Abstract
SIRT3 is a genomically expressed, mitochondrial localized tumor suppressor protein where it directs multiple metabolic processes by deacetylating downstream protein substrates. Genetic deletion of Sirt3 in mice leads to the spontaneous development of mammary tumors starting at one year and decreased SIRT3 mRNA has been observed in several human tumors including breast malignancies. In this investigation we assessed SIRT3 expression in human breast cancer tissue microarray and examined the relationship between SIRT3 expression and outcome in breast cancer patients. SIRT3 protein expression is significantly lower in neoplastic compared to normal breast epithelium, including normal epithelium adjacent to tumors. Breast cancer patients in the lowest quartile of SIRT3 expression had a significantly shorter locoregional relapse free survival [Hazard ratio = 0.53 (0.47–0.61), log rank p=0]. Notably, low SIRT3 expression was associated with reduced locoregional relapse-free survival in all breast cancer subtypes analyzed, including ER+, ER−, HER2+, and basal subtypes (Hazard ratios =0.44 to 0.65; log rank p=0 to 0.0019). These results highlight the importance of the SIRT3 as a tumor suppressor protein in breast cancer and suggest that SIRT3 may be a potential molecular biomarker to identify high risk patients across all molecular subtypes of breast cancer.
Keywords: Sirt3, Breast Cancer, Clinical Outcomes, Sirtuins, Metabolism, Mitochondria
INTRODUCTION
One of the fundamental observations in oncology is, that cancer is a disease of aging, and the rate of malignancies increases significantly with age (1). In fact, the single strongest prognostic variable that predicts the outcome of cancer is increasing age and this is especially true for breast malignancies. While the connection between aging and cancer has long been considered an important area of cancer research, the models to study this relationship have not been available (2, 3).
One idea that has emerged in the last ten years is that aging is a complex cellular process that appears to be regulated, at least in part, by a relatively new gene family referred to as sirtuins (4, 5). As genetic models for longevity advance, numerous works have been expanded to include the development of in vivo models to investigate the connection between sirtuins as tumor suppressor proteins and carcinogenesis (6, 7).
Sirtuins are Nicotinamide adenine dinucleotide (NAD+) dependent class III histone deacetylases are present from bacteria to humans (4). Unlike traditional histone deacetylases, sirtuins dynamically deacetylate a variety of substrates ranging from transcription factors to metabolic enzymes as well as histones (5). Sirtuins require NAD+ as a co-factor which makes them a metabolic sensor and connects their enzymatic activity to the energy and redox state of cells (5, 8). These proteins share a common 275-amino acid catalytic deacetylase domain and are localized to the nucleus (SIRT1, 6, and 7), mitochondria (SIRT3, 4, and 5), and cytoplasm (SIRT2) (9, 10). Unlike histone deacetyl transferases the sirtuins primarily target cellular proteins other than histones suggesting that these proteins are critical in the regulation of cell signaling networks similar to phosphatases and kinases (11). The mammalian sirtuin members are associated with numerous physiological roles, such as stress response, regulation of metabolism, gene silencing, and aging (12). As such, sirtuins appear to function as signaling proteins that post translationally alter the activity of downstream protein targets via acetylation.
Sirtuin activity can be increased in response to metabolic, genotoxic, oxidative, and osmotic stresses. These stress responses appear to link aging [19], oxidative stress, and the free-radical theory of aging (13). In this model it is proposed that there is a mechanistic connection between aberrant cellular reactive oxygen species levels and aging. Thus, it was proposed that an organism ages due to the unrepaired accumulation free radical damage to critical biomolecules as a function of time (14). For most biological structures, free radical damage is closely associated with oxidative damage (15). Therefore, this theory would predict that antioxidants or reducing agents, may limit oxidative damage to biological structures by detoxifying free radicals and preventing aging and age-related human illness (16).
We have recently shown that mice lacking Sirt3 develop breast malignancies that develop by one year of age suggesting that these mice may be a murine model to investigate the genetic and biochemical connection between aging and mammary tumors (6). In addition, it has also been shown that the Sirt3 knockout mice are permissive for other age-related illnesses including fatty liver (17), insulin resistance (18), and cardiac hypertrophy (19)]. These results identified Sirt3 as a more generalized mitochondrial fidelity protein and the mice lacking Sirt3 as useful in vivo models to investigate human diseases.
In addition to demonstrating that Sirt3 as a mitochondrial tumor suppressor protein in mice (6), we (6) and others (20) have shown that SIRT3 expression and protein levels are decreased in human breast cancer samples. In addition, one SIRT3 allele is deleted in roughly 40% of human breast cancer samples (20). Based on these results, it has been proposed that SIRT3 may be a human tumor suppressor protein in breast cancers; however, much more is required to make such a strong biological and physiological scientific argument. Here we examined SIRT3 expression by immunohistochemistry (IHC) of human breast cancer samples and examined the correlation between SIRT3 expression and patient outcome in a relatively large collection of human breast cancer cases.
Materials and Methods
Tissue microarrays (TMAs)
All procedures were performed in compliance with relevant laws and institutional guidelines with approval of institutional review board. Tissue micro-array (TMA) slides purchased from US Biomax (Rockville, MD) were used in the present study. The slides contain normal and malignant breast tissue cores. 1) TMA BR-954 contains invasive ductal carcinoma (IDC) (n=27) with matched ductal carcinoma in situ (DCIS) (n=3), matched lymph node metastasis (n=9) and matched normal breast tissue (n=5) with survival data available. 2) TMA BR-1921 contains invasive carcinomas (n=156), 18 cores of cancer adjacent normal tissue and 3 cores of normal breast tissue. Tumor characteristics were evaluated by a pathologist (M.M.D).
Immunohistochemistry (IHC)
The IHC protocol is as described previously (21). Briefly, the TMA slides were deparaffinized by incubation in xylene and ascending grades of alcohol. For antigen retrieval, slides were steamed for 15 minutes in 10 mM sodium citrate buffer (pH 6.0). Primary antibody (2 μg/ml anti-SIRT3, Cell Signaling, Beverly, MA) incubation was in 10% rabbit serum with biotin at 4°C overnight, followed by incubation with secondary peroxidase labeled anti-rabbit IgG (H+L) antibody in a concentration of 5 μg/ml. Color was developed by incubating slides with DAB kit (Vector Laboratories, Burlingame, CA) followed by counterstaining with Hematoxylin. All sections were examined with Olympus (BX53) microscope. The pictures were processed with cellSens Standard XV Image Processing software (Olympus Corporation of the Americas, Center Valley, PA). IHC scoring was performed by a board certified anatomic pathologist (MMD).
SIRT3 is mainly localized to the cytoplasm of mammary epithelial cells, with minimal staining in the stroma as previously shown (7). Tumor staining intensity was semiquantitatively scored as negative (<1% positive staining), weak/focal (1–10% positive), intermediate (11–50% positive) and strong (>50%) positive cytoplasmic depending on the percentage of positive cells and intensity of staining (7). Simple binary “negative/positive” SIRT3 status was used for analysis of The TMA. In the later, negative cases are those with less than 1% of positive cells and positive cases are those with 1% or more positive cells. Estrogen receptors (ER), Progesterone receptors (PR) and Her2 data by IHC obtained with the commercial TMA slides. Confirmation of the ER and PR status by IHC was done on TMA sections (BR1921) with antibodies to ER (SP1 clone; Ventana, Tucson, AZ; 1 μg/ml) and PR (1E2 clone; Ventana, Tucson, AZ; 1 μg/ml) and evaluated by the pathologist on the study (MMD). ER and PR were classified into positive and negative according to the ASCO/CAP guidelines with 1% or greater positive cells to be considered positive (22, 23)
Analysis of gene expression databases and patient survival
We examined the Breast Cancer Database in KM (24) (http://kmplot.com/analysis/, last accessed Jul 18, 2013). Locoregional relapse free survival was performed by analyzing low and high SIRT3 expressing tumors using the auto select best cutoff and the JetSet best probe set options. Biased arrays were excluded and the 2012 version of the database was used.
Statistical analysis
The mean scores of SIRT3 expression by IHC in normal and invasive/in situ lesions were analyzed by the Student’s t-Test. A linear correlation test was used to find out the correlation between SIRT3 expression and breast markers namely ER, PR and HER2 in invasive carcinomas. The IHC scores were considered nominal to find out significance. P ≤ 0.05 was considered significant. Chi square test was calculated as previously shown (25). An interactive calculation tool for chi-square tests of goodness of fit and independence [Computer software]”, available from http://quantpsy.org, last accessed July 31, 2013.
RESULTS
SIRT3 expression is reduced in breast ductal carcinoma in situ (DCIS) and invasive breast cancer
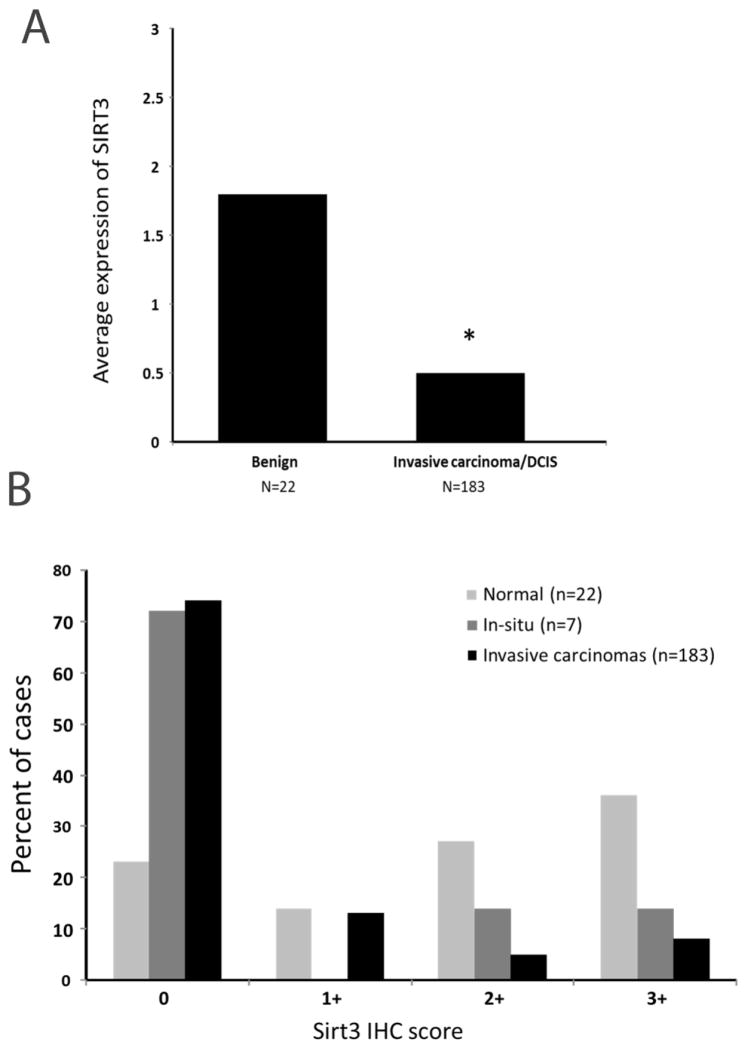
SIRT3 expression by IHC was consistently lower in neoplastic tumor cells compared to normal breast epithelial cells (Figure 1). The overall average score expression in normal breast duct epithelium was significantly higher (Figure 2A), as compared to that in invasive/in situ lesions (1.8 vs. 0.5, p<0.001). For example, while only 23% normal breast tissue were negative for SIRT3 expression, 72% of in situ breast lesions and 74% of invasive lesions were negative for the expression of SIRT3 (Figure 2B). There was no statistically significant difference in SIRT3 IHC expression among different age groups (using different age cut offs), across tumor types (IDC vs. invasive lobular carcinomas), and different tumor grades in invasive carcinomas (Table 1). There is statistically significant difference between SIRT3 expression and tumor sizes (pT) and lymph node status (pN) (Table 1). All metastatic carcinomas in lymph nodes (n=9) had corresponding SIRT3 staining with that of the primary breast tumor except 2 cases with weak/focal staining in the primary tumor and negative staining in the matched lymph node metastasis.
Figure 1.
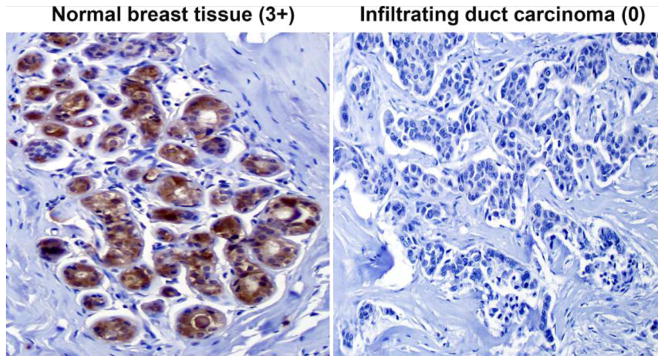
SIRT3 expression in normal ducts and invasive duct carcinoma of the breast (IDC) by IHC. SIRT3 is strongly expressed (3+) in the cytoplasmic component of normal breast ducts compared to negative (0) in IDC. Original magnifications, 200X.
Figure 2.
Average score expression of SIRT3 by IHC in normal and invasive/in situ breast carcinomas (A). Notice more than three-fold expression of SIRT3 in normal compared to breast carcinomas. * p<0.00. Expression of SIRT3 by IHC in normal, in situ and invasive breast carcinomas shows evidence for reduced SIRT3 expression in in-situ and invasive carcinoma compared to normal glands (B).
Table 1.
SIRT3 expression in infiltrating duct carcinoma of the breast according to patient’s age and histopathological characteristics.
| Variable | SIRT3 Immunohistochemistry
|
Total | P value/r | |
|---|---|---|---|---|
| Positive | Negative | |||
|
| ||||
| Patient’s age | NS/−0.05 | |||
| <50 years | 28 (27%) | 77 (73%) | 105 | |
| ≥ 50 years | 20 (25%) | 61 (75%) | 81 | |
|
| ||||
| Tumor type | NS | |||
| Infiltrating duct | 32 (%) | 74 (%) | 106 | |
| Infiltrating lobular | 14 (%) | 62 (%) | 76 | |
|
| ||||
| Tumor grade | NS/−0.16 | |||
| Grade 1–2 | 17 (26%) | 48 (74%) | 65 | |
| Grade 3 | 3 (23%) | 10 (77%) | 13 | |
|
| ||||
| Tumor size (pT) | <0.001/−0.03 | |||
| pT1 | 4 (27%) | 11 (73%) | 15 | |
| pT2 | 35 (27%) | 96 (73%) | 131 | |
| pT3 | 7 (28%) | 18 (72%) | 25 | |
| pT4 | 2 (17%) | 10 (83%) | 12 | |
|
| ||||
| Lymph node status (pN) | 0.02/0.16 | |||
| pN0 | 19 (23%) | 63 (77%) | 82 | |
| pN1 | 17 (28%) | 43 (72%) | 60 | |
| pN2 and pN3 | 10 (40%) | 15 (60%) | 25 | |
Note: NS; not significant, r; correlation coefficient
SIRT3 protein expression in relation to breast cancer hormone receptor status
We also examined SIRT3 expression in relation to estrogen receptor (ER) and progesterone receptor (PR) status. SIRT3 loss occurred with a higher frequency in ER negative (ER−) and PR negative (PR−) tumors. SIRT3 loss was seen in 83% of ER− tumors compared to 62% of ER+ tumors (Table 2, p=0.002). Similarly SIRT3 was lost in 82% of PR− breast cancers compared to 68% of PR+ cases (Table 2, p=0.04). Table 2 summarizes the SIRT3 expression in relation to the HER2 status of breast cancer cases, showing no significant correlation, although the fraction of HER2+ cases was low. Analysis of the expression of SIRT3 according to hormone receptors and HER2 status of breast cancers revealed that 75% of triple negative cases (ER−, PR− and HER2−) showed loss of SIRT3 expression which was comparable to 80% cases with at least one positive marker that showed loss of SIRT3 (Table 2).
Table 2.
SIRT3 expression in infiltrating duct carcinoma of the breast according to hormone receptors and Her2 status.
| Variable | SIRT3 Immunohistochemistry
|
Total | P value/r | |
|---|---|---|---|---|
| Positive | Negative | |||
|
|
||||
| ER | 0.002/0.1 | |||
| Positive | 25 (38%) | 41 (62%) | 66 | |
| Negative | 19 (17%) | 91 (83%) | 110 | |
|
| ||||
| PR | 0.04/0.16 | |||
| Positive | 27 (32%) | 58 (68%) | 85 | |
| Negative | 17 (18%) | 75 (82%) | 92 | |
| Her2 status | NS/0.06 | |||
| Equivocal/Positive (+2,3) | 4 (25%) | 12 (75%) | 16 | |
| Negative (0, +1) | 31 (22%) | 109 (78%) | 140 | |
| ER, PR and Her2 | NS | |||
| Non triple negative | 18 (20%) | 71 (80%) | 89 | |
| Triple negative | 17 (25%) | 50 (75%) | 67 | |
Note: NS; not significant, r; correlation coefficient, ER; estrogen receptors, PR; progesterone receptors
Decreased SIRT3 is associated with poor outcome in multiple subtypes of breast cancer
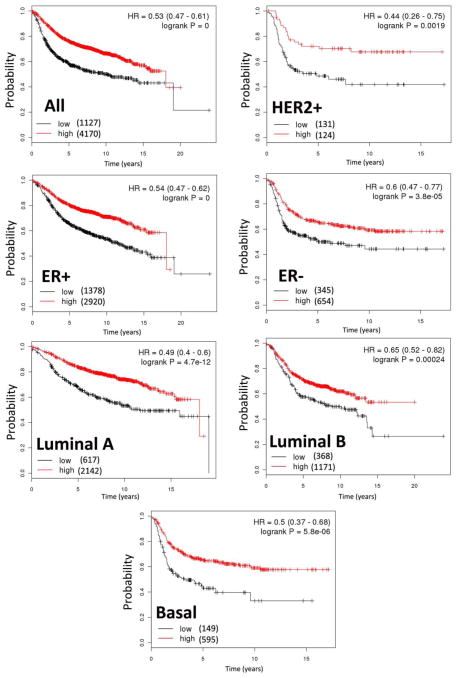
To determine a potential association of low SIRT3 expression with patient outcome, we interrogated data in a publically available breast cancer expression hub, KM Plotter [29,3]. We subdivided samples based on SIRT3 expression, with those in the lowest quartile in one group (low SIRT3) and the rest in another group (high SIRT3) and tested for the relationship to locoregional relapse free survival. Remarkably, low SIRT3 expression was found to be associated with significantly reduced survival in all breast cancers, as well as in ER+, ER−, HER2+, Luminal A (ER+ and/or PR+, HER2−), Luminal B (ER+ and/or PR+, HER2+) or basal-like type (ER−, PR−, HER2−, CK 5/6+, and/or EGFR+) breast cancers (Figure 3). All normal cases with negative SIRT3 expression has no available follow up data to correlate with the outcome.
Figure 3.
Analysis of breast cancer data by Kaplan-Meier Plotter shows that low SIRT3 expression is significantly associated with poor outcome (locoregional relapse free survival) in all types of breast cancer examined. The numbers of samples in each group are indicated in parentheses, and the hazard ratios (HR) and log rank p values are shown.
DISCUSSION
The results presented in this paper established consistent reduction of SIRT3 protein in DCIS as well as invasive breast cancer in human tissue. We examined the expression of SIRT3 in normal, DCIS and primary invasive breast tumors and these results provide supporting evidence for a role of SIRT3 as a tumor suppressor protein in breast cancer carcinogenesis.
Specifically, our study revealed that SIRT3 is low/weakly expressed in 23% of normal breast tissue compared to 72% and 74% complete loss in DCIS and invasive carcinomas, respectively and these results were statistically significant different. The evaluation of the staining was carried out by one pathologist (MMD), thus assessing interobserver variations was not possible in this study. Consistent with our results in breast cancer, other studies reported low expression/downregulation of SIRT3 in head and neck squamous cell carcinomas (26), lung adenocarcinomas (27) and hepatocellular carcinoma (28). Kim et al, 2010 reported that SIRT3 knockout mice developed breast tumors later in life and SIRT3 protein levels are decreased in human breast cancers as well as several other malignancies (6). In this investigation it was also found that loss of SIRT3 is more common in ER negative (83%) and PR negative (84%) compared to ER and PR positive invasive carcinomas with statistically significant difference. Additionally, SIRT3 is lost in 78% of HER2 negative cases, with no statistically significant difference which may be due to low number of cases with available HER2 data. Interestingly, SIRT3 is lost in 75% of triple negative breast cancers which usually carry a bad prognosis (29). The loss of SIRT3 in triple-negative breast cancers is somewhat puzzling. These are aggressive, large tumors that are known to occur in a younger age group. So the mechanism of action might not be the same as other breast cancer types. The preliminary data extracted from the current study encourages further mechanistic studies to elaborate more insight on the mechanisms of SIRT3 and may be other Sirtuins especially in triple-negative breast cancer cases.
SIRT3 expression has been linked in altering sensitivity of breast cancer cells to tamoxifen treatment with up-regulation in the Tamoxifen resistance human breast cancer cell lines. The authors concluded that SIRT3 might be considered as a potential target for overcoming Tamoxifen resistance in treatment of breast cancer (30). Reduced expression of SIRT3 in DCIS and invasive breast tumors with no statistically significant difference between tumor grades reported here suggest that SIRT3 abnormalities are early events in breast tumorigenesis and paves the way for analysis of other sirtuin proteins in breast cancers.
We have also demonstrated for the first time that low SIRT3 expression is associated with poor outcome in multiple subtypes of breast cancer. Remarkably, low SIRT3 expression is found to be associated with reduced survival in all breast cancers, as well as in ER+, ER−, HER2+, Luminal A, Luminal B or basal-like type breast cancers (Figure 3). Taken together, this study underscores the importance of SIRT3 as a potential tumor suppressor gene in breast cancer. Additionally, the study highlights a potential role of SIRT3 as a biomarker to assist in identifying high risk patients across all molecular subtypes of breast cancer. More studies are needed to identify the role of SIRT3 and other sirtuin genes and understand the mechanisms involved in this process.
Acknowledgments
S.A.A. is supported by NCI-RO1CA123484, R01CA167966 and BC093803 from the DOD. DG is supported by NCI-1R01CA152601-01, 1R01CA152799-01A1, 1R01CA168292-01A1, 1R01CA16383801A1, NCI 1R01CA163838-01A1, and BC093803 from the DOD. Micrographs were taken at the Cell Imaging Core at Vanderbilt University Medical Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no conflict of interest in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ershler WB, Longo DL. The biology of aging: the current research agenda. Cancer. 1997;80:1284–1293. [PubMed] [Google Scholar]
- 2.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Molecular cell. 2007;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, Li C, Veenstra TD, Li B, Yu H, Ji J, Wang XW, Park SH, Cha YI, Gius D, Deng CX. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14:1021–1026. [PubMed] [Google Scholar]
- 9.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 10.Hallows WC, Albaugh BN, Denu JM. Where in the cell is SIRT3?--functional localization of an NAD+-dependent protein deacetylase. Biochem J. 2008;411:e11–13. doi: 10.1042/BJ20080336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarente L, Partridge L, Wallace DC. Molecular biology of aging. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- 13.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 14.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 15.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Molecular cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. The Journal of clinical investigation. 2009 doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdulkadir SA, Carvalhal GF, Kaleem Z, Kisiel W, Humphrey PA, Catalona WJ, Milbrandt J. Tissue factor expression and angiogenesis in human prostate carcinoma. Hum Pathol. 2000;31:443–447. doi: 10.1053/hp.2000.6547. [DOI] [PubMed] [Google Scholar]
- 22.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123:21–27. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 23.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyorffy B, Schafer R. Meta-analysis of gene expression profiles related to relapse-free survival in 1,079 breast cancer patients. Breast cancer research and treatment. 2009;118:433–441. doi: 10.1007/s10549-008-0242-8. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham WA, Preacher KJ, Banaji MR. Implicit attitude measures: consistency, stability, and convergent validity. Psychological science. 2001;12:163–170. doi: 10.1111/1467-9280.00328. [DOI] [PubMed] [Google Scholar]
- 26.Park SH, Ozden O, Jiang H, Cha YI, Pennington JD, Aykin-Burns N, Spitz DR, Gius D, Kim HS. Sirt3, Mitochondrial ROS, Ageing, and Carcinogenesis. International journal of molecular sciences. 2011;12:6226–6239. doi: 10.3390/ijms12096226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao K, Jiang J, Wang W, Cao S, Zhu L, Zeng H, Ouyang R, Zhou R, Chen P. Sirt3 is a tumor suppressor in lung adenocarcinoma cells. Oncology reports. 2013 doi: 10.3892/or.2013.2604. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y, Luo X, Ye T, Wang R, Hu H, Li H, Wang L, Pao W, Chen H. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. 2012;18:1947–1953. doi: 10.1158/1078-0432.CCR-11-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 30.Razandi M, Pedram A, Jordan VC, Fuqua S, Levin ER. Tamoxifen regulates cell fate through mitochondrial estrogen receptor beta in breast cancer. Oncogene. 2013;32:3274–3285. doi: 10.1038/onc.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]