Abstract
Depression is a developmental phenomenon. Considerable progress has been made in describing the syndrome, establishing its prevalence and features, providing clues as to its etiology, and developing evidence-based treatment and prevention options. Despite considerable headway in distinct lines of vulnerability research, there is an explanatory gap in the field ability to more comprehensively explain and predict who is likely to become depressed, when, and why. Still, despite clear success in predicting moderate variance for future depression, especially with empirically rigorous methods and designs, the heterogeneous and multi-determined nature of depression suggests that additional etiologies need to be included to advance knowledge on developmental pathways to depression. This paper advocates for a multiple levels of analysis approach to investigating vulnerability to depression across the lifespan and providing a more comprehensive understanding of its etiology. One example of a multiple levels of analysis model of vulnerabilities to depression is provided that integrates the most accessible, observable factors (e.g., cognitive and temperament risks), intermediate processes and endophenotypes (e.g., information processing biases, biological stress physiology, and neural activation and connectivity), and genetic influences (e.g., candidate genes and epigenetics). Evidence for each of these factors as well as their cross-level integration is provided. Methodological and conceptual considerations important for conducting integrative, multiple levels of depression vulnerability research are discussed. Finally, translational implications for how a multiple levels of analysis perspective may confer additional leverage to reduce the global burden of depression and improve care are considered.
Several years ago, I was doing psychotherapy with a depressed adolescent girl, and she described her mood and depression as “an ogre.” A few weeks later while I was watching Shrek with my own children, we laughed at the scene when Shrek is walking through a field with Donkey on their way to battle the dragon, and Shrek explains to Donkey, “Ogres are like onions. Onions have layers; Ogres have layers! Get it?” This juxtaposition leads to a metaphor of clinical depression as an ogre, a potentially dark and scary condition, and its etiology may best be understood as being comprised of multiple layers, or levels. As a field, child and adolescent clinical scientists have made considerable progress in describing depression, establishing its prevalence and features, providing clues as to its etiology, and developing evidence-based treatment and prevention options (for reviews, see Abela & Hankin, 2008a). However, much of this progress has been based on inquiries that usually are at one level of analysis and exclusive of other possible coherent integrations across multiple levels or perspectives. Despite considerable progress in distinct lines of vulnerability research, there is an explanatory gap in our ability to more comprehensively explain and predict who is likely to become depressed, when, and why. Overall, the field lacks a more complete understanding of multiple levels of analysis that can bridge together accessible, observable risks to molecular genetic influences and their connection through various intermediate processes (or endophenotypes; Gottesman & Gould, 2003).
There are likely many reasons for the current state of affairs in which there appears to be a ceiling for the amount of variance of youth depression that we can currently explain. First, there has been excessive and exclusive concentration on one vulnerability theoretical model to the exclusion of meaningful integration with other vulnerabilities. Many investigators focus on their own comfortable set of constructs and processes as well as their familiar measures, methods, and approaches. Clearly, the lack of interdisciplinary training and knowledge base is a primary reason for the paucity of integrated, multidisciplinary research; there is a strong need for improvements in training, including broad and deep skills and knowledge across behavioral sciences, cognitive and affective neurosciences, molecular neuroscience, and psychiatry (Insel & Wang, 2010). Second, many of the methodological features that characterize much of the research on depression vulnerability study preclude a deeper and more rigorous empirical investigation. Third and related, repeated use of the same, traditional conceptual models, instead of considering and testing refined and more advanced, alternative conceptual models, hampers progress, calcifies and reinforces the present state of knowledge, and does not permit testing of newer conceptual vulnerability models across multiple levels of analysis.
One possible approach to addressing these issues involves greater integration across multiple levels of analysis of depression vulnerabilities across the lifespan. The primary purpose of this paper is to advocate for this perspective. By doing so, I hope this stimulates some promising future research that will “unpeel the onion” and promote a more complete understanding of the etiological connections among the multiple levels of risk to depression among youth, or the “ogre,” that this depressed girl described.
Overview of this paper
As many psychiatric disorders, including depression, are multiply determined, a singular focus on one etiological risk at a single level of analysis is unlikely to provide a complete understanding of the many pathways leading to the development of this disorder. I first illustrate this point with a very succinct review of cognitive vulnerability theories of depression (e.g., Abramson, Metalsky, & Alloy, 1989; Beck, 1967, 1987; Nolen-Hoeksema, 1991) and evidence for these models as predictors of depression in youth. These etiological models were initially developed to characterize adult depression, but have more recently been investigated in children and adolescents using fairly rigorous empirical designs and approaches. This review is intended to make the point that even such rich conceptual models that have proven exceptionally successful at prospectively predicting significant variance in depression can only explain a moderate amount of why individuals become depressed. Thus, continuing to exclusively focus on any singular level aspect of vulnerability will not suffice to advance knowledge on the etiological influences contributing to the development of depression.
After this review, I illustrate one example of a multiple levels of analysis model of vulnerabilities to depression. A brief review of the conceptual underpinnings and evidence in support of each of these select vulnerabilities is provided, followed by some empirical examples of studies that have demonstrated cross-level integrations and connections among the different levels of analysis. Next, I discuss some methodological and conceptual considerations that are especially critical for conducting research that integrates multiple levels of depression vulnerability. Finally, translational implications for how a multiple levels of analysis perspective may confer additional leverage to reduce the burden of depression and improve care are considered.
COGNITIVE VULNERABILITIES TO DEPRESSION AMONG YOUTH: THEORIES AND EVIDENCE
Cognitive theories of depression describe characteristic cognitive vulnerabilities that are hypothesized to causally contribute to depression. These models, like many other vulnerability theories, operationalize vulnerability as an internal and stable feature of an individual that predisposes to develop depression following negative events (Ingram, Miranda, & Segal, 1998). Most of the theories include vulnerability-stress components that posit that depression is produced by the interaction between an individual’s cognitive vulnerability and certain environmental adversities that serve to activate this vulnerability, thus leading to depression. In contrast, individuals low in cognitive vulnerability are hypothesized to react to similar adversities with an appropriate level of distress and depressive affect to the event but to not spiral into depression.
A multitude of cognitive vulnerability factors have been posited. This brief review focuses on the most extensively studied vulnerability factors across child, early adolescent, and adolescent populations: (1) depressogenic inferential styles about causes, consequences, and the self from hopelessness theory (HT; Abramson, Metalsky, & Alloy, 1989), (2) dysfunctional attitudes from Beck’s Cognitive Theory (BCT; Beck, 1967, 1983), and (3) the tendency to ruminate in response to depressed mood from Response Styles Theory (RST; Nolen-Hoeksema, 1991).
These main cognitive vulnerability theories have been studied for several decades, and a large corpus of research has accumulated that largely supports their hypothesized propositions in adults (for reviews see Abramson et al., 2002; Joormann, 2009; Ingram, Miranda, & Segal, 1998; Nolen-Hoeksema, Wisco, & Lyubomirksy, 2008). Similarly, many prospective studies have evaluated whether these vulnerabilities can be extended downward and are equally applicable in the prediction of depressive symptoms and clinical depression among children and adolescents. A quantitative review of this literature with youth (Lakdawalla, Hankin, & Mermelstein, 2007) showed that each vulnerability significantly predicts prospective increases in depressive symptoms, with generally medium effect sizes (ES) among adolescents and small ES for children (see Abela & Hankin, 2008b; Jacobs, Reinecke, Gollan, & Kane, 2008, for qualitative reviews).
Overall, the preponderance of studies, especially the more recent and exacting multi-wave designs, provide strong support for negative cognitive style (e.g., Abela et al., 2011; Carter et al., 2011; Hankin, 2008a), dysfunctional attitudes (e.g., Abela et al., 2011; Abela & Skitch, 2007; Hankin, Wetter, Cheely, & Oppenheimer, 2008), and rumination (e.g., Abela & Hankin, 2011; Hankin, 2008b; Skitch & Abela, 2008) as vulnerabilities that interact with stress to predict depressive symptoms and disorder among children and adolescents. Support for cognitive vulnerability-stress components has been obtained in samples across different ages (children as young as 5, Conley et al., 2001, to late adolescents, Hankin, Abramson, Miller, & Haeffel, 2004) and cultures (e.g., Chinese adolescents, Abela et al., 2011, and Spanish youth, Calvete et al., 2008). Also, studies using diagnostic interviews to assess clinical depression have demonstrated that cognitive vulnerabilities interact with stressors to predict the prospective onset of depressive episodes (Abela & Hankin, 2011; Bohon, Stice, Burton, Fudell, & Nolen-Hoeksema, 2008; Carter & Garber, 2011; Hankin et al., 2004; Lewinshohn, Joiner, & Rhode, 2001).
Thus, cognitive vulnerabilities prospectively predict depression (symptoms and disorder) across youth of varying ages and cultures. Still, despite clear success with very conceptually rich theoretical models, and when tested with the most recent research using more empirically rigorous methods and designs, the field has not been able to completely explain why and when individuals become depressed. Here, I illustrated this point with cognitive theories, but the point holds with other single-focus vulnerability models. That only a moderate amount of variance can be explained by a conceptual model focused largely on a sole vulnerability can be understood clearly from a developmental psychopathology perspective (Cicchetti, 2006). Concepts of multiple pathways, especially equifinality (multiple processes can contribute to one outcome) and multifinality (multiple outcomes can result from one starting, initial point) can help solve this problem. As noted next, depression is a heterogeneous, multifactorial disorder, and it is unlikely that any one level of analysis vulnerability model can fully explain its etiology. Overall, more needs to be done.
A MULTIPLE LEVELS OF ANALYSIS MODEL INTEGRATING VULNERABILITIES TO DEPRESSION
One of the primary arguments of this paper is that future research can more significantly advance understanding of the development of depression among youth when a multiple levels of analysis approach is utilized. In this section I will first explicate the advantages of studying multiple vulnerabilities to depression. Then I will present one concrete example of a multiple levels of analysis perspective to depression vulnerability (see Figure 1). This is not intended to represent a full, coherent theory; nor does this example comprehensively include all conceptually possible and empirically supported processes. A comprehensive review of the evidence for the aspects of vulnerability included in this integrative model is beyond the scope of this article; thus, only select, illustrative studies along with reviews are provided.
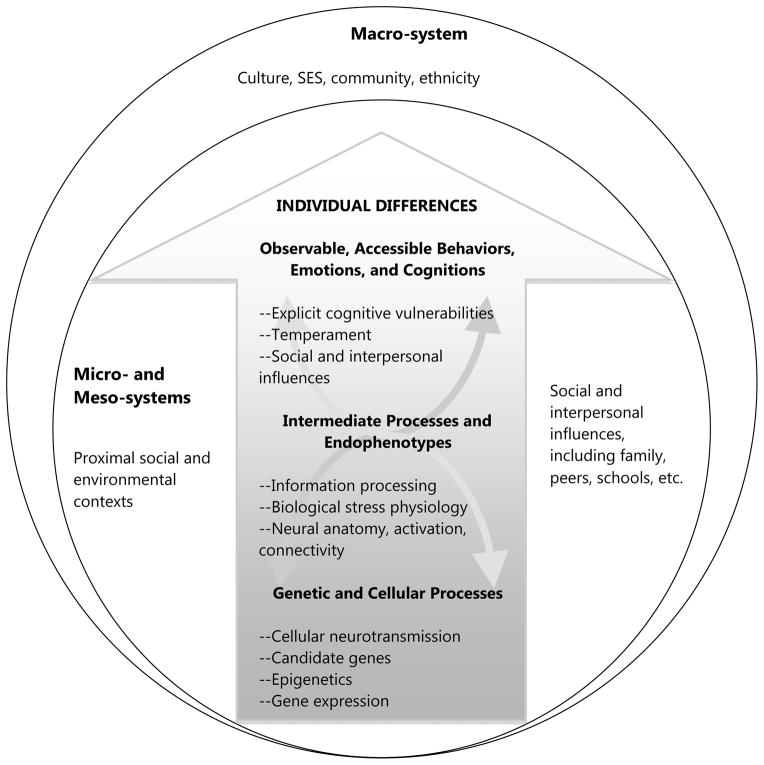
Figure 1.
This conceptual example of a multi-level analysis model of depression is structured and organized from the NAS and NIMH perspective emphasizing multiple levels of analysis ranging from genes, to intermediate traits, to observable and accessible features.
Why Adopt a Multiple Levels of Analysis Perspective?
First, there are many potential vulnerabilities to depression among youth, including genetic, biological, temperament, cognitive, interpersonal, and emotion regulation (see Abela & Hankin, 2008a, for reviews of each). Depression is a prototypical multifactorial disorder, like heart disease, in that one single cause or underlying vulnerability has not been demonstrated. Unlike some other medical diseases (e.g., cystic fibrosis), it is unlikely that a single cause will ever be found to be necessary or sufficient and completely predict who will develop depression. In light of the etiological heterogeneity of depression, future research would benefit from focusing on cross-level mechanisms that more realistically capture the multifactorial nature of depression and many other psychiatric disorders (Kendler, 2012). So, from a scientific perspective (including nosological classification and etiological understanding), it is necessary to consider multiple factors and processes that contribute to onset, maintenance, and recurrence over time. Second, when envisioning future directions that hold promise in scientific inquiry into the study of youth depression, taking a multiple levels of analysis perspective appears paramount. The National Academy of Science (NAS) has called for creation of new nosology based on multiple levels of analysis across medicine (National Research Council, 2011). NIMH has encouraged future investigations based on Research Domain Criteria (RDoC; Insel, 2008) to integrate across areas of genetics, neurobiology, and behavioral science to understand core mechanisms, such as cognitive systems, social processes, executive functioning, positive and negative valence systems, that may be implicated in depression and other psychopathologies. The NAS and NIMH both emphasize multiple levels of analysis, from genes to neural circuits to observable behavior, emotion, and symptoms.
Multiple Levels: From Individual Difference Vulnerabilities to Environmental Contexts
The multiple levels of analysis approach to depression advocated in this article explicitly considers and articulates a conceptual model in which different levels are represented both within the individual (e.g., genetic risks to intermediate traits/endophenotypes to accessible cognition, mood, and behavior) as well as within the environment (e.g., Bronfrenbrenner, 1979). This approach, in which individuals transact and are nested within and across different ecological contexts (e.g., micro-, meso-, macro-, and exo-systems), is consonant with developmental systems (e.g., Bertalanffy, 1950; Hollenstein, 2011; Thelen & Smith, 1994; Waddington, 1957) and developmental psychopathology (Cicchetti, 2006) theories.
Applying such a perspective holds considerable promise for future research aimed at addressing some of the “big facts” of depression (Hankin & Abramson, 2001). For example, the emergence of the sex difference during early adolescence, the overall dramatic surge in rates of depression and first onsets throughout adolescence, as well as cultural and ethnic influences on the development of depression (e.g., Ryder et al., 2012; this issue), could potentially be addressed from a multiple levels of analysis integration that explicitly considers the transactions among the multiple individual differences and various ecological contexts.
Multiple Levels of Analysis for Depression Vulnerability: An Example
Approaches to characterizing depression vulnerability across multiple levels have been proposed previously (e.g., for outstanding examples, see Beck, 2008; Davey, Yucel, & Allen, 2008; DeRaedt & Koster, 2010; Disner, Beevers, Haigh, & Beck, 2011; Goodyer, 2007; Ingram, Atchley, & Segal, 2011; Nelson, Leibenluft, McClure, & Pine, 2005). Here I provide one concrete example of how genetic factors, neural processes, biological stress reactivity, information processing of socially imbued affective cues, and temperament dimensions can be combined with the explicit cognitive vulnerabilities (see Figure 1 for a schematic illustration). In this Figure, different levels of individuals’ environment and context are represented by the circles, consistent with Bronfrenbrenner’s classic ecological model, and the different levels of individuals’ vulnerabilities are represented in the arrow to more explicitly articulate that there are multiple levels of both individual vulnerabilities and environmental ecology and that multiple levels of co-action are likely. Such perspective offers potential for new questions to be asked and addressed in future research inquiries. In the following sections, some of the essential conceptual and empirical points relevant to this multiple levels of analysis model are illustrated followed by initial evidence.
Individual differences in temperament as vulnerabilities to depression
Temperament typically has been broadly defined as individual differences in emotional and behavioral style that appear early in life, are stable across time and situations, and presumably have some biological and/or genetic underpinnings, although temperament characteristics can by modified by experience (Rothbart & Bates, 1998; Shiner, 1998). The basic child developmental literature (Rothbart & Bates, 1998) suggests three higher-order temperament dimensions: negative emotionality (NE), positive emotionality (PE), and effortful control (EC). NE involves a tendency toward negative emotions (e.g., discomfort, fear, anger, sadness) and stress, and PE characterizes the extent to which an individual is outgoing, sociable, and receptive to reward (Rothbart & Bates, 1998). Both NE and PE represent affective reactive dimensions of temperament. Finally, EC taps into self-regulatory, executive functioning (EF), and control processes (e.g., task persistence, attentional control, planfulness, inhibitory control) that can modulate the more affective/reactive dimensions of NE and PE. As a regulatory dimension of temperament, EC permits and can promote flexible modulation of individuals’ reactive responses to aid pursuit of longer-term goals.
Considerable research has examined vulnerability models1 of these temperament dimensions as predicting prospective elevations of depressive symptoms over time among children and adolescents (for reviews, see Compas, Connor-Smith, & Jaser, 2004 Harbaughet al., 2012; Tackett & Krueger, 2005). The preponderance of studies show that the reactive dimensions of temperament, especially NE, are associated with and prospectively predict later depression. The evidence for NE x PE interactions is relatively supportive but still mixed, especially among longitudinal investigations (see Harbaugh et al., 2012 for review). With respect to higher-order interactions involving EC as a self-regulatory dimension capable of reducing risk conferred by reactive temperament dimensions, most cross-sectional studies have demonstrated that the interactions of low EC with high NE, or low EC with low PE, show significant associations with elevated internalizing symptoms (see Harbaugh et al., 2012 for review). Finally, initial research has begun to suggest that the 3-way interaction (high NE, low PE, and low EC) may constitute the highest temperament risk to depression (Dinovo & Vasey, 2011; Vasey, Harbaugh, Lonigan, Phillips, Hankin, Bijttebeir, & Willem, 2012). In essence, reactive temperament risk is a function of high NE x low PE, but this synergistic affective reactivity would likely only manifest itself and contribute to depression among individuals with low EC who do not have the resources or skills to inhibit or interrupt the more toxic combination of high NE and low PE.
Overall, relatively less research has focused on the role of EC in depression compared to NE and PE. Future research would benefit from expanding the scope of inquiry to include EC as an important regulatory temperament dimension for depression vulnerability. This could be accomplished via typical temperament approaches (e.g., child or parent report or observational methods and tasks of EC) as well as through the use of measures of executive functioning (EF). EFs are higher-level cognitive processes that control and regulate lower-level processes to effortfully guide behavior toward a goal (Banich, 2009). EF is impaired among depressed adults (Snyder, 2012) and adolescents (Matthews & Coghill, 2008; Wilkinson & Goodyer, 2006, but see Favre et al., 2008; Halari et al., 2009; Pan et al., 2011 for null findings).
Information processing biases as vulnerabilities to depression
The cognitive vulnerability theories introduced earlier propose that explicit, accessible negative cognitions, such as negative cognitive styles, dysfunctional attitudes, and rumination, predict depression and that implicit, less accessible cognitive processes, such as attention and memory, are also related to depression. These more automatic, implicit models of biased information processing have been studied among adults (see Joormann, 2009; Mathews & MacLeod, 2005 for reviews) relatively more so than with youth. Research with at-risk (Gibb, Benas, Grassia, & McGeary, 2009; Joormann, Talbot, & Gotlib, 2007) and clinically depressed (Hankin et al., 2010) youth have demonstrated attention biases specifically to sad faces. Other research examining overgeneral autobiographic memory biases has shown significant effects among clinically depressed youth (Kuyken & Howell, 2006; Kuyken, Howell, & Dagleish, 2006; Park, Goodyer, & Teasdale, 2004) and at-risk youth (Kuyken & Dagleish, 2011).
These information processing biases can also be placed within modern cognitive science, especially EF, models. For example, impairments in cognitive inhibition or updating, which have been hypothesized as deficits that may underlie information processing biases (Joormann, 2010), may involve difficulties in overriding prepotent responses, removing negative material from working memory, and reducing the influence of task-irrelevant material that captures attention. Thus, one intriguing hypothesis that can be examined in future research is the possibility that youth who exhibit high levels of NE may be at greater risk for depression, especially when they also possess poor EF, because they have more negative emotional material that resides in their working memory, and, they have greater difficulty eliminating negatively toned information. This may be one cross-level interactive process to characterize some risks to depression, such as rumination.
HPA axis dysregulation and biological stress reactivity
Stressful life events are one of the strongest predictors of depression among children and adolescents (Grant et al., 2006 for review), and major stressors have been demonstrated to be a significantly important predictor for first onsets of depressive disorder among adolescents (Lewinsohn, Allen, Seeley, & Gotlib, 1999; Stroud, Davila, & Moyer, 2008). Accessible individual differences, such as cognitive vulnerabilities, have been shown to potentiate the association between stressors and depression. But. how does “stress get under the skin,” and what are biological/physiological processes of stress reactivity?
The association between stress and hypothalamic pituitary adrenal (HPA) – axis activation has been extensively investigated in both animals and humans at all developmental periods and as part of both normative and maladaptive processes (see Gunnar & Quevedo, 2007; Lupien, McEwen, Gunnar, & Heim, 2009, for reviews). Cortisol is the primary hormonal product of the HPA-axis. However, more extended exposure to elevated steroid hormones is physiologically damaging and may contribute to disease (McEwen, 1998). In the concept of allostatic load, or the “wear and tear” on the body that can occur with repeated activation of the adaptive physiological systems as a result of repeated stress over time, there can be both over- and under-activation of the HPA axis (McEwen, 1998). While both too much and too little cortisol can contribute to disease states (Miller, Chen, & Zhou, 2007), hypercortisolism has overwhelmingly received the preponderance of research attention in most investigations of the HPA axis in developmental psychopathology (Gunnar & Vazquez, 2006) and depression (Burke, Davis, Otte, & Mohr, 2005).
Dysregulation of the HPA axis is a potentially robust biomarker for depression among many adults (Burke et al., 2005; Parker, Schatzberg, & Lyons, 2003), who show cortisol hyperreactivity in response to a psychosocial stressor. While most studies of depressed adults demonstrate cortisol hyperreactivity in response to challenge, fewer studies have investigated the role of cortisol, and the direction of effects, among depressed youth (for reviews, see Guerry & Hastings, 2011, and Lopez-Duran, Kovacs, & George, 2009). Indeed, HPA axis dysregulation among youth may be more complex and nuanced than the standard hypercortisolism to challenge pattern found in depressed adults. While research with postpubertal adolescents (e.g., Hankin et al., 2010; Rao, Hammen, Ortiz, Chen, & Poland, 2009) shows that depressed adolescents exhibit cortisol hyperreactivity in response to challenge in a similar manner as do adults, younger prepubertal children tend to demonstrate cortisol hyporesponsivity to laboratory stressors (e.g., Hankin et al., 2010; Luby et al., 2003). Such research suggests a developmental switch in HPA axis dysregulation around puberty (see Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Stroud et al., 2009 with healthy youth). Finally, the few longitudinal studies suggest that HPA axis dysregulation predicts later symptoms and the course of depression (Adam, 2010; Rao, Hammen, & Poland, 2010). For example, youths’ lower levels of cortisol, as a physiological indicator of allostatic load, interacted with prospectively assessed increases in stressful life events to predict elevations of depressive symptoms over one year (Badanes, Watamura, & Hankin, 2011).
Neural activity and connectivity
Considerable research, including neuroimgaging work from basic cognitive and affective neuroscience as well as more clinically oriented research with at-risk or patient populations, suggests a generally consistent picture of how particular brain regions and neural circuits operate and are connected in such a way as to confer risk to depression (for reviews, see Davidson, Pizzagalli, & Nitschke, 2009; Disner, Beevers, Haigh, & Beck, 2011; Gotlib & Hamilton, 2008; Mayberg, 2007). In short, this corpus of research converges to suggest that limbic areas (e.g., the amygdala), which are frequently found to be associated with high negative emotion and affectivity, tend to be overactive, whereas various interconnected prefrontal cortex (PFC) areas (e.g., dorsolateral, ventromedial, orbitofrontal), which commonly are found to be associated with EF and cognitive control, are underactivated. Moreover, some empirical evidence shows strong functional connectivity (inverse association) between PFC and limbic areas. The PFC, as a broad area linked with higher order planning, EF, and cognitive control, can moderate more affective, limbic-driven activity.
In addition, several neural circuitry models of depression include other prominent brain regions hypothesized to be dysregulated in depression. These include the anterior cingulate, which has been associated with emotion processing, attention, and more general cognitive control (e.g., Hamani et al., 2011; Hamilton, Chen, Thomason, Schwartz, & Gotlib, 2011; Wagner et al., 2008), and reward processing areas (e.g., nucleus accumbens and ventral striatum; Forbes et al., 2006; Pizzagalli et al., 2009).
Despite studies showing significant patterns of association consistent with these neural circuitry models, the vast majority of research continues to employ cross-sectional designs. There are relatively few longitudinal studies, especially with children or adolescents, in which baseline neural activity and connectivity are used to predict later increases in depression and retesting of these neural circuits is conducted after depressive episode (Kessler, Traue, & Wiswede, 2011).
Genetic influences
There are genetic influences to the development of depression across the lifespan as demonstrated by significant, moderate heritability estimates from behavioral genetic research conducted with children, adolescents, and adults (e.g., see Lau & Eley, 2008; Levinson, 2009 for reviews). More recent molecular genetic research has produced significant advances in knowledge of how measured, specific genes relate to depression, from either direct main effects associations or through gene-environment interplay (i.e., Gene x Environment interaction, GxE; or Gene-Environment correlation, rGE).
Probably the most investigated candidate gene associated with depression is the serotonin transporter promoter polymorphism (5-HTTLPR). Although there is no consistent direct association between depression and 5-HTTLPR (e.g., Anguelova, Benkelfat, & Turecki, 2003), most evidence shows a significant interaction between 5-HTTLPR and stressful life events (GxE) predicting prospective increases in depression in adults (Karg, Burmeister, Shedden, & Sen, 2011), especially when more reliable and sophisticated assessments of life events are used (Monroe & Reid, 2008; Uher & McGuffin, 2010). The majority of youth GxE studies show significant interactions between 5-HTTLPR and environmental stress, such as maltreatment (Cichetti, Rogosch, & Sturge-Apple, 2007; Kaufman et al., 2006), family environment (Hammen, Brennan, Keenan-Miller, Hazel, & Najman, 2010; Jenness, Hankin, Abela, Young, & Smolen, 2011; Nobile et al., 2009; Sjoberg et al., 2006), and general stressors (Eley et al., 2004; Hankin et al., 2011). In addition to 5-HTTLPR, other well-investigated candidate genes include catechol-o-methyltransferase (COMT), brain-derived neurotrophic factor (BDNF), other broad monoamine-(e.g., MAOA) and serotonin- (e.g., TPH) system related genes, and other physiological stress-system related genes (e.g., glucocorticoid receptor genes—FKBP5, NR3C1; corticotropin releasing hormone genes—CRHR1 and 2).
Another way in which genetic influences work with the environment is through gene-environment correlations (rGE; Plomin, DeFries, & Loehlin, 1977; Scarr & McCartney, 1983). With rGE processes, individuals’ genes influence the likelihood of exposure to particular environments through three mechanisms: (1) passive rGE, in which genetic factors that are common to both a parent and the child influence parenting behaviors or the environment the parent provides, (2) evocative rGE, in which individuals’ genetically influenced behaviors elicit reactions from others, and (3) active rGE, in which individuals’ genetic factors influence their selection of environments. The majority of evidence in support of rGE derives from behavioral genetic studies (Plomin & Daniels, 1987); such research shows that many “environmental” experiences, including negative life events, divorce, marital quality, parental warmth and discipline, and social support are moderately heritable. In contrast to considerable and consistent evidence for rGE from behavioral genetic research, the current evidence for rGE with measured, specific candidate genes is thin (Jaffee & Price, 2007). There is good reason to believe that future research will unearth rGEs with candidate genes, as recent studies have demonstrated rGEs between parenting quality and variation of the dopamine D2 receptor gene (e.g., Hayden et al., 2010; Lucht et al., 2006; Mills-Koonce et al., 2007; Propper et al., 2008).
Taking together both GxE and rGE as forms of gene-environment interplay, future research can investigate which particular genes affect individuals’ exposure to environmental contexts (positive and negative) through rGE processes as well examine which specific candidate genes potentiate reactivity to those positive and negative environmental contexts through GxE processes.
In addition to allelic variation in structural DNA sequences, epigenetics and gene expression are important, as more dynamic sources of genetic influence can also contribute risk to depression. Epigenetics involves biochemical modifications of DNA, without alterations of the base-pair sequence itself, that affect gene expression, such as through the process of methylation, or the silencing of genes. The epigenome is more dynamic and interacts with the environment, which can have a significant impact on gene expression across the lifespan (Meaney, 2010). Epigenetic processes are important because they affect gene expression through dynamic interplay with individuals’ environment, and such epigenetic modifications of genes can result in enduring changes in other biological, cognitive, emotional, and behavioral changes at other levels of analysis (Zhang & Meaney, 2010).
Much of the empirical research on epigenetics, and the demonstration that environmental influence can have profound effects on DNA methylation, and in turn, phenotypical characteristics, has been conducted with animal models (e.g., Weaver et al., 2004; see also Petronis & Mill, 2011; Zhang & Meaney, 2010). For example, Meaney and colleagues (Weaver et al., 2004) have elegantly demonstrated intergenerational transmission of behavioral change through epigenetic processes with their experimental and cross-fostering studies of rats. The quality of maternal care (e.g., licking and grooming behavior), which is associated with the offspring’s stress response, physiology, and brain morphology, can be altered over multiple generations in the glucocorticoid (GR) receptor gene and have ensuing effects for the next generation’s parenting behavior and stress regulation.
In addition to these animal studies, recent research with human development reinforces the importance of incorporating epigenetic processes and gene expression into a multiple levels of analysis perspective to understand how the environment can affect genetic, biological, and psychosocial processes leading to depression. In one groundbreaking study (McGowan et al., 2009), the brains of humans who completed suicide, some of whom had documented cases of maltreatment and others did not, were shown to have different gene expression in the hippocampus that was reduced through methylation. Importantly, this epigenetic effect was observed only in the adults who completed suicide and had been maltreated (i.e., GxE), not in those who committed suicide without abuse or in controls.
Summary
This section has briefly introduced various vulnerabilities to depression at multiple levels of analysis, ranging from those most easily accessed and measured (e.g., cognitive vulnerabilities, temperament), to the intermediate process/endophenotype level (e.g., information processing biases, biological stress reactivity, and neural activation and connectivity), and finally to various genetic influences (e.g., candidate genes, epigenetics and gene expression). Evidence was reviewed showing that each of these vulnerabilities is associated with depression, either as a main effect or through interplay with the environment. The following section provides some empirical examples of how these different vulnerabilities are related to each other based on research that has crossed these typically disparate levels of analysis.
Genetic influences and neural activity
Several exciting, innovative studies have examined connections between candidate genes and neural activity. Indeed, genomic imaging is a burgeoning new area (e.g., for review, see Hariri, 2009) that has emerged to explore these fertile relations (e.g. see Hyde, Bogdan, & Hariri, 2011, for an excellent review of GxE and neural imaging with implications for psychopathology). For example, research with adults has shown that 5-HTTLPR is associated with greater amygdala activity to negative emotional faces (see Munafo, Brown, & Hariri, 2007 for review) as well as reduced functional connectivity in the amygdala-cingulate feedback circuit that is important for regulation of negative emotion (Pezawas et al., 2005) and in frontal-limbic pathway connections (Pacheco et al., 2009). The available research with youth likewise suggests significant associations between amygdala activation to negative affective stimuli and commonly investigated candidate genes, including 5-HTTLPR (e.g., Fortier et al., 2010; Lau et al., 2009) and BDNF (e.g., Lau et al., 2010). 5-HTTLPR correlates with regions of association cortex that underlie attentional control, an aspect of EF (Thomason et al., 2010).
Genetic influences and biological stress reactivity
A variety of studies show that the short allele of 5-HTTLPR is linked to heightened sensitivity and stress reactivity, as operationalized in various ways (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; McGuffin, Alsabban, & Uher, 2011; Thase, 2009). For example, girls carrying the S allele of 5HTTLPR exhibit elevated cortisol in response to a laboratory stressor (Gotlib, Joormann, Minor, & Hallmayer, 2008) and higher wakening diurnal cortisol levels (Chen, Joormann, Hallmayer, & Gotlib, 2009). Other studies have shown that MAOA in interaction with COMT is associated with cortisol stress response differentially for boys versus girls (Bouma, Riese, Doornbos, Ormel, & Oldehinkel, 2012; Jabbi et al., 2007). Last, short allele carries of 5-HTTLPR with high morning cortisol levels were most likely to experience a depressive episode (Goodyer, Bacon, Ban, Croudace, & Herbert, 2009; see also Goodyer, Croudace, Dubridge, Ban, & Herbert, 2010, with 5-HTTLPR and BDNF).
Genetic influences and biased information processing
Several studies suggest that 5-HTTLPR is associated with attentional bias to negative emotional stimuli (Beevers, Gibb, McGeary, & Miller, 2007; Beevers, Wells, Ellis, & McGeary, 2009; Perez-Edgar et al., 2010) and memory biases among children (Hayden et al., 2012). Other research suggests that BDNF is associated with rumination in healthy adults (Beevers, Wells et al., 2010), although the pattern of association may be complex and vary by age (Hilt, Sander, Nolen-Hoeksema, & Simen, 2007). Other research suggests that rumination is linked with BDNF only in the context of stress (Clasen, Wells, Knopik, McGeary, & Beevers, 2011) or maternal depression history (Hayden et al., 2012).
Neural activity and biased information processing
Various studies with adults have examined associations between information processing (attention and memory) tasks and different patterns of neural circuitry. Attentional bias was associated inversely with activation in lateral PFC, and interestingly, this effect was observed only among those carrying the short allele of 5-HTTLPR (Beevers, Pacheco, Clasen, McGeary, & Schnyer, 2010). Other research suggests interesting potential neural links to rumination and difficulties with perseverating on negative emotional material (e.g., Berman et al., 2011; Cooney, Joormann, Eugene, Dennis, & Gotlib, 2011; Eugene, Joormann, Cooney, Atlas, & Gotlib, 2009). Last, a handful of other studies have investigated neural activation in relation to depression-related categorization and memory biases (Chan, Harmer, Goodwin, & Norbury, 2008; Ramel et al., 2007; Wolfensberger et al., 2008) as well as selective attention (Mannie et al., 2008).
Biological stress reactivity and cognitive vulnerabilities
A few studies suggest that cortisol is associated with explicit self-reported cognitive vulnerabilities. Rudolph and colleagues (2011) found that anticipatory cortisol during a social challenge task predicted rumination and depressive symptoms 1 year later among children who experienced peer victimization. Kuehner and colleagues showed that rumination is associated with basal cortisol among adults (2007) and with cortisol response after mood induction only among vulnerable adults (2009).
Other possible links in this hierarchical, multiple levels of analysis model (Figure 1) have not been thoroughly investigated. For example, neither the degree to which information processing tasks relate to biological stress reactivity nor neural activity associates with biological stress reactivity has been investigated. These, and other underinvestigated, links offer promising areas for future research to flesh out the degree to which the different factors and processes outlined in this multiple levels of analysis model cohere and connect. Finally, although many cross-level connections in this emerging model have been examined, fewer studies have investigated how these cross-level associations contribute risk to the development of depression or function differently in depressed relatively to non-depressed individuals.
METHODOLOGICAL AND CONCEPTUAL RECOMMENDATIONS AND CONSIDERATIONS WHEN CONDUCTING MULTIPLE LEVELS OF ANALYSIS VULNERABILITY RESEARCH
Up to now, I have advocated that future research adopt a multiple levels of vulnerability perspective, have conceptually discussed some of these risks, and reviewed some evidence in support of each vulnerability and their potential integration. This section introduces some methodological and conceptual issues and questions to consider when conducting multiple levels of analysis research. Several of these require additional attention beyond those typical methodological recommended approaches (e.g., multiple methods and informants) used in the standard single level-of-analysis vulnerability investigation.
Conceptualizing multiple vulnerabilities: Articulating and testing clear models
It is essential that future research endeavors consider how putatively different vulnerabilities, across different levels of analysis, relate to each other and determine what are the most appropriate conceptual models for these vulnerabilities. There is a strong need for multivariate research on a host of different risks and processes across multiple levels of analysis to chart and place these multiple influences into the relevant multivariate nomological network space for depression vulnerabilities across development.
A primary question when using a multiple levels of analysis perspective is determining whether the different risks and processes are redundant with one another and merely mark the same vulnerability, or whether these different factors represent relatively distinct risks and processes. After all, if the architecture and structure of depression vulnerability reveals the former (different labels marking the same latent vulnerability), then it would seem most efficient, economical, and clinically practical to simply measure the easiest and cheapest marker of this single risk. Moreover, as noted by Cronbach and Meehl (1955), “confidence in a theory is increased as more relevant evidence confirms it, but it is always possible that tomorrow’s investigation will render the theory obsolete.” Future research may reveal that certain risks are redundant with other risks, at varying levels of analysis, and if so, such data would importantly advance a more parsimonious theoretical and empirical knowledge base.
While not much empirical research has systematically investigated the multi-level structure of various vulnerabilities, most evidence suggests that the different vulnerabilities articulated in the multiple levels of analysis vulnerability model (Figure 1) are, in fact, factorially independent and do not entirely overlap. First, as the evidence reviewed earlier on the correspondence between different vulnerabilities across levels shows, many of these various factors and processes (e.g., genetics, neural activity, information processing, etc.) are moderately correlated, but not so strongly to suggest that one influence is entirely redundant with another. Second, factor analytic research examining the structure of cognitive and personality vulnerabilities with young adults (e.g., Hankin, Lakdwalla, Carter, Abela, & Adams, 2007; Joiner & Rudd, 1996) and youth (Adams, Abela, & Hankin, 2007; Gotlib, Lewinsohn, Seeley, Rhode, & Redner, 1993, but see Garber, Weiss, & Shanley, 1993 for exception) suggests that the core cognitive risks are factorially separable, albeit moderately intercorrelated, from each other and from NE. Finally, other research demonstrates the independence between explicit, self-reported cognitive vulnerabilities and implicit information processing biases (e.g., Gotlib et al., 2004), and theoretical models have been articulated to illustrate how explicit and implicit cognitive vulnerabilities at different levels of analysis can culminate in depression (e.g., dual-process models; Beevers, 2005).
Additionally, future research can address the intriguing, but unanswered question, of whether the underlying factor structure of depression vulnerabilities changes substantially across development. It is conceivable that the hierarchical organization and factor structure among different vulnerabilities changes and manifests differently across time and development. For example, the orthogenetic principle of development (Werner, 1957) states that organisms progress from a relatively undifferentiated state to a relatively more complex, distinguishable organization over time. This would predict that younger individuals may not exhibit as many empirically distinct and theoretically separable vulnerabilities as may be identified among adults and as theoretically specified in originally adult-based vulnerability models. Rather, children may be more likely to exhibit an undifferentiated “lump” of fewer, simpler vulnerabilities (e.g., a single broad vulnerability) earlier in childhood, and these vulnerabilities then become more differentiated risks that emerge and stabilize later in adolescence. Future research examining these core questions can importantly advance basic knowledge necessary for a taxonomy of depression vulnerabilities across multiple levels of analysis. Such answers can have important translational implications for what factors and processes to target and when in developmentally sensitive interventions.
Different Conceptual Models of Depression Vulnerabilities
Presuming that the fundamental, prerequisite research has established a taxonomy of depression vulnerabilities that exhibits some degree of factorial independence, then explicit articulation and consideration of different conceptual models that may provide the most accurate representation of the multiple levels of analysis combination of these risks is needed. While there are likely many potential conceptualizations that could characterize different combinations of risks, three meaningfully different conceptual approaches toward conceptualizing the relation among various vulnerabilities across different levels of analysis are articulated here: additive, multiplicative, and weakest link (see Abela & Hankin, 2008b, for greater discussion).
The additive approach assumes that an individual’s degree of vulnerability to depression depends upon the ultimate balance among his/her vulnerability and protective factors. Vulnerability factors exhibit a cumulative effect with the presence of each additional risk leading to a greater degree of risk, whereas, protective factors do the opposite (presence of each additional protective influence subtracts risk). This approach assumes the factors work independently of one another (this is another reason for demonstrating factorial independence among vulnerabilities) and are weighted relatively equally. Thus, an individual with three vulnerabilities (e.g., high negative cognitive style, high NE, and attentional bias to sad faces) should exhibit greater risk than an individual with two vulnerabilities (e.g., average negative cognitive style, high NE, and HPA axis dysfunction). Moreover, protective factors can counterbalance against vulnerability factors, so the additive approach would predict that an individual with two vulnerabilities (e.g., high rumination, memory bias, and average neural activation) is equally at risk as someone with three vulnerabilities and one protective factor (e.g., genetic risk, HPA axis dysregulation, high rumination, and high PE as protection). Note that the additive approach is highly similar to Rutter’s (1977) classic research on indicators of adversity (e.g., family conflict, social class, maternal psychopathology) in which the additive summation best predicted risk to psychopathology.
Next, the multiplicative approach posits that the vulnerability factors will interact synergistically to potentiate the stress-depression relation such that the greatest increases in depressive symptoms following increases in stress will be observed in individuals possessing both (or more) vulnerability factors. For example, in the temperament vulnerabilities reviewed earlier, individuals with high NE, low PE, and low EC may be expected to experience the highest levels of depressive symptoms. Although the multiplicative approach has proven useful in examining the integration and cross-level connection of any given two or three models of vulnerability to depression, such an approach can become increasingly cumbersome, both theoretically and methodologically, when attempting to integrate across a more extensive range of vulnerabilities. As the pool of vulnerabilities across levels increases, the precision of the hypotheses can become so exact that only individuals possessing the complete “depressogenic profile” would be expected to exhibit increases in depressive symptoms. Considerable sample sizes would be needed to have sufficient statistical power to meaningfully test such increasingly higher-order interactions (e.g., high NE x low PE x low EC x high stress x HPA axis dysregulation x genetic risk).
Finally, the weakest link approach (Abela & Sarin, 2002) posits that when multiple vulnerability factors and processes are expected to predict depression through a similar mediating pathway (e.g., overall cognitive vulnerability, including explicit cognitive risks and biased information processing), then an individual’s most depressogenic vulnerability is the best marker of his/her true propensity for developing depression. Thus, when considering similar vulnerabilities (and this can be construed and postulated to be as narrow, e.g., explicit cognitive risks, or as broad, e.g., individual differences in reactivity to environment), this approach predicts that an individual will be as vulnerable to depression as his/her most depressogenic vulnerability makes him/her.
Conceptualization of interdependence with environment: Vulnerability or Susceptibility/Plasticity?
It is essential to evaluate whether each particular hypothesized factor or process across the different levels of analysis operates best according to a traditional vulnerability concept (e.g., Zubin & Spring, 1977; Zuckerman, 1999) or a susceptibility/plasticity perspective (Belsky & Pluess, 2009). According to a pure vulnerability model, heightened level of risk is associated with only negative outcomes. In contrast, the differential susceptibility hypothesis (DSH) suggests the possibility that some individual difference factors and processes may confer increased sensitivity to the relevant environmental context, and outcomes can result “for better and for worse” depending on that environment. Under negative environmental contexts and high risk (or susceptibility) status, the vulnerability and DSH models make the same prediction that maladaptive outcomes may occur. Yet the DSH makes the novel prediction that positive outcomes can be attained when high susceptibility individuals experience positive environments. Thus, careful consideration of the meaning, definition, and operationalization of vulnerabilities (or susceptibilities) at different levels of analysis can yield important new insights into pathways leading to the development of depression, especially dependent on particular environments across different ecological contexts. These points are illustrated with respect to 5-HTTLPR as a genetic influence interacting with different environments, how such GxE processes may enhance risk to depression, and how these vulnerability conceptualizations can suggest very different underlying processes depending on the particular form of the conceptual model.
In Caspi and colleagues’ original, seminal investigation (2003) showing that 5-HTTLPR interacted with negative events to predict depression, this GxE was initially interpreted from the traditional vulnerability-stress perspective (e.g., Hankin & Abela, 2005; Zuckerman, 1999). More recently, this specific GxE, and 5-HTTLPR as a candidate gene, has been reframed and reconceptualized from a susceptibility/plasticity model (see Belsky & Pluess, 2009; see Caspi et al. 2010 for a related environment sensitivity model). Overall, there has been considerable controversy and mixed evidence surrounding GxE processes in depression, especially with respect to 5-HTTLPR and negative events (e.g., Karg et al., 2010; Risch et al., 2009). Unfortunately, many prior studies examining 5-HTTLPR x stress used cross-sectional designs with poor environment measures (Monroe & Reid, 2008; Uher & McGuffin, 2010), and this contributed to the mixed GxE literature. With inadequate measures and designs, misleading conclusions can be reached. To enable proper empirical tests of these different underlying conceptual models (e.g., DSH or vulnerability), rigorous and appropriate methodological approaches are necessary.
Guided by theory and evidence suggesting that 5-HTTLPR enhances susceptibility and sensitivity to one’s environment (Caspi et al. 2010), rather than only vulnerability to depression per se, Hankin and colleagues (2010) reasoned that youth carrying short 5-HTTLPR alleles would be more reactive to increases in stressful life events relative to their own typical stress level, consistent with an idiographic perspective (see Abela & Hankin, 2008b for greater discussion). From an idiographic conceptual and analytic model, it is within-person fluctuations and changes in the environment from the individual’s perspective (i.e., individual is experiencing high levels of stress relative to his/her own individual average level) that are most important. In contrast, from a nomothetic perspective it is between-subjects differences in environment (i.e., individual is experiencing high levels of stress in comparison to the sample’s, or population’s, average stress level) that are most salient. A design with multiple waves of data is necessary to disentangle more precisely within-subjects from between-subjects effects (Curran & Bauer, 2011) and to investigate whether a GxE (or other vulnerability-stress processes) best conforms to an idiographic or nomothetic model (see Carter & Garber, 2011, for another illustration of these points with cognitive vulnerabilities to depression). However, all of the prior GxE studies used either cross-sectional or two-time point designs that only allowed for a test of GxE from a nomothetic approach; thus, an exacting test of DSH or vulnerability models could not be achieved. To remedy this gap, Hankin, Jenness and colleagues (2011) collected multiple waves of stress and symptom data to evaluate whether 5-HTTLPR interacts with stressors from an idiographic or nomothetic approach and evaluate whether the expected GxE best conforms to DSH or vulnerability. Consistent with the DSH model, multilevel modeling showed that there was a significant GxE effect for 5-HTTLPR when interacting with idiographic, but not nomothetic, stressors in the prospective prediction of depressive symptoms.
While this multi-wave study with idiographic analysis was consistent with a DSH, rather than traditional vulnerability, model, only half of the DSH conceptualization was examined. Recall that both vulnerability and DSH posit that negative outcomes (e.g., depression) would result when susceptible (e.g., short carriers of 5-HTTLPR) individuals experience negative environments (e.g., stress), yet DSH uniquely predicts that enhanced outcomes would be observed among susceptible individuals who have experienced positive outcomes. Only one study has tested the full DSH conceptualization and prediction. Hankin, Nederhoff and colleagues (2011) examined whether 5-HTTLPR functioned as a vulnerability or susceptibility gene in three independent studies of youth with measures of the environment (parenting) and an outcome (positive emotion) that fully ranged from positive to negative. Consistent with DSH, youth carrying the short allele of 5-HTTLPR experienced lower positive emotion under negative environment conditions (unsupportive, less warm parenting), and higher positive emotion was reported under positive environments (supportive, warm parenting).
Given this differential susceptibility GxE pattern, how might S carriers who experience negative parenting (and, likely, other negative environmental experiences) be at enhanced risk for depression? Establishing which youth are more likely to experience low positive emotion can inform mechanisms contributing to depression. Affective neuroscience and reward-processing models that implicate low positive affect and difficulties upregulating positive emotion act as potent risks to depression, including at the most observable, accessible (e..g, temperament [PE], emotion regulation) and neural levels (e.g., Davey et al., 2008; Davidson et al., 2009; Forbes & Dahl, 2005; Sheeber et al., 2009).
While this evidence suggests that 5-HTTLPR may conform best to a DSH perspective, the data reviewed earlier show that explicit cognitive risks correspond to a traditional vulnerability model (e.g., Abela et al., 2011; Carter & Garber, 2011). Thus, investigators need to evaluate carefully whether particular factors and processes function solely as vulnerabilities or more subtle, differential susceptibilities, and examine which do so across the multiple levels of analysis2.
In sum, future research is needed to examine how different potential individual difference risks at each level of analysis operate, what conceptual model best applies to that particular risk (e.g., susceptibility, traditional vulnerability), at what developmental stage (e.g., pre vs post puberty; cognitive, social, emotional level), and how these different individual difference factors may transact across levels—both within individual vulnerabilities and across environment contexts (e.g., parents, peers, romantic partners). Thus, examining individual difference factors and processes across multiple levels of analysis from fresh conceptual, theoretical perspectives promises to yield exciting new advances.
Methodological considerations to enable rigorous empirical tests of vulnerabilities
To allow for clear, thoughtful articulation of the conceptualization of vulnerability, it is necessary that methodologically sophisticated and developmentally appropriate measures, designs, and analyses are used (e.g., Rutter, 2005). Here I focus on using longitudinal designs, prospective follow-ups with systematically varied time courses, multiple measures of different aspects of psychopathology, and precise assessment of key phenomenological features of depression.
First, longitudinal designs should be used to more accurately and rigorously test developmental processes and establish temporal ordering among key variables. Unfortunately, the preponderance of research investigating many depression vulnerabilities (e.g., information processing biases, biological stress reactivity, neural activation and connectivity) has used cross-sectional designs. As a result, differentiating among basic alternative models relating putative risk factors to depression, including predisposition, concomitant, or consequence, cannot be accomplished. Thus, future research should collect at least two time points at a minimum, as a second assessment does not require substantially more resources relative to the significant advances in knowledge obtained. Still, while two-time point panel designs can provide the most basic demonstration that a vulnerability precedes later depression, such designs are not much more informative than simple cross-sectional designs (Curran & Willoughby, 2003). With designs involving multiple time points, various conceptual models with importantly different underlying processes involved can be rigorously tested and compared. These include: (1) idiographic versus nomothetic approaches to vulnerability-stress processes (Abela & Hankin, 2008b), (2) within versus between subjects effects (Curran & Bauer, 2011), (3) dynamic, bi-directional and transactional models (Sameroff & MacKenzie, 2003), and (4) developmental cascade models (e.g., Hankin et al., 2010; Masten et al., 2005). Finally, with multiple waves of data, the temporal ordering and time course of depressive symptoms and the covariation between vulnerabilities and environmental influences can be more rigorously investigated using modern data analytic approaches, such as growth curve modeling, trajectory analyses/growth mixture modeling, and multi-level modeling (e.g., Collins & Horn, 2003; Singer & Willet, 2003).
Second, by using longitudinal designs, especially multiple time points, future research can investigate developmental origins of different depression vulnerabilities. This topic remains an important, but relatively understudied goal and can be advanced by consideration of the interplay among vulnerabilities at different levels of analysis and a developmental systems theoretical perspective within an ecological framework. Prior research has examined some predictors of vulnerabilities and shown that negative environmental experiences, ranging from peer rejection and victimization to negative parenting styles and behaviors to frank child maltreatment, are associated with and predict the emergence of psychosocial risks, such as explicit cognitive vulnerabilities (e.g., Alloy, Abramson, Smith, Gibb & Neeren, 2006; Gibb, 2002; Hankin et al., 2009) and biased information processing (e.g., Gibb, Schofield, & Coles, 2009; Pollak & Tolley-Schell, 2003), as well as biological stress reactivity (e.g., Badanes et al., 2011; Cicchetti, Rogosch, Gunnar, & Toth, 2010; Heim, 2007; Lupien et al., 2009) and methylation of candidate genes (McGowan et al., 2009).
Related, future research would benefit from investigating the degree of stability and change in different vulnerabilities across the multiple levels of analysis along with factors and processes that contribute to the stabilization and crystallization of these vulnerabilities at different points across development. A fundamental assumption of most vulnerability theories of depression, whether psychosocial (Ingram & Luxton, 2005) or possible biomarkers and endophenotypes (Gottessman & Gould, 2003), is that the risk factors and/or processes are relatively stable, trait-like, and enduring. To exactingly investigate degree of stability and change, several time points are required as a two-time point design is inadequate (Fraley & Roberts, 2005).
Some rigorous, multi-wave research has supported these hypothesized tenets of stability for cognitive vulnerabilities with adolescents (Hankin, 2008c) and young adults (Hankin, Fraley, & Abela, 2005) and for temperament dimensions (Caspi, Roberts, & Shiner, 2005). Basic test-retest reliability research has been conducted with methylation status for some commonly investigated candidate genes (Wong et al., 2010). Yet, no research has examined such fundamental properties, especially degree of trait-like stability, which is necessary for demonstrating that a hypothesized factor or process is in fact an enduring vulnerability, including biased information processing, cortisol indices for biological stress reactivity to challenge, or neural activation patterns.
The extant research suggests that explicit cognitive vulnerabilities (e.g., negative cognitive style, dysfunctional attitudes, and rumination) are already relatively stable and trait-like by early adolescence (e.g., Cole et al., 2008; Hankin, 2008c). Even for temperament and personality traits, which are constructs that are typically believed to exhibit strong continuity, it has been demonstrated that that the degree of stability changes across development, including in the first few years of life (Lemery, Goldsmith, Klinnert, & Mrazek, 1999) and over decades in adulthood (Roberts & DelVecchio, 2000). Significant continuity co-exists alongside change (Caspi et al., 2005). Other than DNA allelic variation, all of the vulnerabilities across a multiple levels of analysis model likely exhibit both continuity and change across the lifespan. Future research is needed to continue to investigate when vulnerabilities across levels of analysis begin to coalesce and crystallize into enduring, trait-like risks to depression. Knowing when during development various vulnerabilities begin to stabilize can provide important clues that can inform future research investigating the developmental origins of these risk factors and processes as well as offer essential information regarding developmentally sensitive periods that may be optimal for delivering preventions to forestall the crystallization of these risks into enduring vulnerabilities so that individuals’ likelihood of developing depression later in the lifespan (e.g., during middle to late adolescence) is significantly diminished.
Third, when using longitudinal designs, there is a need for careful consideration of the length of time for the follow-up assessments. Unfortunately, there seems to have been little thoughtful, theoretical consideration to this critical design feature of follow-up length in most depression studies that have employed longitudinal designs. The time course in which different vulnerabilities to depression across levels of analysis relate to changes in depressed mood and symptoms across systematically varying time frames (e.g., minutes, to days, to weeks, to several months) is dramatically understudied. For several of the vulnerabilities reviewed in this paper (e.g., information processing, biological stress reactivity, neural activation, genetic influence), the majority of studies employed cross-sectional designs, so understanding the duration or persistence of depression across different time frames cannot be achieved. Thus, it is unknown whether information processing biases, for example, predict mood and symptom changes over a matter of minutes to hours, days, and/or weeks. Future research can examine the time course (e.g., on a distal to proximal dimension) of various vulnerabilities and how well these risk factors and processes predict depressed mood, symptoms, and episodes across systematically different time frames. This becomes important because evidence consistently shows that almost all individuals, regardless of vulnerability or clinical status, experience increases in negative affect/depressed mood immediately after stressful life events (Rottenberg, 2007; Weiner, 1985).
Future research that systematically examines the strength of associations between vulnerabilities and depression across varying time courses can explicate more carefully how and when various vulnerability factors and processes, at different levels of analysis, predict enduring elevations in depressed mood and symptoms for different youth. Such work promises to be valuable for understanding which etiological factors and processes contribute to the development of clinical depression (i.e., persistent, sustained negative mood and other symptoms) and its associated distress and impairment, in contrast to temporary, short-lived, normative increases in negative affect that would be expected after negative events and challenges. Future research can investigate the association between different vulnerability factors and the varying time course (from minutes to days to weeks to months) using different approaches, such as Ecological Momentary Analysis (EMA) for minutes to hours, to daily diaries for days, to prospective multi-wave follow-ups of symptoms and episodes for weeks to months. For example, prior research shows that cognitive vulnerabilities predict depressive mood and symptoms after stress exposure after a few days (e.g., daily diaries, Hankin et al., 2005; naturalistic stressors, Hankin et al., 2004; Metalsky, Joiner, Hardin, & Abramson, 1993), several weeks (e.g., Abela & Skitch, 2007; Hankin, 2008a, b), and over several years (e.g., Abela & Hankin, 2011; Carter & Garber, 2011; Hankin et al., 2004). While some research has investigated these critical questions from cognitive vulnerability and affective neuroscience (Davidson, Jackson, & Kalin, 2000) perspectives, future research can and should be extended to investigate different time courses and the maintenance and intensification of negative affect into enduring, persistent clinical depression for multiple vulnerabilities.
Fourth, future research on depression vulnerability needs to routinely incorporate multiple measures of psychopathology. Comorbidity of depression with commonly occurring emotional and behavioral problems, especially anxiety, conduct problems, and ADHD, has been well-established (Angold, Costello, & Erkanli, 1999). However, the degree to which supposedly depression-specific vulnerability factors and processes predict prospective increases in depression, as opposed to these and other frequently co-occurring, symptoms, has been understudied. Some research has demonstrated that particular vulnerabilities, such as HT’s negative cognitive style (e.g., Hankin, 2008a) or 5-HTTLPR (Hankin et al., 2010) interacting with stressors, predict depressive symptoms specifically relative to anxious or behavioral problems, whereas other cognitive vulnerabilities, such as RST’s ruminative response style, more generally predict depressive symptoms along with other general emotional forms of distress, including eating pathology (Holm-Denoma & Hankin, 2010; Nolen-Hoeksema, Stice, Wade, & Bohon, 2007) and anxiety symptoms (e.g., Abela & Hankin, 2011; Hankin, 2008b).
The degree to which various vulnerabilities across multiple levels of analysis operate as relatively specific predictors of depression versus more general psychopathology (i.e., “transdiagnostic” factors; Nolen-Hoeksema & Watkins, 2011) can be informed by recent hierarchical, structural models of psychopathology among youth (e.g., Lahey et al., 2004; Lahey, D’Onofrio, & Waldman, 2009) and adults (e.g., Krueger & Markon, 2006). Future research is needed to examine how vulnerabilities across the multiple levels of analysis relate to which particular hierarchical level in these structural psychopathological models (e.g. rumination predicting an overarching internalizing distress factor; negative cognitive style predicting depression specifically, etc.). Certain vulnerabilities, across multiple levels of analysis, likely relate to differing levels in the structural hierarchy of common psychopathological symptoms. Knowledge of both transdiagnostic and depression-specific factors and processes can be better advanced by examining at which level of this hierarchical structural model the various vulnerabilities best connect.
Last, it is essential that research properly and fully assess key phenomenological features of depression, such as duration, severity, time to episode onset, and chronicity (e.g., first onset vs. recurrence; single episode versus first lifetime episode, Monroe & Harkness, 2005; 2011). Remarkably little attention has been paid to these critical phenomenological qualities. Indeed, few studies have considered and carefully investigated whether particular factors and processes predict anything beyond variability in depressive symptoms (typically self-reported depression questionnaire) or dichotomous onset of disorder at any point over the lifecourse.
Many of the studies in the depression literature have indiscriminately lumped together all individuals who have experienced a depressive disorder and have not explicitly considered and analyzed critical depression features (e.g. separating first onsets vs recurrent episodes). There are likely important differences in the processes and factors that may contribute to a first depressive onset (e.g., major stressful event; Lewinsohn et al., 1999; Stroud et al., 2008), a recurrent episode (e.g., kindling or neural sensitization; Post, 1992), or potentially to both (e.g., cognitive vulnerabilities; Abela & Hankin, 2011).
This becomes especially salient depending on the age and developmental status of participants (e.g., child, adolescent, or adult) that depression investigators select, and such choices can have important implications and affect conclusions. With younger samples, especially children or early adolescents, it is most likely that first-onsets of depression are being measured, and thus, the factors and processes associated with risk to first onsets are under investigation. With middle to late adolescent samples, the preponderance of the sample is likely experiencing a first episode (given that most individuals experience their first depressive episode between ages 14–18; Hankin et al., 1998), although there will be an admixture of recurrences of those who had early-onset episodes in childhood or early adolescence. In contrast, with adult samples, it is most likely that investigators are studying an even more complex mixture of first-episodes, single recurrences, or multiple recurrences. Unfortunately, many investigations do not clearly report such essential facts and do not analyze their data accordingly. As a result, it is unknown precisely which aspects of depression phenomenology (e.g., first onset vs recurrence; single vs. first lifetime episode) the set of risk factors and processes under investigation relate to.
Future research on depression vulnerability across the lifespan requires an explicit developmental rationale for the age of participants, and investigators should provide descriptive statistics, and conduct moderator analyses, for the phenemonological features of depression in the sample. Just as any investigator should carefully select the measures and methods to rigorously test underlying hypotheses based on the theoretical model, thoughtful selection a priori of the appropriate developmental nature of the sample (e.g., pubertal status; cognitive, social, and emotional level), including other salient demographic characteristics (e.g., gender, race, ethnicity, culture, SES) is necessary so that the field can more accurately understand and have an accumulating knowledge base of which vulnerability factors and processes, across the different levels of analysis, relate best to first onsets, single recurrences, or multiple recurrent episodes (cf., Monroe & Harkness, 2005; 2011) as well as other features (e.g., severity, duration, etc.).
As one example, we (Technow, Hankin, Hazel, & Abela, 2012) have recently completed a 2-year multi-wave prospective study. We purposefully selected a group of early adolescents (ages 11–14 at baseline) and followed them every 3 months for two years into middle adolescence in order to examine the beginning of the surge of depression across the adolescent transition. Multilevel modeling analyses showed that baseline negative cognitive style interacted with idiographic increases in dependent stressors over the 2-year follow-up to predict elevations of depressive symptoms, consistent with prior cognitive vulnerability-stress studies. But this effect was particularly pronounced among the sub-group of youth with a prior history of clinical depression. Those youth who were previously depressed, had a negative cognitive style, and experienced more dependent stressors exhibited chronically high levels of depressive symptoms across the two years. By explicating considering important phenomenological depression features (i.e., depression history) into the design and analysis, we unearthed potentially important new knowledge that may inform which particular factors and processes predict and differentiate those youth on a chronic, recurrent depression pathway versus those youth who may be on a more resilient path and recover from an initial depressive episode. Several longitudinal investigations using trajectory analyses (Costello, Swendsen, Rose, & Dierker, 2008; Galambos, Leadbeater, & Barker, 2004; Wickrama, Wickrama, & Lott, 2009) have now demonstrated different depression trajectories, including a high-stable/chronic, a low-stable, and often an initially high then decreasing group. Our results suggest that cognitive risk processes and ongoing dependent stressful environments are two, likely of many, processes that can predict which youth are more likely to constitute the high-stable/chronic group of youth. Prior research has shows there is a sub-set of depressed individuals who constitute the highly recurrent depressed group over the lifecourse (cf., Monroe & Harkness, 2011). Clearly, being able to identify which youth are at maximal risk for a chronic, recurrent trajectory of depression over their lifetime is important for informing basic etiological theories and enhancing translational knowledge.
Translational implications
What are some of the more applied, clinical applications of these future directions, especially as the majority of this paper has focused on the etiology of depression and vulnerabilities from a multiple levels of analysis perspective? Especially with respect to depression, the scope, burden, and pain of the syndrome are enormous. The National Comorbidity Study (1992; Kessler et al., 1994) and its Replication (2002; Kessler et al., 2008), spanning a 10 year period, showed overall no change in the prevalence of overall mental illness, an increase in the rates of treatment, but no improvement in decreasing disability (Kessler et al., 2008). While more adults are getting treatment, especially for depression, many receive non-evidence based interventions. Specifically for depression, while it may be positively viewed that 50% of adults receive treatment, it is disheartening that only 20% receive care that is deemed minimally adequate (Kessler et al., 2003). Moreover, when appropriate care is delivered with real-world adult patients as typically seen in clinical practice, the results are relatively disappointing: only 31% of patients remit (Warden, Rush, Trivedi, Fava, & Wisniewski, 2007). Overall, this indicates that many individuals receive sub-optimal care even when seeking treatment, and for those receiving appropriate care, many do not recover. Moreover, effect sizes in most evidence-based psychosocial interventions for adults (e.g., CBT, IPT) seem to have hit a ceiling in that ~65% in the intervention arm improve relative to ~40–50% response rate in placebo/control conditions (Kirsch, Moore, Scoboria, & Nicholls, 2002).
What are some possible solutions to the enormous need to improve evidence-based interventions? How can a basic child clinical scientific understanding of the etiological pathways leading to depression inform and advance progress in depression interventions? Here, I comment on two. These can be broadly construed as need for enhancing knowledge on mechanisms (i.e., mediation) as well as personalized treatment and prevention (i.e., moderation).
First, we know very little about the actual processes of successful treatment and mechanisms of action, especially in the best and most tested treatments for child and adolescent depression (e.g., CBT; see Webb et al., 2012, this issue). Of interest, there are recent reviews in the adult psychotherapy literature indicating some of the cognitive and neural changes underlying successful treatment (e.g., DeRubeis, Siegle, & Hollon, 2008; Roiser, Elliott, & Sahakian, 2012), and these adult-based models and mechanisms could be applied downward to youth for relevant, developmentally appropriate examination of process. So, while CBT works for many youth, although clearly not sufficient numbers, as a field we do not know why CBT may work or what else could be done to improve various CBT treatments, whether by adding on elements or refining CBT processes (or other evidence-based treatments, such as IPT, Young & Mufson, 2008; or Behavioral Activation, Martell, Dimidjian, & Herman-Dunn 2010), to improve overall care. Such modifications could be accomplished through either (or both) reducing individuals’ deficits (i.e. vulnerabilities) or enhancing their strengths (i.e., resilience). Knowing better the causal chain and etiological processes leading to depression, or those influences that enhance resilience, would surely inform the next generation of treatment development, including what treatment elements to include, whether from a deficit reducing or strength building perspective, and for whom.
Second, personalizing interventions (i.e., “what works for whom”; Insel, 2008; Shoham & Insel, 2011) is essentially a question of moderation and individual differences. Enhancing the basic knowledge on individual difference moderators from multiple levels, including genetic, neural, biological stress reactivity, information processing, interpersonal, temperament, cognitive control, etc., holds great promise for improving individualized treatment and prevention. The field of personalized psychological interventions is in its relative infancy, so there are presently few empirical examples available to demonstrate the strong potential of enhanced effect sizes when treatments and preventions are matched to the particular individuals’ strengths and/or risks. One example comes from Gillham and colleagues (2012; this issue) who demonstrated substantially larger effect sizes for their CBT-based prevention approach for reducing adolescent depression when delivered to youth with high levels of initial hopelessness.
SUMMARY
This is an exciting time to investigate vulnerabilities to the development of depression among children and adolescents. Child clinical science and developmental psychopathology have produced many significant, impressive foundational theories and knowledge sets. Inquiry into vulnerability to depression among youth is on a very strong, firm footing based on considerable progress examining descriptive features, epidemiology, course, etiology, and intervention options over the past several decades. Building on this solid foundation, future research holds great potential to continue to yield important, significant, and innovative contributions to understand the different developmental etiological pathways that ultimately contribute to the first onset, maintenance, recurrence, and resolution, of depression over the lifespan and to translate this basic knowledge into new and refined clinical interventions that can reduce the significant global burden and distress of the syndrome of clinical depression (cf., Collins et al., 2011). Through future research efforts that seek to integrate knowledge on etiological risks and processes, across multiple levels of analysis, that contribute to the ontogeny of depression among children and adolescents, we, as developmentally informed clinical scientists, will hopefully have improved the degree to which we can metaphorically slay the ogre of clinical depression.
Acknowledgments
This work was supported by NIMH grant 5R01 MH077195 (Hankin).
Footnotes
I quickly note here that theorists have postulated several conceptual models that may characterize associations among temperament and psychopathology, including: (a) vulnerability, (b) pathoplasty, (c), scar, and (d) spectrum or continuity (see Clark, Watson, & Mineka, 1994; Klein, Kotov & Bufferd, 2011 for discussion).
For this paper, I will continue to use the term “vulnerability,” although it should be clear that care needs to be taken to determine whether results are best understood from the traditional vulnerability model or a susceptibility model.
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
References
- Abela JRZ, Hankin BL, editors. Handbook of Child and Adolescent Depression. New York, NY: Guilford Press; 2008a. [Google Scholar]
- Abela JRZ, Hankin BL. Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology approach. In: Abela JRZ, Hankin BL, editors. Handbook of Child and Adolescent Depression. New York, NY: Guilford Press; 2008b. pp. 35–78. [Google Scholar]
- Abela JRZ, Hankin BL. Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: A multi-wave longitudinal study. Journal of Abnormal Psychology. 2011;120:259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- Abela JZ, Sarin S. Cognitive vulnerability to hopelessness depression: A chain is only as strong as its weakest link. Cognitive Therapy and Research. 2002;26(6):811–829. doi: 10.1023/A:1021245618183. [DOI] [Google Scholar]
- Abela JZ, Skitch SA. Dysfunctional attitudes, self-esteem, and hassles: Cognitive vulnerability to depression in children of affectively ill parents. Behaviour Research and Therapy. 2007;45(6):1127–1140. doi: 10.1016/j.brat.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Abela JRZ, Stolow D, Mineka S, Yao S, Zhu XX, Hankin BL. Cognitive vulnerability to depressive symptoms in urban and rural Hunan, China: A multi-wave longitudinal study. Journal of Abnormal Psychology. 2011;120:765–778. doi: 10.1037/a0025295. [DOI] [PubMed] [Google Scholar]
- Abramson LY, Alloy LB, Hankin BL, Haeffel GJ, MacCoon D, Gibb BE. Cognitive vulnerability-stress models of depression in a self-regulatory and psychobiological context. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. NY: The Guilford Press; 2002. pp. 268–294. [Google Scholar]
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96:358–372. doi: 10.1037/0033-295X.96.2.358. [DOI] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P, Abela JZ, Hankin BL. Factorial categorization of depression-related constructs in early adolescents. Journal Of Cognitive Psychotherapy. 2007;21(2):123–139. doi: 10.1891/088983907780851540. [DOI] [Google Scholar]
- Alloy LB, Abramson LY, Smith JM, Gibb BE, Neeren AM. Role of parenting and maltreatment histories in unipolar and bipolar mood disorders: Mediation by cognitive vulnerability to depression. Clinical Child and Family Psychology Review. 2006;9:23–64. doi: 10.1007/s10567-006-0002-4. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:57–87. [PubMed] [Google Scholar]
- Anguelova MM, Benkelfat CC, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Molecular Psychiatry. 2003;8(6):574–591. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. doi: 10.1111/j.1467-8721.2009.01615.x. [DOI] [Google Scholar]
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. NY: Harper & Row; 1967. [Google Scholar]
- Beck AT. Cognitive models of depression. Journal of Cognitive Psychotherapy. 1987;1:5–37. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. The American Journal of Psychiatry. 2008;165(8):969–977. doi: 10.1176/appi.ajp.2008.08050721. doi:10.1176/ [DOI] [PubMed] [Google Scholar]
- Beevers CG. Cognitive vulnerability to depression: A dual process model. Clinical Psychology Review. 2005;25(7):975–1002. doi: 10.1016/j.cpr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. Journal of Abnormal Psychology. 2007;116(1):208212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Pacheco J, Clasen P, McGeary JE, Schnyer D. Prefrontal morphology, 5-HTTLPR polymorphism and biased attention for emotional stimuli. Genes, Brain and Behavior. 2010;9:224–233. doi: 10.1111/j.1601-183X.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118(3):670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, McGeary JE. The BDNF Val66Met polymorphism is associated with rumination in healthy adults. Emotion. 2009;9(4):579–584. doi: 10.1037/a0016189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Berman MG, Nee DE, Casement M, Kim H, Deldin P, Kross E, Jonides J. Neural and behavioral effects of interference resolution in depression and rumination. Cognitive, Affective, and Behavioral Neursocience. 2011;11(1):85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertalanffy L. The theory of open systems in physics and biology. Science. 1950;3:23–29. doi: 10.1126/science.111.2872.23. [DOI] [PubMed] [Google Scholar]
- Bohon C, Stice E, Burton E, Fudell M, Nolen-Hoeksema S. A prospective test of cognitive vulnerability models of depression with adolescent girls. Behavior Therapy. 2008;39:79–90. doi: 10.1016/j.beth.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma EC, Riese HH, Doornbos BB, Ormel JJ, Oldehinkel AJ. Genetically based reduced MAOA and COMT functioning is associated with the cortisol stress response: A replication study. Molecular Psychiatry. 2012;17(2):119–121. doi: 10.1038/mp.2011.115. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner B. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychosocial stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Calvete E, Villardon L, Estevez A. Attributional style and depressive symptoms in adolescents: An examination of the role of various cognitive vulnerabilities. Behaviour Research and Therapy. 2008;46:944–953. doi: 10.1016/j.brat.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Carter J, Garber J. Predictors of the first onset of a major depressive episode and changes in depressive symptoms across adolescence: Stress and negative cognitions. Journal of Abnormal Psychology. 2011;120(4):779–796. doi: 10.1037/a0025441. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: Stability and change. Annual Review of Psychology. 2005;56:453–84. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chan SY, Harmer CJ, Goodwin GM, Norbury R. Risk for depression is associated with neural biases in emotional categorisation. Neuropsychologia. 2008;46(12):2896–2903. doi: 10.1016/j.neuropsychologia.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Chen MC, Joormann J, Hallmayer J, Gotlib IH. Serotonin transporter polymorphism predicts waking cortisol in young girls. Psychoneuroendocrinology. 2009;34:681–686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Development and psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 1: Theory and method. 2. Hoboken, NJ US: John Wiley & Sons Inc; 2006. pp. 1–23. [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Developmental Psychopathology. 2007;19:1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;10:103–116. [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Knopik VS, McGeary JE, Beevers CG. 5-HTTLPR and BDNF Val66Met polymorphisms moderate effects of stress on rumination. Genes, Brain and Behavior. 2011;10:740–746. doi: 10.1111/j.1601-183X.2011.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, Ciesla JA, Dallaire DH, Jacquez FM, Pineda AQ, LaGrange B, Felton JW. Emergence of attributional style and its relation to depressive symptoms. Journal of Abnormal Psychology. 2008;117(1):16–31. doi: 10.1037/0021-843X.117.1.16. [DOI] [PubMed] [Google Scholar]
- Collins LM, Horn JL. Best methods for the analysis of change. Washington, DC: American Psychological Association; 2003. [Google Scholar]
- Compas BE, Connor-Smith J, Jaser SS. Temperament, stress reactivity, and coping: Implications for depression in childhood and adolescence. Journal of Clinical Child and Adolescent Psychology. 2004;33:21–31. doi: 10.1207/S15374424JCCP3301_3. [DOI] [PubMed] [Google Scholar]
- Conley CS, Haines BA, Hilt LM, Metalsky GI. The children’s attributional style interview: Developmental tests of cognitive diathesis-stress theories of depression. Journal of Abnormal Child Psychology. 2001;29(5):445–463. doi: 10.1023/A:1010451604161. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cognitive, Affective, and Behavioral Neuroscience. 2011;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DM, Swedsen J, Rose JS, Dierker LC. Risk and protective factors associated with trajectories of depressed mood from adolescence to early adulthood. Journal of Counseling and Clinical Psychology. 2008;76(2):173–183. doi: 10.1037/0022-006X.76.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychological Bulletin. 1955;52:281–302. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology. 2011;62:583–619. doi: 10.1146/annurev.psych.093008.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Willoughby MT. Implications of latent trajectory models for the study of developmental psychopathology. Development & Psychopathology. 2003;15:581–612. doi: 10.1017/s0954579403000300. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Review. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126(6):890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB. The representation and regulation of emotion in depression: perspectives from affective neuroscience. In: Gotlib I, Hammen C, editors. Handbook of Depression. 2. Guilford Press; New York: 2009. pp. 218–248. [Google Scholar]
- De Raedt R, Koster EW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective & Behavioral Neuroscience. 2010;10(1):50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nature Neuroscience Reviews. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinovo SA, Vasey MW. Reactive and self-regulatory dimensions of temperament: Interactive relations with symptoms of general distress and anhedonia. Journal of Research in Personality. 2011 doi: 10.1016/j.jrp.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico AA, Gregory AM, Sham PP, McGuffin PP, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Moecular Psychiatry. 2004;9(10):908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Eugene F, Joormann J, Cooney RE, Atlas LY, Gotlib IH. Neural correlates of inhibitory deficits in depression. Psychiatry Research: Neuroimaging. 2009;181:30–35. doi: 10.1016/j.pscychresns.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre T, Hughes C, Emslie G, Stavinoha P, Kennard B, Carmody T. Executive functioning in children and adolescents with major depressive disorder. Child Neuropsychology. 2008;15(1):85–98. doi: 10.1080/09297040802577311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, May JC, Siegle GJ, Ladouceur CD, Ryan ND, Carter &, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: An fMRI study. Journal of Child Psychology and Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier E, Noreau A, Lepore F, Boivin M, Pérusse D, Rouleau GA, Beauregard M. Early impact of 5-HTTLPR polymorphism on the neural correlates of sadness. Neuroscience Letters. 2010;485(3):261–265. doi: 10.1016/j.neulet.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Roberts BW. Patterns of continuity: A dynamic model for conceptualizing the stability of individual differences in psychological constructs across the life course. Psychological Review. 2005;112:60–74. doi: 10.1037/0033-295X.112.1.60. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137(2):201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos NL, Leadbeater BJ, Barker ET. Gender differences in and risk factors for depression in adolescence: A 4-year longitudinal study. International Journal of Behavioral Development. 2004;28(1):16–25. doi: 10.1080/01650250344000235. [DOI] [Google Scholar]
- Garber J, Weiss B, Shanley N. Cognitions, depressive symptoms, and development in adolescents. Journal of Abnormal Psychology. 1993;102(1):47–57. doi: 10.1037/0021-843X.102.1.47. [DOI] [PubMed] [Google Scholar]
- Gillham JE, Reivich KJ, Brunwasser SM, Freres DR, Chajon ND, Kash-MacDonald VM, Chaplin TM, Abenavoli RM, Matlin SL, Gallop RJ, Seligman MEP. Preventing depressive symptoms in middle school students: A randomized effectiveness trial. Journal of Clinical Child and Adolescent Psychology. 2012 doi: 10.1080/15374416.2012.706517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE. Childhood maltreatment and negative cognitive styles: A quantitative and qualitative review. Clinical Psychology Review. 2002;22:223–246. doi: 10.1016/s0272-7358(01)00088-5. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults’ information-processing biases for facial displays of emotion. Child Maltreatment. 2009;14(2):148–156. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38:415–426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Current Directions in Psychological Science. 2008;17:159–163. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and Specificity of Information-Processing Biases in Depression and Social Phobia. Journal of Abnormal Psychology. 2004;113(3):386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Lewinsohn PM, Seeley JR, Rhode P, Redner JE. Negative cognitions and attributional style in depressed adolescents: An examination of stability and specificity. Journal of Abnormal Psychology. 1993;102(4):607–615. doi: 10.1037/0021-843X.102.4.607. [DOI] [PubMed] [Google Scholar]
- Goodyer IH. Emanuel Miller Lecture: Early onset depressions—meanings, mechanisms, and processes. Journal of Child Psychology and Psychiatry. 2007;49:1239–1256. doi: 10.1111/j.1469-7610.2008.01964.x. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Bacon A, Ban M, Croudace T, Herbert J. Serotonin transporter genotype, morning cortisol and subsequent depression in adolescents. The British Journal of Psychiatry. 2009;195:39–45. doi: 10.1192/bjp.bp.108.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Croudace T, Dubridge F, Ban M, Herbert J. Polymorphisms in BDNF (Val66Met) and 5-HTTLPR, morning cortisol and subsequent depression in at-risk adolescents. The British Journal of Psychiatry. 2010;197:365–371. doi: 10.1192/bjp.bp.110.077750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY, Campbell AJ, Westerholm RI. Stressors and child and adolescent psychopathology: Evidence of moderating and mediating effects. Clinical Psychology Review. 2006;26(3):257–283. doi: 10.1016/j.cpr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Family Psychology Review. 2011;14(2):135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The Neurobiology of Stress and Development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. Developmental Psychopathology. 2006;2:533–547. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Developmental Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. Journal of Child Psychology and Psychiatry. 2009;50(3):307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biological Psychiatry. 2011;69(4):301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: Multivariate Granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry. 2011;16(7):763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene environment interactions predicting depression symptoms in youth. Journal of Child Psychology and Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Cognitive vulnerability-stress model of depression during adolescence: Investigating symptom specificity in a multi-wave prospective study. Journal of Abnormal Child Psychology. 2008a;36:999–1014. doi: 10.1007/s10802-008-9228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Response styles theory and symptom specificity in adolescents: A multi-wave prospective study. Journal of Clinical Child and Adolescent Psychology. 2008b;37:701–713. doi: 10.1080/15374410802359627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Stability of cognitive vulnerabilities to depression: A short-term prospective multi-wave study. Journal of Abnormal Psychology. 2008c;117:324–333. doi: 10.1037/0021-843X.117.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abela JRZ, editors. Development of Psychopathology: A Vulnerability-Stress Perspective. Thousand Oaks, CA: Sage Publishing; 2005. [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Miller N, Haeffel G. Cognitive vulnerability-stress theories of depression: Examining affective specificity in the prediction of depression versus anxiety in three prospective studies. Cognitive Therapy and Research. 2004;28:309–345. doi: 10.1023/B:COTR.0000031805.60529.0d. [DOI] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic pituitary adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychological stress from preschool through middle adolescence. Biological Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Fraley RC, Abela JRZ. Daily depression and cognitions about stress: Evidence for a trait-like depressogenic cognitive style and the prediction of depressive symptoms trajectories in a prospective daily diary study. Journal of Personality and Social Psychology. 2005;88:673–685. doi: 10.1037/0022-3514.88.4.673. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Gibb BE, Abela JRZ, Flory K. Selective attention to affective stimuli and clinical depression among youth: Role of comorbid anxiety and specificity of emotion. Journal of Abnormal Psychology. 2010;119:491–501. doi: 10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Jenness J, Abela JRZ, Smolen A. Interaction of 5-HTTLPR and idiographic stressors predicts prospective depressive symptoms specifically among youth in a multiwave design. Journal of Clinical Child and Adolescent Psychology. 2011;40:572–585. doi: 10.1080/15374416.2011.581613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Lakdawalla Z, Carter IL, Abela JRZ, Adams P. Are neuroticism, cognitive vulnerabilities and self-esteem over- lapping or distinct risks for depression? Evidence from confirmatory factor analyses. Journal of Social & Clinical Psychology. 2007;26:29–63. doi: 10.1521/jscp.2007.26.1.29. [DOI] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness JL, Young JF, Abela JRZ, Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect “for better and worse. Translational Psychiatry. 2011;1:1–7. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Oppenheimer C, Jenness J, Barrocas A, Shapero BG, Goldband J. Developmental origins of cognitive vulnerabilities to depression: Review of processes contributing to stability and change across time. Journal of Clinical Psychology. 2009;65:1327–1338. doi: 10.1002/jclp.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Stone LB, Wright PA. Co-rumination, interpersonal stress generation, and internalizing symptoms: Sex differences and transactional influences in a multi-wave study of adolescents. Development and Psychopathology. 2010;22:217–235. doi: 10.1017/S0954579409990368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Wetter E, Cheely C, Oppenheimer C. Beck’s cognitive theory of depression in adolescence: Specific prediction of depressive symptoms and transactional influences in a multi-wave prospective study. International Journal of Cognitive Therapy. 2008;1:313–332. doi: 10.1521/ijct.2008.1.4.313. [DOI] [Google Scholar]
- Harbaugh CN, Vasey MW, Lonigan CJ, Bijttebier P, Hankin BL, Buffington AG. The Synergistic Emotional and Regulatory Systems Model: An Interactive Model of Depressive Symptoms and Temperamental Risk for Depressive Disorders. 2012. Manuscript submitted for publication. [Google Scholar]
- Hariri AR. The Neurobiology of Individual Differences in Complex Behavioral Traits. Annual Review Neuroscience. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Dougherty LR, Olino TM, Laptook RS, Dyson MW, Singh S. The dopamine D2 receptor and depressive and anxious symptoms in childhood: Associations and evidence for gene-environment correlation and gene-environment interaction. Psychiatric Genetics. 2010;20(6):304–310. doi: 10.1097/YPG.0b013e32833adccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Olino TM, Bufferd SJ, Miller A, Dougherty LR, Sheikh HI, Singh SM, Klein DN. The 5-HTTLPR and BDNF Val66Met polymorphisms and maternal history of depression: Associations with cognitive vulnerability to depression in childhood. 2012. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Sander LC, Nolen-Hoeksema S, Simen AA. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neuroscience Letters. 2007;429:12–16. doi: 10.1016/j.neulet.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Hollenstein T. Twenty years of dynamic systems approaches to development: Significant contributions, challenges, and future directions. Child Development Perspectives. 2011;5:256–259. [Google Scholar]
- Holm-Denoma JM, Hankin BL. Physical appearance competence as a mediator of the rumination and bulimic symptom link in female adolescents. Journal of Child and Adolescent Clinical Psychology. 2010;39:537–544. doi: 10.1080/15374416.2010.486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene–environment interactions. Trends in Cognitive Sciences. 2011;15:417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, Atchley R, Segal ZV. Vulnerability to depression: From cognitive neuroscience to prevention and treatment. New York, NY US: Guilford Press; 2011. [Google Scholar]
- Ingram RE, Luxton DD. Vulnerability-stress models. In: Hankin BL, Abela JRZ, editors. Development of psychopathology: A vulnerability-stress perspective. Thousand Oaks, CA: Sage; 2005. pp. 32–46. [Google Scholar]
- Ingram RE, Miranda J, Segal ZV. Cognitive vulnerability to depression. NY: Guilford; 1998. [Google Scholar]
- Insel TR. Translating scientific opportunity into public health impact: A strategic plan for research on mental illness. Archives of General Psychiatry. 2008;66(2):128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- Insel TR. Translating scientific opportunity into public health impact. A strategic plan for research on mental illness. Archives of General Psychiatry. 2009;66:128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang PS. Rethinking mental illness. JAMA. 2010;303:1970–1971. doi: 10.1001/jama.2010.555. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema I, Hartman C, van der Pompe G, Minderaa R, den Boer J. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry. 2007;12(5):483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: A review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Reinecke MA, Gollan JK, Kane P. Empirical evidence of cognitive vulnerability for depression among children and adolescents: A cognitive science and developmental perspective. Clinical Psychology Review. 2008;28:759–782. doi: 10.1016/j.cpr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness J, Hankin BL, Abela JRZ, Young JF, Smolen A. Chronic family stress interacts with 5-HTTLPR to predict prospective depressive symptoms among youth. Depression and Anxiety. 2011;28(12):1074–1080. doi: 10.1002/da.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE, Rudd DM. Toward a categorization of depression-related psychological constructs. Cognitive Therapy and Research. 1996;20(1):51–68. doi: 10.1007/BF02229243. [DOI] [Google Scholar]
- Joormann J. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York, NY: Guilford Press; 2009. pp. 298–321. [Google Scholar]
- Joormann J. Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science. 2010;19(3):161–166. doi: 10.1177/0963721410370293. [DOI] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited. Archives of General Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatr.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Gelernter J. Brain-derived neurotrophic factor 5-HHTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Levels of explanation in psychiatric and substance use disorders: implications for the development of an etiologically based nosology. Molecular Psychiatry. 2012;17:11–21. doi: 10.1038/mp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. National comorbidity survey replication. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Heeringa S, Lakoma MD, Petukhova M, Rupp AE, Schoenbaum M, Zaslavsky AM. Individual and societal effects of mental disorders on earnings in the United States: results from the national comorbidity survey replication. American Journal of Psychiatry. 2008;165:703–711. doi: 10.1176/appi.ajp.2008.08010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao A, Nelson CB, Huges M, Eshelman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Study. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler H, Traue H, Wiswede D. Why we still don’t understand the depressed brain – Not going beyond snapshots. GMS Psycho-Social-Medicine. 2011;8 doi: 10.3205/psm000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor’s new drugs: An analysis of antidepressant medication data submitted to the U.S. Food and Drug Administration. Prevention & Treatment. 2002;5(1) doi: 10.1037/1522-3736.5.1.523a. [DOI] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and depression: Explanatory models and review of the evidence. Annual Review of Clinical Psychology. 2011;7:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE. Understanding psychopathology: Melding behavior genetics, personality, and quantitative psychology to develop an empirically based model. Current Directions in Psychological Science. 2006;15(3):113–117. doi: 10.1111/j.0963-7214.2006.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C, Holzhauer S, Huffziger S. Decreased cortisol response to awakening is associated with cognitive vulnerability to depression in a nonclinical sample of young adults. Psychoneuroendocrinology. 2007;32(2):199–209. doi: 10.1016/j.psyneuen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Kuehner C, Huffziger S, Liebsch K. Rumination, distraction and mindful self-focus: Effects on mood, dysfunctional attitudes and cortisol stress response. Psychological Medicine. 2009;39(2):219–228. doi: 10.1017/S0033291708003553. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Dalgleish T. Overgeneral autobiographical memory in adolescents at risk for depression. Memory. 2011;19(3):241–250. doi: 10.1080/09658211.2011.554421. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Howell R. Facets of autobiographical memory in adolescents with major depressive disorder and never-depressed controls. Cognition and Emotion. 2006;20(3–4):466–487. doi: 10.1080/02699930500342639. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Howell R, Dalgleish T. Overgeneral autobiographical memory in depressed adolescents with, versus without, a reported history of trauma. Journal of Abnormal Psychology. 2006;115:387–398. doi: 10.1037/0021-843X.115.3.387. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Waldman ID, Loft JD, Hankin BL, Rick J. The structure of child and adolescent psychopathology: Generating new hypotheses. Journal of Abnormal Psychology. 2004;113(3):358–385. doi: 10.1037/0021-843X.113.3.358. [DOI] [PubMed] [Google Scholar]
- Lahey BB, D’Onofrio BM, Waldman ID. Using epidemiologic methods to test hypotheses regarding causal influences on child and adolescent mental disorders. Journal of Child Psychology and Psychiatry. 2009;50(1–2):53–62. doi: 10.1111/j.14697610.2008.01980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakdawalla Z, Hankin BL, Mermelstein R. Cognitive theories of depression in children and adolescents: A conceptual and quantitative review. Child Clinical and Family Psychology Review. 2007;10:1–24. doi: 10.1007/s10567-006-0013-1. [DOI] [PubMed] [Google Scholar]
- Lau JF, Eley TC. New behavioral genetic approaches to depression in childhood and adolescence. In: Abela JZ, Hankin BL, editors. Handbook of Depression in Children and Adolescents. New York, NY US: Guilford Press; 2008. pp. 124–148. [Google Scholar]
- Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson &, Ernst M. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biological Psychiatry. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JYF, Goldman D, Buzas B, Hodgkinson C, Leibenluft E, Nelson E, Ernst M. BDNF gene polymorphism (val66met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 2010;53:952–961. doi: 10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery KS, Goldsmith H, Klinnert MD, Mrazek DA. Developmental models of infant and childhood temperament. Developmental Psychology. 1999;35(1):189–204. doi: 10.1037/0012-1649.35.1.189. [DOI] [PubMed] [Google Scholar]
- Levinson DF. Genetics of major depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. 2. New York, NY: Guilford Press; 2009. pp. 165–186. [Google Scholar]
- Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: Differential processes of psychosocial risk. Journal of Abnormal Psychology. 1999;108(3):483–489. doi: 10.1037/0021-843X.108.3.483. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Joiner TE, Rohde P. Evaluation of cognitive diathesis-stress models in predicting major depressive disorder in adolescents. Journal of Abnormal Psychology. 2001;110:203–215. doi: 10.1037/0021-843X.110.2.203. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Lucht M, Barnow S, Schroeder W, Grabe HJ, Finckh U, John U, Herrman FH. Negative perceived paternal parenting is associated with dopamine D2 receptor exon 8 and GABA(A) Alpha 6 receptor variants. American Journal of Medical Genetics Part B. 2006;141:167–172. doi: 10.1002/ajmg.b.30255. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior, and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Norbury R, Murphy SE, Inkster B, Harmer CJ, Cowen PJ. Affective modulation of anterior cingulate cortex in young people at increased familial risk of depression. British Journal of Psychiatry. 2008;192(5):356–361. doi: 10.1192/bjp.bp.107.043398. [DOI] [PubMed] [Google Scholar]
- Martell CR, Dimidjian S, Herman-Dunn R. Behavioral activation for depression: A clinician’s guide. New York, NY: Guilford Press; 2010. [Google Scholar]
- Masten AS, Roisman GI, Long JD, Burt KB, Obradovic J, Riley JR, Tellegen A. Developmental cascades: Linking academic achievement and externalizing and internalizing symptoms over 20 years. Developmental Psychology. 2005;41(5):733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- Matthews K, Coghill D. Neuropsychological functioning in depressed adolescent girls. Journal of Affective Disorders. 2008;111:113–118. doi: 10.1016/j.jad.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mayberg H. Defining the neural circuitry of depression: Toward a new nosology with therapeutic implications. Biological Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Alsabban S, Uher R. The truth about genetic variation in the serotonin transporter gene and response to stress and medication. British Journal of Psychiatry. 2011;198(6):424–427. doi: 10.1192/bjp.bp.110.085225. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene-environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Metalsky GI, Joiner TE, Jr, Hardin TS, Abramson LY. Depressive reactions to failure in a naturalistic setting: A test of the hopelessness and self-esteem theories of depression. Journal of Abnormal Psychology. 1993;102:101–109. doi: 10.1037/0021-843X.102.1.101. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Propper CB, Gariepy JL, Blair C, Garrett-Peters P, Cox MJ. Bidirectional genetic and environmental influences on mother and child behavior: The family system as the unit of analyses. Development and Psychopathology. 2007;19:1073–1087. doi: 10.1017/S0954579407000545. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the ‘kindling’ hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review. 2005;112(2):417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Recurrence in major depression: A conceptual analysis. Psychological Review. 2011;118(4):655–674. doi: 10.1037/a0025190. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis–stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2007;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, Battaglia M. The influence of family structure, the TPH2 G-703-T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. Journal of Child Psychology and Psychiatry. 2009;50(3):317–325. doi: 10.1111/j.1469-7610.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Stice E, Wade E, Bohon C. Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. Journal of Abnormal Psychology. 2007;116:198–207. doi: 10.137/0021-843X.116.1.198. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6(6):589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco B, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. The Journal of Neuroscience. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LA, Batezati-Alves SC, Almeida RC, Segreti A, Akkal D, Hassel S, Phillips ML. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(6):602–611. doi: 10.1016/j.jaac.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RJ, Goodyer IM, Teasdale JD. Effects of induced rumination and distraction on mood and overgeneral autobiographical memory in adolescent major depressive disorder and controls. Journal of Child Psychology and Psychiatry. 2004;45:996–1006. doi: 10.1111/j.1469-7610.2004.t01-1-00291.x. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Hormones and Behavior. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott J, Gorodetsky E, Hodgkinson CA, Goldman D, Fox NA. Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biological Psychology. 2010;83(3):269–271. doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Petronis A, Mill J. Brain, Behavior, and Epigentics. NY: Springer-Verlag Publishing; 2011. [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Daniels D. Why are children in the same family so different from one another? Behavioral and Brain Sciences. 1987;10:1–60. [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112(3):323–338. doi: 10.1037/0021-843X.112.3.323. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. The American Journal of Psychiatry. 1992;149(8):99–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Propper C, Moore G, Mills-Koonce W, Halpern C, Hill-Soderlund AL, Calkins SD, Cox M. Gene-environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Development. 2008;79(5):1377–1394. doi: 10.1111/j.1467-8624.2008.01194. [DOI] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biological Psychiatry. 2007;61(2):231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2009;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE. Longitudinal course of adolescent depression: Neuroendocrine and psychosocial predictors. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(2):141–151. doi: 10.1097/00004583-201002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Merikangas K. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126(1):3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Rosier JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–136. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of Child Psychology. NJ: John Wiley & Sons, Inc; 1998. [Google Scholar]
- Rottenberg J. Major depressive disorder: emerging evidence for emotion context insensitivity. In: Rottenberg J, Johnson SL, editors. Emotion and Psychopathology: Bridging Affective and Clinical Science. Washington, DC US: American Psychological Association; 2007. pp. 151–165. [DOI] [Google Scholar]
- Rudolph KD, Troop-Gordon W, Granger DA. Individual differences in biological stress responses moderate the contribution of early peer victimization to subsequent depressive symptoms. Psychopharmacology. 2011;214(1):209–219. doi: 10.1007/s00213-010-1879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Environmentally mediated risks for psychopathology: Research strategies and findings. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:3–18. doi: 10.1097/01.chi.0000145374.45992.c9. [DOI] [PubMed] [Google Scholar]
- Rutter M, Quinton D. Psychiatric disorder: Ecological factors and concepts of causation. In: McGurk H, editor. Ecological Factors in Human Development. Amsterdam, the Netherlands: North-Holland Publishing Co; 1977. pp. 173–187. [Google Scholar]
- Ryder AG, Sun J, Zhu X, Yao S, Dutton-Chentsova YE. Depression in China: Integrating developmental psychopathology and cultural-clinical psychology. Journal of Clinical Child and Adolescent Psychology. 2012 doi: 10.1080/15374416.2012.710163. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, MacKenzie MJ. Research strategies for capturing transactional models of development: The limits of the possible. Development and Psychopathology. 2003;15:613–640. doi: 10.1017/s0954579403000312. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype-environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Sheeber LB, Allen NB, Leve C, Davis B, Shortt JW, Katz LF. Dynamics of affective experience and behavior in depressed adolescents. Journal of Child Psychology and Psychiatry. 2009;50:1419–1427. doi: 10.1111/j.1469-7610.2009.02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiner RL. How shall we speak of children’s personalities in middle childhood? A preliminary taxonomy. Psychological Bulletin. 1998;124(3):308–332. doi: 10.1037/0033-2909.124.3.308. [DOI] [PubMed] [Google Scholar]
- Shoham V, Insel TR. Rebooting for whom?: Portfolios, technology, and personalized intervention. Perspectives on Psychological Science. 2011;6(5):478–482. doi: 10.1177/1745691611418526. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Development of depression: Sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. International Journal of Neuropsychopharmacology. 2006;9(4):443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- Skitch SA, Abela JZ. Rumination in response to stress as a common vulnerability factor to depression and substance misuse in adolescence. Journal of Abnormal Child Psychology. 2008;36(7):1029–1045. doi: 10.1007/s10802-008-9233-9. [DOI] [PubMed] [Google Scholar]
- Snyder HR. Executive function is broadly impaired in major depressive disorder: A meta-analysis and review. Psychological Bulletin. 2012 doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud CB, Davila J, Moyer A. The relationship between stress and depression in first onsets versus recurrences: A meta-analytic review. Journal of Abnormal Psychology. 2008;117(1):206–213. doi: 10.1037/0021-843X.117.1.206. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Walport M. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, Krueger RF. Interpreting personality as a vulnerability for psychopathology: A developmental approach to the personality-psychopathology relationship. In: Hankin BL, Abela JZ, editors. Development of psychopathology: A vulnerability-stress perspective. Thousand Oaks, CA US: Sage Publications, Inc; 2005. pp. 199–214. [Google Scholar]
- Technow JR, Hankin BL, Hazel N, Abela JRZ. Mechanisms contributing to exacerbation of depressive symptoms in youth: The interplay among depression history, negative cognitive style, and dependent stressors. 2012 Manuscript in preparation. [Google Scholar]
- Thase ME. Neurobiological aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 187–217. [Google Scholar]
- Thelen E, Smith LB. A Dynamic Systems Approach to the Development of Cognition and Action. Cambridge, Mass: MIT Press; 1994. [Google Scholar]
- Thomason ME, Henry ML, Hamilton J, Joormann J, Pine DS, Ernst M, Gotlib IH. Neural and behavioral responses to threatening emotion faces in children as a function of the short allele of the serotonin transporter gene. Biological Psychology. 2010;85(1):38–44. doi: 10.1016/jbiopsycho.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15(1):18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Harbaugh CN, Lonigan CJ, Phillips BM, Hankin BL, Bijttebier P, Willem Positive emotionality and effortful control synergistically moderate the association between negative emotionality and depressive symptoms: Evidence from five samples. 2012 Manuscript in preparation. [Google Scholar]
- Waddington CH. The Strategy of the Genes. London: Allen and Unwin; 1957. [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Sauer H, Schlösser RGM. Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. Journal of Psychiatry & Neuroscience. 2008;33(3):199–208. [PMC free article] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D project results: A comprehensive review of findings. Current Psychiatry Reports. 2007;9(6):449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- Weaver IG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Webb CA, Auerbach RP, DeRubeis RJ. Process of change in CBT of adolescent depression: Review and recommendation. Journal of Clinical Child and Adolescent Psychology. 2012 doi: 10.1080/15374416.2012.704842. [DOI] [PubMed] [Google Scholar]
- Weiner B. An attributional theory of achievement motivation and emotion. Psychological Review. 1985;92:548–573. [PubMed] [Google Scholar]
- Werner H. The concept of development from a comparative and organisismic point of view. University of Minnesota Press; 1957. [Google Scholar]
- Wickrama KS, Wickrama T, Lott R. Heterogeneity in youth depressive symptom trajectories: Social stratification and implications for young adult physical health. Journal of Adolescent Health. 2009;45(4):335–343. doi: 10.1016/j.jadohealth.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Wilkinson PO, Goodyer IM. Attention difficulties and mood-related ruminative response style in adolescents with unipolar depression. Journal of Child Psychology and Psychiatry. 2006;47(12):1284–1291. doi: 10.1111/j.1469-7610.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Wolfensberger SA, Veltman DJ, Hoogendijk WG, De Ruiter MB, Boomsma DI, de Geus EC. The neural correlates of verbal encoding and retrieval in monozygotic twins at low or high risk for depression and anxiety. Biological Psychology. 2008;79(1):80–90. doi: 10.1016/j.biopsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wong CCY, Caspi A, Williams B, Craig IW, Houts R, Ambler A, Mill J. A longitudinal study of epigenetic variation in twins. Epigenetics. 2010;5(6):1–11. doi: 10.4161/epi.5.6.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Mufson L. Interpersonal psychotherapy for treatment and prevention of adolescent depression. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. New York: Guilford Press; 2008. pp. 288–306. [Google Scholar]
- Zhang TY, Meaney MJ. Epigenetics and the environmental regulation of the genome and its function. Annual Review Psychology. 2010;61:439–466. doi: 10.1146/annurev.psych.60.110707.163625. [DOI] [PubMed] [Google Scholar]
- Zubin J, Spring B. Vulnerability: A new view of schizophrenia. Journal of Abnormal Psychology. 1977;86(2):103–126. doi: 10.1037/0021-843X.86.2.103. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Vulnerability to psychopathology: A biosocial model. Washington, DC US: American Psychological Association; 1999. [DOI] [Google Scholar]