Abstract
Rationale
Cocaine use is associated with cognitive impairment which impacts treatment outcome. A clearer understanding of those deficits, and whether particular environments exacerbate them, is needed.
Objectives
This study evaluated whether previously observed domain-specific cognitive deficits persisted following a three month cessation from chronic cocaine self-administration, as well as the impact of novel and cocaine-associated attentional distractors.
Methods
Control and experimental groups of monkeys performed stimulus discrimination, stimulus reversal, and delayed match-to-sample (DMS) tasks. After establishing post-cocaine baseline performance, we examined general distractibility in both groups, using brief novel distractors counterbalanced across each task. After testing the novel distractor, an identical approach was used for exposure to an appetitive distractor previously associated with cocaine in the experimental group, or water in the control group.
Results
Post-administration baseline performance was equivalent between groups on all tasks. In the cocaine group, stimulus discrimination was unaffected by either distractor, whereas reversal performance was disrupted by both the novel and appetitive distractors. DMS performance was impaired in the cocaine group in the presence of the novel distractor. The control group’s performance was not affected by the presentation of either distractor on any task.
Conclusion
Our results reveal that despite normalized performance between groups, there exists in the cocaine group a domain-specific latent vulnerability of cognitive performance to impairment by environmental distractors. The pattern of vulnerability recapitulates the frank impairments seen in drug free animals during an active self-administration phase. A greater impact of the cocaine-associated distractor over the novel one was not observed.
Keywords: cocaine, chronic, self-administration, reversal, cognition, macaque, cue, attention, attentional control, distractor
Introduction
Cocaine dependent individuals display selective cognitive deficits (Beatty et al. 1995; Bolla et al. 1998; Bolla et al. 2003; Ersche et al. 2010; Ersche and Sahakian 2007; Kaufman et al. 2003; O'Malley et al. 1992). Some are relatively specific, such as impairments in reversal performance, a measure of cognitive control, whereas others are more general, such as attentional impairments (Jovanovski et al. 2005). It might be predicted that there would be a selective interaction between attentional and other domain-specific cognitive deficits, however, this has been little studied outside of one report employing a working memory task (Hester et al. 2006). We previously demonstrated large impairments in cognitive control/flexibility, assessed using stimulus reversal performance, and more modest impairments in visual working memory in drug-free rhesus monkeys after extended chronic cocaine self-administration (Porter et al. 2011). Stimulus discrimination was not affected. Reversal impairments likely reflect damage to the orbitofrontal cortex, an area repeatedly shown to be abnormal in cocaine dependence (Bolla et al. 1998; Lucantonio et al. 2012; Volkow and Fowler 2000; Volkow et al. 1991) and addiction in general (Schoenbaum and Shaham 2008). The orbitofrontal cortex is implicated in the valuation of rewards and stimuli that represent them (Haber and Knutson 2010; Padoa-Schioppa and Assad 2006; Roesch and Olson 2004; Rolls 1996; Schoenbaum et al. 2003; Schultz 2000), but is also implicated in decision making and inhibitory control (Rolls and Grabenhorst 2008). Given the importance of these functions in maintenance of sobriety, it is important to evaluate whether environmental events, associated with drug use or otherwise, could selectively impair the proper function of a key brain region whose integrity is necessary for good decisions and self-control.
In the current study, following extended cessation from cocaine in a subset of the previous group we reported on (Porter et al. 2011), we again evaluated domain-specific cognitive performance. Unlike our previous work, animals were tested with and without two types of attentional distractors. One distractor was an appetitive compound contextual cue previously paired with either cocaine (in the experimental group), or water (in the control group). The other distractor was a novel compound stimulus. Given the importance of attention to cognitive function in general (Maunsell 2004), we predicted that an attentional challenge, such as the presentation of a distractor, would produce a selective pattern of impairment that would recapitulate the pattern of impairments seen during active cocaine self-administration, despite the fact that performance between the control and cocaine groups was equivalent following the extended cessation. Selective impairments by the distractors, despite equivalent performance in their absence, would represent a latent vulnerability consistent with long term dysfunction. Because of reports of greater attentional bias in favor of drug-related cues (Carpenter et al. 2006; Copersino et al. 2004; Ersche et al. 2010; Hester et al. 2006; Hester and Garavan 2009), we also predicted that a cocaine associated distractor would produce a greater impairment than the novel distractor in the cocaine experienced animals.
Methods
Subjects
Young adult (6–7 years old at the time of testing) male rhesus macaque monkeys (n=11) with previous drug exposure and extensive behavioral training were used for the present study. These monkeys participated in a previous study, which looked at the effects of chronic cocaine self-administration on cognition (Porter et al. 2011). We were only able to use a subset of animals for this study due to unexpected illness and loss of motivation to work. From the original group of 8 cocaine and 6 control animals, one cocaine animal was deceased due to lymphoma, and another was removed from the study due to behavioral problems. For the discrimination/reversal task, one cocaine animal failed to reach criteria during the discrimination blocks, leaving 6 control and 5 cocaine animals. For the DMS task, one of the controls was not cooperative, leaving 6 cocaine and 5 control monkeys. Animals were water-regulated 5 days a week and were supplemented to meet physiological needs at the end of each day following training and testing. Animal use conformed to the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Apparatus
All cognitive assessments took place in a sound attenuated chamber (Eckel Industries, Ontario, Canada, model AB4240) fitted with a 40 W house light and background white noise. The E-prime software suite (Psychology Software Tools, Pittsburgh, PA), coupled with a 15” touch screen (Elo systems CarrolTouch), was used for all stimulus presentation, recording of responses, and initial data processing.
Surgery
Prior to self-administration, all animals had a vascular access port placed midscapula from which a catheter extended subcutaneously to an internal jugular vein. The vascular access port allows percutaneous non-stressful access to vasculature for cocaine self-administration without the need for a protective jacket and with reduced risk of infection because nothing is external to the skin (Wojnicki et al. 1994).
Self-administration
The self-administration protocol and cognitive characterization of animals has been previously published (Porter et al. 2011). Animals self-administered by touching an abstract shape on the touch screen for the required number of touches. Once the response requirement was met, either cocaine (cocaine group) was administered intravenously via the vascular access port or water (control group) via a sipper tube. During the cocaine self-administration sessions, animals were allowed to self-administer up to 6 infusions of cocaine at a unit dose of 0.5 mg/kg, which they typically did. The cumulative amount of cocaine self-administered over a 12-month period ranged from 528–546 mg/kg.
Stimuli used as distractors
During the chronic cocaine or water self-administration, an audiovisual compound stimulus had been present for the entirety of each self-administration session, except during the time of reward delivery. This stimulus consisted of a distinct sequence of tones (rising or falling) and a distinct visual border around the screen. Because the stimulus was always present in the background during the self-administration session, it is considered to be a contextual cue. In that regard, it is important to note as well, that all self-administration and cognitive testing took place in identical chambers. Two compound stimuli used as distractors were counterbalanced across groups: half of the cocaine group and half of the control group saw an abstract “blue sun” border with descending tones (stimulus set 1). The other half of the each group saw a red “Navajo blanket” border and heard ascending tones (stimulus set 2). The stimulus set present during self-administration was the appetitive distractor, the other was the novel distractor.
Experimental timeline: counterbalancing of distractors across cognitive tasks
Animals had not self-administered cocaine for 3 months before we assessed whether novel or appetitive distractors would interfere with associative learning, reversal performance and/or working memory. Because the aim of the study was to assess selective attentional vulnerability across cognitive domains, we counterbalanced stimulus presentation across the different cognitive tasks. As a conservative approach, we chose to use an ascending order of predicted influence to avoid possible accelerated habituation of a novel distractor by an appetitive one. As a result, we did not counterbalance across cue types. We first evaluated the novel distractor across all domains, then repeated that with the appetitive distractor. Six baseline sessions for both cognitive tasks (no distractor presentation) were collected. Then, the novel distractor was presented during the stimulus discrimination/reversal and delayed match-to-sample (DMS) tasks. Table 1 shows a daily schedule for how the distractors were distributed across the cognitive tasks. We followed the same daily cue presentation schedule during cognitive assessment for both novel stimuli and appetitive distractors (Table 1). Establishing the post-cessation baseline, the novel distractor effects, and the appetitive distractor effects each took approx. one month, beginning at three months after cocaine self-administration ceased.
Table 1.
Schedule for counterbalancing stimulus presentations across tasks.
| Weekday: | Stimulus presented during this task: |
|---|---|
| Monday | Stimulus discrimination |
| Tuesday | Stimulus reversal |
| Wednesday | DMS (block 2, delay period only) |
| Thursday | Stimulus reversal |
| Friday | Stimulus discrimination |
Distractor presentation during stimulus discrimination and reversal tasks
We probed associative learning and cognitive control/flexibility using a stimulus discrimination/reversal task, similar to the task used previously to show reversal deficits resulting from cocaine exposure (Porter et al. 2011). For this experiment, we used a 2-object stimulus discrimination task instead of the 3-object stimulus discrimination task used previously because of motivational issues in some of the monkeys from both groups. Given inherent variability in cognitive performance data, we needed to ensure compliance in the small number of sessions used for distractor presentations. In brief, two stimuli were associated with a high or low water reward. A correct response was recorded when the monkey touched the stimulus associated with the high reward. Once a criterion of 27/30 correct responses on consecutive trials was reached, the reward contingency was reversed. Reaching the same performance criterion after the reversal resulted in presentation of a new set of stimuli for a discrimination block. Distractor presentations during the stimulus discrimination and reversal blocks were alternated across sessions. Animals performed 6 sessions per distractor type (novel or appetitive); 3 sessions with a distractor presented during the first 20 trials of stimulus discrimination component and 3 sessions in which the cues were presented during the first 20 trials of the reversal component of the task. This allowed us to counterbalance distractor presentations across trial types and minimize habituation (Table 1).
Distractor presentation during DMS task
We intermixed associative learning and reversal performance assessment with working memory assessment. The DMS task employed here is the same task used previously to assess working memory during self-administration (Porter et al. 2011). In brief, a sample stimulus would appear on the touch screen to start each trial. Pressing the sample stimulus accurately and holding it for 1s led to its offset and the start of a delay period (randomly selected from 0, 10, 20, or 40s). Following the delay period, the sample and a novel stimulus (randomly selected from the image pool) appeared, randomly assigned to the left or right side of the screen. Choosing the sample stimulus within 10 s following the presentation of the two stimuli led to a water reward (0.075 ml/kg). No reward was delivered for choosing the wrong stimulus or pressing the area outside of the choice stimuli. The inter-trial intervals for a correct response and incorrect response were two seconds and seven seconds, resp. Not responding within a 10 second window resulted in an omission. This task was performed once a week (Table 1), and was broken up into three blocks, in order to limit distractor exposure time. The first 60 trials (block 1) were performed in the absence of a distractor, trials 61–100 (block 2) were performed with a distractor presented for 7s during the delay period) and trials 101–160 (block 3) were again performed in the absence of a distractor.
Behavioral analysis
Stimulus discrimination and reversal task accuracy was averaged over the first 10 trials of the stimulus discrimination and reversal component, during which the distractor was presented. Because of the few number of sessions, we could not evaluate performance across the first fifteen trials in bins of three as previously reported in Porter et al., (2011), but for comparison purposes, data for the reversal task is shown in that format in Fig. 2c. For both distractor types, performance over the three sessions with distractors was compared with baseline performance (mean of six consecutive sessions without distractor stimulus presentation). A main effect of distractor type on stimulus discrimination and reversal performance was determined using repeated measures analysis of variance (RM ANOVA; SigmaStat 3.5). Post-hoc analysis was used for comparing each distractor type to baseline. All statistical analyses were conducted using absolute numbers. Results for stimulus discrimination and reversal tasks are presented as percent of baseline performance to facilitate visualization of effects.
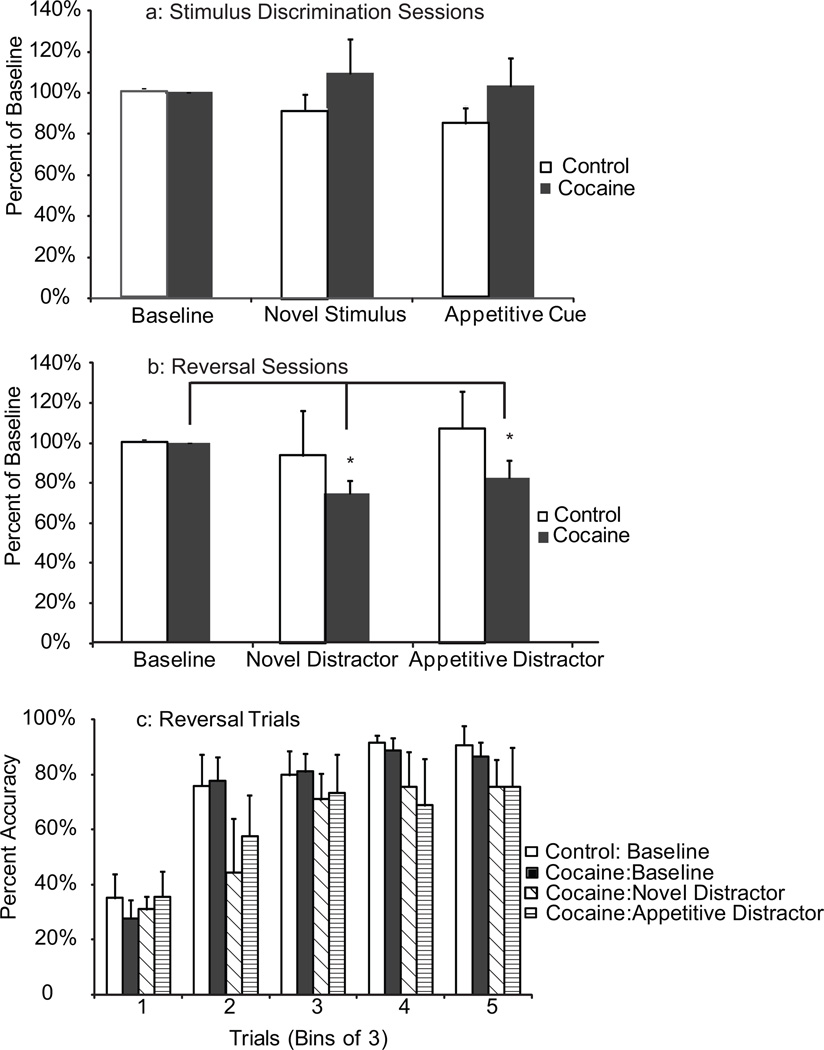
Fig. 2.
Accuracy on the stimulus discrimination and reversal tasks in the presence of distractors. (a) Neither the cocaine group nor the control group showed an impairment in stimulus discrimination performance in the presence of either distractor relative to baseline. (b) Within-group comparison of reversal performance during distractor presentation over the first 10 trials. (c) Progression of performance after reversal for both groups at baseline, and in presence of distractors within the cocaine group. white = control baseline, black = cocaine baseline, diagonal = cocaine novel, horizontal = cocaine appetitive
For the DMS task, baseline data were averaged over six consecutive sessions for comparison of performance between groups following cessation. Distractor effects on accuracy were determined over 3 sessions for each type (novel or appetitive). We conducted a within session analysis, comparing performance in the absence of distractor (block 1 and block 3) to performance in the presence of environmental distractors (block 2). Accuracy was analyzed over the first 20 trials during each block. For each stimulus type, we conducted a two way-RM ANOVA with two factors: block and delay interval. Differences between blocks at given delays were evaluated with Tukey post-hoc tests.
Results
Baseline cognitive assessments
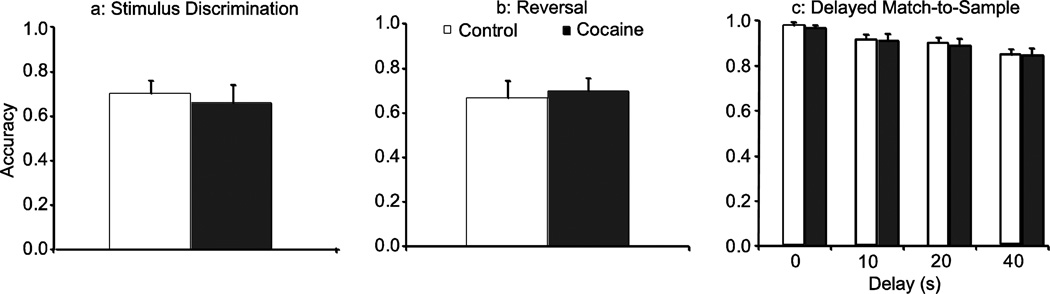
There were no significant between group differences in accuracy on stimulus discrimination (Fig. 1a), reversal performance (Fig. 1b), or the DMS task (Fig. 1c). It should be noted that because we employed a two stimulus discrimination/reversal task, there is a quicker increase in accuracy following reversal than previously observed with the three stimulus task used in Porter et al. (2011). Compare Fig. 2b from that paper to baseline performance in Fig 2c herein.
Fig. 1.
Baseline performance following 3 months cessation from cocaine on (a) stimulus discrimination, (b) reversal, and (c) DMS performance. There was no significant difference in performance between the groups on any task prior to distractor presentations
The effect of novel and appetitive distractors on stimulus discrimination and reversal performance
There was no significant effect of either the novel or appetitive distractor on stimulus discrimination in either of the groups (Fig. 2a). However, there was a main effect of distractor on reversal performance (Fig. 2b) relative to baseline in the cocaine group (F(4,2)=7.08; p=0.02). A follow up post hoc test revealed that performance during the novel (p<0.01) and the appetitive distractor (p=0.04) was decreased relative to baseline in the cocaine group. Fig. 2c illustrates the impact of each distractor across the first fifteen trials following stimulus reversal in the cocaine group relative to baseline performance, and also shows the control group baseline performance. Neither distractor had an effect on reversal performance in the control group.
The effect of novel and appetitive distractors on working memory performance
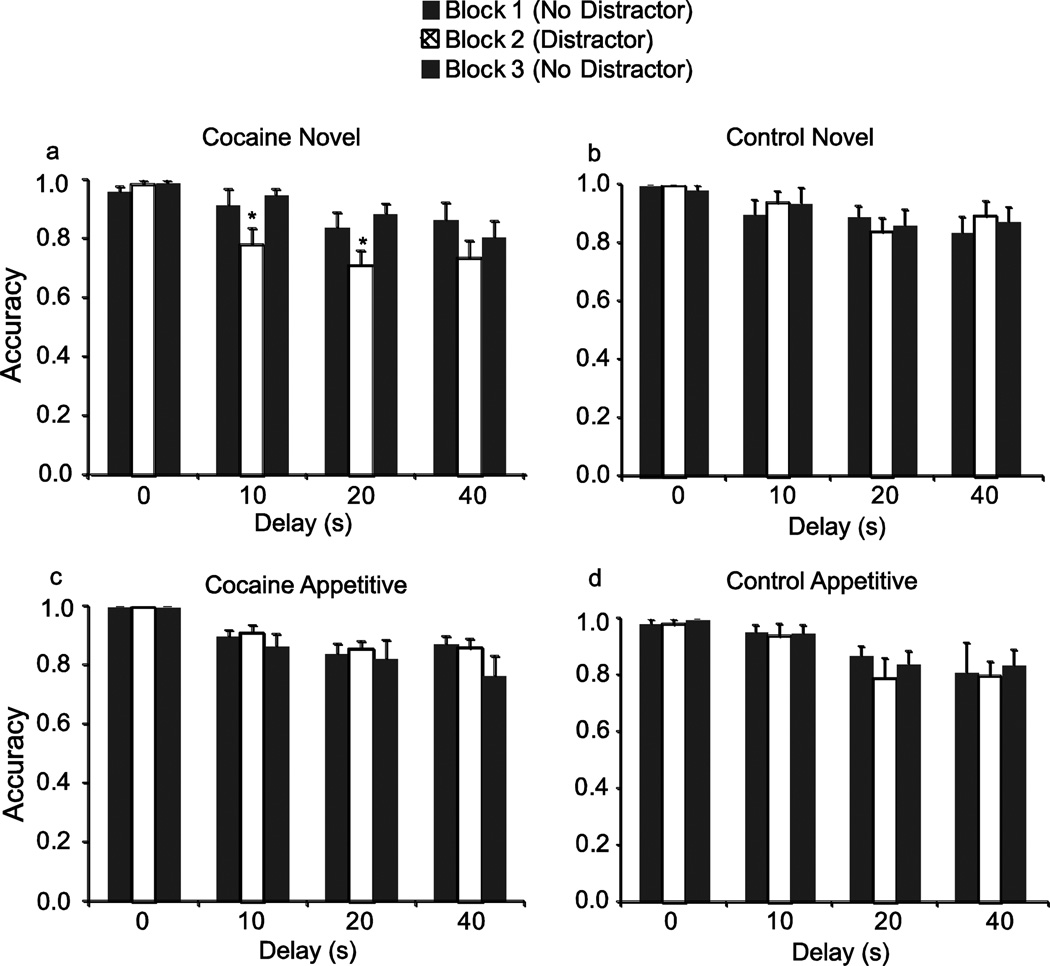
There was a main effect of the novel distractor (Fig. 3a) on working memory in the cocaine group (F(5,2)=5.02; p=0.031) relative to non-distractor blocks. There was no effect of the novel distractor on the control’s group performance (Fig. 3b). We did not observe an effect of the appetitive distractor on performance in either the cocaine group (Fig. 3d) or the control group (Fig. 3d).
Fig. 3.
Within session effect of attentional distractors on DMS performance. (a) In the cocaine group, there was a main effect of block (F(5,2)=5.02; p=0.031), with a decrease in performance in the presence of the novel distractor (block 2) relative to the non-stimulus blocks 1 and 3. (b) In the control group, there was no effect of the novel distractor on working memory. In the presence of appetitive cues, neither the (c) cocaine group nor (d) control group showed impaired performance. *p< 0.05 relative to non-cue blocks. Solid bars are blocks of trials without distractors, cross-hatched bars are distractor blocks.
Discussion
The primary aim of this study was to examine the effects of attentional distractors on domain specific cognition in control and cocaine exposed monkeys following an extended cessation, after which there were no differences in performance. Secondly, we assessed whether the presentation of “appetitive” distractors previously paired with either drug availability in the cocaine group, or water availability in the control group would disrupt cognitive performance more than a novel, though similar distractor. Our data show a selective vulnerability to attentional challenge in the cocaine group across the same cognitive domains that were impaired even without distractors when tested (in a drug-free state) during chronic cocaine self-administration. This indicates continuing long-term dysfunction consistent with orbitofrontal cortex damage. Given the role of anterior cingulate cortex in attentional control, it is potentially implicated as well. We did not see greater impairments with the cocaine associated distractor compared to the novel stimulus.
Long term domain-specific impairments
Following the long term cessation, cognitive performance across the three tasks employed was equivalent between the cocaine and control groups. Thus, the pattern of selective impairments in which reversal performance was greatly impaired and DMS performance was modestly impaired was no longer evident. However, given clinical observations of cocaine-associated structural changes in the orbitofrontal and anterior cingulate cortex (Bartzokis et al. 2002; Ersche et al. 2011; Franklin et al. 2002; O'Neill et al. 2001; Sim et al. 2007), we hypothesized that an attentional challenge could reveal latent continuing dysfunction. Our main finding is that there remains a latent impairment in reversal performance that can be revealed by an attentional challenge. In the cocaine group, reversal performance was impaired by environmental distractors, whether they were novel or drug associated, whereas there was no effect of either on stimulus discrimination performance. Neither the novel, nor the appetitive distractor had an effect on stimulus discrimination or reversal performance in the control group. These data are in line with previous findings which showed that chronic cocaine resulted in impaired reversal performance, while having no effect on stimulus discrimination performance in monkeys (Gould et al. 2012; Jentsch et al. 2002; Porter et al. 2011). An important question also is whether baseline performance was equivalent due to cessation of cocaine, or whether continued practice helped the animals to overcome deficits previously seen during active self-administration using a three stimulus task (different from the two stimulus task used in the present report). The difference in task structure, and the lack of a comparison group that continued to self-administer cocaine to control for practice effects makes this impossible to answer.
We also examined working memory performance in the presence of novel and appetitive distractors. The presence of the novel distractor impaired working memory performance in the cocaine group, relative to the non-distractor blocks. There was no effect of the novel distractor on working memory in the control group, and no effect of the appetitive distractor on working memory in either group. In our previous report examining the effect of chronic cocaine self-administration on working memory, the impairment was less robust than seen on reversal performance (Porter et al. 2011), similar to another recent report (Gould et al. 2012). In general, there are inconsistencies in the literature on whether working memory is impaired by cocaine exposure, with some reports indicating impairments in working memory (Bechara and Martin 2004; Hoff et al. 1996; Kubler et al. 2005; O'Malley et al. 1992; Verdejo-Garcia and Perez-Garcia 2007) whereas others do not (Bolla, 1999 #4540; Pace-Schott, 2008 #5884).
Thus, regardless of distractor type (novel or appetitive), we see a pattern of latent vulnerability in the cocaine group relative to the control group that recapitulates the frank dysfunction apparent previously, when chronically self-administering animals were tested drug free (72 hours post-cocaine). At that time, reversal performance was strongly impaired, DMS performance was weakly impaired, and stimulus discrimination performance was unimpaired.
Comparison of novel and appetitive distractors
We failed to see evidence of an attentional bias toward the appetitive distractor on any task. Despite the greater attention novel stimuli attract over habituated ones, we hypothesized that distractor stimuli repeatedly paired with drug exposure would be even more intrusive. Our intent to assess the impact of the distractors across multiple cognitive domains resulted in a design limitation in which the distractor order was not counterbalanced. Our conservative choice to initially expose animals to the novel distractor meant that evidence of greater impairment by the appetitive distractor in the cocaine group would have been strong evidence of attentional bias. However, the pattern of results we observed does not permit strong interpretive statements. There is the possibility of generalization of the novel and appetitive distractors. The same frequency tones were used as audio components in both compound stimuli used as distractors, with the only difference being whether they were ascending or descending. Likewise, though the visual components were distinct, there was some similarity in that each formed a border around the touchscreen. The pattern of results seen in the DMS task in particular suggests a habituation in that the weak impairment seen with the novel distractor was lost when the studies progressed to the appetitive distractor presentation. The observation that an impairment in reversal performance remained during appetitive distractor presentation, whereas the DMS performance was unimpaired, suggests the appetitive distractor was more intrusive on cognitive control/flexibility (reversal performance) than on working memory (DMS). In a relevant clinical study, Hester et al., (Hester and Garavan 2009) conducted a study into neural mechanisms underlying attentional bias to cocaine cues in cocaine users, while varying working memory load. When cocaine stimuli were presented, active cocaine users showed a significant decrease in accuracy and increase in response time under high working memory load compared to controls. In addition to the impact of the design limitations discussed above, our experiment differed from that of Hester et al. in that our monkeys had not received cocaine for at least 3 months before we conducted the experiments, whereas Hester et al. examined active cocaine users (Hester and Garavan 2009). In another clinical study, when cocaine dependent subjects performed a color-word drug Stroop fMRI task, hypoactivations in the rostro-ventral anterior cingulate and medial orbitofrontal cortex were associated with a greater likelihood of errors (Goldstein et al. 2007). The pattern of hypoactivation of orbitofrontal cortex and anterior cingulate cortex has been seen in many types of tasks (Bolla et al. 2004; Hester and Garavan 2004; Li et al. 2008). The rostral anterior cingulate has been implicated in attentional control and the orbitofrontal is critical for proper reversal performance. The enduring latent vulnerability to impairment by environmental distraction we observed in this controlled animal study is consistent with long lasting damage to these regions associated with drug exposure per se. Given that performance on cognitive tasks dependent on these areas is predictive of treatment outcome (Aharonovich et al. 2006; Aharonovich et al. 2003; Patkar et al. 2004; Streeter et al. 2008), understanding the neurobiological basis of the impairments we observed, and whether they respond to behavioral or pharmacotherapeutic approaches has the potential to improve clinical outcomes.
Acknowledgments
Supported by NIH/NIDA DA025636 and VA BLR&D 1IO1BX000782
Footnotes
conflict of interest: none
Experiments conducted herein comply with current US law.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug and Alcohol Dependence. 1995;37:247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. Journal of Neuropsychiatry & Clinical Neurosciences. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Serper MR, Vadhan N, Goldberg BR, Richarme D, Chou JC, Stitzer M, Cancro R. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Res. 2004;128:209–218. doi: 10.1016/j.psychres.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain : a journal of neurology. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Muller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Archives of General Psychiatry. 2010;67:632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Sahakian BJ. The Neuropsychology of Amphetamine and Opiate Dependence: Implications for Treatment. Neuropsychol Rev. 2007 doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Gage HD, Nader MA. Effects of Chronic Cocaine Self-Administration on Cognition and Cerebral Glucose Utilization in Rhesus Monkeys. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol Biochem Behav. 2009;93:270–277. doi: 10.1016/j.pbb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, Wang GJ, Volkow N. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60:167–176. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De la Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nature Neuroscience. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- O'Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18:131–144. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:1673–1682. [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis. 2004;23:109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal Activity Related to Reward Value and Motivation in Primate Frontal Cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex. [Review] [103 refs] Philosophical transactions of the Royal Society of London. 1996;351:1433–1443. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog Neurobiol. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33:827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal [see comments] American Journal of Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci. 1994;44:491–494. [PubMed] [Google Scholar]