SUMMARY
A patient with a 20-year history of recurrent respiratory papillomatosis had progressive, bilateral tumor invasion of the lung parenchyma. We used conditional reprogramming to generate cell cultures from the patient’s normal and tumorous lung tissue. Analysis revealed that the laryngeal tumor cells contained a wild-type 7.9-kb human papillomavirus virus type 11 (HPV-11) genome, whereas the pulmonary tumor cells contained a 10.4-kb genome. The increased size of the latter viral genome was due to duplication of the promoter and oncogene regions. Chemosensitivity testing identified vorinostat as a potential therapeutic agent. At 3 months after treatment initiation, tumor sizes had stabilized, with durable effects at 15 months.
Recurrent respiratory papillomatosis is a common benign neoplasm of the larynx.1 Most cases are due to infection by HPV type 6 (HPV-6) or HPV-11 that is acquired at birth. HPV-11 is associated with a more aggressive clinical course,2 including involvement of the lung. The standard treatment of recurrent respiratory papillomatosis involves repeated surgical debulking of the laryngeal tumors. In rare cases (3 to 5%), recurrent respiratory papillomatosis can extend below the vocal cords into the trachea and main-stem bronchi. However, when it progresses into the lung parenchyma (in less than 1% of cases), there are no effective therapies3 and it is almost invariably fatal. The precise cellular or viral alterations that promote lung invasion are unknown, although there has been speculation that HPV mutations may contribute to the progression.4
One of the limitations of studying tumor progression in recurrent respiratory papillomatosis and treatment of the disorder is the lack of an appropriate cell-culture system. We used a new cell-culture technology (conditionally reprogrammed cells)5 to generate continuous cell cultures from tumor samples and corresponding normal lung tissue in a patient with recurrent respiratory papillomatosis. This technique allowed us to detect a mutation of HPV-11 that may have contributed to the observed aggressive clinical behavior and to identify vorinostat as a potential new therapeutic approach to the treatment of advanced pulmonary extension in patients with recurrent respiratory papillomatosis.
CASE REPORT
The patient was a 24-year-old man with a 20-year history of recurrent respiratory papillomatosis. He had undergone more than 350 laryngeal ablation surgeries to control viral-induced tumors. In addition, the patient has been treated with several drug therapies: interferon (1996 to 2010), methotrexate (2001 to 2003), intralesional cidofovir (2007 to 2010), and intralesional bevacizumab (2010). None of these treatments slowed tumor growth or its progression into the lung, and thoracotomy was required in 1993, 2000, and 2007 to excise the tumors. In 2010, computed tomographic (CT) scanning revealed that there were multiple pulmonary nodules that had accelerated in growth, with three index tumors increasing in size by 21%, 72%, and 130% in 11 months. At the initiation of this study, a fourth thoracotomy was performed to remove tumors from the right upper lobe, and the patient was classified clinically as having chemoresistant, progressive disease.
METHODS
CELL ISOLATION AND PROPAGATION
Samples of normal and tumor tissue were obtained after the patient provided written informed consent according to a protocol of the Georgetown University Hospital institutional review board. Tissue samples were dispersed into single cells by digestion with collagenase plus trypsin, and the cells were propagated by means of a new cell-culture technique5 that uses coculture with J2 murine fibroblast cells and medium containing the Rho-kinase inhibitor Y-27632 (Enzo Life Sciences). This method has been shown to rapidly generate epithelial-cell cultures that can be propagated indefinitely in vitro. The new culture technique is described in the Supplementary Appendix, available with the full text of this article at NEJM.org.
DNA ISOLATION, CLONING, AND SEQUENCING
Total DNA was isolated from the patient’s cultured cells or tissues with the use of the DNeasy Blood and Tissue Kit (Qiagen) and was amplified with the use of a rolling circle amplification (RCA) kit (Illustra TempliPhi, GE Healthcare Life Sciences), as described in the Supplementary Appendix. The products were digested with HindIII enzyme and cloned into the pUC19 vector. Viral genomes were sequenced from two directions with the use of Primer Walking Services (Genewiz).
DETECTION OF VIRAL GENOME WITH DUPLICATION
DNA was isolated as described above or from formalin-fixed, paraffin-embedded tissue samples with the use of the QuickExtract FFPE DNA Extraction Kit (Epicentre). The DNA preparation was subjected to polymerase-chain-reaction (PCR) analysis with the use of HPV-11 general primers to the L1 gene or duplication-specific primers targeting the junction of the E1(A) and L1(B) regions. Sequences of primers and details of the PCR method are provided in the Supplementary Appendix.
DRUG TESTING
Cells isolated from the lung tumor and adjacent normal lung were plated in triplicate with J2 feeder cells in 24-well microtiter plates (BD Falcon) at 104 cells per well. Twenty-four hours later, the cells were treated with vehicle (dimethylsulfoxide), cidofovir, dihydroartemisinin, or vorinostat dissolved in dimethylsulfoxide at various concentrations for 48 hours. After removal of feeder cells, lung-cell viability was measured with the use of the CellTiter-Glo Luminescent Cell Viability Assay (Promega). A sigmoidal concentration-response curve was fitted to the data with the use of Graphpad Prism, version 4.03.
RESULTS
GENERATION OF NORMAL-CELL AND TUMOR-CELL CULTURES
Histologic examination of samples of the surgically excised lung tumor revealed papillomatosis with focal koilocytotic atypia, a finding consistent with spread from the preceding laryngeal lesions (Fig. 1A). Small fragments of the tumor and adjacent normal lung tissue were dispersed into single cells, and the cells were propagated with the use of the new cell-culture technique.5 The 8-day time course for establishing cell cultures and evaluating HPV gene expression and tumor-cell chemosensitivity is shown in Figure 1B. The typical morphologic features of early-stage epithelial colonies (1.5 days in culture) are shown in Figure 1C; there were no obvious morphologic differences between the normal cells and tumor cells derived from this patient. These primary cultures reached confluence in 4 days. The growth of the normal cells and that of the tumor cells were also very similar; the cells were maintained for more than 72 doublings, as shown in Figure 1C, to verify continuous growth. The normal-cell and tumor-cell cultures were used for molecular analysis of the HPV-11 genome and for in vitro testing of chemotherapy.
Figure 1. Generation of Conditionally Reprogrammed Cells from Normal and Neoplastic Lung.
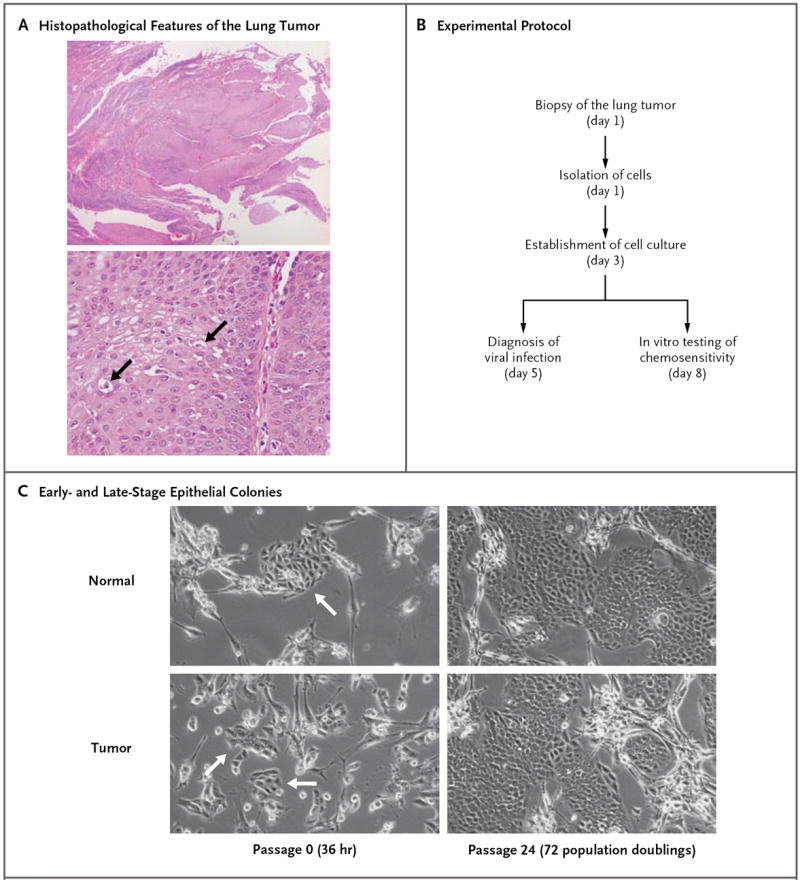
Panel A shows the histopathological features of the lung tumor (hematoxylin and eosin): typical papillomatosis without evidence of dysplasia (top) and cells with koilocytotic atypia (bottom, arrows). Panel B summarizes the experimental protocol. Panel C shows the morphologic features of conditionally reprogrammed lung tumor cells cocultured with feeder cells for 36 hours and after continued proliferation for 72 population doublings. The arrows at the left indicate the colonies of cells isolated from the patient.
HPV-11 GENOME MUTATIONS AND TUMOR PROGRESSION
Although this patient had long-term disease, the HPV in the tumor had not been typed. Using general HPV detection primers and HPV type–specific primers, we identified HPV-11 in the cultured pulmonary tumor cells (Fig. 2A). Sequencing of the PCR products showed that all the products matched HPV-11 DNA. We also evaluated viral gene expression in the cells and detected messenger RNA (mRNA) for the E6 and E7 genes but not for the L1 gene (Fig. 2B). To further evaluate the HPV-11 infection, we used RCA to amplify episomal HPV DNA. Surprisingly, the pattern of restriction-enzyme digestion did not match that anticipated for HPV-11 (Fig. 2C). All the indicated restriction enzymes (BamHI, AatII, AgeI, FspI, XcmI, and PpuMI) should cut the viral DNA once to generate a linearized genome of 7931 base pairs (GenBank accession number, M14119.1).6 Instead, these enzymes cut the DNA twice, generating fragments of about 8 kb and 2 kb. In addition, the size of the viral genome was estimated to be more than 10 kb when it was linearized by HindIII, XbaI, or SphI. This suggested potential duplication of the L1-LCR-E6-E7-E1 region.
Figure 2. Detection of Mutant HPV-11 in Lung Tumor and Derivative Cell Cultures.
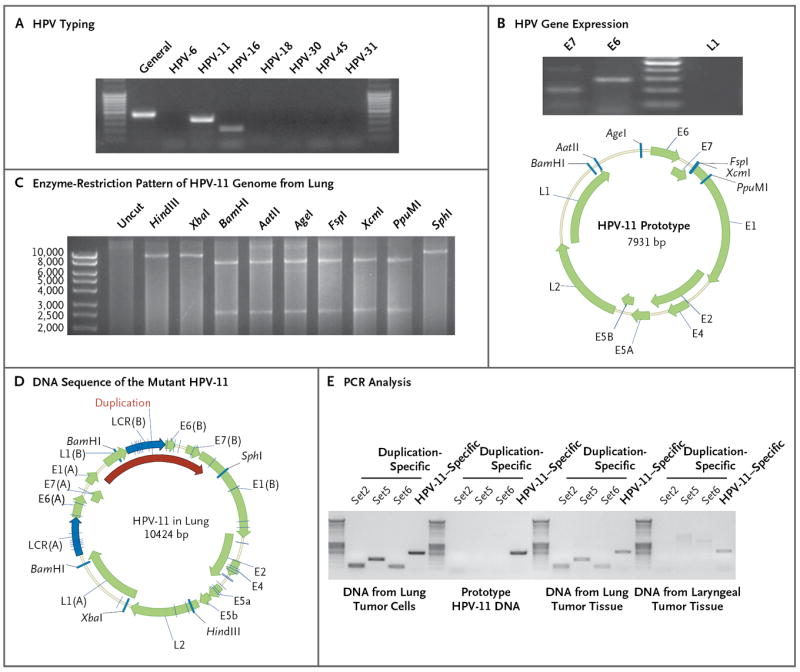
Panel A shows typing for human papillomavirus (HPV) in the lung tumor cells by means of a polymerase-chain-reaction (PCR) assay. DNA was isolated from cultured tumor cells, and a general HPV primer set (MY09/MY11), as well as type-specific primer sets, was used for HPV typing. Only the primers for HPV type 11 (HPV-11) yielded the expected PCR products, which were confirmed by DNA sequencing. Panel B shows HPV gene expression in the tumor cells. The messenger RNA (mRNA) transcripts of HPV-11 E6 and E7, but not L1, were detected in the tumor cells by reverse-transcriptase PCR. Panel C shows an altered enzyme-restriction pattern in the HPV-11 genome in the tumor cells. All indicated enzymes cut the prototype HPV-11 genome only once (GenBank accession number, M14119.1). After rolling circle amplification, HPV-11 from lung cells exhibited two digestion sites for enzymes BamHI, AatII, AgeI, FspI, XcmI, and PpuMI. Panel D shows the DNA sequence of the mutant HPV-11 in the tumor cells. Viral DNA was cloned and sequenced. Open reading frames were predicted with the use of ORF Finder (National Center for Biotechnology Information), and the genome map was constructed with the use of Vector NTI software (Invitrogen). The duplicated sequence included L1-LCR-E6-E7-E1 sequences, although the L1 and E1 sequences were only partially duplicated. We annotated the duplicated sequence as L1(B), LCR(B), E6(B), E7(B), and E1(B). Duplication-specific or general HPV-11 PCR primers, shown in Panel E, were used for the detection of corresponding HPV-11 in the DNA isolated from lung tumor cells or from paraffin-embedded lung and laryngeal tissues.
To further evaluate this possibility, we cloned the viral genome into the vector pUC19 and sequenced it from two directions. According to sequencing data, the mutant HPV-11 genome contained 10,424 bp (GenBank accession number, JN644141) owing to duplication of 2493 bp that include partial L1-LCR-E6-E7-partial E1 sequences (Fig. 2D). Duplication-specific primers were used to verify the presence of the mutant virus genome in the lung tumor cells and corresponding fixed lung tumor tissue (Fig. 2E). The pulmonary tumor cells contained approximately 40 viral genomes per cell. Somewhat surprisingly, PCR analysis revealed that the primary laryngeal tumor lacked the mutant viral genome. To confirm that the laryngeal site lacked this mutant genome, we used RCA to amplify and sequence the viral genome from the larynx. The generated HPV-11 genome (GenBank accession number, JN644142) had the prototype size of 7933 bp, a finding that suggests that duplication had occurred during tumor extension into the lung. The HPV genomes present in the lung and larynx, in addition to differing in genome size, contained several mutations throughout the genome that differ from the prototype HPV-11 sequence (Table S1 in the Supplementary Appendix).
IDENTIFICATION OF VORINOSTAT AS A THERAPEUTIC AGENT
Because there are no effective therapies for treating lung tumors in patients with recurrent respiratory papillomatosis and the disease was progressing rapidly in this patient, we used the generated HPV-11 lung-cell cultures to screen for potential drug therapies. We selected cidofovir, dihydroartemisinin, and vorinostat for evaluation. Cidofovir, the most commonly used drug for the treatment of recurrent respiratory papillomatosis, was administered in this patient without effect. Both dihydroartemisinin and vorinostat have been shown to be effective against HPV-16–positive cancer cell lines.7 Cidofovir (Fig. 3A) was ineffective in vitro even at very high concentrations (50 to 200 μM), corresponding to its lack of clinical efficacy in this patient. Dihydroartemisinin exhibited moderate killing of tumor cells (Fig. 3B) but required doses significantly higher than those used for cervical cancer cells.7 Vorinostat is a histone deacetylase inhibitor that is approved by the Food and Drug Administration for the treatment of cutaneous T-cell lymphoma,8 and similar inhibitors have been shown to be toxic to HPV-positive cervical cancer cells.9 We found that the median curative dose of vorinostat was 4.2 μM for tumor cells and 15.9 μM for normal cells (Fig. 3C), with selectivity for tumor cells over normal cells.
Figure 3. Sensitivity of the Lung Tumor Cells to Three Selected Drugs.
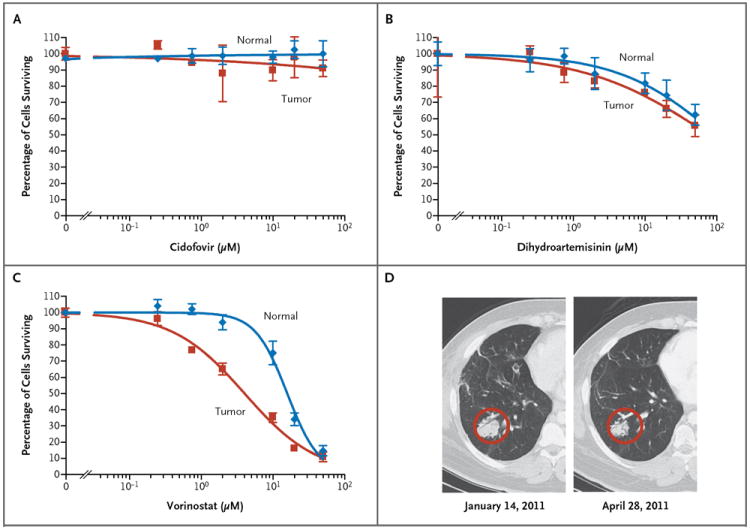
Normal lung cells and lung tumor cells were treated with cidofovir (Panel A), dihydroartemisinin (Panel B), or vorinostat (Panel C) at the indicated concentrations for 48 hours. Cell viability was measured with the use of the CellTiter-Glo Luminescent Cell Viability Assay. Vorinostat had a median curative dose of 4.2 μM in tumor cells (95% confidence interval [CI], 3.2 to 5.4) versus 15.9 μM in normal cells (95% CI, 13.2 to 19.2), with selectivity for tumor versus normal cells (P<0.001). Panel D shows images from a computed tomographic scan of a representative lung tumor mass in the right lower lobe before and after a 3-month course of treatment with vorinostat. This mass remained reduced in size 9 months later, when the yearlong course of therapy was terminated.
On the basis of the in vitro sensitivity data, the patient was treated with vorinostat (at a dose of 400 mg daily), administered in 4-week cycles (3 weeks on and 1 week off) for 1 year. A CT scan obtained after 3 months of treatment revealed encouraging results. There was shrinkage of both small and large tumors, and no new lesions were identified. The mass in the right lower lobe showed a measurable decrease between January 2011 and April 2011 (Fig. 3D). Two lung tumors and one right hilar lymph node were considered to be measurable target lesions, according to Response Evaluation Criteria in Solid Tumors, in January 2011. The sum of the long and short measurements was 7.9 cm. In April 2011, the sum was 6.6 cm, a 16% decrease from baseline. During the 12-month treatment, the tumor sizes stabilized, and they remained stable after treatment, with the last measurements being made in April 2012 (Fig. S1 in the Supplementary Appendix).
DISCUSSION
Despite its generally benign nature, recurrent respiratory papillomatosis may cause serious complications due to local scarring in the larynx. In rare cases (approximately 3%), the disorder can extend below the larynx into the trachea and bronchi,10 and in even fewer cases (less than 1%), it can extend into the pulmonary parenchyma, where it almost invariably proves fatal.11 Malignant transformation has also been observed in recurrent respiratory papillomatosis. We recently described a new method of reprogramming cells, which we call conditionally reprogrammed cells.5 In this study, we used the method to establish long-term cultures of tumor cells from a patient with recurrent respiratory papillomatosis. The cultures helped us to identify a mutant HPV-11 in the lung tumors and to screen a limited number of drugs for potential clinical application. This represents an example of personalized medicine, and since our cell-reprogramming technique can efficiently generate cell cultures from many epithelia, it may be applicable to the study of human cancers and other diseases. The screening of both normal and tumor cells from a given patient has advantages for rapidly identifying appropriate single or combination therapies and reducing the risk of adverse effects. In the clinical case we describe, an appropriate therapy was identified in less than 2 weeks.
Cidofovir is the most commonly used drug in patients with recurrent respiratory papillomatosis. However, the efficacy in patients with lung involvement is still controversial.2,3,12 In our patient, cidofovir had little or no effect in vitro, which correlated with the lack of clinical response. Vorinostat, or suberoylanilide hydroxamic acid, is a histone deacetylase inhibitor that functions by multiple mechanisms, including alteration of histone and protein acetylation, which leads to cell-cycle arrest and apoptosis.13,14 Vorinostat has been used to treat cutaneous T-cell lymphoma, the Sézary syndrome, recurrent glioblastoma multiforme, and advanced non–small-cell lung cancer but not progressive cases of recurrent respiratory papillomatosis. Further studies are warranted to determine whether the current findings can be generalized to other cases of advanced recurrent respiratory papillomatosis.
We speculate that the duplication of the HPV-11 promoter, E6, and E7 oncogenes is associated with the clinical aggressiveness of the tumor in this patient. Despite its aggressiveness, the patient’s lung tumor maintained well-differentiated, benign histologic features. In contrast, two earlier studies showed duplications of HPV genomes in recurrent respiratory papillomatosis, but only in cases that had progressed to squamous-cell carcinoma and metastasized. In 1987, an HPV-11 genome of 10 kb was observed in a squamous-cell carcinoma that had evolved from recurrent respiratory papillomatosis and metastasized to the liver.15 Although there was no information on the viral DNA sequence, the published pattern of restriction-enzyme digestion was exactly the same as the HPV-11 DNA in the current patient. A study in 1992 showed a duplication in the HPV-6 genome in a squamous-cell carcinoma in the lung of a patient with recurrent respiratory papillomatosis. The genome was larger (14 kb) and the duplication included the late region, the regulatory region, and a portion of the early region.16 The precise sequences duplicated were not delineated.
These studies suggest that a similar mechanism or selection force may be responsible for the genetic modification. It is well established that the E6 and E7 genes of the “high risk” HPVs play major roles in cell immortalization, transformation, and carcinogenesis.17 Detection of intragenomic duplication in viral genomes in patients with recurrent respiratory papillomatosis might be predictive of a poor clinical outcome, and additional studies are warranted to determine whether this mutation converts benign HPV genomes to more aggressive phenotypes.
For several reasons, it is unlikely that the episomal HPV-11 genome in the lung tumor from our patient generated infectious virus. The size of the genome was 10.4 kb, and studies have shown that HPV DNA larger than 8 kb cannot be efficiently packaged by viral capsid proteins.18 The largest known packaged papillomavirus genome is 8.6 kb.19 In addition, the lung tumor cells from our patient did not synthesize the capsid L1 mRNA in vitro (Fig. 2B), and we have not detected capsid protein on immunohistochemical analysis of the lung tumor. Thus, the spread of the tumor in the lung was most likely due to the distal aspiration of tumor cells rather than reinfection of new cells. Finally, the finding that the laryngeal tumor lacked the 10.4-kb genome suggests that duplication in the viral genome did not precede extension into the lung.
Supplementary Material
Acknowledgments
Supported in part by a grant from the Office of the Director, National Institutes of Health (R01 OD011168, to Dr. Schlegel).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope. 2008;118:1236–47. doi: 10.1097/MLG.0b013e31816a7135. [DOI] [PubMed] [Google Scholar]
- 2.Donne AJ, Hampson L, Homer JJ, Hampson IN. The role of HPV type in recurrent respiratory papillomatosis. Int J Pediatr Otorhinolaryngol. 2010;74:7–14. doi: 10.1016/j.ijporl.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Goon P, Sonnex C, Jani P, Stanley M, Sudhoff H. Recurrent respiratory papillomatosis: an overview of current thinking and treatment. Eur Arch Otorhinolaryngol. 2008;265:147–51. doi: 10.1007/s00405-007-0546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook JR, Hill DA, Humphrey PA, Pfeifer JD, El-Mofty SK. Squamous cell carcinoma arising in recurrent respiratory papillomatosis with pulmonary involvement: emerging common pattern of clinical features and human papillomavirus serotype association. Mod Pathol. 2000;13:914–8. doi: 10.1038/modpathol.3880164. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dartmann K, Schwarz E, Gissmann L, zur Hausen H. The nucleotide sequence and genome organization of human papilloma virus type 11. Virology. 1986;151:124–30. doi: 10.1016/0042-6822(86)90110-8. [DOI] [PubMed] [Google Scholar]
- 7.Disbrow GL, Baege AC, Kierpiec KA, et al. Dihydroartemisinin is cytotoxic to papillomavirus-expressing epithelial cells in vitro and in vivo. Cancer Res. 2005;65:10854–61. doi: 10.1158/0008-5472.CAN-05-1216. [DOI] [PubMed] [Google Scholar]
- 8.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–9. doi: 10.1182/blood-2006-06-025999. Erratum, Blood 2007;109: 5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzer P, Kuntzen C, Soto U, zur Hausen H, Rösl F. Inhibitors of histone deacetylase arrest cell cycle and induce apoptosis in cervical carcinoma cells circumventing human papillomavirus oncogene expression. Oncogene. 2001;20:4768–76. doi: 10.1038/sj.onc.1204652. [DOI] [PubMed] [Google Scholar]
- 10.Gélinas JF, Manoukian J, Côté A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: a systematic review of the literature. Int J Pediatr Otorhinolaryngol. 2008;72:433–52. doi: 10.1016/j.ijporl.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Silver RD, Rimell FL, Adams GL, Derkay CS, Hester R. Diagnosis and management of pulmonary metastasis from recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg. 2003;129:622–9. doi: 10.1016/j.otohns.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Pransky SM, Albright JT, Magit AE. Long-term follow-up of pediatric recurrent respiratory papillomatosis managed with intralesional cidofovir. Laryngoscope. 2003;113:1583–7. doi: 10.1097/00005537-200309000-00032. [DOI] [PubMed] [Google Scholar]
- 13.Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. doi: 10.1038/icb.2011.100. [DOI] [PubMed] [Google Scholar]
- 14.Wiegmans AP, Alsop AE, Bots M, et al. Deciphering the molecular events necessary for synergistic tumor cell apoptosis mediated by the histone deacetylase inhibitor vorinostat and the BH3 mimetic ABT-737. Cancer Res. 2011;71:3603–15. doi: 10.1158/0008-5472.CAN-10-3289. [DOI] [PubMed] [Google Scholar]
- 15.Byrne JC, Tsao MS, Fraser RS, Howley PM. Human papillomavirus-11 DNA in a patient with chronic laryngotracheobronchial papillomatosis and metastatic squamous-cell carcinoma of the lung. N Engl J Med. 1987;317:873–8. doi: 10.1056/NEJM198710013171406. [DOI] [PubMed] [Google Scholar]
- 16.DiLorenzo TP, Tamsen A, Abramson AL, Steinberg BM. Human papillomavirus type 6a DNA in the lung carcinoma of a patient with recurrent laryngeal papillomatosis is characterized by a partial duplication. J Gen Virol. 1992;73:423–8. doi: 10.1099/0022-1317-73-2-423. [DOI] [PubMed] [Google Scholar]
- 17.Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010;118:422–49. doi: 10.1111/j.1600-0463.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 18.Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–62. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 19.Delius H, Van Ranst MA, Jenson AB, et al. Canine oral papillomavirus genomic sequence: a unique 1.5-kb intervening sequence between the E2 and L2 open reading frames. Virology. 1994;204:447–52. doi: 10.1006/viro.1994.1552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


