Abstract
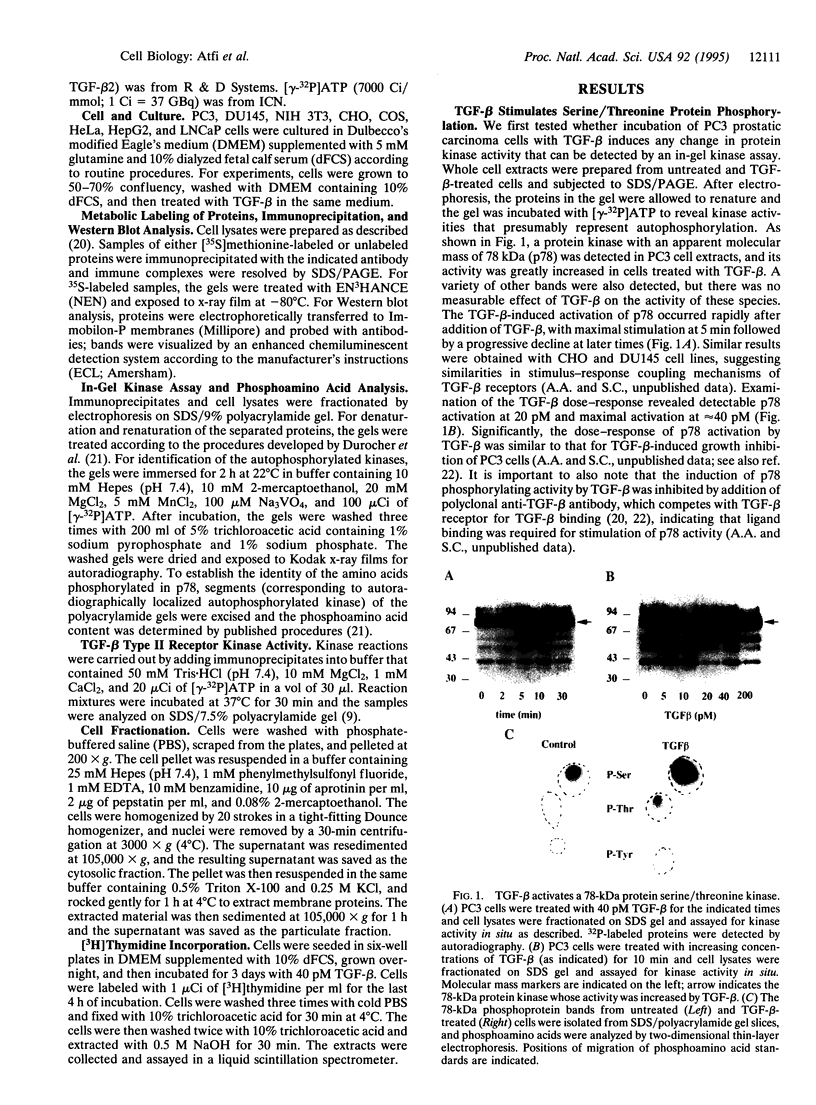
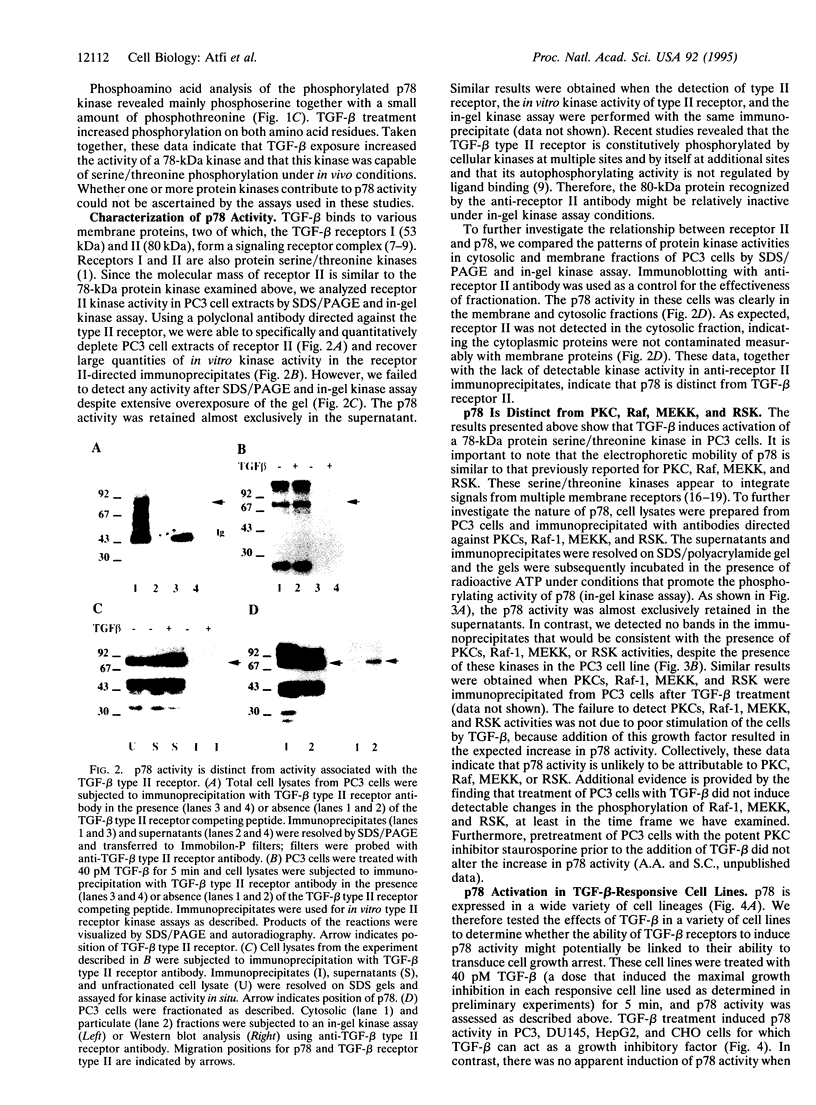
Transforming growth factor type beta (TGF-beta) is a multifunctional factor that regulates proliferation and differentiation of many cell types. TGF-beta mediates its effects by binding to and activating cell surface receptors that possess serine/threonine kinase activity. However, the intracellular signaling pathways through which TGF-beta receptors act remain largely unknown. Here we show that TGF-beta activates a 78-kDa protein (p78) serine/threonine kinase as evidenced by an in-gel kinase assay. Ligand-induced activation of the kinase was near-maximal 5 min after TGF-beta addition to the cells and occurred exclusively on serine and threonine residues. This kinase is distinct from TGF-beta receptor type II, as well as several cytoplasmic serine/threonine kinases of similar size, including protein kinase C, Raf, mitogen-activated protein kinase kinase kinase, and ribosomal S6 kinase. Indeed, these kinases can be separated almost completely from p78 kinase by immunoprecipitation with specific antibodies. Furthermore, using different cell lines, we demonstrate that p78 kinase is activated only in cells for which TGF-beta can act as a growth inhibitory factor. These data raise the interesting possibility that protein serine/threonine kinases contribute to the intracellular relay of biological signals originating from receptor serine/threonine kinases such as the TGF-beta receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atfi A., Drobetsky E., Boissonneault M., Chapdelaine A., Chevalier S. Transforming growth factor beta down-regulates Src family protein tyrosine kinase signaling pathways. J Biol Chem. 1994 Dec 2;269(48):30688–30693. [PubMed] [Google Scholar]
- Atfi A., Samperez S., Jouan P. Secretion of transforming growth factor-beta by the immature rat prostate. Prostate. 1994;24(3):149–155. doi: 10.1002/pros.2990240309. [DOI] [PubMed] [Google Scholar]
- Bassing C. H., Yingling J. M., Howe D. J., Wang T., He W. W., Gustafson M. L., Shah P., Donahoe P. K., Wang X. F. A transforming growth factor beta type I receptor that signals to activate gene expression. Science. 1994 Jan 7;263(5143):87–89. doi: 10.1126/science.8272871. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Durocher Y., Chapdelaine A., Chevalier S. Identification of cytosolic protein tyrosine kinases of human prostate by renaturation after SDS/PAGE. Biochem J. 1992 Jun 15;284(Pt 3):653–658. doi: 10.1042/bj2840653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Whitehouse L. L., Livingston D. M. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993 Sep 24;74(6):1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Franzén P., ten Dijke P., Ichijo H., Yamashita H., Schulz P., Heldin C. H., Miyazono K. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell. 1993 Nov 19;75(4):681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995 Jan 27;80(2):213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995 Jan 27;80(2):187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Lin H. Y., Lodish H. F. Receptors for the TGF-beta superfamily: multiple polypeptides and serine/threonine kinases. Trends Cell Biol. 1993 Jan;3(1):14–19. doi: 10.1016/0962-8924(93)90195-7. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995 Jan 27;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Massagué J., Attisano L., Wrana J. L. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994 May;4(5):172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Bicknell R. Responses of pertussis toxin-treated microvascular endothelial cells to transforming growth factor beta 1. No evidence for pertussis-sensitive G-protein involvement in TGF-beta signal transduction. J Biol Chem. 1992 Oct 25;267(30):21617–21622. [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Ohtsuki M., Massagué J. Evidence for the involvement of protein kinase activity in transforming growth factor-beta signal transduction. Mol Cell Biol. 1992 Jan;12(1):261–265. doi: 10.1128/mcb.12.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Segarini P. R. Cell type specificity of TGF-beta binding. Ann N Y Acad Sci. 1990;593:73–90. doi: 10.1111/j.1749-6632.1990.tb16101.x. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992 Mar 20;68(6):1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Wieser R., Ventura F., Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994 Aug 4;370(6488):341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]