Abstract
Objective:
Patients with small intestine bacterial overgrowth (SIBO) have chronic intestinal and extraintestinal symptomatology which adversely affects their quality of life. Present treatment of SIBO is limited to oral antibiotics with variable success. A growing number of patients are interested in using complementary and alternative therapies for their gastrointestinal health. The objective was to determine the remission rate of SIBO using either the antibiotic rifaximin or herbals in a tertiary care referral gastroenterology practice.
Design:
One hundred and four patients who tested positive for newly diagnosed SIBO by lactulose breath testing (LBT) were offered either rifaximin 1200 mg daily vs herbal therapy for 4 weeks with repeat LBT post-treatment.
Results:
Three hundred ninety-six patients underwent LBT for suspected SIBO, of which 251 (63.4%) were positive 165 underwent treatment and 104 had a follow-up LBT. Of the 37 patients who received herbal therapy, 17 (46%) had a negative follow-up LBT compared to 23/67 (34%) of rifaximin users (P=.24). The odds ratio of having a negative LBT after taking herbal therapy as compared to rifaximin was 1.85 (CI=0.77-4.41, P=.17) once adjusted for age, gender, SIBO risk factors and IBS status. Fourteen of the 44 (31.8%) rifaximin non-responders were offered herbal rescue therapy, with 8 of the 14 (57.1%) having a negative LBT after completing the rescue herbal therapy, while 10 non-responders were offered triple antibiotics with 6 responding (60%, P=.89). Adverse effects were reported among the rifaximin treated arm including 1 case of anaphylaxis, 2 cases of hives, 2 cases of diarrhea and 1 case of Clostridium difficile. Only one case of diarrhea was reported in the herbal therapy arm, which did not reach statistical significance (P=.22).
Conclusion:
SIBO is widely prevalent in a tertiary referral gastroenterology practice. Herbal therapies are at least as effective as rifaximin for resolution of SIBO by LBT. Herbals also appear to be as effective as triple antibiotic therapy for SIBO rescue therapy for rifaximin non-responders. Further, prospective studies are needed to validate these findings and explore additional alternative therapies in patients with refractory SIBO.
Key Words: Irritable bowel syndrome (IBS), rifaximin, Antibiotics, Small Intestine Bacterial Overgrowth (SIBO), Dysbiosis, Complementary and Alternative Medicine (CAM), Herbal Therapies
INTRODUCTION
Small intestinal bacterial overgrowth (SIBO) is defined as an increase in the concentration of bacteria of more than 100 000 colony-forming units per mL of proximal jejunal fluid.1 Although the overall prevalence of SIBO in the general public is unknown, a metaanalysis has shown that the prevalence of SIBO is approximately 56% among patients with irritable bowel syndrome (IBS).2 At present, the prevalence of SIBO in the community or in a tertiary-care gastroenterology referral practice remains unknown. SIBO is becoming an increasingly significant problem in clinical practice as its manifestations can be protean and range from a full-blown enteropathy causing profound malabsorption and malnutrition simulating celiac disease to mild symptoms that overlap with IBS.1 There are several factors involved in the pathogenesis of SIBO that have in common a disruption in the natural protective antibacterial mechanisms of the gut as shown in Tables 1–3.3 These factors, alone or in combination, can predispose the gut toward dysbiosis and favor the growth and colonization of aerobes and anaerobes in the proximal jejunum and may provide barrier(s) from achieving durable remission of symptoms following treatment.10 Currently, lactulose breath testing (LBT) is the most commonly used procedure for diagnosing SIBO.11,12 LBT is useful as an indirect testing method for SIBO, given that the human gastrointestinal (GI) tract does not absorb or use lactulose, which is metabolized by bacteria to produce hydrogen and methane gas. These gases are then measured by gas chromatography.13 Once suspected SIBO is diagnosed, it should be treated to prevent its myriad of adverse consequences.14–17
Table 1.
| Achlorhydria (surgical, iatrogenic, autoimmune) |
| Motor abnormalities |
| Scleroderma |
| Intestinal pseudo-obstruction |
| Diabetic enteropathy |
| Vagotomy |
| Abnormal communication between colon and small bowel |
| Fistulas between colon and small bowel |
| Resection of ileocecal valve |
| Structural abnormalities |
| Systemic and intestinal immune deficiency states |
| Surgical loops (Billroth II, entero-entero anastomosis, Rou-en-Y) |
| Duodenal or jejunal diverticula |
| Partial obstruction of small bowel (stricture, adhesions, tumors) |
| Large small Intestine diverticulosis |
| Systemic diseases (celiac disease, cirrhosis, pancreatic exocrine insufficiency, non-alcoholic fatty liver disease) |
| Alcoholism |
Table 2.
Protective Factors That Protect Against the Development of Small Intestine Bacterial Overgrowth4,8,9
| • Gastric acid |
| • Pancreatic enzymes |
| • Bile acids |
| • Cholecystectomy |
| • Motility |
| • Migrating motor complex |
| • Biofilm |
| • Secretory immunoglobulin A |
Table 3.
Extrinsic Factors That Alter the Gut Microbiome and May Influence the Development of Small Intestine Bacterial Overgrowth4
| FODMAPsa (fructose, lactose, galactans, fructans, sugar alcohols) |
| Proton pump inhibitors |
| Anti-motility agents |
| Fiber |
| Prebiotics |
| Probiotics |
| Antibiotics |
FODMAPs is an acronym for a group of highly fermentable foods (Fermentable Oligo-, Di-, Monosaccharides and Polyols).
What is the current knowledge?
Small intestinal bacterial overgrowth (SIBO) is widely prevalent among patients with the irritable bowel syndrome (IBS) and is associated with a worsening of symptoms and quality of life.
Current treatment of SIBO is presently limited to antibiotics with a variable response rate and none have been approved by the US Federal Drug Administration (FDA).
SIBO is often treated with a course of rifaximin 1200 mg/ day for 10-14 days, which is expensive and not routinely covered by many commercial health plans in the United States.
The majority of IBS patients use complementary and alternative therapies to control symptoms and are often looking for more “natural” options to treat their disease.
What is new here?
The high prevalence rate for SIBO of 64% in a tertiary care referral gastroenterology practice.
The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies vs 34% for Rifaximin.
Herbal therapy may be an effective treatment for patients with SIBO.
Patients with SIBO refractory to rifaximin can be given the choice of herbal therapy as rescue therapy.
The current state-of-the-art treatment of SIBO is the provision of a short course (10-14 days) of antibiotics (Table 4). Thus far, rifaximin, a rifampin derivative, is the antibiotic that has been most widely recognized and published for its use in the treatment of SIBO.19 Additionally, SIBO has been implicated as a major contributing pathophysiological factor in IBS. Over the past decade, patients with IBS are increasingly turning to complementary and alternative medicine (CAM) options for symptoms relief and disease management.20 Herbal remedies and nutraceutical supplements continue to dominate as the most common forms of CAM used for IBS patients.21 A number of herbs have a long tradition of antimicrobial activity,22 thus we hypothesized that the use of plant extracts possessing antimicrobial activity would be as effective as antibiotic therapy for patients with an abnormal LBT and the diagnosis of SIBO.
Table 4.
Antibiotic Regimens Used for Small Intestine Bacterial Overgrowth18
| Agent | Dose | Frequency |
|---|---|---|
| Amoxicillin-clavulanate | 500 mg PO | 3 times/day |
| Cephalexin | 250 mg PO | 4 times/day |
| Chloramphenicol | 250 mg PO | 4 times/day |
| Ciprofloxacin | 500 mg PO | twice daily |
| Doxycycline | 100 mg PO | twice daily |
| Metronidazole | 250 mg PO | 3 times/day |
| Neomycin | 500 mg PO | twice daily |
| Norfloxacin | 400 mg PO | twice daily |
| Rifaximin | 400 mg PO | 3 times/day |
| Tetracycline | 250 mg PO | 4 times/day |
| Trimethoprim- sulfamethoxazole | 1 double-strength tablet PO | twice daily |
Abbreviation: PO, per os (by mouth).
METHODS
Study Participants
The medical records at a single tertiary-care referral center from October 2006 to November 2010 were reviewed as part of an institutional IRB-approved protocol. Study participants were included in the analysis if they had: (1) symptoms suggestive of SIBO, (2) an initial positive LBT, (3) completed a prescribed regimen of either rifaximin or herbal therapy, and (4) received a post-treatment LBT. Symptoms considered to be suggestive of SIBO included otherwise unexplained abdominal discomfort, cramping, bloating, flatulence, eructation, diarrhea, worsening of symptoms after meal ingestion, and low serum B12. Criteria considered to be risk factors for SIBO included prior history of gastric bypass surgery, known gastrointestinal motility disorder, collagen vascular disease, IBS (association), pancreatic insufficiency, and chronic proton-pump inhibitor (PPI) use. Exclusion criteria included those patients aged <18 or >85 years, use of antibiotics within 3 months, and failure to comply with regimen or to have a follow-up LBT. A subset of patients who (1) completed the rifaximin regimen, (2) had persistently positive post-treatment LBT, (3) subsequently completed a prescribed herbal or triple antibiotic regimen, and (4) had a post-herbal therapy LBT were defined as “rifaximin non-responders.”
Lactulose Breath Testing Protocol
Study participants followed a standard preparation protocol prior to the procedure.23 No laxatives were to be taken for at least a week prior to the test. One day prior, test subjects were advised to avoid high-fiber foods, butter, margarine, and sodas and asked to fast for 12 hours before the test, consuming no food except water. Subjects were advised not to smoke, sleep, or exercise vigorously up to 30 minutes before or at any time during the test.
A QuinTron Gas Microanalyzer (QuinTron Instrument Company, Inc, Milwaukee, Wisconsin) was used to detect breath hydrogen in samples. Baseline breath hydrogen was obtained on all subjects prior to the ingestion of 10 gm of lactulose in 30 cc of water. Serial breath hydrogen analysis was obtained every 20 minutes after ingestion of lactulose up to a maximum of 3 hours. The test was considered positive if it showed one or more of the following24: (1) a baseline breath concentration of >10 parts per million (ppm) for hydrogen or >7 ppm for methane only if patients were compliant with their preparation or (2) an increase within 90 minutes (small intestine) that was followed by a larger peak (colonic), indicative of a positive study (with a decrease of at least 5 ppm following the first peak). The first increase had to have one of the following to be considered positive: (1) an increase of at least 12 ppm methane over the baseline by 90 minutes or (2) if producing hydrogen only, an increase of at least 20 ppm hydrogen over the baseline by 90 minutes. All breath tests were evaluated by a single experienced reader who was blinded to the treatment regimen (GM).
Treatment
Subjects with newly diagnosed SIBO by LBT were given two open-label treatment choices based upon individualized treatment preference; either two 200 mg rifaximin tablets three times daily (TID) or 2 capsules twice daily of the following commercial herbal preparations; Dysbiocide and FC Cidal (Biotics Research Laboratories, Rosenberg, Texas) or Candibactin-AR and Candibactin-BR (Metagenics, Inc, Aliso Viejo, California) for 4 consecutive weeks immediately followed by a repeat LBT. Table 5 illustrates the details regarding the composition for each herbal preparation. The cost of herbal therapy was no more than $120 for a 30-day supply. The primary outcome was the proportion of patients in each group who had a negative post-treatment LBT. Rifaximin non-responders were then prescribed either the herbal protocol or triple antibiotics (clindamycin 300 mg TID, metronidazole 250 mg TID, neomycin 500 mg TID) for 4 additional weeks. The statistical methods applied to analyze the results were the student t-test and chi-squared test. The study was approved by the Johns Hopkins University (Baltimore, Maryland) Internal Review Board.
Table 5.
Herbal Preparations for the Treatment of Small Intestine Bacterial Overgrowth
| FC Cidal | Dysbiocide | Candibactin-AR | Candibactin-BR |
|---|---|---|---|
| Proprietary blend - 500 mg: 1 capsule | Proprietary Blend 950 mg per 2 capsules | One Capsule contains: | Two Capsules contain: |
| Tinospora cordifolia (stem) | Dill seed | Red Thyme oil (thymus vulgaris, providing 30%-50% thymol) 0.2 mL | Coptis root and rhizome extract (coptis chinensis, containing berberine) 30 mg |
| Equisetum arvense (stem) | Stemona Sessilifolia powder and extract | Oregano Oil (origanum vulgare, providing 55% to 75% carvacrol) 0.1 mL | Indian Barberry root extract (berberis aristata, containing berberine) 70 mg |
| Pau D'Arco (inner bark) | Artemisia Absinthium shoots and leaves extract, | Sage leaf 5.5:1 extract (salvia officinalis) 75 mg | Berberine Sulfate 400 mg • Proprietary 4:1 Extract 300 mg: Coptis root and rhizome (coptis chinensis) |
| Thymus vulgaris (aerial part) | Pulsatilla Chinensis rhizome powder and extract | Lemon Balm leaf 5:1 extract (melissa officinalis) 50 mg | Chinese Skullcap root (scutellaria baicalensis) |
| Artemisia dracunculus (leaf) | Brucea Javanica powder and extract | Philodendron bark (phellodendron chinense) | |
| Sida cordifolia (aerial part) | Picrasma Excelsa bark extract | Ginger rhizome (zingiber officinale) | |
| Olea europaea (leaf) | Acacia Catechu stem extract | Chinese Licorice root (glycyrrhiza uralensis) | |
| Hedyotis Diffusa powder and extract | Chinese Rhubarb root and rhizome (rheum officinale) | ||
| Yarrow leaf and flower extract (achillea millefolium). | Chinese Rhubarb root and rhizome (rheum officinale). |
RESULTS
A flow chart of the study design and results are shown in the Figure. Three hundred ninety-seven patients were offered LBT for suspected SIBO, of which 252 (63.5%) were positive; 87 of these patients did not pursue treatment, and 61 of those who underwent therapy did not undergo repeat LBT. The remaining 104 patients were provided the option of two treatment arms as per the patients' choice and underwent post-treatment LBT. Rifaximin was completed by 67 patients, and 37 completed herbal therapy. Results were tabulated only for those who completed therapy. There was no difference in the mean age of patients who chose rifaximin (47.55 ± 15.73) and those on herbal therapy (41.6 ffl 16.3) (P=.30). There was a gender skewing of participants as 48/67 (71%) of the rifaximin arm and 29/37 (78%) of the herbal arm were females (Table 6). Of the 67 patients who completed the rifaximin arm, 9 (13%) had risk factors for SIBO (diabetes mellitus, gastroparesis, narcotics, connective tissue disease, etc) while 7 (19%) in the herbal arm had risk factors (P=.46). Of the 252 individuals who tested positive for SIBO by LBT, 160 (63.5%) had IBS as diagnosed by the Rome III criteria. Of the 37 patients who received herbs, 17 (46%) had a negative follow-up LBT compared to 23/67 (34%) of rifaximin users (P=.24) (Table 7). The odds ratio of having a negative LBT if on herbal therapy as compared to rifaximin was 1.85 (CI=0.77-4.41, P=.17) once adjusted for age, gender, SIBO risk factors, and IBS status (Table 8). Fourteen of the 41 (34.1%) rifaximin non-responders were offered herbal rescue therapy; 8 of the 14 (57.1%) had a negative LBT after completing the rescue herbal therapy. Ten of the 41 (24.4%) rifaximin non-responders were offered rescue therapy with triple antibiotics, and 6 of the 10 (60%) had a negative LBT after completing the rescue herbal therapy. Rescue therapy with herbals in rifaximin non-responders was not different (P=1.0). The average age of those SIBO patients who cleared the LBT post-rescue herbal therapy was 37.4 ± 17 years, which was not different than non-responders (41.5 ± 11.6, P=.62) (Table 9). The prevalence of IBS subtype, known risk factor(s) for SIBO, or gender status did not predict response to herbal rescue therapy (Table 9). Adverse effects (AE) were reported among the rifaximin treated arm (6/67; 9.0%) including 1 case of anaphylaxis, 2 cases of hives, 2 cases of diarrhea, and 1 case of Clostridium difficile (post-treatment). One case of diarrhea (non–Clostridium difficile) was reported in the herbal therapy arm (1/37; 2.7%) (P=.22)
Figure.
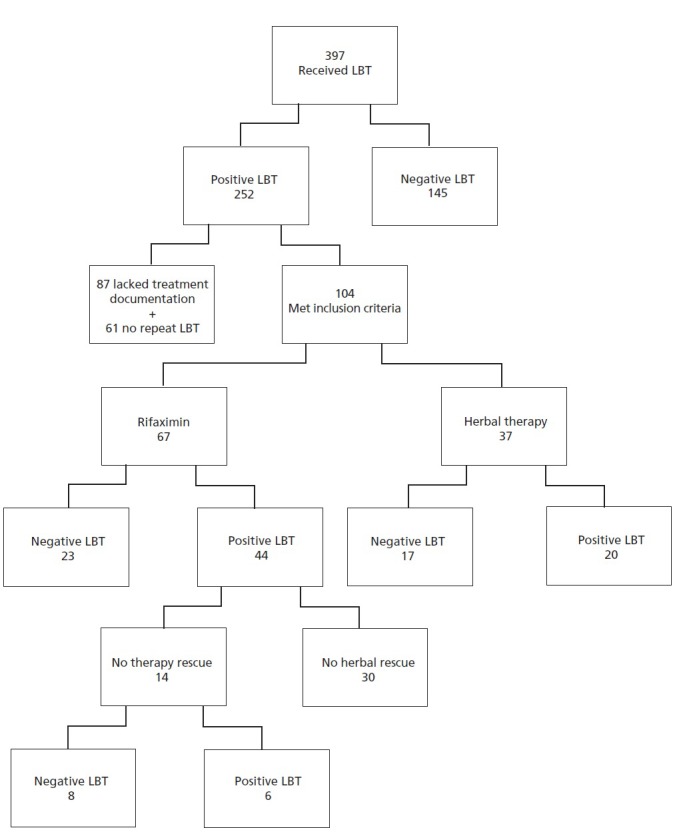
Flow chart of the study design: 397 patients were recruited to participate in the study and underwent lactulose breath hydrogen testing (LBT), 252 were found to be LBT positive while 145 were negative. Due to lack of protocol adherence, only 104 of those who were had positive LBT met our inclusion criteria for the study, with 67 opting for Rifaximin therapy and 37 preferring herbals. Forty-four of the 67 (65.7%) subjects treated with Rifaximin failed to respond and had positive LBT after therapy with only 34.3% responding. Herbal therapies were associated with a (17/37) 45.9% response rate with (20/37) 54.1% having post-treatment positive LBT. For those who failed the first round of Rifaximin therapy, a crossover trial showed a 8/14 (57.1%) response rate for herbals in those who failed Rifaximin.
Table 6.
Demographic Information of Rifaximin vs Herbal Users
| Rifaximin (n=67) | Herbal (n=37) | P value | |
|---|---|---|---|
| Age (yrs) | 47.55 ± 15.73 | 41.6 ± 16.3 | .07 |
| Percent female | 48 (71%) | 29 (78%) | .45 |
| Risk Factors for SIBO | 9 (13%) | 7 (19%) | .46 |
| Diabetes mellitus | 4 (6%) | 0 (0%) | .12 |
| Gastroparesis | 2 (3%) | 1 (3%) | .93 |
| Narcotic | 1 (1%) | 3 (8%) | .09 |
| CTD | 2 (3%) | 4 (11%) | .1 |
| Any risk factor | 9 (13%) | 7 (19%) | .46 |
| IBS | 53 (79%) | 25 (68%) | .19 |
| Distribution of IBS by Subtypes | .08 | ||
| None | 14 (21%) | 12 (32%) | |
| Diarrhea predominant | 22 (32%) | 6 (16%) | |
| Constipation predominant | 16 (24%) | 12 (32%) | |
| Mixed | 14 (21%) | 4 (11%) | |
| Unclassified IBS | 1 (1%) | 3 (8%) | |
| Negative LBT after Rx | 23 (34%) | 17 (46%) | .24 |
Table 7.
Results of IBS Subjects Undergoing Intervention for Positive Lactulose Breath
| Characteristics | Rifaximin | Herbs | P value |
|---|---|---|---|
| Number | 67 | 37 | N/A |
| Age (y), SD, range | 44.4 ± 14.8 (19-81) | 41.3 ± 14.8 (19-76) | .33 |
| Gender | |||
| Female, n (%) | 48 (71) | 29 (78) | .97 |
| Male, n (%) | 19 (29) | 8 (22) | |
| Responses (n) | 26 | 15 | N/A |
| Response Rate (%) | 34 | 46 | .24 |
| Adverse Events (n, %) | 2, 2.9 | 1, 2.7 | .83 |
Table 8.
Odds Ratios (OR) for Negative LBT Comparing Herbal Therapy to Rifaximin
| OR | Confidence Interval | P value | |
|---|---|---|---|
| Unadjusted | 1.63 | 0.72 - 3.70 | .24 |
| Adjusted for Age and Gender | 1.62 | 0.70 - 3.77 | .16 |
| Fully Adjusted* | 1.85 | 0.77 - 4.41 | .17 |
Adjusted for age, gender, small intestine bacterial overgrowth risk factors, and irritable bowel syndrome status.
Table 9.
Demographic Information of Rifaximin Nonresponders After Herbal Rescue Therapy
| Responder (n=8) | Nonresponder (n=6) | P value | |
|---|---|---|---|
| Age +/− SD | 37.4 +/− 17 | 41.5 +/− 11.57 | 0.62 |
| Female (%) | 5 (62.5%) | 4 (67%) | 0.87 |
| IBS subtype | 0.74 | ||
| None | 1 (12.5%) | 1 (16.7%) | |
| Diarrhea predominant | 1 (14%) | 2 (40%) | |
| Constipation predominant | 3 (43%) | 1 (20%) | |
| Mixed | 3 (60%) | 2 (40%) | |
| Any Risk Factor for SIBOa | 2 (25%) | 1 (17%) | 0.71 |
Defined as narcotic use, gastroparesis, diabetes mellitus, and connective tissue disease.
DISCUSSION
There is an ongoing resurgence of interest in the role of gut microbiota-host interactions in both health and disease.25 The gut microbiota is a finely balanced ecosystem that helps regulate key vital functions for the host, including immunity, barrier defense, biotransformation of toxins and carcinogens, and much more.26 As discussed in more detail by Okeke et al in this issue of Global Advances in Health and Medicine, imbalances in the gut microbiome (also known as dysbiosis) have been linked to the development of disorders of mood and behavior, Alzheimer's disease, and numerous gastrointestinal and systemic disorders including inflammatory bowel disease, diabetes, obesity, and cardiovascular disease.27,28 Bacterial dysbiosis in SIBO can disrupt epithelial tight junctions increasing small intestine paracellular permeability, translocation of endotoxin, and induction of proinflammatory cytokines.29–31 SIBO can have a number of extraintestinal manifestations such as rosacea, restless legs syndrome,17,32 arthralgias, anemia, interstitial cystitis,33 chronic prostatitis,34 and polyneuropathy.1 A large body of work has linked bacterial dysbiosis of the small bowel and endotoxemia to non-alcoholic steatohepatitis and progression of alcoholic liver disease, non-alcoholic fatty liver disease, obesity, and others.35–38 SIBO is a widely prevalent condition in the practice of gastroenterology that is chronic and recurrent for patients and lacks clear treatment guidelines for practitioners. Given the role of the gut microbiome in health and dysbiosis in disease, SIBO is an important entity in clinical practice to recognize and treat.
Rifaximin is the most commonly studied antibiotic treatment for SIBO, with an overall breath test resolution rate of 49.5% (95% confidence interval, CI 44.0-55.1) in 8 clinical trials.18 A recent meta-analysis reported that the therapeutic efficacy of rifaximin to treat SIBO in the setting of IBS showed benefit; however, the therapeutic gain was only 9.8%, and the number needed to treat is 10.2 with mild heterogeneity (P=.25, I(2)=26%).39 Additionally, rifaximin is currently not approved by the US Food and Drug Administration (FDA) for the treatment of SIBO and is only labeled for hepatic encephalopathy. Furthermore, a month's supply of rifaximin retails for $1247.39, and according to Medicare part D, a patient's copay in 2014 will be $638.09 for preferred ($703.70, non-preferred) pharmacy or mail-in.40 Antibiotics may also produce a wide range of toxicity (Clostridium difficle colitis, antibiotic induced diarrhea, anaphylaxis, Steven's Johnson reactions, hemolytic-uremic syndrome, etc).41 Antibiotics have also been postulated to have pervasive adverse effects on the gut microbiome and protective biofilm layer.42 In fact, a 7-day course of clindamycin has been shown to reduce commensal flora and gut microbial diversity and select antibiotic-resistant genes for over 2 years.43 Thus, research into more effective and safer therapeutic options for SIBO are ongoing.
The present study demonstrates that herbal therapy may be as effective as antibiotic therapy in the treatment of SIBO, as indirectly measured by normalization of the lactulose breath test abnormalities. Complementary and alternative medicine has gained wide popularity among the United States population, with up to 50% of patients using at least one form of alternative therapy.44 In addition, it has been noted that the majority of alternative medicine users are patients with chronic conditions. Examples include relaxation techniques and acupuncture for migraines, chiropractic for back problems, and herbal therapy and relaxation for digestive problems.44 Drossman et al reported based on an international survey that approximately 37% of patients with functional digestive disorders seek complementary and alternative therapies to control their symptoms.45 More recent data indicates that up to 51% of patients with IBS report using CAM.20,21 Since CAM has gained popularity among patients with gastrointestinal diseases, especially functional disorders, physicians should familiarize themselves with available CAM therapies.46 Alternative therapies commonly used by IBS patients that have some level of scientific basis in the literature include acupuncture, fiber, peppermint oil, herbal, traditional, yoga, massage, meditation, mind, relaxation, probiotics, hypnotherapy, psychotherapy, cognitive therapy, and behavioral therapies.47 The use of antimicrobial herbs to treat SIBO has not been previously reported in the literature. A number of herbs exist that have known antimicrobial activity.48–53 In the current study, we chose a combination of herbal preparations to provide broad-spectrum coverage against enteric coliforms.54,55 Oil of oregano (Origanum vulgare) is a well-documented botanical that directly kills or strongly inhibits the growth of intestinal microbes.56,57 Oil of oregano has other beneficial properties such as inducing apoptosis in human colon cancer caco2 cells.58 Berberine extracts and thymus vulgaris are also well known for their broad antibacterial activities.56,59–61 Wormwood (Artemisia absinthium) has substantial antimicrobial and anti-inflammatory properties that may be important to the pathogenesis of SIBO and has been used to successfully induce remission of Crohn's Disease.37,62 There are other herbals used in our study that have noteworthy properties. Lemon balm offers anti-anxiety and antidepressant effects that may benefit patients with IBS, while coptis root has growth-inhibitory effects on human bacteria.63–65 Red thyme essential oil inhibits the growth of Escherichia coli O157: H7 and Staphylococcus aureus.66 Indian Barbarry root extract (Berberis aristata) contains berberine and has antimicrobial, anti-inflammatory, and antidiarrheal proper-ties.67 Equisetum arvense L. was shown to possesses a broad spectrum of a very strong antimicrobial activity against a variety of enteric microorganisms including Staphylococcus aureus (S aureus), Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Salmonella enteritidis and the fungi Aspergillus nigerand Candida albicans.68 Thymus vulgaris has potent antimicrobial and anti-inflammatory actions.69–72 Olea europaea inhibits the growth of a number of staphylococcal species including S aureus.73
Rifaximin is a non-absorbable oral antibiotic, first introduced in Italy in 1987 and in the United States in 2004, that has been approved for a variety of indications and is presently an antibiotic of choice for SIBO.74 Rifaximin is presently the best studied antibiotic with an overall breath test resolution rate of 49.5% (95% confidence interval, CI 44.0-55.1).18 Lauritano et al in 2009 showed that rifaximin is superior to metronidazole in the treatment of SIBO; however, recurrence rates after treatment with antibiotics is high as evidenced by high breath test positivity.75 In our study, we demonstrated that 46% of patients normalized their LBT with herbal therapy. There was no statistical difference between antimicrobial herbs (46%) and rifaximin (34%) (P=.24). Patients were offered the choice of either therapy. SIBO tends to be a recurrent disease, and frequent antibiotic use may have longterm adverse effects on the gut microbiome and be costly; thus, herbal therapy may be a reasonable treatment option for patients with SIBO. In our study, the side effect profile of herbs when compared to rifaximin was not statistically different with a P=.22. However, the prevalence of side effects in the Rifaximin group was 9% (6/67) including C difficle and 2 non-C difficle-associated diarrhea. In the herbal group, only 1 case of non-C difficle–associated diarrhea (1%) was observed. These observations are contrary to popular beliefs. Perhaps the herbal therapies are less disruptive to the gut microbiome and while producing efficacy in resolving SIBO there appears to be less risk of C difficle; extended trials will need to confirm this hypothesis. The fear of bacterial resistance including opportunistic infections such as Clostridium difficile raises concerns among recurrent antibiotic users. The rifaximin non-responders who received herbal therapy were equally successful in resolving SIBO via the LBT as were triple antibiotics, which had much higher cost and risk of toxicity. Thus, herbal therapy appears to be effective in the treatment of SIBO patients that is initially refractory to rifaximin. Patients who were treated with herbs were not different than those who received rifaximin with respect to the number of predisposing factors for SIBO, and thus the equivalence in response was not due to disparities in underlying conditions. Induction of achlorhydria by acid-suppressive medications (eg, PPIs) has been studied as a potential risk factor for SIBO, with a recent meta-analysis of 11 studies revealing that a pooled OR of SIBO in PPI users vs nonusers was 2.282 (95% confidence interval [CI], 1.238-4.205) when duodenal/jejunal aspirate culture was used to diagnose SIBO but not for glucose hydrogen breath testing.76 In contrast, in our study, concurrent PPI use did not appear to influence response to either herbal therapies (P=.27) or rifaximin (P=.10).
Limitations of this study include the fact that it was a retrospective chart review and not a prospective, randomized controlled trial. Furthermore, there was heterogeneity and multiplicity of herbs used and, additionally, diet was not controlled. Our patients were also not formally assessed for symptom resolution using a standardized questionnaire. The cost of the herbal regimen is traditionally not covered by commercial insurance; however, the cost of the herbals is relatively low and many individuals did find coverage through their flexible spending accounts.
In summary, we conclude that in the setting of SIBO, patients can be given the choice of antibiotic or herbal therapy depending on their individual preference with similar response rates and safety profiles. In addition, patients who are refractory to rifaximin can receive herbal therapy as a potential rescue therapy with equivalent results to triple antibiotics. This decision is left at the discretion of the treating physician and the patient depending on the clinical setting and the patient's inclination. Future prospective studies that consolidate the antimicrobial herbal regimen to a single agent would potentially enable randomization and blinding, improve patient compliance, and diminish associated costs in this challenging gastrointestinal disorder.
Disclosures The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none was reported.
Contributor Information
Victor Chedid, University of Pittsburgh Medical Center, Department of Internal Medicine, Pittsburgh, Pennsylvania (Dr Chedid), United States..
Sameer Dhalla, The Johns Hopkins Hospital, Department of Internal Medicine, Division of Gastroenterology, Baltimore, Maryland (Dr Dhalla), United States..
John O. Clarke, The Johns Hopkins Hospital, Department of Internal Medicine, Division of Gastroenterology, Baltimore, Maryland (Dr Clarke), United States..
Bani Chander Roland, The Johns Hopkins Hospital, Department of Internal Medicine, Division of Gastroenterology, Baltimore, Maryland (Dr Roland), United States..
Kerry B. Dunbar, University of Texas Southwestern, Department of Internal Medicine, Division of Gastroenterology, Dallas, Texas (Dr Dunbar), United States..
Joyce Koh, The Johns Hopkins Hospital, Department of Internal Medicine, Division of Gastroenterology, Baltimore, Maryland (Dr Koh), United States..
Edmundo Justino, Trinity Health Center-Department of Internal Medicine-Division of Gastroenterology, Minot, North Dakota (Dr Justino)., United States..
Eric Tomakin, The Johns Hopkins Hospital, Department of Internal Medicine, Division of Gastroenterology, Baltimore, Maryland (Mr Tomakin), United States..
Gerard E. Mullin, The Johns Hopkins Hospital, Department of Internal Medicine, Division of Gastroenterology, Baltimore, Maryland (Dr Mullin), United States..
REFERENCES
- 1.Bures J, Cyrany J, Kohoutova D, et al. Small intestinal bacterial over-growth syndrome. World J Gastroenterol. 2010;16(24):2978–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7(12):1279–86 [DOI] [PubMed] [Google Scholar]
- 3.Choung RS, Ruff KC, Malhotra A, et al. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33(9):1059–67 [DOI] [PubMed] [Google Scholar]
- 4.Bohm M, Siwiec RM, Wo JM. Diagnosis and management of small intestinal bacterial overgrowth. Nutr Clin Pract. 2013;28(3):289–99 [DOI] [PubMed] [Google Scholar]
- 5.Batt RM. Exocrine pancreatic insufficiency. Vet Clin North Am Small Anim Pract. 1993;23(3):595–608 [DOI] [PubMed] [Google Scholar]
- 6.Jacobs C, Coss Adame E, Attaluri A, Valestin J, Rao SS. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther. 2013;37(11):1103–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98(4):839–43 [DOI] [PubMed] [Google Scholar]
- 8.Gabbard SL, Lacy BE, Levine GM, Crowell MD. The Impact of alcohol consumption and cholecystectomy on small intestinal bacterial over-growth. Dig Dis Sci. 2013. [DOI] [PubMed]
- 9.Macfarlane S. Microbial biofilm communities in the gastrointestinal tract. J Clin Gastroenterol. 2008;42Suppl 3 Pt 1:S142–3 [DOI] [PubMed] [Google Scholar]
- 10.Scarpellini E, Gabrielli M, Lauritano CE, et al. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25(7):781–6 [DOI] [PubMed] [Google Scholar]
- 11.Park JS, Yu JH, Lim HC, et al. [Usefulness of lactulose breath test for the prediction of small intestinal bacterial overgrowth in irritable bowel syndrome]. Korean J Gastroenterol. 2010;56(4):242–8 [DOI] [PubMed] [Google Scholar]
- 12.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98(2):412–9 [DOI] [PubMed] [Google Scholar]
- 13.Corazza GR, Menozzi MG, Strocchi A, et al. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98(2):302–9 [DOI] [PubMed] [Google Scholar]
- 14.Pimentel M, Wallace D, Hallegua D, et al. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63(4):450–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6(7):759–64 [DOI] [PubMed] [Google Scholar]
- 16.Shanab AA, Scully P, Crosbie O, et al. Small intestinal bacterial over-growth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56(5):1524–34 [DOI] [PubMed] [Google Scholar]
- 17.Weinstock LB, Fern SE, Duntley SP. Restless legs syndrome in patients with irritable bowel syndrome: response to small intestinal bacterial overgrowth therapy. Dig Dis Sci. 2008;53(5):1252–6 [DOI] [PubMed] [Google Scholar]
- 18.Shah SC, Day LW, Somsouk M, Sewell JL. Meta-analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2013;38(8):925–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadi M, McCallum RW. Rifaximin in irritable bowel syndrome: rationale, evidence and clinical use. Ther Adv Chronic Dis. 2013;4(2):71–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong SC, Hurlstone DP, Pocock CY, Walkington LA, Farquharson NR, Bramble MG, et al. The Incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol. 2005;39(2):138–41 [PubMed] [Google Scholar]
- 21.Haas L, McClain C, Varilek G. Complementary and alternative medicine and gastrointestinal diseases. Curr Opin Gastroenterol. 2000;16(2):188–96 [DOI] [PubMed] [Google Scholar]
- 22.Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004;11(11):1451–60 [DOI] [PubMed] [Google Scholar]
- 23.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95(12):3503–6 [DOI] [PubMed] [Google Scholar]
- 24.Lupascu A, Gabrielli M, Lauritano EC, et al. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22(11-12):1157–60 [DOI] [PubMed] [Google Scholar]
- 25.Aitken JD, Gewirtz AT. Gut microbiota in 2012: Toward understanding and manipulating the gut microbiota. Nat Rev Gastroenterol Hepatol. 2013;10(2):72–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6(4):295–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azad MB, Konya T, Maughan H, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013. [DOI] [PMC free article] [PubMed]
- 28.Major G, Spiller R. Irritable bowel syndrome, inflammatory bowel disease and the microbiome. Curr Opin Endocrinol Diabetes Obes. 2014;21(1):15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauritano EC, Valenza V, Sparano L, et al. Small intestinal bacterial over-growth and intestinal permeability. Scand J Gastroenterol. 2010;45(9):1131–2 [DOI] [PubMed] [Google Scholar]
- 30.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48(2):206–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riordan SM, McIver CJ, Thomas DH, Duncombe VM, Bolin TD, Thomas MC. Luminal bacteria and small-intestinal permeability. Scand J Gastroenterol. 1997;32(6):556–63 [DOI] [PubMed] [Google Scholar]
- 32.Weinstock LB, Walters AS. Restless legs syndrome is associated with irritable bowel syndrome and small intestinal bacterial overgrowth. Sleep Med. 2011;12(6):610–3 [DOI] [PubMed] [Google Scholar]
- 33.Weinstock LB, Klutke CG, Lin HC. Small intestinal bacterial overgrowth in patients with interstitial cystitis and gastrointestinal symptoms. Dig Dis Sci. 2008;53(5):1246–51 [DOI] [PubMed] [Google Scholar]
- 34.Weinstock LB, Geng B, Brandes SB. Chronic prostatitis and small intestinal bacterial overgrowth: effect of rifaximin. Can J Urol. 2011;18(4):5826–30 [PubMed] [Google Scholar]
- 35.Imajo K, Yoneda M, Ogawa Y, Wada K, Nakajima A. Microbiota and non-alcoholic steatohepatitis. Semin Immunopathol. 2013. [DOI] [PubMed]
- 36.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imajo K, Yoneda M, Ogawa Y, Wada K, Nakajima A. Microbiota and non-alcoholic steatohepatitis. Semin Immunopathol. 2014;36(1):115–32 [DOI] [PubMed] [Google Scholar]
- 38.Jouet P, Coffin B, Sabate JM. Small intestinal bacterial overgrowth in patients with morbid obesity. Dig Dis Sci. 2011;56(2):615; author reply –6 [DOI] [PubMed] [Google Scholar]
- 39.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107(1):28–35; quiz 6 [DOI] [PubMed] [Google Scholar]
- 40.Tillisch K. Complementary and alternative medicine for functional gastrointestinal disorders. Gut. 2006;55(5):593–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Dong J, Qiao Y, He J, Wang T, Ma S. Efficacy and safety profile of antibiotic prophylaxis usage in clean and clean-contaminated plastic and reconstructive surgery: a meta-analysis of randomized controlled trials. Ann Plast Surg. 2014;72(1):121–30 [DOI] [PubMed] [Google Scholar]
- 42.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156(Pt 11):3216–23 [DOI] [PubMed] [Google Scholar]
- 44.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569–75 [DOI] [PubMed] [Google Scholar]
- 45.Drossman DA, Morris CB, Schneck S, et al. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol. 2009;43(6):541–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullin GE, Pickett-Blakely O, Clarke JO. Integrative medicine in gastrointestinal disease: evaluating the evidence. Expert Rev Gastroenterol Hepatol. 2008;2(2):261–80 [DOI] [PubMed] [Google Scholar]
- 47.Magge SS, Wolf JL. Complementary and alternative medicine and mind-body therapies for treatment of irritable bowel syndrome in women. Womens Health (Lond Engl). 2013;9(6):557–67 [DOI] [PubMed] [Google Scholar]
- 48.Lee MH, Kwon HA, Kwon DY, Park H, Sohn DH, Kim YC, et al. Antibacterial activity of medicinal herb extracts against Salmonella. Int J Food Microbiol. 2006;111(3):270–5 [DOI] [PubMed] [Google Scholar]
- 49.Zhu CL, Li MY. [Inhibition of extracts from 17 Chinese herbs on periodontal pathogenic microbes]. Shanghai Kou Qiang Yi Xue. 2006;15(4):434–6 [PubMed] [Google Scholar]
- 50.Nielsen PV, Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol. 2000;60(2-3):219–29 [DOI] [PubMed] [Google Scholar]
- 51.Eftekhar F, Nariman F, Yousefzadi M, Hadiand J, Ebrahimi SN. Anti-Helicobacter pylori activity and essential oil composition of Thymus caramanicus from Iran. Nat Prod Commun. 2009;4(8):1139–42 [PubMed] [Google Scholar]
- 52.Gutierrez J, Barry-Ryan C, Bourke P. Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009;26(2):142–50 [DOI] [PubMed] [Google Scholar]
- 53.Talei GR, Meshkatalsadat MH. Antibacterial activity and chemical constitutions of essential oils of Thymus persicus and Thymus eriocalyx from west of Iran. Pak J Biol Sci. 2007;10(21):3923–6 [DOI] [PubMed] [Google Scholar]
- 54.Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10(10):813–29 [DOI] [PubMed] [Google Scholar]
- 55.Schillaci D, Napoli EM, Cusimano MG, Vitale M, Ruberto A. Origanum vulgare subsp. hirtum essential oil prevented biofilm formation and showed antibacterial activity against planktonic and sessile bacterial cells. J Food Prot. 2013;76(10):1747–52 [DOI] [PubMed] [Google Scholar]
- 56.Arcila-Lozano CC, Loarca-Pina G, Lecona-Uribe S, Gonzalez de Mejia E. [Oregano: properties, composition and biological activity]. Arch Latinoam Nutr. 2004;54(1):100–11 [PubMed] [Google Scholar]
- 57.Saeed S, Tariq P. Antibacterial activity of oregano (Origanum vulgare Linn.) against gram positive bacteria. Pak J Pharm Sci. 2009;22(4):421–4 [PubMed] [Google Scholar]
- 58.Savini I, Arnone R, Catani MV, Avigliano L. Origanum vulgare induces apoptosis in human colon cancer caco2 cells. Nutr Cancer. 2009;61(3):381–9 [DOI] [PubMed] [Google Scholar]
- 59.Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17(7):RA164–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Ye XL, Li XG, Zhen J, Zhang B, Yuan L. Synthesis and antimicrobial activity of 8-alkylberberine derivatives with a long aliphatic chain. Planta Med. 2007;73(6):602–4 [DOI] [PubMed] [Google Scholar]
- 61.Baser KH. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr Pharm Des. 2008;14(29):3106–19 [DOI] [PubMed] [Google Scholar]
- 62.Juteau F, Jerkovic I, Masotti V, et al. Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 2003;69(2):158–61 [DOI] [PubMed] [Google Scholar]
- 63.Raines T, Jones P, Moe N, Duncan R, McCall S, Ceremuga TE. Investigation of the anxiolytic effects of luteolin, a lemon balm flavonoid in the male Sprague-Dawley rat. AANA J. 2009;77(1):33–6 [PubMed] [Google Scholar]
- 64.Taiwo AE, Leite FB, Lucena GM, et al. Anxiolytic and antidepressant-like effects of Melissa officinalis (lemon balm) extract in rats: Influence of administration and gender. Indian J Pharmacol. 2012;44(2):189–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chae SH, Jeong IH, Choi DH, Oh JW, Ahn YJ. Growth-inhibiting effects of Coptis japonica root-derived isoquinoline alkaloids on human intestinal bacteria. J Agric Food Chem. 1999;47(3):934–8 [DOI] [PubMed] [Google Scholar]
- 66.Zarringhalam M, Zaringhalam J, Shadnoush M, Safaeyan F, Tekieh E. Inhibitory Effect of Black and Red Pepper and Thyme Extracts and Essential Oils on Enterohemorrhagic Escherichia coli and DNase Activity of Staphylococcus aureus. Iran J Pharm Res. 2013;12(3):363–9 [PMC free article] [PubMed] [Google Scholar]
- 67.Joshi PV, Shirkhedkar AA, Prakash K, Maheshwari VL. Antidiarrheal activity, chemical and toxicity profile of Berberis aristata. Pharm Biol. 2011;49(1):94–100 [DOI] [PubMed] [Google Scholar]
- 68.Radulovic N, Stojanovic G, Palic R. Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother Res. 2006;20(1):85–8 [DOI] [PubMed] [Google Scholar]
- 69.Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, Carvalho MD, Cunha JM, Grespan R, et al. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid Based Complement Alternat Med. 2012;2012:657026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gancevici GG, Popescu C. Natural inhibitors of complement. III. Inactivation of the complement cascade in vitro by vegetal spices (Ocimum basilicum, Artemisia dracunculus and Thymus vulgaris). Arch Roum Pathol Exp Microbiol. 1987;46(4):321–31 [PubMed] [Google Scholar]
- 71.Hammad M, Sallal AK, Darmani H. Inhibition of Streptococcus mutans adhesion to buccal epithelial cells by an aqueous extract of Thymus vulgaris. Int J Dent Hyg. 2007;5(4):232–5 [DOI] [PubMed] [Google Scholar]
- 72.Esmaeili D, Mobarez AM, Tohidpour A. Anti-helicobacter pylori activities of shoya powder and essential oils of thymus vulgaris and eucalyptus globulus. Open Microbiol J. 2012;6:65–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ali NH, Faizi S, Kazmi SU. Antibacterial activity in spices and local medicinal plants against clinical isolates of Karachi, Pakistan. Pharm Biol. 2011;49(8):833–9 [DOI] [PubMed] [Google Scholar]
- 74.Pimentel M. Review of rifaximin as treatment for SIBO and IBS. Expert Opin Investig Drugs. 2009;18(3):349–58 [DOI] [PubMed] [Google Scholar]
- 75.Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Novi M, Sottili S, et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103(8):2031–5 [DOI] [PubMed] [Google Scholar]
- 76.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(5):483–90 [DOI] [PubMed] [Google Scholar]


