Introduction
Approximately 70% of breast cancers express estrogen receptor alpha (ER) and rely on this hormone receptor signaling pathway for tumor growth [1]. ER-targeting therapy represents the most effective and commonly used treatment strategy for ER positive (+) breast cancer patients. The ER pathway can be effectively inhibited by using either selective ER modulators (SERMs) such as tamoxifen (Tam) or selective ER downregulators (SERDs) such as fulvestrant (Ful), or by depriving the ER of its ligand estrogen (E2) with aromatase inhibitors (AIs) in postmenopausal women or ovarian ablation in younger patients [2, 3]. However, many tumors exhibit de novo resistance to these endocrine treatments, while others acquire resistance after an initial response [4].
Compelling preclinical and clinical evidence suggests that in the presence of chronic ER blockade, the human epidermal growth factor receptor (HER) family plays a critical role in mediating endocrine therapy resistance [5–9]. It has been postulated that the up-regulation of HER receptor signaling, even when modest, modulates ER activity by enhancing ER’s transcriptional activity, by causing ligand-independent receptor activation, by altering its transcriptional program, or by suppressing ER expression and its classic transcriptional function, thereby rendering the cells endocrine resistant [10–12].
The HER receptor tyrosine kinase family is comprised of four receptors: epidermal growth factor receptor (EGFR or HER1), HER2, HER3, and HER4 [13]. Activation of the HER pathway results from HER2 overexpression and HER2 homo-dimerization, or by hetero-dimerization of HER2 with other HER receptors after they are activated by ligand binding [14, 15]. After receptor dimerization, the HER tyrosine kinase domains are activated via phosphorylation which ultimately activates downstream pathways that regulate cell survival and proliferation as well as other biological processes important for breast tumor progression [16, 17].
About 10–15% of ER+ tumors are HER2+, and patients with these tumors have a worse prognosis and higher risk of de novo resistance to endocrine therapies [7, 18, 19]. Furthermore, in ER+/HER2-negative tumors, compelling preclinical in vitro and in vivo studies have shown that acquired resistance to endocrine treatments is associated with an adaptive up-regulation of EGFR and HER2 as well as increased expression of HER ligands [6, 20–22]. Thus, combined therapies that target both the HER receptor pathway and the ER pathway have recently been used to overcome endocrine resistance in clinical trial settings [23, 24]. Phase II clinical trials using the EGFR inhibitor gefitinib in combination with either anastrozole [25] or tamoxifen [26] in ER+ metastatic breast cancer patients showed that gefitinib enhanced the efficacy of anastrozole and tamoxifen at least in certain subsets such as those with tumors low in ER.
The dual EGFR/HER2 tyrosine kinase inhibitor lapatinib was evaluated in combination with the AI letrozole in ER+ metastatic breast tumors [27]. This combination prolonged progression-free survival in patients with ER+/HER2+ tumors compared to letrozole treatment alone. It was however, ineffective in patients with ER+/HER2- tumors [28].
Several studies have shown that expression of HER ligands and activation of HER receptors and downstream pathways can modulate ER transcriptional activity resulting in endocrine resistance [22, 29]. Several reports have shown that lapatinib is less effective in the presence of HER ligands, in ER+/HER2+ and ER+/HER2- tumors [30, 31]. This suggests, that in scenarios of acquired endocrine resistance, where ligand expression is still critical to activate the HER pathway, there is a need to develop new and more potent anti-HER treatment strategies with more efficacy in inhibiting HER ligand-dependent signaling.
AZD8931 is a novel dual EGFR and HER2 tyrosine kinase inhibitor that targets EGFR, HER2, and HER3. Its mechanism of action is distinct from current anti-HER therapies, and under ligand stimulated conditions, AZD8931 is more potent than lapatinib [32]. Its ability to delay or overcome acquired resistance in ER+/HER2- breast tumors however, has not yet been evaluated. Herein, we show that AZD8931 is significantly more effective than lapatinib in tamoxifen resistance that is mediated by increased HER ligands and receptors using both in vitro and in vivo experimental models of tamoxifen resistance.
Materials and Methods
Cell Lines and Establishment of Resistant Lines & Reagents
MCF7 (MCF7L, originally from Dr. Marc Lippman’s lab) and T47D breast cancer cells were grown in RPMI-1640 medium (Lonza, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin and glutamine. Parental MCF7 and T47D (have already become resistant to estrogen deprivation (ED)) cells were continuously treated with tamoxifen (Tam, 10−7 M, >6 months), and the resistant derivatives (TamRes) were selected when the initially sensitive cells resumed comparable growth to the parental cells. The TamRes cell lines were maintained as previously described (5). Lapatinib (GlaxoSmithKline, Research Triangle Park, NC), AZD8931, or gefitinib (AstraZeneca, UK) were dissolved in DMSO and added to cell culture at 1 μM concentration. 17β estradiol (E2), 4-hydroxy tamoxifen (for all in vitro studies) from Sigma (St Louis, MO) and ICI 182,780 (Fulvestrant, AstraZeneca, UK), were dissolved in ethanol. Tamoxifen citrate (Sigma) was used in all in vivo treatments as previously described [5].
Cell Growth Assays
Parental and TamRes cells were starved in medium with 5% charcoal-stripped FBS for 48 hours, then plated in 96-well plates (5,000 cells per well) for a further 48 hours before beginning additional treatments. The different treatment groups were estradiol (E2, 10−9 M), ED, Tam (10−7 M), or Ful (10−7 M), alone or in combination with either lapatinib (1 μM) or AZD8931 (1 μM). The number of cells at days 0 and 6 was assessed using an in situ Cell Cytometer (Celigo, Brooks Life Sciences Systems, Chelmsford, MA). Growth experiments: all treatment groups were plated in quadruplicate. Experiments in all cell models were repeated and analyzed 3 separate times. A representative experiment is shown.
Immunoblotting Assays
Cells were plated in 6 well plates and treated as previously described in cell growth assays. Cells were lysed in RIPA buffer and equivalent amounts of protein (15 μg) from each sample were used as previously described [33]. Primary antibodies were: phospho-EGFR-Tyr1173, EGFR, phospho-HER2-Tyr1221, phospho-HER2-Tyr1248, HER2, phospho-HER3-Tyr1289, phospho-AKT-Ser473, AKT, phospho-p44/42 MAPK-Thr202/Tyr204, p44/42 MAPK, β-actin, cleaved PARP, (Cell Signaling Technology, Beverly, MA), ERα 6F11 (Abcam, Fremont, CA), and progesterone receptor (PR) (Santa Cruz Biotechnology, Santa Cruz, CA). Western blots were performed at least three independent times.
RNA Interference
SMARTpool Small interfering RNAs (siRNA) against EGFR, HER2, HER3, and scrambled nonspecific siRNA (control) were purchased from Dharmacon (Lafayette, CO). Cells were transfected following the manufacturer’s instructions (Dharmacon). Transfection protocol for growth assay used was previously described [33]. For parallel protein expression, parental and TamRes cells were plated into 24-well plates and subjected to transfection protocol as above.
Ligand Stimulation
Cells were plated in 6-cm dishes for 3 days in their growth media, and serum-starved overnight. Cells were hormonally treated (E2) or treated with Tam, or Ful alone or in combination with the TKIs (lapatinib, AZD8931, and gefitinib all at 1uM concentration) for 90 mins followed by stimulation with 20 uM HRG or EGF for 10 mins. Lysates were collected and protein levels were detected by immunoblotting as indicated above.
In vitro Cell Proliferation and Apoptosis Assays
The cell proliferation assay was performed using the Click-iT EdU (5-ethynyl-2′-deoxyuridine) Microplate Assay (Invitrogen, Carlsbad, CA) and apoptosis assays were performed using the Annexin V-FITC Apoptosis Detection Kit (Abcam, Cambridge, MA) following 48 hours treatment, as described in cell growth assays. Assays were performed and analyzed as described previously [33]. All measurements were performed in quadruplicate. T47D apoptosis under our experimental conditions was not associated with elevated annexin V levels and was therefore measured by c-PARP expression after 48hrs of treatment as described in cell growth and immunoblotting assays.
Xenograft Studies
The MCF7 TamRes xenograft tumors were generated and maintained by continuously harvesting and transplanting TamRes tumors that have reached 1000 mm3 in the presence of Tam from donors to recipients as previously described [34]. Studies were conducted using ovariectomized 5–6 week-old athymic mice (Harlan Sprague Dawley, Madison, Wisconsin). Tumors were then transplanted into Tam-treated mice and when tumors reached 150–200 mm3 (1–2weeks), the mice bearing these MCF7 TamRes xenografts were randomly allocated to four treatment groups: continued Tam, Tam+AZD8931 (100mg/kg by oral gavage daily), ICI 182,780 (Ful (100ug once a week)), and Ful+AZD8931. Each treatment group contained a minimum of 8 mice. Tumor volumes were measured weekly as previously described [5]. All tumors were harvested 4hrs post treatment, after 11days of treatment (Short-term) or after longer treatment (1000mm3). Each tumor included in the statistical analysis came from a different mouse.
Statistical Analysis
Cell growth, proliferation, and apoptosis experiments were analyzed within each E2 stimulated or endocrine therapy group using one-way ANOVA with the Bonferonni post hoc test. Error bars on plots represent +/− standard error of mean (SEM). Kaplan-Meier estimators and Wilcoxon tests were used for in vivo xenograft tumor analysis. Pairwise comparisons with p-value adjustment were performed to compare the difference between treatments. Statistical analyses were done using SAS 9.2 (SAS institute Inc., Cary, NC).
Results
HER ligand expression is up-regulated in cells acquiring tamoxifen resistance
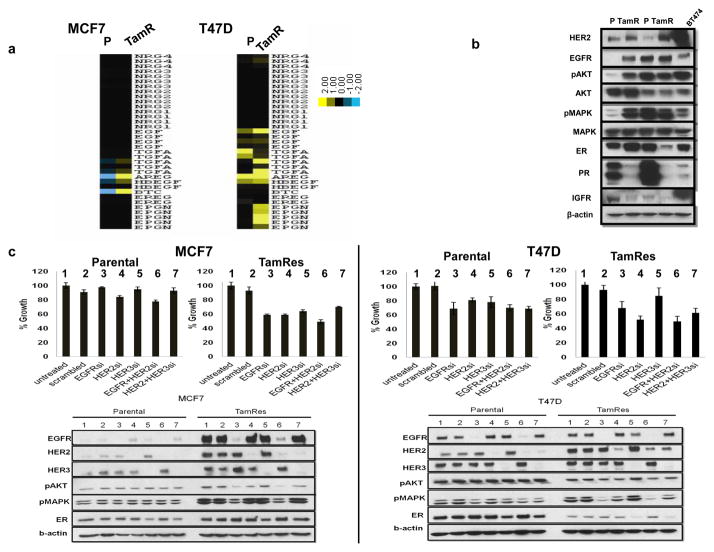
Several studies have shown that increased expression of HER ligands and/or their receptors activates downstream pathways that can modulate ER function resulting in endocrine therapy resistance [21, 22, 29]. We have used RNA-Seq to investigate more thoroughly the change in expression of several HER ligands upon the acquisition of tamoxifen resistance by comparing MCF7 and T47D parental cell lines with their tamoxifen-resistant derivatives (Fig 1A). We found that EGFR ligands were up-regulated in MCF7 TamRes cells compared with parental cells. T47D parental cells show higher expression of several ligands in comparison to MCF7 parental cells, and the ligand expression was further increased in the T47D TamRes cells.
Figure 1. Side by side comparison of ligand and HER receptor levels and pathway activation in parental and TamRes cells.
a) RNA Seq data were used to analyze HER ligand expression across HER2-low models. Expression patterns are represented using heat maps (yellow, high expression; blue low expression). Each column represents a cell model. P, parental; TamR, tamoxifen-resistant. b) HER and ER expression and signaling were compared by western blotting in low-HER cells MCF7 and T47D (P and TamRes) and high-HER BT474 cells. c) MCF7 parental and resistant cells, T47D parental and resistant cells were treated with non-targeting control siRNA (scrambled), EGFR, HER2, HER3 siRNA, and their combination, for 6 days to evaluate effect of downregulation on growth. To confirm protein knockdown, cells were treated with siRNA for 72 hours and down-regulation of EGFR, HER2, and, HER3 was detected by Western blot. Whole-cell extracts were analyzed with the indicated antibodies, including downstream signaling markers. Unlike MCF7parental cells, T47D Parental cells were slightly sensitive to EGFR and EGFR+HER2 inhibition. Both MCF7 and T47D TamRes cells were sensitive to EGFR and EGFR+HER2 siRNA and showed downregulation of downstream effectors such as pAKT and pMAPK.
We next investigated the levels of ER and HER and downstream signaling in our cell models (Fig 1B). In MCF7 cells, levels of HER2, EGFR, and downstream effectors were up-regulated in resistant compared to parental cells. In the T47D model both parental and TamRes cells expressed moderate levels of EGFR, but HER2 was up-regulated only in the TamRes cells. Although HER2 was increased at the time of endocrine resistance in both of these cell lines, the levels were still substantially less than those observed in HER2-gene amplified BT474 cells. Overall, these results are consistent with previous studies [5, 35, 36] and suggest that the up-regulation of HER ligands and receptors and the concomitant decrease in classic ER activity may contribute to endocrine resistance. Using siRNA inhibition, (Fig 1C), we find that both TamRes models were more sensitive to EGFR and HER2 siRNA than parental cells, indicating their partial dependence on the HER pathway for their growth.
AZD8931 and lapatinib inhibit tamoxifen-resistant growth in vitro
We next investigated whether AZD8931 combined with endocrine therapy is more effective than either treatment alone in inhibiting growth of parental cells (Fig 2A). As expected, MCF7 parental cell growth was significantly inhibited by endocrine therapy. With the exception of ED, neither AZD8931 nor lapatinib enhanced the effect of endocrine therapy. Using a second ER+ breast cancer cell model that expresses higher levels of EGFR, we found that the T47D parental cells were dependent on both the ER and HER pathways and thus sensitive to each of these treatments alone under E2 treatment.
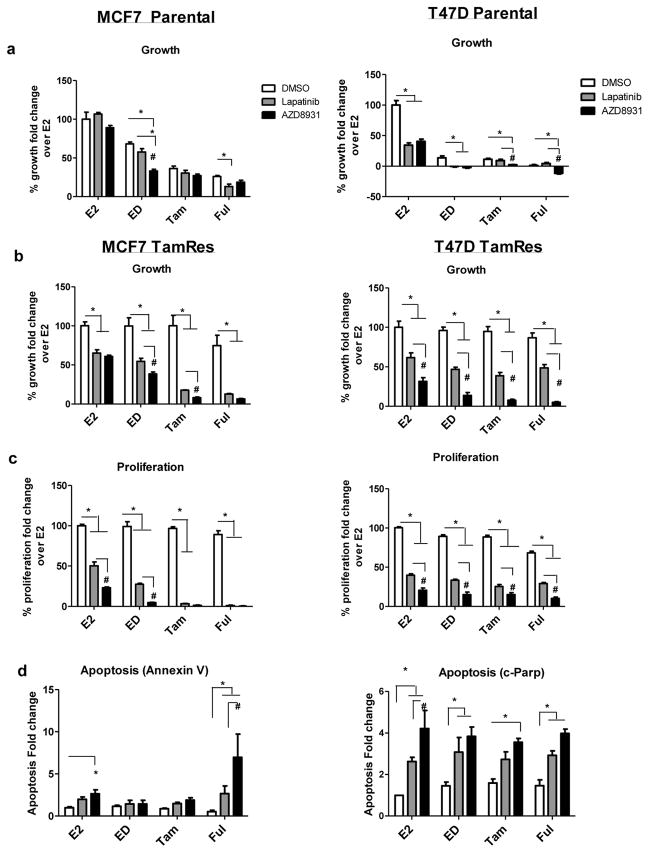
Figure 2. Effects of AZD8931 or lapatinib alone or in combination with endocrine therapy in TamRes MCF7 and T47D cells.
a,b) Cell Growth Assays: prestarved parental and TamRes MCF7 and T47D cells were treated with either estrogen (E2), estrogen deprivation (ED), tamoxifen (Tam), or fulvestrant (Ful), all in the presence of vehicle (DMSO), lapatinib, or AZD8931. Cells were counted using the in situ cell cytometer Celigo at day 6, post treatment. c) Proliferation Assays: cells were treated under conditions defined in A. After 48 hours of treatment, proliferation was measured using the Click-iT EdU (5-ethynyl-2′-deoxyuridine) Microplate Assay. d) Apoptosis Assays: MCF7 TamRes cells were incubated with Annexin V-FITC after 48 hours of treatment as in A. Positive cells from C and D were visualized and quantitated by the Celigo cytometer. Graphs show mean ± SE of quadruplicate wells and are a representative of 3 independent experiments. In the T47D parental model, cells lysates were collected after 48 hours of treatment. c-PARP levels were assessed by western blotting and normalized to β-actin using Image J. Graphs show mean ± SE of 3 independent experiments. *, p<0.01 comparing DMSO with either lapatinib or AZD8931 treatment groups, #, p<0.01 comparing lapatinib with AZD8931
We next assessed the effect of AZD8931 in the TamRes models that are characterized by a modest up-regulation of HER ligands and HER receptors (Fig 1A, B). Although the combination of lapatinib with endocrine therapy significantly inhibited MCF7 TamRes cell growth, AZD8931 combinations were slightly more effective than lapatinib when combined with ED and Tam, with a similar trend in combination with Ful (p<.07) in MCF7 (Fig 2B). Likewise, in the T47D TamRes model, AZD8931 was superior to lapatinib when combined with endocrine therapy. AZD8931 inhibited cell proliferation more effectively than lapatinib in MCF7 E2 and ED combinations and in all combinations in the T47D TamRes models (Fig 2C). Ful in combination with AZD8931 significantly induced apoptosis in MCF7 TamRes cells to a greater extent than lapatinib. In the T47D TamRes model, lapatinib significantly induced apoptosis in combination with only ED or Ful, whereas AZD8931 induced apoptosis in combination with all 3 endocrine treatments (Fig 2C). Taken together, these data suggest that in parental models, where the cells are less dependent on the HER pathway, AZD8931 does not enhance endocrine therapy activity. On the other hand, AZD8931 shows significant activity in the TamRes cell models, where the modestly increased levels of HER receptors and/or ligands contribute to endocrine resistance.
AZD8931 is a potent inhibitor of EGF- and HRG-mediated signaling and growth
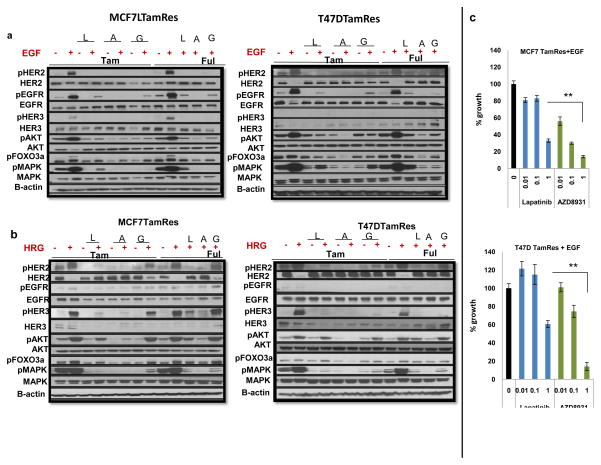
We next explored the conditions under which AZD8931 manifests its greatest effects to inhibit HER signaling in the context of endocrine therapy. AZD8931 has been shown to block ligand-induced activation of HER receptors in several solid tumor cell models [32]. We evaluated AZD8931 effects on both EGF and HRG-mediated signaling, since members of both ligand families were up-regulated in our TamRes models (Fig 1A), and since overexpression of HRG results in de novo endocrine resistance [22]. As expected, EGF treatment increased phosphorylation of HER2 and EGFR, AKT and MAPK in both MCF7 and T47D TamRes models (Fig 3A). In EGF-mediated signaling, which induces EGFR homo and hetero-dimerization, gefitinib in combination with Tam decreased the levels of pAKT and pMAPK much more effectively than lapatinib, a weaker EGFR inhibitor. Importantly, AZD8931 combined with Tam or Ful consistently showed stronger inhibition of downstream signaling than either gefitinib or lapatinib. As expected, HRG also strongly activated AKT and MAPK signaling in both MCF7 and T47D TamRes cells (Fig 3B). AZD8931 was the most potent agent in blocking phosphorylation of HER2 and HER3, as well as downstream effectors AKT and MAPK in both Tam and Ful conditions although both lapatinib and, to a much lesser extent gefitinib, inhibited AKT and MAPK activation as well. Finally, AZD8931 significantly inhibited cell growth under ligand-stimulated conditions in a dose dependent manner (Fig 3C). These data suggest that AZD8931 is a more effective inhibitor of ligand-dependent HER receptor signaling and growth in combination with endocrine therapy than lapatinib and gefitinib. The results also suggest that both EGFR and HER2 need to be blocked for maximal growth inhibition.
Figure 3. Effects of anti-HER inhibitors on EGF and HRG-stimulated HER signaling.
a) MCF7 and T47D TamRes cells were serum-starved for 24 hours and treated with endocrine therapy, with and without lapatinib (L), AZD8931 (A), or gefitinib (G) as described in Materials and Methods. Cells were treated with EGF for 10 minutes and effects of treatment on HER signaling were assessed by western blotting. b) MCF7 and T47D TamRes cells were serum-starved for 24 hours and treated as described in Materials and Methods. Cells were treated with HRG for 10 minutes and effects of treatment on HER signaling were assessed by western blotting. Both EGF and HRG stimulation further increase the expression of phospho-HER receptors as well as their downstream signals (pAKT and pMAPK). AZD8931 treatments more potently inhibited the levels of phospho-(HER2, EGFR, HER3, AKT, and MAPK) than lapatinib in both TamRes models. c) Both Cell line models were starved and treated with different concentrations of lapatinib or AZD8931 under EGF stimulation. Effect on growth was analyzed after 6days. **<.001
AZD8931 significantly delays Tam-resistant tumor growth in vivo
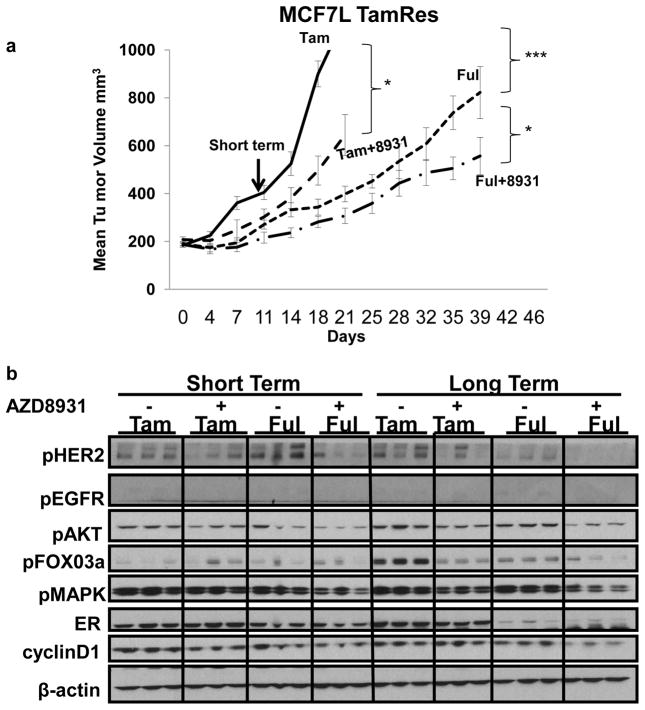
While our data clearly indicate that AZD8931 can inhibit TamRes breast cancer cells in vitro, it is still unclear whether AZD8931 treatment can circumvent tamoxifen resistance in vivo. Therefore, we investigated whether AZD8931 treatment could delay the growth of MCF7 TamRes xenografts where resistance is associated with up-regulation of the HER pathway (36). We transplanted established MCF7 TamRes tumor xenografts into nude mice in the presence of Tam. When the tumors reached 200 mm3, mice were randomized to continued Tam, Tam+AZD8931, Ful, or Ful+AZD8931. In accordance with our in vitro results, the addition of AZD8931 to Tam significantly delayed TamRes tumor growth. Ful treatment alone was more potent than Tam+AZD8931 but the combination of AZD8931 plus Ful was most effective in delaying tumor growth (Fig 4A). It should be emphasized, however, that although these treatments slowed tumor growth, none of them resulted in significant tumor regression, suggesting that other survival pathways must also be functioning to cause tamoxifen resistance in these models.
Figure 4. Effects of AZD8931 in overcoming endocrine resistance in vivo.
a) TamRes tumor xenografts were transplanted into nude mice and continuously grown with Tam. After reaching 200mm3, tumors were randomized to continued Tam, Tam+AZD8931, Ful, or Ful+AZD8931. Tumor size tripling from the randomization was considered as the survival event of tumor progression.*p<0.05 (Tam vs Tam+8931, Tam+8931 vs Ful+8931), ***p<0.0001 (Tam vs Ful). b) Immunoblotting analysis of phospho-HER receptors and their downstream effectors, phospho-(MAPK, AKT and FOXO3a) in three representative individual tumor extracts per group, treated by Tam and Ful alone, or in combination with AZD8931 at 11 days of treatment (Short Term), or after long term treatment (harvested at 1000mm3). All tumors were harvested 4 hrs. post treatment and extracts were on the same gel and b-actin is shown as the control for equivalent loading.
To evaluate the mechanisms by which AZD8931 delays tamoxifen resistance, tumors harvested after short (11days) or long (when tumors reached 1000mm3) term treatment, were subjected to western blot analysis (Fig 4B). Levels of pEGFR and HER2, as well as their downstream effectors pMAPK, pAKT, pFOXO3a and Cyclin D1 were analyzed. AZD8931+ Tam inhibited pHER2, pMAPK, and pAKT levels in both short and long term treated tumors compared to tumors treated with Tam alone. Ful reduced ER levels particularly in long-term treated tumors. Ful alone in long-term treated tumors reduced pHER2, but had little effect on pMAPK and pAKT compared to Tam in long-term tumors. AZD8931 combined with Ful reduced the levels of pHER2, pMAPK, pAKT, and pFOX03a. Furthermore, AZD8931 in combination with Ful inhibited cyclin D1 levels more than the other treatment groups.
Discussion
Crosstalk between ER and HER2 pathways has been shown to play an important role in endocrine resistance [20, 37] and it is known that HER2+ tumors are more intrinsically resistant to endocrine therapy [19, 20]. Similarly, in ER+ HER2- (HER2-low) tumors, modestly elevated levels of the EGFR and HER2 receptors and/or their ligands upon chronic endocrine treatment have been suggested to play an important role in acquired endocrine resistance [5, 6, 23, 38]. Previous studies showed that both the EGFR TKI gefitinib and the dual EGFR/HER2 TKI lapatinib only modestly and/or transiently delayed the development of endocrine resistance [25, 26, 39–41]. This modest response to HER inhibitors may at least partly be due to the incomplete blockade of the HER receptor layer by gefitinib (EGFR inhibitor) and because lapatinib is most effective in a HER2-amplified breast cancer and is less effective in inhibiting EGFR and ligand dependent activation of the HER pathway [5, 32]. Here, we used two parental ER+/HER2-negative (or HER2-low) cell line models and their corresponding tamoxifen-resistant derivatives and compared the therapeutic effect of lapatinib with the equipotent dual EGFR/HER2 TKI AZD8931, which more effectively blocks EGFR and ligand-dependent HER signaling, when both are combined with endocrine therapies (32).
Our in vitro data show that AZD8931 does not enhance endocrine therapy sensitivity in the parental cells with low HER ligand and signaling (i.e. MCF7-like). In contrast, both the MCF7 and T47D tamoxifen-resistant models were characterized by upregulation of HER ligands and receptors. In this setting, with increased dependence on the HER pathway for growth and survival, AZD8931 was slightly more effective than lapatinib in enhancing the response of these cells to multiple endocrine therapies. Fulvestrant, which results in ER degradation and would be expected to inhibit ligand-dependent and independent ER activity, had the most synergistic effect. In our in vivo model of tamoxifen resistance we also found that AZD8931 in combination with tamoxifen significantly slowed growth. Fulvestrant alone also significantly slowed tamoxifen- resistant tumor growth in vivo as has been previously shown [42]. This suggests that in tamoxifen-resistant breast cancer cells, ER still plays a role in promoting growth, As previously shown, increased HER signaling can either modify ER activity to promote endocrine resistance [5, 29], or can promote additional alternative signaling to bypass ER [43]. Indeed, we found that the combination of fulvestrant with AZD8931 inhibited tamoxifen resistant tumor growth significantly better than either treatment alone, emphasizing the importance of dual-pathway blockade in overcoming endocrine resistance. Of note, although AZD8931 in combination with fulvestrant inhibited tumor growth, tumor regression did not occur. Western blot analyses of xenografts harvested at time of tumor progression on fulvestrant combined with AZD8931, showed that even with potent and sustained inhibition of pHER2, downstream pAKT and pMAPK levels were still maintained, suggesting the presence of additional compensatory pathways contributing to endocrine resistance in these cells.
AZD8931 remains effective in the presence of HER ligands, distinguishing itself as a more potent inhibitor of HRG and EGF-induced activation of HER signaling, while the anti-tumor effects of other inhibitors such as lapatinib [31, 44] and gefitinib [45] are decreased by these HER ligands. Previous data showed that the drug lapatinib effectively blocks HER signaling and tumor growth in HER2+ tumors [41]. Our data suggests that in ER+ settings of acquired tamoxifen resistance, where tumors express increased levels of HER ligands and receptors, a TKI such as AZD8931 may be a better choice, as it is more effective in global inhibition of ligand-dependent activation of the HER pathway. Importantly, our in vitro and in vivo data suggests that an effective combination to study is a HER inhibitor such as AZD8931 with fulvestrant which degrades ER and would block ER that has been activated by growth factor signaling in an estrogen-independent way.
A Phase II clinical study, the MINT trial, with AZD8931 in ER+ breast cancer has recently been reported [46]. In this trial, AZD8931 was combined with anastrozole in women with endocrine therapy-naive advanced breast cancer where growth factor signaling would be expected to be less and overall there was no evidence of significant therapeutic benefit. It is interesting to speculate based on our preclinical data whether the use of AZD8931 in a different group of patients might have resulted in greater clinical benefit. Indeed, a recently published study correlated levels of ER to response in a trial of letrozole versus letrozole plus lapatinib in metastatic ER-positive, HER2 negative breast cancer patients. That study showed that the subgroup of patients with lower levels of ER benefited significantly from the addition of lapatinib to letrozole, while those with high ER content did not [28]. This demonstrates the importance of patient selection when designing trials studying the role of drugs targeting pathways associated with endocrine therapy resistance. Patients who are likely to be resistant to endocrine-therapy-alone should be selected for these trials or we would risk having negative trial results driven by the inclusion of patients with endocrine therapy sensitive tumors and diluting out the benefit experienced in patients with resistant tumors.
Our data also suggests that resistance to endocrine therapy in a tumor is likely to be multi-factorial and might require inhibition of multiple signaling pathways in addition to ER and HER2.
Acknowledgments
Grant Support: This work was supported in part by Dan L. Duncan Cancer Center Grant P30CA125123, Breast Cancer Research Foundation (R. Schiff and C.K. Osborne), AstraZeneca Research Grant and Susan G. Komen for the Cure Foundation Promise Grant PG12221410
We thank T. Mitchell for assistance with the mouse studies and Dr. G. Chamness for discussing and reviewing this manuscript.
Footnotes
Disclosure of Possible Conflicts of Interest
R. Schiff, M. Rimawi and C.K. Osborne have received research funding from AstraZeneca. T. Klinowska is an employee of AstraZeneca.
References
- 1.Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2(10):1102–1109. doi: 10.1200/JCO.1984.2.10.1102. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90 (Suppl 1):S2–6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark GM. Prognostic and Predictive Factors for Breast Cancer. Breast Cancer. 1995;2(2):79–89. doi: 10.1007/BF02966945. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer research. 2008;68(3):826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 6.Drury SC, Detre S, Leary A, Salter J, Reis-Filho J, Barbashina V, Marchio C, Lopez-Knowles E, Ghazoui Z, Habben K, et al. Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocr Relat Cancer. 18(5):565–577. doi: 10.1530/ERC-10-0046. [DOI] [PubMed] [Google Scholar]
- 7.Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators. Cancer chemotherapy and pharmacology. 2005;56 (Suppl 1):10–20. doi: 10.1007/s00280-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson RI, Hutcheson IR, Britton D, Knowlden JM, Jones HE, Harper ME, Hiscox SE, Barrow D, Gee JM. Growth factor signalling networks in breast cancer and resistance to endocrine agents: new therapeutic strategies. The Journal of steroid biochemistry and molecular biology. 2005;93(2–5):257–262. doi: 10.1016/j.jsbmb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, Nicholson RI. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12 (Suppl 1):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 10.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95(5):353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 11.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20(14):5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, Shou J, Malorni L, Schiff R. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer research. 2008;68(18):7493–7501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 14.Karunagaran D, Tzahar E, Beerli RR, Chen X, Graus-Porta D, Ratzkin BJ, Seger R, Hynes NE, Yarden Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15(2):254–264. [PMC free article] [PubMed] [Google Scholar]
- 15.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 16.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 12(8):553–563. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 17.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21(2):177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008;26(7):1059–1065. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen BB, Regan MM, Lykkesfeldt AE, Dell’Orto P, Del Curto B, Henriksen KL, Mastropasqua MG, Price KN, Mery E, Lacroix-Triki M, et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1–98 randomised trial. Lancet Oncol. 2008;9(1):23–28. doi: 10.1016/S1470-2045(07)70386-8. [DOI] [PubMed] [Google Scholar]
- 20.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 21.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 22.Tang CK, Perez C, Grunt T, Waibel C, Cho C, Lupu R. Involvement of heregulin-beta2 in the acquisition of the hormone-independent phenotype of breast cancer cells. Cancer Res. 1996;56(14):3350–3358. [PubMed] [Google Scholar]
- 23.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10(1 Pt 2):331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res. 16(7):1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 25.Cristofanilli M, Valero V, Mangalik A, Royce M, Rabinowitz I, Arena FP, Kroener JF, Curcio E, Watkins C, Bacus S, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 16(6):1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 26.Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, Schiff R, Gutierrez C, Migliaccio I, Anagnostou VK, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 17(5):1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartzberg LS, Franco SX, Florance A, O’Rourke L, Maltzman J, Johnston S. Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancer. Oncologist. 15(2):122–129. doi: 10.1634/theoncologist.2009-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn RS, Press MF, Dering J, O’Rourke L, Florance A, Ellis C, Martin AM, Johnston S. Quantitative ER and PgR Assessment as Predictors of Benefit from Lapatinib in Postmenopausal Women with Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-1260. [DOI] [PubMed] [Google Scholar]
- 29.Lupien M, Meyer CA, Bailey ST, Eeckhoute J, Cook J, Westerling T, Zhang X, Carroll JS, Rhodes DR, Liu XS, et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 2010;24(19):2219–2227. doi: 10.1101/gad.1944810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer IA, Arteaga CL. Does lapatinib work against HER2-negative breast cancers? Clin Cancer Res. 16(5):1355–1357. doi: 10.1158/1078-0432.CCR-09-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11(3):582–593. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 32.Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, Beck S, Marshall G, Davenport S, Callis R, et al. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB receptor blockade in cancer. Clin Cancer Res. 16(4):1159–1169. doi: 10.1158/1078-0432.CCR-09-2353. [DOI] [PubMed] [Google Scholar]
- 33.Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, Botero MF, Healy NA, Hilsenbeck SG, Phillips GL, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers--role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 13(6):R121. doi: 10.1186/bcr3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborne CK, Coronado E, Allred DC, Wiebe V, DeGregorio M. Acquired tamoxifen resistance: correlation with reduced breast tumor levels of tamoxifen and isomerization of trans-4-hydroxytamoxifen. J Natl Cancer Inst. 1991;83(20):1477–1482. doi: 10.1093/jnci/83.20.1477. [DOI] [PubMed] [Google Scholar]
- 35.Emde A, Mahlknecht G, Maslak K, Ribba B, Sela M, Possinger K, Yarden Y. Simultaneous Inhibition of Estrogen Receptor and the HER2 Pathway in Breast Cancer: Effects of HER2 Abundance. Transl Oncol. 4(5):293–300. doi: 10.1593/tlo.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Britton DJ, Hutcheson IR, Knowlden JM, Barrow D, Giles M, McClelland RA, Gee JM, Nicholson RI. Bidirectional cross talk between ERalpha and EGFR signalling pathways regulates tamoxifen-resistant growth. Breast Cancer Res Treat. 2006;96(2):131–146. doi: 10.1007/s10549-005-9070-2. [DOI] [PubMed] [Google Scholar]
- 37.Johnston SR, Martin LA, Leary A, Head J, Dowsett M. Clinical strategies for rationale combinations of aromatase inhibitors with novel therapies for breast cancer. J Steroid Biochem Mol Biol. 2007;106(1–5):180–186. doi: 10.1016/j.jsbmb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11(2 Pt 2):865s–870s. [PubMed] [Google Scholar]
- 39.Leary AF, Drury S, Detre S, Pancholi S, Lykkesfeldt AE, Martin LA, Dowsett M, Johnston SR. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 16(5):1486–1497. doi: 10.1158/1078-0432.CCR-09-1764. [DOI] [PubMed] [Google Scholar]
- 40.Chu I, Blackwell K, Chen S, Slingerland J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65(1):18–25. [PubMed] [Google Scholar]
- 41.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(33):5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 42.Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer. 2000;89(4):817–825. doi: 10.1002/1097-0142(20000815)89:4<817::aid-cncr14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Kirkegaard T, Hansen SK, Larsen SL, Reiter BE, Sørensen BS, Lykkesfeldt AE. T47D breast cancer cells switch from ER/HER to HER/c-Src signaling upon acquiring resistance to the antiestrogen fulvestrant. Cancer Letters. doi: 10.1016/j.canlet.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Gwin W, Liu L, Zhao S, Xia W, Spector N. The impact of the heregulin-HER receptor signaling axis on response to HER tyrosine kinase inhibitors. Cancer Research. 2012;72(24 Suppl):Abstract nr P4-08-03. [Google Scholar]
- 45.Hutcheson IR, Knowlden JM, Hiscox SE, Barrow D, Gee JM, Robertson JF, Ellis IO, Nicholson RI. Heregulin beta1 drives gefitinib-resistant growth and invasion in tamoxifen-resistant MCF-7 breast cancer cells. Breast Cancer Res. 2007;9(4):R50. doi: 10.1186/bcr1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston SRD. Phase II randomized study of the EGFR, HER2, HER3 signaling inhibitor AZD8931 in combination with anastrozole (A) in women with endocrine therapy (ET) naive advanced breast cancer (MINT) J Clin Oncol. 2013 doi: 10.1007/s10549-016-3979-5. [DOI] [PubMed] [Google Scholar]