex, Vaccinex, Genzyme, Genentech; and has received grant support from EMD-Serono, Teva, Biogen-IDEC, Genentech, Bayer, Abbott, and Vertex.
Dr. Elias Sotirchos, Stephanie Syc, Michaela Seigo, Navid Shiee, Dr. Christopher Eckstein, Teresa Frohman, & Dr. Dzung Pham have no disclosures.
Author Contributions:
Study concept and design: Shiv Saidha, Laura Balcer, Elliot Frohman, Daniel Reich, Peter Calabresi
Acquisition of data: Shiv Saidha, Elias Sotirchos, Jiwon Oh, Stephanie Syc, Michaela Seigo, Navid Shiee, Mary Durbin, Jonathan Oakley, Scott Meyer, Dzung Pham, Daniel Reich, Peter Calabresi
Analysis and interpretation of data: Shiv Saidha, Ciprian Crainiceanu, Elias Sotirchos, Jiwon Oh, Daniel Reich, Peter Calabresi
Drafting of the manuscript: Shiv Saidha, Elias Sotirchos, Jiwon Oh, Stephanie Syc, Michaela Seigo, Navid Shiee, Christopher Eckstein, Mary Durbin, Jonathan Oakley, Scott Meyer, Teresa Frohman, Scott Newsome, John Ratchford, Laura Balcer, Elliot Frohman, Dzung Pham, Ciprian Crainiceanu, Daniel Reich, Peter Calabresi
Critical revision of the manuscript for important intellectual content: Shiv Saidha, Elias Sotirchos, Jiwon Oh, Stephanie Syc, Michaela Seigo, Navid Shiee, Christopher Eckstein, Mary Durbin, Jonathan Oakley, Scott Meyer, Teresa Frohman, Scott Newsome, John Ratchford, Laura Balcer, Elliot Frohman, Dzung Pham, Ciprian Crainiceanu, Daniel Reich, Peter Calabresi
Administrative, technical and material support: Shiv Saidha, Elias Sotirchos, Jiwon Oh, Stephanie Syc, Michaela Seigo, Navid Shiee, Mary Durbin, Jonathan Oakley, Scott Meyer, Dzung Pham, Daniel Reich, Peter Calabresi
Study supervision: Daniel Reich, Peter Calabresi
Competing interests
The authors report no conflicts of interest
References
- 1.Saidha S, Syc SB, Ibrahim MA, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134(Pt 2):518–533. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- 2.Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17(12):1449–1463. doi: 10.1177/1352458511418630. [DOI] [PubMed] [Google Scholar]
- 3.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(Pt 2):521–533. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seigo MA, Sotirchos ES, Newsome S, et al. In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol. 2012 doi: 10.1007/s00415-012-6466-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: A window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008;4(12):664–675. doi: 10.1038/ncpneuro0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saidha S, Eckstein C, Ratchford JN. Optical coherence tomography as a marker of axonal damage in multiple sclerosis. CML - Multiple Sclerosis. 2010;2(2):33–43. [Google Scholar]
- 7.Toussaint D, Perier O, Verstappen A, Bervoets S. Clinicopathological study of the visual pathways, eyes, and cerebral hemispheres in 32 cases of disseminated sclerosis. J Clin Neuroophthalmol. 1983;3(3):211–220. [PubMed] [Google Scholar]
- 8.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the united states. Neurology. 1976;26(6 PT 2):26–28. doi: 10.1212/wnl.26.6_part_2.26. [DOI] [PubMed] [Google Scholar]
- 9.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133(6):1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerrison JB, Flynn T, Green WR. Retinal pathologic changes in multiple sclerosis. Retina. 1994;14(5):445–451. doi: 10.1097/00006982-199414050-00010. [DOI] [PubMed] [Google Scholar]
- 11.Shindler KS, Ventura E, Dutt M, Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp Eye Res. 2008;87(3):208–213. doi: 10.1016/j.exer.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naismith RT, Xu J, Tutlam NT, Trinkaus K, Cross AH, Song SK. Radial diffusivity in remote optic neuritis discriminates visual outcomes. Neurology. 2010;74(21):1702–1710. doi: 10.1212/WNL.0b013e3181e0434d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papakostopoulos D, Fotiou F, Hart JC, Banerji NK. The electroretinogram in multiple sclerosis and demyelinating optic neuritis. Electroencephalogr Clin Neurophysiol. 1989;74(1):1–10. doi: 10.1016/0168-5597(89)90045-2. [DOI] [PubMed] [Google Scholar]
- 15.Forooghian F, Sproule M, Westall C, et al. Electroretinographic abnormalities in multiple sclerosis: Possible role for retinal autoantibodies. Doc Ophthalmol. 2006;113(2):123–132. doi: 10.1007/s10633-006-9022-0. [DOI] [PubMed] [Google Scholar]
- 16.Gundogan FC, Demirkaya S, Sobaci G. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis?--A structural and functional evaluation. Invest Ophthalmol Vis Sci. 2007;48(12):5773–5781. doi: 10.1167/iovs.07-0834. [DOI] [PubMed] [Google Scholar]
- 17.Gills JP., Jr Electroretinographic abnormalities and advanced multiple sclerosis. Invest Ophthalmol. 1966;5(6):555–559. [PubMed] [Google Scholar]
- 18.Hollander H, Bisti S, Maffei L, Hebel R. Electroretinographic responses and retrograde changes of retinal morphology after intracranial optic nerve section. A quantitative analysis in the cat. Exp Brain Res. 1984;55(3):483–493. doi: 10.1007/BF00235279. [DOI] [PubMed] [Google Scholar]
- 19.Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D'Anna SA, Kerrigan D, Pease ME. Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2001;42(5):975–982. [PubMed] [Google Scholar]
- 20.Williams RR, Cusato K, Raven MA, Reese BE. Organization of the inner retina following early elimination of the retinal ganglion cell population: Effects on cell numbers and stratification patterns. Vis Neurosci. 2001;18(2):233–244. doi: 10.1017/s0952523801182088. [DOI] [PubMed] [Google Scholar]
- 21.Dawson WW, Maida TM, Rubin ML. Human pattern-evoked retinal responses are altered by optic atrophy. Invest Ophthalmol Vis Sci. 1982;22(6):796–803. [PubMed] [Google Scholar]
- 22.Kaufman D, Celesia GG. Simultaneous recording of pattern electroretinogram and visual evoked responses in neuro-ophthalmologic disorders. Neurology. 1985;35(5):644–651. doi: 10.1212/wnl.35.5.644. [DOI] [PubMed] [Google Scholar]
- 23.Seiple W, Price MJ, Kupersmith M, Siegel IM, Carr RE. The pattern electroretinogram in optic nerve disease. Ophthalmology. 1983;90(9):1127–1132. doi: 10.1016/s0161-6420(83)80057-8. [DOI] [PubMed] [Google Scholar]
- 24.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69(16):1603–1609. doi: 10.1212/01.wnl.0000295995.46586.ae. [DOI] [PubMed] [Google Scholar]
- 25.Grazioli E, Zivadinov R, Weinstock-Guttman B, et al. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J Neurol Sci. 2008;268(1–2):12–17. doi: 10.1016/j.jns.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Siger M, Dziegielewski K, Jasek L, et al. Optical coherence tomography in multiple sclerosis: Thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol. 2008;255(10):1555–1560. doi: 10.1007/s00415-008-0985-5. [DOI] [PubMed] [Google Scholar]
- 27.Dorr J, Wernecke KD, Bock M, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6(4):e18132. doi: 10.1371/journal.pone.0018132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68(18):1488–1494. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- 29.Syc SB, Warner CV, Hiremath GS, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 2010;16(7):829–839. doi: 10.1177/1352458510371640. [DOI] [PubMed] [Google Scholar]
- 30.Warner CV, Syc SB, Stankiewicz AM, et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS One. 2011;6(8):e22947. doi: 10.1371/journal.pone.0022947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: Implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22(8):1483–1489. [PMC free article] [PubMed] [Google Scholar]
- 32.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: Results of an international survey. national multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996;46(4):907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 34.Carass A, Cuzzocreo J, Wheeler MB, Bazin PL, Resnick SM, Prince JL. Simple paradigm for extra-cerebral tissue removal: Algorithm and analysis. Neuroimage. 2011;56(4):1982–1992. doi: 10.1016/j.neuroimage.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiee N, Bazin PL, Ozturk A, Reich DS, Calabresi PA, Pham DL. A topologypreserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage. 2010;49(2):1524–1535. doi: 10.1016/j.neuroimage.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 37.Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA. Intracortical lesions in multiple sclerosis: Improved detection with 3D double inversionrecovery MR imaging. Radiology. 2005;236(1):254–260. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese M, Atzori M, Bernardi V, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol. 2007;254(9):1212–1220. doi: 10.1007/s00415-006-0503-6. [DOI] [PubMed] [Google Scholar]
- 39.Calabrese M, De Stefano N, Atzori M, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol. 2007;64(10):1416–1422. doi: 10.1001/archneur.64.10.1416. [DOI] [PubMed] [Google Scholar]
- 40.Rucker CW. Sheathing of the retinal veins in multiple sclerosis. review of pertinent literature. Mayo Clin Proc. 1972;47(5):335–340. [PubMed] [Google Scholar]
- 41.Engell T, Hvidberg A, Uhrenholdt A. Multiple sclerosis: Periphlebitis retinalis et cerebro-spinalis. A correlation between periphlebitis retinalis and abnormal technetium brain scintigraphy. Acta Neurol Scand. 1984;69(5):293–297. doi: 10.1111/j.1600-0404.1984.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 42.Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2010;9(9):921–932. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 43.Werner JS, Keltner JL, Zawadzki RJ, Choi SS. Outer retinal abnormalities associated with inner retinal pathology in nonglaucomatous and glaucomatous optic neuropathies. Eye (Lond) 2011;25(3):279–289. doi: 10.1038/eye.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.