Summary
Improving muscle precursor cell (MPC, muscle-specific stem cells) function during aging has been implicated as a key therapeutic target for improving age-related skeletal muscle loss. MPC dysfunction during aging can be attributed to both the aging MPC population and the changing environment in skeletal muscle. Previous reports have identified elevated levels TNF-α in aging, both circulating and locally in skeletal muscle. The purpose of the present study was to determine if age-related differences existed between TNF-α-induced NF-κB activation and expression of apoptotic gene targets. MPCs isolated from 32-mo-old animals (32-mo) exhibited an increased NF-κB activation in response to 1, 5, and 20 ng/ml TNF-α, compared to MPCs isolated from 3-mo-old animals (3-mo). No age differences were observed in the rapid canonical signaling events leading to NF-κB activation or in the increase in mRNA levels for TNF receptor 1, TNF receptor 2, TNF receptor-associated factor 2 (TRAF2), or Fas (CD95) observed after 2 hr of TNF-α stimulation. Interestingly, mRNA levels for TRAF2 and the cell death inducing receptor, Fas (CD95) were persistently upregulated in response to 24 hr TNF-α treatment in MPCs isolated from 32-mo, compared to 3-mo. Our data indicate that age-related differences may exist in the regulatory mechanisms responsible for NF-κB inactivation, which may have an effect on TNF-α-induced apoptotic signaling. These findings improve our understanding of the interaction between aged MPCs and the changing environment associated with age, which is critical for the development of potential clinical interventions aimed at improving MPC function with age.
Keywords: Satellite cells, aging, cytokine, apoptosis, NF-κB signaling
Introduction
Aging is associated with decreased strength and skeletal muscle mass, a condition known as sarcopenia, leading to decreased quality of life and increased mortality in our aging population. One contributing factor to sarcopenia is its diminished capacity for skeletal muscle regeneration (Brooks and Faulkner, 1990), hypertrophy (Alway et al, 2002), and regrowth after a bout of atrophy (Chakravarthy et al, 2000).
In skeletal muscle, the resident stem cells responsible for growth and repair exist primarily as a quiescent population of cells located between the basal lamina and plasmalemma. Based on their anatomical location, these stem cells were first termed satellite cells in 1961 (MAURO, 1961). In response to activation signaling events, the satellite cells enter the cell cycle. The progeny of dividing satellite cells become a heterogenic population of muscle precursor cells (MPCs) (Sinanan et al, 2006), with a large population that fuses with the myofiber to form new myonuclei and a smaller subpopulation of self-renewing stem cells that forms as a result of asymmetric cell divisions (Cooper et al, 2006). Importantly, Conboy and Rando (Conboy and Rando, 2005) concluded that an age-associated loss of MPC functionality is the primary factor responsible for the loss of regenerative potential and atrophy of aged skeletal muscle. It has been shown that the age-associated changes in the systemic and local environments contribute to skeletal muscle regenerative capacity (Carlson and Faulkner, 1989;Gutmann and Carlson, 1976) and MPC dysfunction (Conboy et al, 2005;Brack et al, 2007). However, the differences in the intrinsic function of MPCs generated from aged skeletal muscle satellite cells should not be overlooked (Conboy et al, 2003;Machida and Booth, 2004;Barani et al, 2003;Lorenzon et al, 2004). Taken together, it is important to consider both the environmental cues and the MPCs themselves while investigating the functional ability of MPCs to contribute to skeletal muscle growth and repair.
Aging is associated with chronic low-grade systemic inflammation, which is a condition that is identified by a ∼2- to 3-fold increase in circulating levels of certain cytokines including TNF-α, IL-1, and IL-6 (Bruunsgaard et al, 2003;Bruunsgaard et al, 2001). Chronic low-grade systemic inflammation has been linked to the development of atherosclerosis (Libby, 2002) and insulin resistance (Dandona et al, 2004;Plomgaard et al, 2005). In terms of skeletal muscle, chronic low-grade systemic inflammation has been associated with the characteristic loss of skeletal muscle mass in sarcopenia (Roubenoff, 2007). Importantly, elevated levels of TNF-α mRNA (Leger et al, 2008;Greiwe et al, 2001) and protein (Greiwe et al, 2001) have been observed in aged human skeletal muscle. Despite the importance of TNF-α as a critical mediator of inflammatory signaling and the vital role MPCs play in muscle repair and maintenance, very little is known about the effects of TNF-α on MPCs isolated from aged skeletal muscle.
TNF-α activates nuclear factor-kappaB (NF-κB) transcription factor family members, which consists of the five members RelA (p65), c-Rel, RelB, NF-κB1 (p105/p50), and NF-κB2 (p100/p52) (Hayden and Ghosh, 2008). Notably, aged tissue including heart, liver, kidney, and brain exhibit increased NF-κB nuclear binding activities (Helenius et al, 1996). It was recently reported that MPCs isolated from aged skeletal muscle exhibit an increased apoptotic response to TNF-α in the presence of a stress inducing agent, compared to MPCs isolated from both young and adult rat muscle (Jejurikar et al, 2006). The purpose of the present study was 2-fold. First, to determine the effects of TNF-α on NF-κB activation in MPCs isolated from young and old rat skeletal muscle. Second, to determine if age influences the TNF-α-induced transcriptional effects on the NF-κB apoptosis target genes, FasL and Fas (CD95).
Results
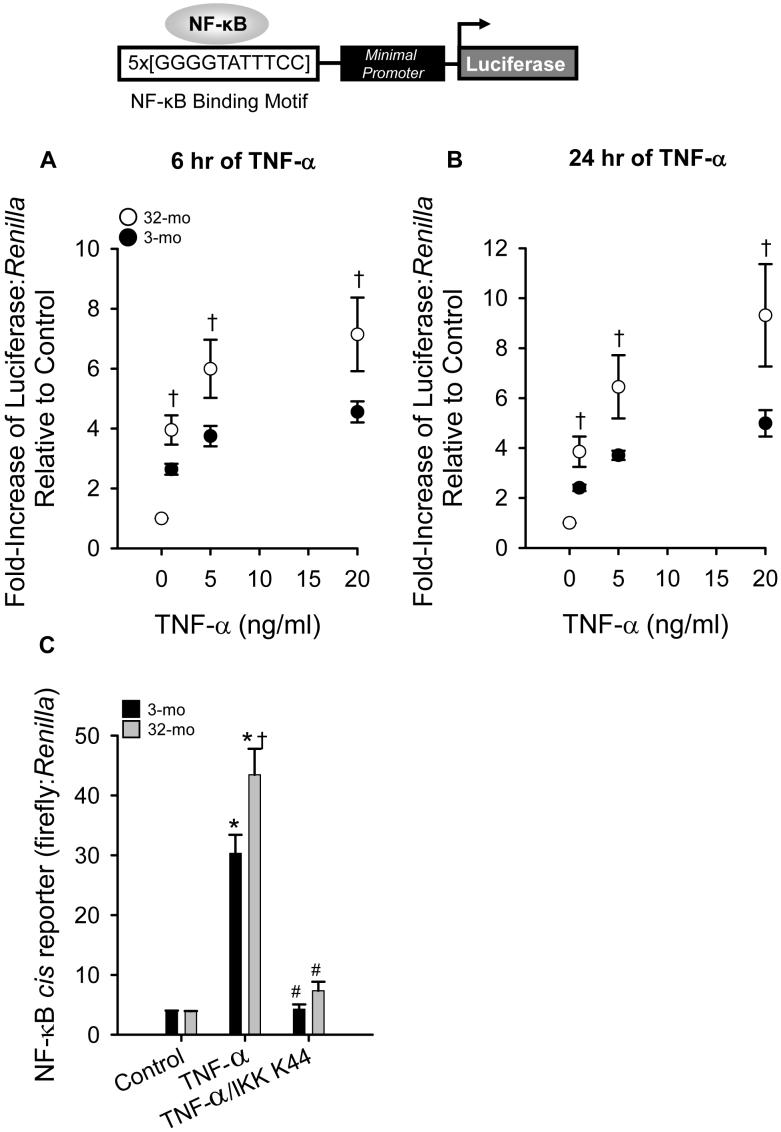
Our first aim was to determine if age-dependent differences exist in response to TNF-α stimulation in MPCs. In order to assess NF-κB activation in response to TNF-α, a NF-κB cis-reporter construct was used. The NF-κB cis-reporter construct consists of 5 tandem repeats of a conserved NF-κB binding motif linked to the firefly luciferase gene, which allows the detection of changes in NF-κB activation (schematic representation in Figure 1). Sample sizes are indicated in the figure legends where n represents independent isolations from separate animals. TNF-α-induced NF-κB activation was increased to a greater extent in MPCs isolated from old, compared to young animals, at both 6 (Figure 1A) and 24 hrs (Figure 1B) after the addition of TNF-α, at all doses tested. The 6 and 24 hr time points represent intermediate and long-term NF-κB activation, respectively. The luciferase assay system is not a sensitive measurement of rapid inactivation because it is dependent on the half-life of the luciferase protein (which is approximately 3-6 hr) (Bronstein et al, 1994;Thompson et al, 1991). In order to better understand potential mechanisms that caused the age-dependent difference in NF-κB activation, we next determined whether the elevated NF-κB response observed in MPCs isolated from aged animals was activated via the canonical (classical) NF-κB signaling pathway.
Figure 1.
Tumor necrosis factor-alpha (TNF-α) stimulation induces a greater nuclear factor-kappaB (NF-κB) response at both A) 6 hr and B) 24 hr after treatment in muscle precursor cells (MPCs) isolated from 32-mo-old animals (32-mo), compared to 3-mo-old animals (3-mo). MPCs were treated with a vehicle (0 ng/ml, designed as control) or 1, 5, or 20 ng/ml TNF-α for the specified time. Data are presented as fold-induction relative to control ± SEM (n=4). C) Overexpression of an IKK-β mutant, in which a conserved lysine-44 (K44) residue is mutated to a methionine (IKK K44M), prevents the activation of NF-κB in MPCs isolated from 3-mo and 32-mo. Data are presented as the ratio of firefly to Renilla luciferase. Values are means ± SEM (n=2). †, significantly different from 3-mo within dose (panels A and B) or within TNF-α treatment (panel C). *, significantly different from vehicle control (panel C). #, significantly different from TNF-α (panel C).
Canonical NF-κB signaling acts via IKK-β (the noncanonical pathway depends on IKKα, (Hayden and Ghosh, 2008)). Therefore, we next determined if overexpression of an IKK-β mutant, in which a conserved lysine-44 (K44) residue is mutated to a methionine (IKK K44M) was used (Mercurio et al, 1997). Previously, it was reported that overexpression of IKK K44M blocked TNF-α-induced nuclear localization of p65 in normal cells (Laszlo and Endre, 1976). As shown in Figure 1C, IKK K44M completely blocked the TNF-α-induced NF-κB activation observed in MPCs isolated from aged animals. Therefore, we have demonstrated that MPCs isolated from aged animals exhibit an increased NF-κB activation in response to TNF-α that can be abolished with overexpression of a mutant IKK-β. We next decided to determine if age was associated with differential expression levels of key molecules involved in canonical NF-κB signaling.
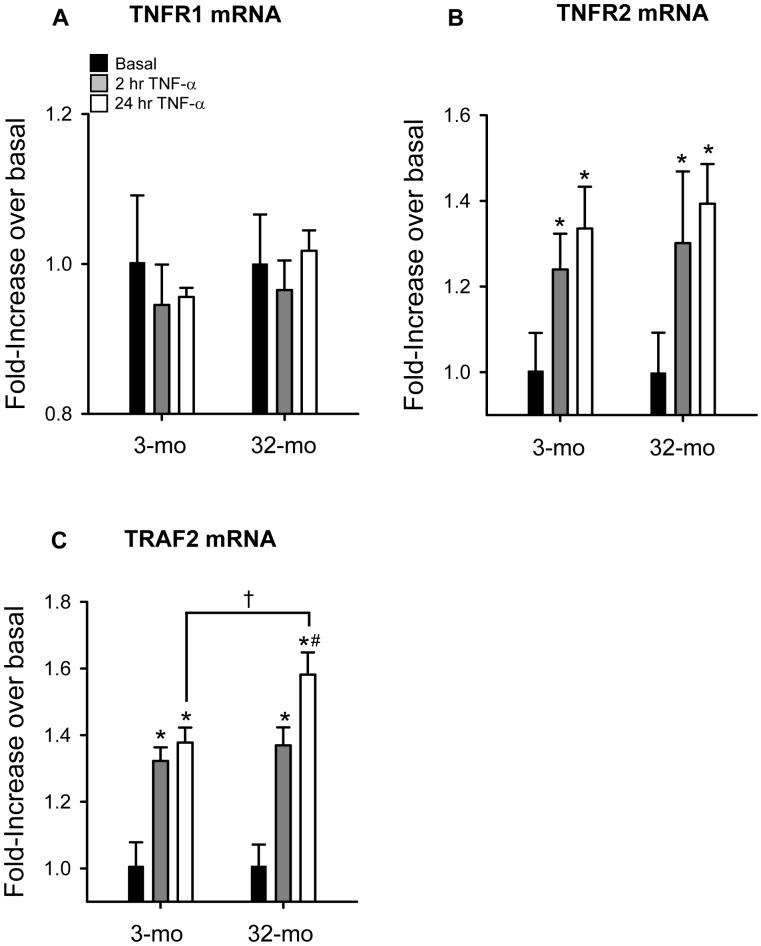
Increased TNF-α-induced apoptosis in human T-cells was associated with increased expression of TNFR1 and decreased expression of TNFR2 (Aggarwal et al, 1999). In rat skeletal muscle, TNFR mRNA was increased in both soleus and plantaris in old, compared to young animals (Pistilli et al, 2006). In order to determine if the age-dependent increase in NF-κB activation could be explained by differences in TNF-receptor expression in MPCs, we measured basal (unstimulated) mRNA levels for both TNFR1 and TNFR2. We found no difference in basal expression levels in the transcripts for either TNF-receptor (data not shown). We also measured the basal mRNA level for the TNF-receptor adaptor protein, TRAF-2. TRAF-2 was of particular interest for three reasons 1) it facilitates cell signaling for both TNFR1 and TNFR2 (Gupta and Gollapudi, 2005), 2) it was shown to be decreased in T-cells from aged humans (Aggarwal et al, 1999), and 3) it is a known target of NF-κB (Wang et al, 1998). Taken together, we hypothesized that higher TRAF-2 expression in MPCs isolated from old animals might help explain the age-specific response to TNF-α in MPCs. However, unlike aged T-cells, basal TRAF-2 mRNA levels were not different in MPCs isolated from young, compared to old animals (data not shown). The aforementioned results led us to next determine if TNF-α stimulation altered mRNA levels of TNFR1, TNFR2, and TRAF-2, relative to basal levels (values were normalized to basal expression within each age group). TNFR1 mRNA was not affected at either 2 hr or 24 hr after the onset of TNF-α stimulation (Figure 2A). However, both TNFR2 and TRAF-2 transcripts were increased in response to TNF-α at both 2 hr and 24 hr (Figure 2B and C). Moreover, TRAF-2 mRNA is increased to a greater extent at 24-hr TNF-α stimulation in MPCs isolated from old animals, compared to young (Figure 2C). These findings are significant due to the fact that TRAF-2 is both involved as a key adapter molecule for signaling downstream of TNFR1 and TNFR2 and a known target of NF-κB (Gupta and Gollapudi, 2005). We next measured the signaling events downstream of TNF receptors and their adaptor proteins.
Figure 2.
Tumor necrosis factor-alpha (TNF-α)-induced mRNA expressions of A) tumor necrosis factor receptor 1 (TNFR1), B) tumor necrosis factor receptor 2 (TNFR2), and C) TNF receptor-associated factor 2 (TRAF2) in muscle precursor cells (MPCs) isolated from 3-mo-old animals (3-mo) and 32-mo-old animals (32-mo). Data are presented as fold-increase over basal ± SEM (n=4). *, significantly different from basal. †, significantly different from 3-mo. #, significantly different from 2 hr TNF-α.
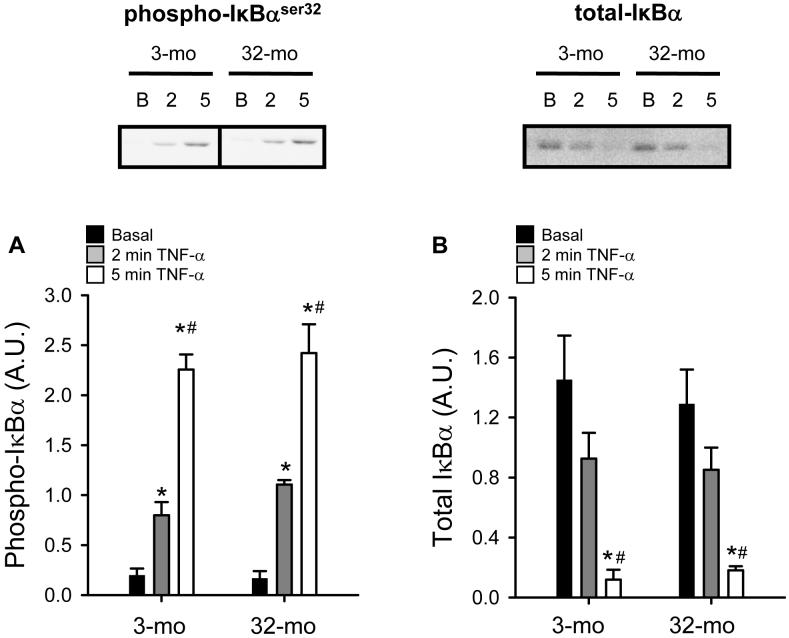
IκBα is a key protein that is normally bound to and inhibits NF-κB. Once activated by TNF-α, IKK-β rapidly phosphorylates IκBα at serine-32 and serine-36, which in turn triggers two events. First, phosphorylation of IκBα allows the release of NF-κB family members allowing them to translocate to the nucleus. Second, phosphorylated IκBα is then ubiquitinated and rapidly degraded via the ubiquitin-proteasome pathway. Phosphorylation of IκBα-serine-32 is significantly increased after 2 minutes of TNF-α stimulation in MPCs isolated from both young and old animals (Figure 3A). After 5 minutes of stimulation, phosphorylation of IκBα-serine-32 is further increased in MPCs isolated from animals of both ages (Figure 3A). As mentioned above, phosphorylated IκBα is rapidly degraded and we demonstrate that the vast majority of IκBα has been degradated within 5 minutes of stimulation with TNF-α (Figure 3B). However, no age-dependent differences were observed in the phosphorylation or degradation of IκBα.
Figure 3.
Tumor necrosis factor-alpha (TNF-α)-induced phosphorylation and degradation of IκBα in muscle precursor cells (MPCs) isolated from 3-mo-old animals (3-mo) and 32-mo-old animals (32-mo). A) Serine-32 phosphorylation of IκBα for basal MPCs and after 2 min and 5 min stimulation with TNF-α (n=4). B) Total IκBα protein for basal MPCs and after 5 min stimulation with TNF-α. Representative Western blot images of blots are presented above group mean data. Group mean data are presented ± SEM in arbitrary units (A.U.) (n=3 for basal and 5 min, n=2 for 2 min). *, significantly different from basal. #, significantly different from 2-min TNF-α.
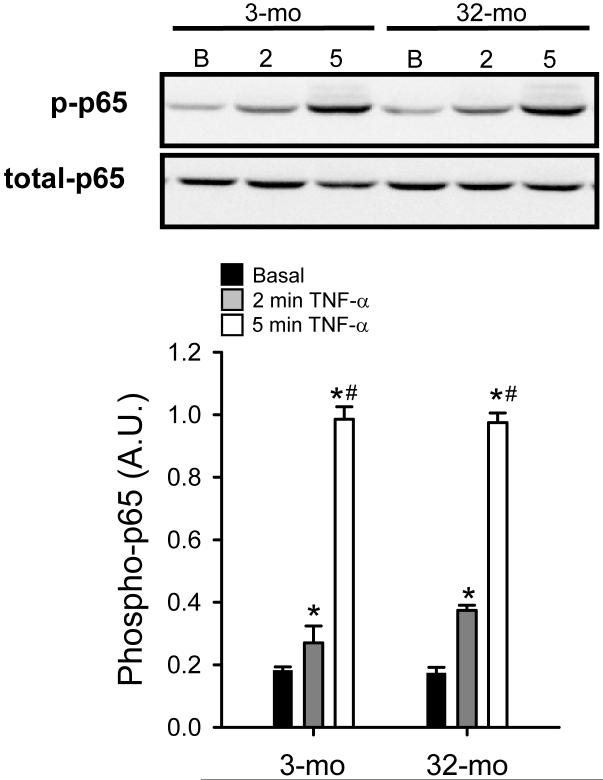
In response to stimulation with TNF-α, NF-κB heterodimers translocate to the nucleus (e.g., p65/p50). Phosphorylation of these dimers induces DNA binding to conserved κB elements of target genes. The NF-κB family member, p65, is phosphorylated by a number of kinases including protein kinase A, mitogen- and stress-activated protein kinase, IKKα, and IKKβ. Phosphorylation at serine-536 of p65 has been demonstrated to promote its interaction with transcriptional co-activators (Hayden and Ghosh, 2008). As shown in Figure 4, phosphorylation of p65 is increased after 2 minutes of TNF-α stimulation in MPCs isolated from both young and old animals. Moreover, after 5 minutes of TNF-α stimulation, phosphorylation of p65 was increased several fold, compared to basal levels (Figure 4). However, there were no age differences detected in phosphorylation of p65 in MPCs isolated from young, compared to old animals. Also, there were no differences in total p65 between either treatment time or age groups (representative bands in Figure 4). Therefore, it seems as though rapid signaling events leading to NF-κB activation are not affected by age. However, even though the events regulating inactivation of NF-κB are poorly understood, another regulatory step is the re-synthesis of IκBα in order to re-sequester NF-κB in the cytoplasm.
Figure 4.
Tumor necrosis factor-alpha (TNF-α)-induced phosphorylation of the NF-κB family member, p65, in muscle precursor cells (MPCs) isolated from 3-mo-old animals (3-mo) and 32-mo-old animals (32-mo). A) Serine-536 phosphorylation of p65 for basal MPCs and after 2 min and 5 min stimulation with TNF-α. Total p65 protein was not different between either age group of any treatment level. Representative Western blot images of blots are presented above group mean data. Group mean data are presented ± SEM in arbitrary units (A.U.) (n=4). *, significantly different from basal. #, significantly different from 2 min TNF-α.
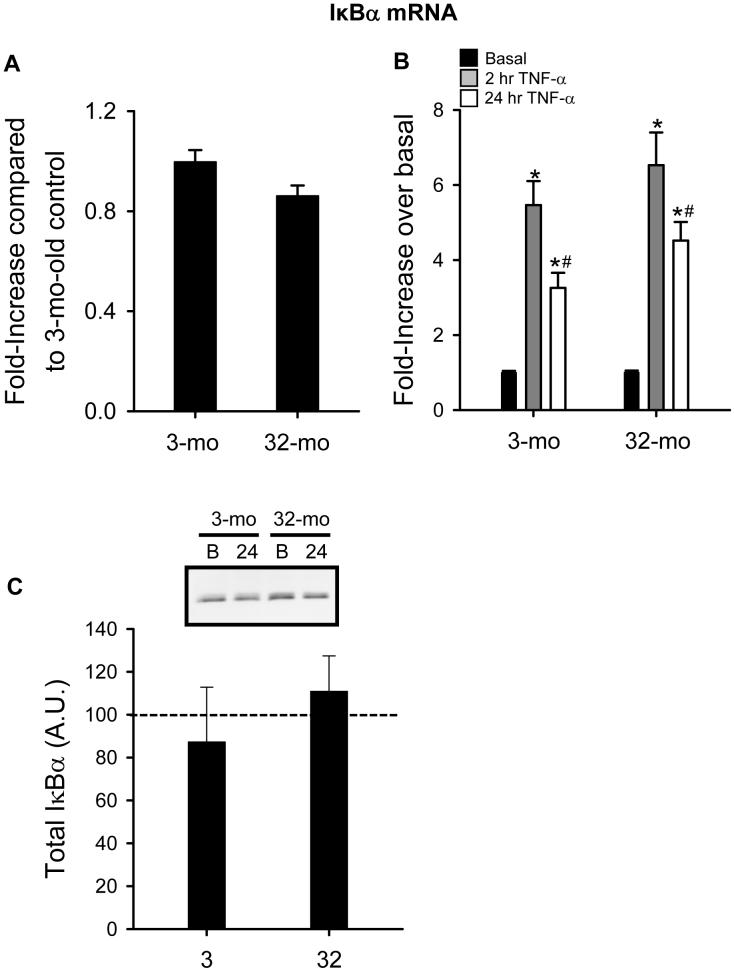
In order to better understand potential age-related differences on NF-κB inactivation, we measured IκBα mRNA after stimulation with TNF-α as an indicator of resynthesis. First, basal expression of IκBα mRNA was not different between MPCs isolated either young or old animals (Figure 5A). In response to TNF-α, there is a marked increased in IκBα mRNA in MPCs from both age groups at 2 hr. Although IκBα mRNA decreased from 2 hr to 24 hr after stimulation in both age groups, it remained elevated over basal levels after 24 hr of TNF-α stimulation without discernable age differences (Figure 5B). This response, which may approximate mRNA resynthesis, is expected since IκBα protein was almost completely degraded after TNF-α stimulation (Figure 3B) and needs to be replenished. Moreover, after 24 hr, IκBα protein had recovered to pre-TNF-α stimulation levels in MPCs isolated from both age groups (Figure 5C). These data indicate that the age-dependent increase in NF-κB activation observed do not appear to be a result of defective up-regulation of IκBα transcriptional events, which allows for resynthesis of IκBα and sequestration of NF-κB in the cytoplasm.
Figure 5.
Basal and tumor necrosis factor-alpha (TNF-α)-induced IκBα mRNA levels. A) Basal IκBα mRNA levels in muscle precursor cells (MPCs) isolated from 3-mo-old animals (3-mo) and 32-mo-old animals (32-mo). Data are presented as group means relative to 3-mo ± SEM (n=4). B) TNF-α-induced IκBα mRNA expression after 2 and 24 hr stimulation with TNF-α in MPCs isolated from 3-mo and 32-mo. Data are presented as fold-increase over basal ± SEM (n=4). C) IκBα protein level 24 hr after TNF-α stimulation expressed relative to non-stimulated levels (n=2). *, significantly different from basal. #, significantly different from 2-hr TNF-α.
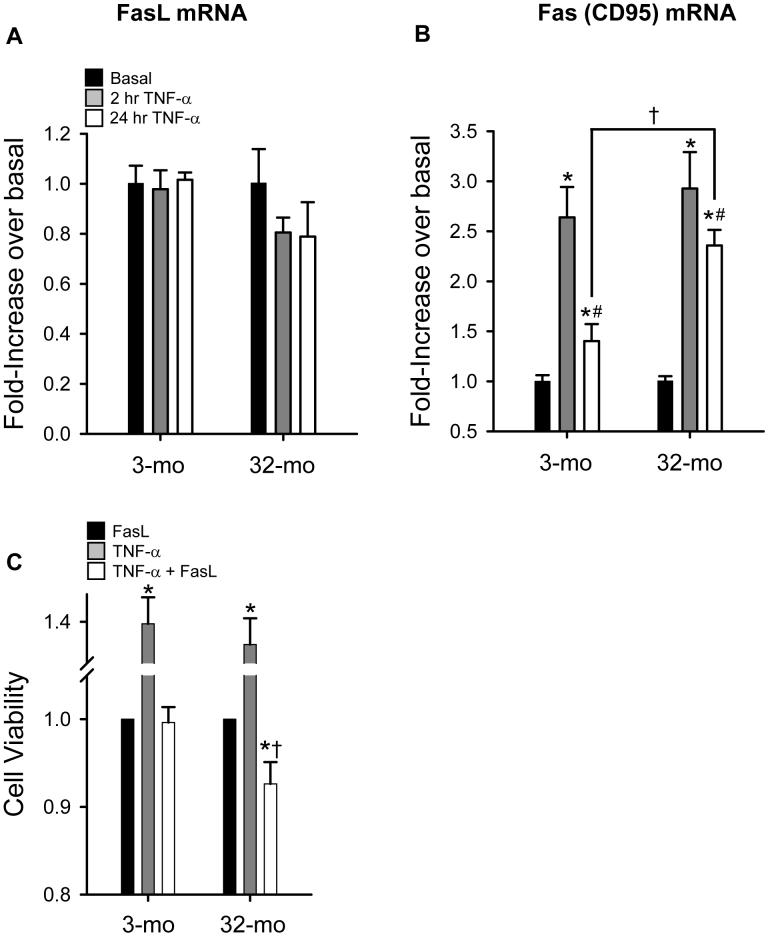
NF-κB activation can result in the upregulation of both anti-apoptotic or pro-apoptotic pathways (Perkins, 2007). Based on the finding that MPCs isolated from aged rats exhibit an increased apoptotic response to TNF-α in the presence of a stress inducing agent (Jejurikar et al, 2006), we measured two target genes of NF-κB involved in apoptotic signaling, FasL and Fas (CD95) (Kasibhatla et al, 1999;Chan et al, 1999). Under basal conditions, mRNA levels were not different for either FasL or Fas in MPCs isolated from young, compared to old, animals (data not shown). After stimulation with TNF-α, MPCs from both young and old animals did not increase FasL mRNA expression (values were normalized to basal expression within each age group). Therefore, unlike the response observed in T cells (Kasibhatla et al, 1999), it does not appear that MPCs are responsive to NF-κB-induced FasL expression (Figure 6A). However, after 2 hr of TNF-α exposure, Fas mRNA increased >2.5-fold in MPCs from both young and old animals (Figure 6B). Therefore, it does appear that MPCs are responsive to NF-κB-induced expression of the pro-apoptotic member of the TNF superfamily of receptors, Fas (CD95). By 24 hrs, Fas differentially declines between ages. In MPCs isolated from young animals, 24 hr after TNF-α exposure, Fas mRNA level had returned to 1.4-fold increase over basal levels. Importantly, 24 hr after TNF-α exposure, Fas mRNA in MPCs isolated from old animals remained at ∼2.5-fold increase over basal levels, which was greater than the level observed in MPCs isolated from young animals (Figure 6B). Next we verified that the observed age-associated difference in Fas mRNA after 24 hr TNF-α differentially affected cell viability in MPCs isolated from young and old animals. In MPCs isolated from young animals, TNF-α did not alter cell viability in response to FasL treatment. In contrast, in MPCs isolated from old animals, TNF-α treatment with FasL did decrease cell viability, compared to FasL only (Figure 6C). TNF-α alone does not induce a decrease in cell viability and similar to previous findings, it seems to have a mitogenic effect (Li, 2003) (Figure 6C). These findings verify a specific apoptotic gene target of NF-κB that exhibits a persistently unregulated, expression after prolonged exposure to TNF-α in MPCs isolated from old, compared to young animals.
Figure 6.
Tumor necrosis factor-alpha (TNF-α)-induced FasL and Fas (CD95) mRNA expression in muscle precursor cells (MPCs) isolated from 3-mo-old animals (3-mo) and 32-mo-old animals (32-mo). A) TNF-α-induced FasL mRNA levels after 2 and 24 hr stimulation with TNF-α in MPCs isolated from 3-mo-old animals (3-mo) and 32-mo-old animals (32-mo). Data are presented as fold-increase over basal ± SEM (n=4). B) TNF-α-induced Fas (CD95) mRNA expression after 2 and 24 hr stimulation with TNF-α in MPCs isolated from 3-mo and 32-mo. Data are presented as fold-increase over basal ± SEM (n=4). C) TNF-α reduced cell viability in response to FasL treatment in MPCs isolated from 32-mo, however, TNF-α did not change cell viability in response to FasL in MPCs isolated from 3-mo. Cell viability data are presented relative to FasL treatment only (n=4). *, significantly different from basal (in panels A and B) or FasL only (in panel C). †, significantly different from 3-mo. #, significantly different from 2 hr TNF-α.
Discussion
Aging is associated with chronically elevated circulating levels of certain cytokines, including TNF-α, which has been termed chronic low-grade systemic inflammation. Moreover, elevated TNF-α mRNA and protein have been reported in aged human skeletal muscle. In the present study, we sought to determine if MPCs isolated from aged skeletal muscle exhibited an increased response to TNF-α. To our knowledge, we are the first to demonstrate that TNF-α causes an exaggerated increase in NF-κB activation in MPCs isolated from old animals, compared to young. The rapid, canonical signaling events leading to NF-κB activation were not different in MPCs isolated from either age. In contrast however, prolonged TNF-α exposure (24 hr) revealed two persistently elevated NF-κB responses. The first response revealed that MPCs isolated from old animals exhibited increased mRNA levels for the key receptor adapter protein, TRAF-2, compared to MPCs isolated from young animals. As samples were not collected at intermediate time points between 2 and 24 hr, it is not known if TNF-α treatment causes an increase in TRAF-2 expression earlier than 24 hr. The second response revealed a prolonged maintenance of increased mRNA levels for the cell death inducing receptor, Fas (CD95) in MPCs isolated from old, compared to young animals. Taken together, our findings support the notion that there are intrinsic differences in the response to TNF-α as a result of age and that they could play a role in increased pro-apoptotic signaling in MPCs in aged skeletal muscle (Krajnak et al, 2006).
Here we show for the first time that MPCs isolated from aged skeletal muscle exhibit an increased NF-κB activation in response to TNF-α stimulation, compared to MPCs isolated from young animals. These findings are particularly important for two reasons. First, TNF-α is a key cytokine that is known to be elevated both systemically (Phillips and Leeuwenburgh, 2005) and in the local environment of skeletal muscle with age (Greiwe et al, 2001). It has been shown that the age-associated change in the systemic environment contributes to the impaired skeletal muscle regenerative response observed with age (Conboy et al, 2005;Carlson and Faulkner, 1989;Gutmann and Carlson, 1976). Moreover, neural, vascular, and interstitial factors have been suggested to contribute to an age-associated change in the local environment that may influence the impaired skeletal muscle regenerative response (Gopinath and Rando, 2008). Second, it is important to consider the possibility that MPCs isolated from old animals are intrinsically different from those in a young animal. There are many examples of tissue specific adult stem cell populations that exhibit impaired intrinsic function with age (Roobrouck et al, 2008). Our data support a previous report by Jejurikar et al. (Jejurikar et al, 2006) that in the presence of the stress inducing agent, actinomycin D, TNF-α caused an increased apoptotic response in MPCs isolated from old, compared to young and adult animals. Since both the present data and those reported by Jejurikar et al. (Jejurikar et al, 2006) were done in culture, our favored interpretation is that the cells isolated from the old animals are intrinsically different. Therefore, in order to best understand impaired MPC function and skeletal muscle regenerative capacity with age, one must consider both the changing environment (e.g., increased TNF-α) and the differences intrinsic to the cells (e.g., increased response to TNF-α).
Although TNF-α caused selective increases in NF-κB response in MPCs isolated from old animals, compared to young, it is interesting that those rapid signaling events involved in NF-κB activation that were determined here were not different with age. One possible explanation for these findings may involve other mechanisms than determined here that may regulate the magnitude of the NF-κB response downstream of the signaling through IKK and IκBα. In the present study, we demonstrated that there was no age effect in the phosphorylation at serine-536, which is in the transactivation domain of p65 and has been shown to be induced by the IKKβ in response to TNF-α stimulation (Sakurai et al, 1999;Schmitz et al, 1995). However, in addition to serine-536, p65 is phosphorylated at serines-276, -311, and -529 which can also modulate its ability to interact with DNA and co-activators (Chen and Greene, 2004). Moreover, the post-translational modifications are not limited to phosphorylation, acetylation of p65 has been shown to regulate the NF-κB response as well. Acetylation of different lysine residues of p65 by acetyltransferases has been demonstrated to influence the magnitude of transcriptional activation and inhibit association with IκBα (Chen and Greene, 2004). The complex regulation of NF-κB transcriptional activation is further modulated by co-activators. CBP and p300 are both versatile co-activators that have been shown to directly interact with NF-κB and potentiate its transcriptional activation (Gerritsen et al, 1997). In addition to their role as co-activators, CBP and p300 are both histone acetyltransferases (HATs) for NF-κB target genes, which is one aspect of histone modification that determines which promoter regions will be transcriptionally active (Zhong et al, 2002;Jorquera et al, 2001). Besides the mechanisms regulating the magnitude of the NF-κB response, our current findings suggest a selective failure to “shut off” NF-κB activation of Fas (CD95) mRNA, in MPCs isolated from old animals. These findings are important because Fas (CD95) is a member of the TNF-α superfamily of receptors involved in the formation of the death-inducing signaling complex and is involved in pro-apoptotic signaling (Peter and Krammer, 2003).
Even though Fas (CD95) mRNA increased similarly in MPCs isolated from both young and old animals after 2 hours TNF-α stimulation, Fas (CD95) mRNA was ∼2-fold higher 24 hours after stimulation in MPCs isolated from old animals, as compared to young animals. These findings suggest that MPCs isolated from young animals were better able to reverse the upregulation of Fas (CD95) mRNA, compared to MPCs isolated from old animals. Mechanisms that regulate inactivation of NF-κB are not completely understood, however, IκBα re-synthesis is considered a critical element to “shutting-off” NF-κB (Hayden and Ghosh, 2008). Once NF-κB is activated, it stimulates the re-synthesis of IκBα as part of a negative feedback mechanism. The newly expressed IκBα binds NF-κB and sequesters it in the cytoplasm (Li and Lin, 2008). However, our current findings of IκBα mRNA 2 and 24 hours after stimulation with TNF-α, as a measure of IκBα mRNA re-synthesis, do not indicate an age-related dysfunction in this negative feedback mechanism. Two hours after stimulation, IκBα mRNA is increased ∼6-fold in MPCs isolated from both young and old animals (Figure 5). Twenty-four hours after stimulation, IκBα mRNA levels in MPCs isolated from both young and old animals had decreased compared to the level observed after 2 hours, but remained elevated ∼4-fold over basal levels. These findings indicate that persistent Fas (CD95) expression observed in MPCs isolated from old animals cannot be explained by deficient re-formation of IκBα mRNA. Other putative mechanisms that “shut off” NF-κB activation, not determined here, include proteasomal degradation in the nucleus (Saccani et al, 2004) and post-translational modification of NF-κB. As mentioned previously, p65 is subject to acetylation, which can inhibit association with IκBα (Chen and Greene, 2004). It is possible that the age-associated increase in Fas (CD95) expression after 24 hr TNF-α stimulation may involve persistent acetylation of p65. Further investigation into mechanisms responsible for NF-κB inactivation in aging will need to be the focus of future studies.
A better understanding of how age-related changes in the environmental milieu of skeletal muscle are vital to understanding the mechanisms responsible for age-associated impaired function of MPCs. Just as important, the intrinsic differences between MPCs isolated from young and old animals must be considered in terms of determining the age-related differences of the interactions between the MPC and the environment. Aging has been previously shown to cause an increase in NF-κB/DNA binding in multiple tissues (Helenius et al, 1996), increased TNF-α/apoptosis signaling in aged muscle (Pistilli et al, 2006;Krajnak et al, 2006;Phillips and Leeuwenburgh, 2005), and increased TNF-α-induced apoptosis in aged satellite cells when co-treated with an agent that blocks translation (Jejurikar et al, 2006). However, very little is known about the implications of elevated TNF-α observed in aged skeletal muscle on NF-κB signaling events in MPCs isolated from old animals. Here we show for the first time that MPCs isolated from old animals exhibit an increased TNF-α-induced activation of a consensus NF-κB reporter construct, a persistent increase in Fas (CD95) mRNA, and a decreased FasL-induced MPC viability. Future studies are needed to better establish the potential role of TNF-α in increasing the susceptibility MPCs to FasL-induced apoptosis in aging. Taken together, the current findings support the hypothesis of increased TNF-α-induced NF-κB apoptotic signaling in old MPCs. These findings are particularly important because during aging, MPCs are considered to be a primary factor contributing to failed skeletal muscle repair (Conboy and Rando, 2005). An improved understanding of the interactions between the MPCs in aged skeletal muscle and the changing environment associated with age will best facilitate the development of clinical interventions aimed at improving skeletal muscle repair and, therefore, quality of life.
Experimental procedures
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia. 3-mo-old (3-mo) and 32-mo-old (32-mo), Fischer 344 x Brown Norway F1 hybrid, male rats were obtained from the National Institute on Aging. Animals were housed at 21°C on a 12-hr light/12-hr dark cycle and allowed free access to food and water. At the time of sacrifice, animals were given an intraperitoneal injection of ketamine (80 mg · kg-1), xylazine (10 mg · kg-1), and acepromazine (4 mg · kg-1) and then muscles were excised.
MPC isolation and culture
MPC isolation was modified from Allen et al. (Allen et al, 1997), as described (Lees et al, 2006;Lees et al, 2008). Briefly, cells isolated from the gastrocnemius and plantaris muscles by pronase digestion were pre-plated for 24 hours on tissue-culture treated 150-mm plates. After the 24-hour pre-plate, cells were seeded onto Matrigel (BD Biosciences, San Jose, CA) coated 150-mm plates (0.1 mg/ml Matrigel, 60 minutes at 37°C) and cultured for 3 days in growth media (GM, 20% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamicin in Ham’s F-10) in a humidified incubator with 5% O2, 5% CO2, and 95% N2 at 37° C (HERAcell, Thermo Scientific). After 3 days, the cells reach ∼80% confluence. Cells were then passaged one time and seeded onto appropriate Matrigel tissue-culture plates. Greater than 95% desmin and MyoD positive cells are obtained using this isolation protocol (data not shown). As media depth is an important concern for 5% O2 culture conditions (Tokuda et al, 2000), 1.5 and 10 ml of GM were used for experiments carried out in 6-well culture plates (25,000 cells/well) and 100 mm culture plates (125,000 cells/plate), respectively.
Recombinant human TNF-α was obtained from Peprotech (Rocky Hill, NJ). For dose-response experiments in Figure 1, TNF-α was added at a dose of 1, 5, or 20 ng/ml in 0.1% BSA in PBS (Sigma, St. Louis). In all other experiments, TNF-α was added at 20 ng/ml. Recombinant rat FasL was obtained from R&D Systems (Minneapolis, MN) and cells were treated with 40 ng/ml FasL (vehicle was 0.15% BSA in sterile PBS). Control conditions were given the appropriate vehicle.
DNA constructs, transfection, and promoter activity
The nuclear factor-kappa B (NF-κB) cis-reporter construct contains 5 repeats of the transcription recognition sequence (TGGGGACTTTCCGC) linked to a basic promoter element (TATA box) and the firefly luciferase gene (Stratagene, La Jolla, CA). The IKK-2 K44M mutant (IKK K44, Addgene plasmid 11104), which had been created by mutation of a conserved lysine44 residue to a methionine and was previously shown to inhibit TNF-induced NF-κB activation (Dr. Anjana Rao) (Mercurio et al, 1997), was obtained from Addgene (Cambridge, MA). Transient transfections were carried out immediately after the cells were seeded in antibiotic-free GM using Fugene® 6 (Roche Applied Science, Indianapolis, IN), following the manufacturer’s instructions. The phRK-null Renilla luciferase reporter vector (Promega, Madison, WI) was co-transfected in each experiment and used as an internal control promoter in order to normalize for transfection efficiency. A total of 0.7 ug of DNA for each well on a 6-well plate was used for both firefly and Renilla luciferase reporter constructs at a firefly:Renilla ratio of 20:1. Cells were lysed using passive lysis buffer (Promega, Madison, WI) and stored at -80°C. Firefly and Renilla luminescence were measured using the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI) on a Veritas™ microplate luminometer (Turner BioSystems, Sunnyvale, CA).
RNA isolation and real-time PCR
Samples were lysed at 24 hr and 48 hr post-seeding in RLT-lysis buffer with 1% β-mercaptoethanol and passed through a QIAshredder (Qiagen, Valencia, CA). RNA purification was performed with the on-column DNase I digestion using the RNeasy® micro kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA was quantified spectrophotometrically by measuring the absorbance at 260 nm and the purity was assessed by measuring the ratio of the absorbance at 260 nm and 280 nm. RNA integrity was verified by denaturing agarose electrophoresis and ethidium bromide staining. RNA was reverse transcribed using SuperScript™ III first-strand cDNA synthesis system (Invitrogen, Carlsbad, CA) with random hexamer primers.
Real-time quantitative PCR was performed using Sybr Green Master Mix and an ABI Prism 7000 (Applied Biosystems, Foster City, CA). The sequences for the target primers are listed in Table 1. The specificity of the primer pair was evaluated using agarose gel electrophoresis; only a single product of appropriate size was observed. 18S rRNA was also determined in each sample as the endogenous control (product number 4319413E, Applied Biosystems, Foster City, CA). 25 ng of cDNA for each sample was used. Standard curves for all targets and 18S rRNA were run to determine amplification efficiency. Data analysis was performed using the comparative method (ΔΔCT) and results are reported as fold change.
Table 1.
Real-time quantitative PCR primers
| mRNA Target | Primer(s) |
|---|---|
| TNFR1 | F: 5′-CTGGAGGACCGTACCCTGATT-3′ R: 5′-GAGCCCCGGGTTAGAAAGG -3′ |
| TNFR2 | F: 5′-GTGCATGTCCGGGTTATGC-3′ R: 5′-GCGTGGGCCCTTCAACT-3′ |
| TRAF2 | F: 5′-GCAGTGACTGCAGAGGCTTGT-3′ R: 5′-TGTTGCTTAGGGCCTCAATCTT-3′ |
| IκBα | F: 5′-GCTGCCCGAGAGTGAGGAT-3′ R: 5′- GTCATCGTAGGGCAACTCATCTT-3′ |
| FasL | F: 5′-GAGCTGTGGCTACCGGTGAT-3′ R: 5′-TTGATACATTCCTAACCCCATTCC-3′ |
| Fas | F: 5′-TGCAGATATGCTGTGGATCATG-3′ R: 5′-TCCCTTGCATTCGAACATTTAA-3′ |
Western Blot Analysis
Cells were treated with TNF-α at the specified times and lysed with RIPA buffer containing 1.04 mM 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), 800 nM aprotinin, 20 μM leupeptin, 40 μM bestatin, 15 μM pepstatin A, 14 μM E-64, and the phosphatase inhibitor cocktail 1 (P2850, Sigma-Aldrich, St. Louis, MO, a proprietary mix of Cantharidin, Bromotetramisole, and Microcystin LR used at 1:100 dilution). Cell lysates were then frozen and stored at -80°C. The samples were thawed and then centrifuged at 12,000 x g (4°C) for 15 minutes, the supernatant was collected, protein concentration was determined using the Bradford assay, and samples were diluted to equal concentrations (0.4 mg/ml) in SDS reducing buffer. Equal amounts of protein were loaded and separated on SDS-PAGE and transferred to nitrocellulose membranes (Osmonics Inc.). To ensure equal loading, nitrocellulose membranes were stained with Ponceau S (Sigma-Aldrich), which allows for both the qualitative visualization and quantitation of the amount or protein in a given lane (Klein et al, 1995). The total IκBα, phospho- IκBαser32, total p65, and phospho-p65ser536 antibodies were purchased from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase (HRP)-conjugated secondary IgG antibody was purchased from Pierce (Pierce Biotechnology, Rockford, IL). Immunocomplexes were visualized using Supersignal West Dura Extended Duration Substrate (Pierce Biotechnology, Rockford, IL). The signal bands were scanned using a Kodak Image Station 4000R Digital Imaging System (Eastman Kodak Company, Rochester, NY) and quantified using Kodak molecular imaging software (version 4.0).
Cell Viability
For cell viability experiments, cells were washed once with phosphate-buffered saline and replaced with low serum media (2% HS in DMEM) prior to FasL treatment (40 ng/ml) (Sandri and Carraro, 1999). Cell number was quantified using the CyQUANT® Cell Proliferation Assay Kit following the manufacturer’s instructions (Invitrogen-Molecular Probes, Carlsbad, CA).
Statistics
Data are presented as mean ± SE. Sample sizes are indicated for each measurement in the figure legends, where n represents independent isolations from separate animals. Comparisons between groups were done using the ANOVA. (SigmaStat, version 3.1). Significance was accepted at p≤0.05.
Acknowledgments
We thank Tom E. Childs for his technical assistance. This work was supported by NIH RO1 AG18780 (FWB) from the National Institute on Aging. K. A. Zwetsloot was supported by US Department of Education H133P050005.
Literature Cited
- Aggarwal S, Gollapudi S, Gupta S. Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J Immunol. 1999;162:2154–2161. [PubMed] [Google Scholar]
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 1997;52:155–176. doi: 10.1016/s0091-679x(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol. 2002;283:C66–C76. doi: 10.1152/ajpcell.00598.2001. [DOI] [PubMed] [Google Scholar]
- Barani AE, Durieux AC, Sabido O, Freyssenet D. Age-related changes in the mitotic and metabolic characteristics of muscle-derived cells. J Appl Physiol. 2003;95:2089–2098. doi: 10.1152/japplphysiol.00437.2003. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Bronstein I, Fortin J, Stanley PE, Stewart GS, Kricka LJ. Chemiluminescent and bioluminescent reporter gene assays. Anal Biochem. 1994;219:169–181. doi: 10.1006/abio.1994.1254. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol. 1990;258:C436–C442. doi: 10.1152/ajpcell.1990.258.3.C436. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- Chan H, Bartos DP, Owen-Schaub LB. Activation-dependent transcriptional regulation of the human Fas promoter requires NF-kappaB p50-p65 recruitment. Mol Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Cooper RN, Butler-Browne GS, Mouly V. Human muscle stem cells. Curr Opin Pharmacol. 2006;6:295–300. doi: 10.1016/j.coph.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci U S A. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- Gupta S, Gollapudi S. Molecular mechanisms of TNF-alpha-induced apoptosis in aging human T cell subsets. Int J Biochem Cell Biol. 2005;37:1034–1042. doi: 10.1016/j.biocel.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Carlson BM. Regeneration and transplantation of muscles in old rats and between young and old rats. Life Sci. 1976;18:109–114. doi: 10.1016/0024-3205(76)90280-0. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Helenius M, Hanninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318(Pt 2):603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jejurikar SS, Henkelman EA, Cederna PS, Marcelo CL, Urbanchek MG, Kuzon WM., Jr. Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Exp Gerontol. 2006;41:828–836. doi: 10.1016/j.exger.2006.06.053. [DOI] [PubMed] [Google Scholar]
- Jorquera F, Almar M, az-Golpe V, Olcoz JL, Garcia-Fernandez A, Gonzalez-Gallego J. Impairment of metabolic function in chronic hepatitis C is related to factors associated with resistance to therapy. Am J Gastroenterol. 2001;96:2456–2461. doi: 10.1111/j.1572-0241.2001.04053.x. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Genestier L, Green DR. Regulation of fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor kappaB. J Biol Chem. 1999;274:987–992. doi: 10.1074/jbc.274.2.987. [DOI] [PubMed] [Google Scholar]
- Klein D, Kern RM, Sokol RZ. A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int. 1995;36:59–66. [PubMed] [Google Scholar]
- Krajnak K, Waugh S, Miller R, Baker B, Geronilla K, Alway SE, Cutlip RG. Proapoptotic factor Bax is increased in satellite cells in the tibialis anterior muscles of old rats. Muscle Nerve. 2006;34:720–730. doi: 10.1002/mus.20656. [DOI] [PubMed] [Google Scholar]
- Laszlo S, Endre S. Gultamate pyruvate transaminase and its use in doubtful paternity cases. Orv Hetil. 1976;117:1567–1570. [PubMed] [Google Scholar]
- Lees SJ, Childs TE, Booth FW. p21(Cip1) expression is increased in ambient oxygen, compared to estimated physiological (5%) levels in rat muscle precursor cell culture. Cell Prolif. 2008;41:193–207. doi: 10.1111/j.1365-2184.2008.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2006;290:C609–C615. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- Leger B, Derave W, De BK, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11:163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- Li H, Lin X. Positive and negative signaling components involved in TNFalpha-induced NF-kappaB activation. Cytokine. 2008;41:1–8. doi: 10.1016/j.cyto.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Li YP. TNF-alpha is a mitogen in skeletal muscle. Am J Physiol Cell Physiol. 2003;285:C370–C376. doi: 10.1152/ajpcell.00453.2002. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lorenzon P, Bandi E, de GF, Pietrangelo T, Schafer R, Zweyer M, Wernig A, Ruzzier F. Ageing affects the differentiation potential of human myoblasts. Exp Gerontol. 2004;39:1545–1554. doi: 10.1016/j.exger.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Machida S, Booth FW. Increased nuclear proteins in muscle satellite cells in aged animals as compared to young growing animals. Exp Gerontol. 2004;39:1521–1525. doi: 10.1016/j.exger.2004.08.009. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Jackson JR, Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis. 2006;11:2115–2126. doi: 10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007;65:S208–S212. doi: 10.1111/j.1753-4887.2007.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Saccani S, Marazzi I, Beg AA, Natoli G. Degradation of promoter-bound p65/RelA is essential for the prompt termination of the nuclear factor kappaB response. J Exp Med. 2004;200:107–113. doi: 10.1084/jem.20040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Sandri M, Carraro U. Apoptosis of skeletal muscles during development and disease. Int J Biochem Cell Biol. 1999;31:1373–1390. doi: 10.1016/s1357-2725(99)00063-1. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Stelzer G, Altmann H, Meisterernst M, Baeuerle PA. Interaction of the COOH-terminal transactivation domain of p65 NF-kappa B with TATA-binding protein, transcription factor IIB, and coactivators. J Biol Chem. 1995;270:7219–7226. doi: 10.1074/jbc.270.13.7219. [DOI] [PubMed] [Google Scholar]
- Sinanan AC, Buxton PG, Lewis MP. Muscling in on stem cells. Biol Cell. 2006;98:203–214. doi: 10.1042/BC20050050. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Hayes LS, Lloyd DB. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991;103:171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- Tokuda Y, Crane S, Yamaguchi Y, Zhou L, Falanga V. The levels and kinetics of oxygen tension detectable at the surface of human dermal fibroblast cultures. J Cell Physiol. 2000;182:414–420. doi: 10.1002/(SICI)1097-4652(200003)182:3<414::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]