Abstract
Saposins or sphingolipid activator proteins (SAPs) are small, nonenzymatic glycoproteins that are ubiquitously present in lysosomes. SAPs comprise the five molecules saposins A–D and the GM2 activator protein. Saposins are essential for sphingolipid degradation and membrane digestion. On the one hand, they bind the respective hydrolases required to catabolize sphingolipid molecules; on the other hand, saposins can interact with intralysosomal membrane structures to render lipids accessible to their degrading enzymes. Thus, saposins bridge the physicochemical gap between lipid substrate and hydrophilic hydrolases. Accordingly, defects in saposin function can lead to lysosomal lipid accumulation. In addition to their specific functions in sphingolipid metabolism, saposins have membrane-perturbing properties. At the low pH of lysosomes, saposins get protonated and exhibit a high binding affinity for anionic phospholipids. Based on their universal principle to interact with membrane bilayers, we present the immunological functions of saposins with regard to lipid antigen presentation to CD1-restricted T cells, processing of apoptotic bodies for antigen delivery and cross-priming, as well as their potential antimicrobial impact.
1. INTRODUCTION
Cells perform endocytosis to capture extracellular material and to remove used plasma membrane components. From endosomes, macromolecules can be either recycled to the plasma membrane or delivered to lysosomes for subsequent destruction. Lysosomes are acidic membrane-enclosed organelles representing the terminal degradative compartment of the endocytic pathway. They contain more than 60 different soluble hydrolytic enzymes specialized in the degradation of macromolecules. In addition, lysosomes are equipped with sphingolipid activator proteins (SAPs) that belong to the large and divergent family of saposin-like proteins (SAPLIPs). SAPLIP domains have been identified in relatively small proteins of about 80 amino acids in length, including the lung surfactant-associated protein B (SP-B), the tumorolytic proteinsNK-lysin and granulysin, cytolytic proteins from amoeba, and several plant aspartic proteases. SAPs comprise the five molecules saposins A–D and the GM2 activator protein. Saposins A–D are produced in acidic endosomal compartments upon sequential proteolytic cleavage of their single polypeptide precursor termed prosaposin (pSAP). Saposins are nonenzymatic, acidic, heat-stable, and protease-resistant molecules of about 8–11 kDa with essential functions in sphingolipid degradation and membrane digestion. The modes of action of SAPs include binding and stimulation of glycosidases required for sphingolipid degradation, as well as interaction with intralysosomal membranes to render lipids accessible to their respective degrading enzymes. Thus, saposins bridge the physicochemical gap between membrane lipid substrates and water-soluble hydrolases. Moreover, the multimolecular association of saposins, lipid bilayers, and CD1 glycoproteins facilitates the loading of CD1 molecules with lipid antigens for subsequent activation of lipid-reactive T cells. In this chapter, we present the current knowledge pertaining to the biology of saposins in lysosomal sphingolipid degradation and membrane digestion and highlight its implications for immunological processes, such as lipid antigen presentation, processing of apoptotic bodies for cross-priming, and direct antibiotic function.
2. SAPOSINS IN LYSOSOMAL GLYCOSPHINGOLIPID DEGRADATION AND MEMBRANE DIGESTION
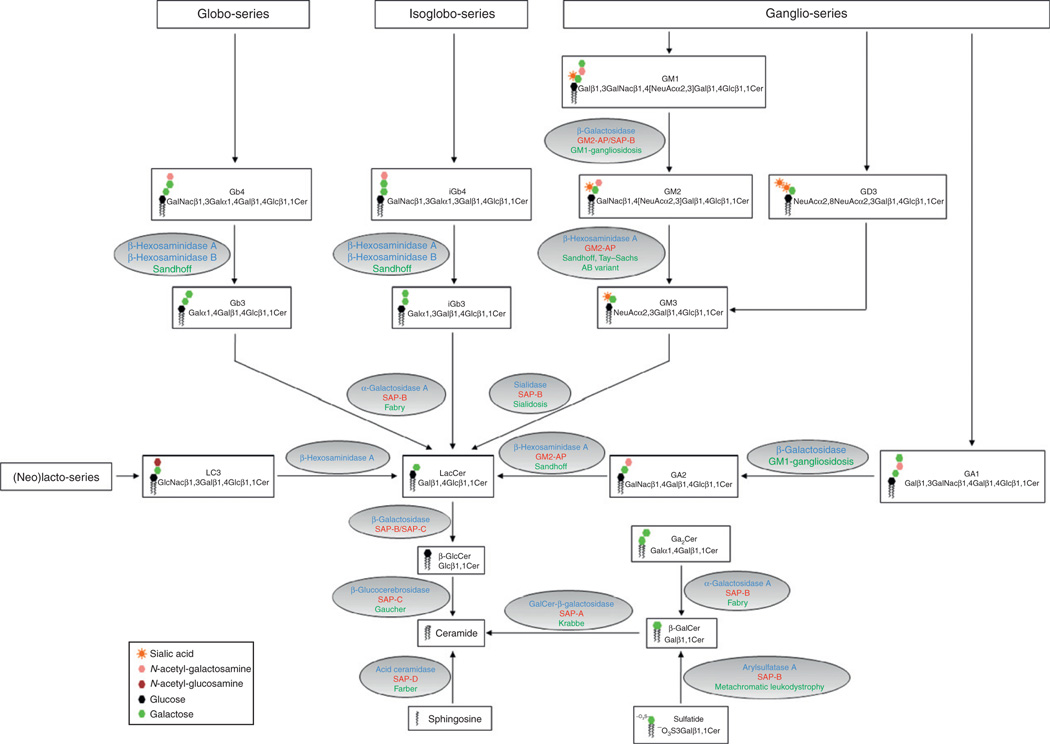
Glycosphingolipids (GSLs) are a class of lipids present in the plasma membrane of eukaryotic cells (Kolter and Sandhoff, 2005). Structurally, GSLs share a common hydrophobic ceramide moiety that acts as a membrane anchor, which is coupled to a hydrophilic oligosaccharide chain. GSLs play important roles in the structural organization of membranes and cellular interactions with microbes or toxins (Hakomori, 1981; Karlsson, 1989). GSLs are generated along the secretory pathway by the sequential, combinatorial addition of monosaccharide units, starting with the initial elongation of ceramide by glycosidic binding of either d-glucose or d-galactose in β-configuration. In particular, β-d-glucosylceramide undergoes elongation upon the stepwise action of specific glycosyltransferases to produce GSLs with complex oligosaccharide chains, such as gangliosides, globosides, and cerebrosides (Ichikawa and Hirabayashi, 1998). Alternatively, the addition of a phosphorylcholine moiety to ceramide produces sphingomyelin, a major constituent of the membrane of nerve cells and a dominant species among sphingolipids (Hooghe-Peters et al., 1979). Eventually, sphingolipids reaching the plasma membrane become a part of structural microdomains enriched in cholesterol (Simons and Ikonen, 1997). Degradation of sphingolipids is initiated upon internalization of membrane patches through diverse mechanisms including endocytosis, phagocytosis, or autophagy, and commences in acidified compartments of the endosomal route for terminal degradation in lysosomes (Luzio et al., 2007). In contrast to soluble molecules, degradation of membranes is a more delicate process since the limiting membrane of lysosomes must remain intact to avoid the leakage of potentially hazardous enzymes into the cytosol. Thus, prior to reaching lysosomes, sphingolipids are sorted to intraendosomal membranes and degraded on the surface of intralysosomal vesicles (ILVs) upon exposure to soluble glycosidases (Sandhoff and Kolter, 1996). Sphingolipid degradation proceeds in a sequential pathway that assures the stepwise removal of monosaccharide units from the nonreducing end of the oligosaccharide chain (Fig. 2.1). Ultimately, ceramide is disassembled to sphingosine and fatty acid for subsequent reuse in metabolic pathways. As sphingolipid degradation proceeds, the length of the sugar headgroup inevitably shrinks in size, thereby becoming less accessible to water-soluble glycosidases. To overcome this physicochemical obstacle and to bring sphingolipids and their respective enzymes in close proximity, mammalians possess five saposins encoded by two genes (Rorman et al., 1992). The first gene encodes pSAP, the common precursor to the four saposins A–D. The second gene encodes the GM2AP. The physiological significance of saposins in stimulating sphingolipid degradation is underscored by multiple reports of human sphingolipidoses with mutations in the pSAP gene leading to deficiencies of saposin function (O’Brien and Kishimoto, 1991). The five SAPs share a high degree of structural homology. However, SAPs show diverse ligand-binding properties and exist in multiple structural states that account for their distinct modes of action in sphingolipid degradation and membrane interaction.
Figure 2.1.
Pathways of GSL degradation. The graph depicts the degradative pathways of various GSL species, including the globo-, isoglobo-, ganglio-, and (neo)lacto-series. The names, formula, and structural icons of the respective GSLs are shown in the boxes. The ovals contain the enzymes (depicted in blue) and the saposins (shown in red) that are involved in the corresponding lipid degradation step. The associated lysosomal storage disease due to enzyme or saposin deficiency or both is indicated in green.
2.1. Prosaposin
pSAP is a 524-amino acid glycoprotein that contains a 16-residue signal peptide sequence and five glycosylation sites. Importantly, pSAP is the common precursor to the four saposins A–D (Furst et al., 1988; O’Brien et al., 1988). In humans, pSAP exists as an intracellular molecule of 68 kDa and a major extracellular form of 73 kDa. Accordingly, pSAP is intracellularly targeted to lysosomes either via mannose-6-phosphate receptors or by sortilin (Lefrancois et al., 2003). Alternatively, pSAP can be secreted and reendocytosed by mannose-6-phosphate receptors, low density lipoprotein receptor-related protein (LRP), or mannose receptors (Hiesberger et al., 1998). Expression of pSAP and individual saposins is virtually ubiquitous and conserved among mammalian species (Kishimoto et al., 1992). This is not surprising considering their important functions in sphingolipid degradation. Protein expression analyses revealed high concentrations of pSAP in the adult liver and body fluids, especially in the brain, semen, milk, serum, pancreatic juice, and bile (Kolter and Sandhoff, 2005). Further, pSAP and saposins are expressed in cells of hematopoietic origin and nerve cells (Kondoh et al., 1993; Sano et al., 1989). pSAP is sequentially processed from its N-terminal end, starting with the cleavage of the SAP-A domain (Hiraiwa et al., 1993). The mechanism through which pSAP is processed to the four saposins remains incompletely understood. Early studies suggested that pSAP proteolysis occurs at low pH and requires the action of proteases susceptible to inhibition by pepstatin A (Hiraiwa et al., 1993). One of these candidate proteases was later shown to be cathepsin D (Hiraiwa et al., 1997). In vitro, unprocessed pSAP can stimulate the degradation of sphingolipids to a similar extent like SAP-B, SAP-C, and SAP-D act on their respective enzymes (Kishimoto et al., 1992). However, considering that pSAP proteolysis occurs in acidic compartments, its contribution to lysosomal sphingolipid degradation might be of limited physiological relevance. However, pSAP has been proposed to represent a neurotrophic factor and might also be involved in the transport of gangliosides (Hiraiwa et al., 1992; O’Brien et al., 1994). Point mutations in pSAP have been identified in patients lacking all four saposins, a disease referred to as combined SAP deficiency (Harzer et al., 1989; Hulkova et al., 2001). pSAP-deficient individuals and pSAP−/− mice show similar clinicopathological features and die during the neonatal period or at the age of 3–4 weeks, respectively, due to multiple organ failure (Fujita et al., 1996). Analysis of pSAP-deficient cells reveals numerous electron-dense membrane inclusions, and pSAP−/− mice show massive accumulation of GSLs such as ceramide, β-glucosylceramide (β-GlcCer), β-galactosylceramide (β-GalCer), sulfatides, galabiaosylceramide (Ga2Cer), lactosylceramide (LacCer), globotriaosylceramide (Gb3), and the ganglioside GM3 (Bradova et al., 1993; Fujita et al., 1996).
2.2. Saposin A
SAP-A activates the hydrolysis of β-GalCer by galactosylceramide b-galactosidase (Fig. 2.1) (Morimoto et al., 1989). Accordingly, mice that carry a point mutation in the SAP-A domain of pSAP, and thus lack SAP-A expression, show tissue accumulation of β-GalCer (Matsuda et al., 2001). SAP-A deficiency forms the basis of a chronic late-onset form of globoid cell leukodystrophy that resembles the disease of patients carrying genetic deficiency in galactosylceramide β-galactosidase (Krabbe disease) (Spiegel et al., 2005). Under acidic conditions, SAP-A mobilizes lipids from liposomes in a reaction that is enhanced by bis(monoacylglycero) phosphate (BMP) or decreased by cholesterol (Locatelli-Hoops et al., 2006). The crystal structure of human SAP-A has been resolved in its closed monomeric saposin-fold conformation and consists of four amphipathic α-helices folded in the shape of an oblate ellipsoid (Ahn et al., 2006). Charged residues are located on the surface of the molecule, whereas conserved hydrophobic residues are directed toward a small cavity. Elucidation of the SAP-A structure in its open, lipid-binding conformation remains to be determined.
2.3. Saposin B
SAP-B has been the first activator protein identified (Mehl and Jatzkewitz, 1964). SAP-B is required to stimulate the breakdown of sulfatide by arylsulfatase A, Gb3 and Ga2Cer by α-galactosidase A, and LacCer by galactosylceramide β-galactosidase (Fig. 2.1). Accordingly, the latter GSLs are present in abnormally high concentrations in the urine of SAP-B-deficient patients and accumulate in the tissues of SAP-B−/− mice (Li et al., 1985; Sun et al., 2008). Further, SAP-B cooperates with GM2AP in the degradation of the ganglioside GM2 in vitro (Wilkening et al., 2000). SAP-B can be considered as a nonspecific activator protein. Accordingly, SAP-B stimulates the hydrolysis of ceramide-free glycolipids by diverse glycosidases from animals, plants, and microorganisms (Li et al., 1988). These special properties might explain as to why SAP-B stimulates the degradation of a broader spectrum of sphingolipids compared to other SAPs. Additionally, SAP-B binds and transfers the anionic phospholipid phosphatidylinositol (PI) between biological membranes (Ciaffoni et al., 2006). Inherited defects of SAP-B lead to an atypical form of metachromatic leukodystrophy (MLD) with late infantile or juvenile onset (Kretz et al., 1990; Schlote et al., 1991; Wenger et al., 1989). The crystal structure of human SAP-B displays a shell-like homodimer that consists of two V-shaped monomers (Ahn et al., 2003). The concave inner surface of each monomer is lined with hydrophobic residues that create a large lipid-binding cavity when two monomers are associated. In its open conformation, the SAP-B dimer can directly interact with lipid membranes, promote the reorganization of lipid alkyl chains, and extract lipid substrates upon transition to the closed conformation.
2.4. Saposin C
SAP-C has been primarily described by O’Brien and colleagues as an activator of β-GlcCer degradation by glucosylceramide β-glucosidase (Fig. 2.1) (Ho and O’Brien, 1971). SAP-C might also stimulate the degradation of ceramide, β-GalCer, and galactosylsphingosine (Harzer et al., 1997). Inherited deficiency of SAP-C causes a variant juvenile form of Gaucher disease (type III) with marked storage of β-GlcCer (Christomanou et al., 1989; Matsuda et al., 2004; Schnabel et al., 1991). SAP-C exhibits a characteristic dual mode of action in sphingolipid degradation. Similar to SAP-A, SAP-C stimulates its respective enzyme partner in an allosteric manner (Berent and Radin, 1981). In parallel, SAP-C can directly bind anionic phospholipids to destabilize membranes and to promote the association of β-glucosylceramidase with its lipid substrate for subsequent degradation (Vaccaro et al., 1993). At low pH, SAP-C additionally triggers the fusion of vesicles containing anionic phospholipids in vitro (Vaccaro et al., 1994, 1995; Wang et al., 2003). Using SAP-C mutants, the fusogenic activity could be mapped to the lysine-rich amino-terminal portion of the molecule, indicating that electrostatic interactions between negatively charged lipids and positively charged saposin residues might be required (Qi and Chu, 2004). Finally, evidence suggests that SAP-C functions as a neurotrophic factor by stimulating neurite outgrowth and increasing choline acetyltransferase activity in neurons (O’Brien et al., 1995). The structure of SAP-C in its closed conformation reveals a homodimer of boomerang-shaped intertwining monomers in an open, extended conformation, with solvent-exposed hydrophobic pockets (Hawkins et al., 2005; Rossmann et al., 2008). Accordingly, a “clip-on” model of vesicle fusion has been proposed. At lysosomal pH, SAP-C dimers unfold their hydrophobic pockets to interact with anionic phospholipids of membrane bilayers. Ultimately, SAP-C molecules, inserted into opposing lipid vesicles, clip one another through domain swapping, thus bringing the vesicles close enough for fusion.
2.5. Saposin D
SAP-D is the most abundant saposin in normal tissues with concentrations threefold higher than other SAPs (O’Brien and Kishimoto, 1991). SAP-D promotes the hydrolysis of ceramide by acid ceramidase in vivo (Fig. 2.1), as demonstrated by the accumulation of α-hydroxyl fatty acid ceramides in the kidneys and the cerebellum of SAP-D−/− mice (Matsuda et al., 2004). Consequently, SAP-D-deficient animals show renal tubular degeneration and hydronephrosis, as well as progressive loss of Purkinje cells in the cerebellum, leading to ataxia. To date, inherited SAP-D deficiency has not been identified in humans. Of note, the phenotype of SAPD−/− mice does not resemble human ceramidase deficiency (Farber disease) or its murine counterpart characterized by early embryonic lethality (Li et al., 2002; Matsuda et al., 2007). Similar to SAP-C, SAP-D poorly binds sphingolipids, but displays high affinity for anionic phospholipids at lysosomal pH (Tatti et al., 1999). However, SAP-C and SAP-D are functionally different. SAP-D is a membrane disrupter, whereas SAP-C fuses lipid bilayers (Ciaffoni et al., 2001). SAP-D spontaneously binds to membranes that contain anionic lipids, including BMP, PI, and phosphatidylserine (PS), in a reversible, pH-driven fashion (Ciaffoni et al., 2003). By destabilizing membranes, SAP-D allows the formation of small vesicles derived from larger liposomes in vitro (Ciaffoni et al., 2001). Multivesicular or multilamellar bodies found in the lumen of acidic cellular compartments are specifically enriched for BMP (Kobayashi et al., 1998, 2002). Therefore, it is tempting to speculate that SAP-D might regulate the homeostasis of internal endolysosomal membranes. SAP-D exists as a substrate-free closed helix bundle or in a V-shaped, open, and ligand-bound configuration in the presence of lipids. Prior to interaction with lysosomal membranes, SAP-D remains in a monomer–dimer equilibrium in the closed conformation. The acidic pH of lysosomes dramatically increases the surface hydrophobicity of SAP-D, thereby allowing the positively charged amino acids at the bottom of SAP-D to associate with the surface of intralysosomal membranes enriched in negatively charged lipids. The top of SAP-D subsequently rotates by 180° along its axis, thus positioning its hydrophobic residues into the membrane bilayer, and exposing positively charged residues to the solvent. Thereafter, SAP-D changes its configuration to the closed conformation, which forms the mechanistic basis for lipid extirpation from the bilayer. Ultimately, SAP-D leaves the membrane with bound lipid (Rossmann et al., 2008).
2.6. The GM2 activator
The GM2AP is the fifth member of the SAP family. GM2AP is larger than saposins A–D, with a molecular weight of 20 kDa in its mature lysosomal form (Furst and Sandhoff, 1992). To reach lysosomes, newly synthesized GM2AP uses the major intracellular mannose-6-phosphate-mediated trafficking route (Rigat et al., 1997). Mannose-6-phosphate receptors also allow the endosomal recapture of GM2AP from extracellular fluids upon endocytosis (Rigat et al., 1997). The saposin function of GM2AP is required to stimulate the degradation of GM2 by β-hexosaminidase A (Hex-A) in vivo (Fig. 2.1) (Conzelmann and Sandhoff, 1979). Deficiency in GM2AP leads to the AB variant of GM2 gangliosidosis, an atypical form of Tay–Sachs disease with characteristic tissue accumulation of GM2 and related GSLs in neuronal lysosomes (Conzelmann and Sandhoff, 1978). GM2AP acts as a lipid transfer protein in vitro as indicated by its capacity to extract and carry GSLs from donor to acceptor liposomes (Conzelmann et al., 1982). The structure of monomeric GM2AP consists of an eight strand, cup-shaped, antiparallel β sheet (Wright et al., 2000). The monomer contains a hydrophobic cavity with dimensions that can accommodate the ceramide portion of GM2 and other lipids, lined by surface loops and a single short helix (Wright et al., 2003). The most flexible of the loops contains the substrate-binding site and controls the entrance to the cavity to facilitate an open or a closed conformation. Accordingly, open, empty GM2AP binds to target membranes by using its hydrophobic loops and penetrates into the hydrophobic region of the bilayer. Subsequently, the lipid recognition site of the activator can interact with the substrate and insert its ceramide portion into the hydrophobic cavity. At this point, the lipid-loaded activator may change to the closed conformation, allowing the complex to leave the membrane in a soluble state. Finally, GM2AP exposes GM2 to the water-soluble enzyme Hex-A for subsequent degradation in the lysosomal lumen (Kolter and Sandhoff, 2005).
2.7. Topology
The inner leaflet of the limiting lysosomal membrane is covered with a thick glycocalix composed of glycoproteins. This layer allows the lysosome to resist the low lumenal pH, and protects the inner limiting membrane from digestion by acid hydrolases or destabilization through lipid-binding molecules. Therefore, membrane constituents targeted for lysosomal degradation have to be sorted to small lumenal vesicles formed from endosomes by the inward budding of the limiting membrane into the lumen. Vesicle-rich endosomes are referred to as multivesicular endosomes or multivesicular bodies (MVBs) (Piper and Luzio, 2001). This topological transition exposes macromolecules originating from the outer leaflet of the plasma membrane to the endosomal lumen. Hence, the subsequent fusion of an MVB with a lysosome renders macromolecules accessible to hydrolases. Following this principle, intraendosomal vesicles serve as important devices in the delivery of used plasma membrane proteins and lipids to lysosomes (Futter et al., 1996; Sandhoff and Kolter, 1996). Recently, the biogenesis and function of MVBs and ILVs has become a major focus for cell biologists, especially since the discovery of endosomal sorting complexes required for transport (ESCRT) (Luzio et al., 2007). Yet, the sequence of events leading to their formation remains poorly understood. Three lipids play critical roles in the biogenesis and function of MVBs and ILVs. Firstly, phosphatidylinositol-3-phosphate is enriched on the cytosolic face of endosomes, and evidence suggests that it might be required for the formation of MVBs (Futter et al., 2001; Odorizzi et al., 1998). Secondly, cholesterol is highly enriched on intraendosomal vesicles, where it stabilizes the membrane (Hornick et al., 1985). By contrast, cholesterol is almost absent from ILVs as it is removed from lysosomes by the Niemann–Pick disease protein C1 (NPC1) and NPC2 (Friedland et al., 2003; Mobius et al., 2003). Considering the negative impact of cholesterol on SAP-A- and SAP-B-mediated lipid mobilization from liposomes, physiological depletion of cholesterol from ILVs might favor lipid mobilization by SAPs (Locatelli-Hoops et al., 2006; Remmel et al., 2007). Thirdly, the anionic lipid BMP plays a crucial role in the topology of ILVs and is derived from mitochondrial cardiolipin during the process of autophagy, or BMP is produced from phosphatidylglycerol (PG) in the endoplasmic reticulum (ER) (Hullin-Matsuda et al., 2009). In contrast to cholesterol, BMP is specifically enriched on ILVs, where it can account for up to 70% of the total membrane phospholipid content (Kobayashi et al., 1998, 2002). BMP functions in the formation of ILVs since BMP-containing liposomes can spontaneously form inward-budding profiles in a pH-inducible manner (Matsuo et al., 2004). Remarkably, the presence of BMP in liposomes strongly enhances the stimulatory capacity of all five SAPs (Chu et al., 2005; Ciaffoni et al., 2003; Locatelli-Hoops et al., 2006; Remmel et al., 2007; Wilkening et al., 2000). Taken together, these findings support a model in which the gradual depletion of cholesterol and subsequent integration of BMP into ILVs renders them more accessible for SAPs. Moreover, BMP enhances SAP-stimulated degradation of GSLs by specific glycosidases. Notably, the membrane-perturbing properties of SAP-C potentially mediate the fusion of multiple ILVs in order to promote their subsequent disruption by SAP-D.
3. SAPOSINS FACILITATE LIPID PRESENTATION TO CD1-RESTRICTED T LYMPHOCYTES
The majority of studies on antigen presentation have concentrated on molecules encoded by the major histocompatibility complex (MHC). However, over the past two decades, it has become evident that other molecules can also trigger T cell responses, including the family of lipid-presenting CD1 molecules. In addition to proteins, lipid antigens extend the spectrum of determinants that are potentially recognized by the immune system, and thus amplify the diversity of immune responses to fight intruders, for example, in the context of infection of the host with lipid-rich pathogens. In contrast to protein processing to peptides, cellular lipid acquisition challenges the host due to the physicochemical properties of fats. Accordingly, hydrophobic lipid antigens have to be extracted from aggregates or membranes and solubilized for subsequent transport. Subsequently, lipids are loaded onto specific antigen-presenting molecules such as CD1 proteins. For both steps, helper molecules like saposins are required (Fig. 2.2). On the other hand, some lipids have to be further subjected to structural editing, or processing, to reveal antigenic epitopes otherwise unavailable for recognition by T cells. In the following, we highlight the predominant roles played by saposins and lysosomal glycosidases in both processes.
Figure 2.2.
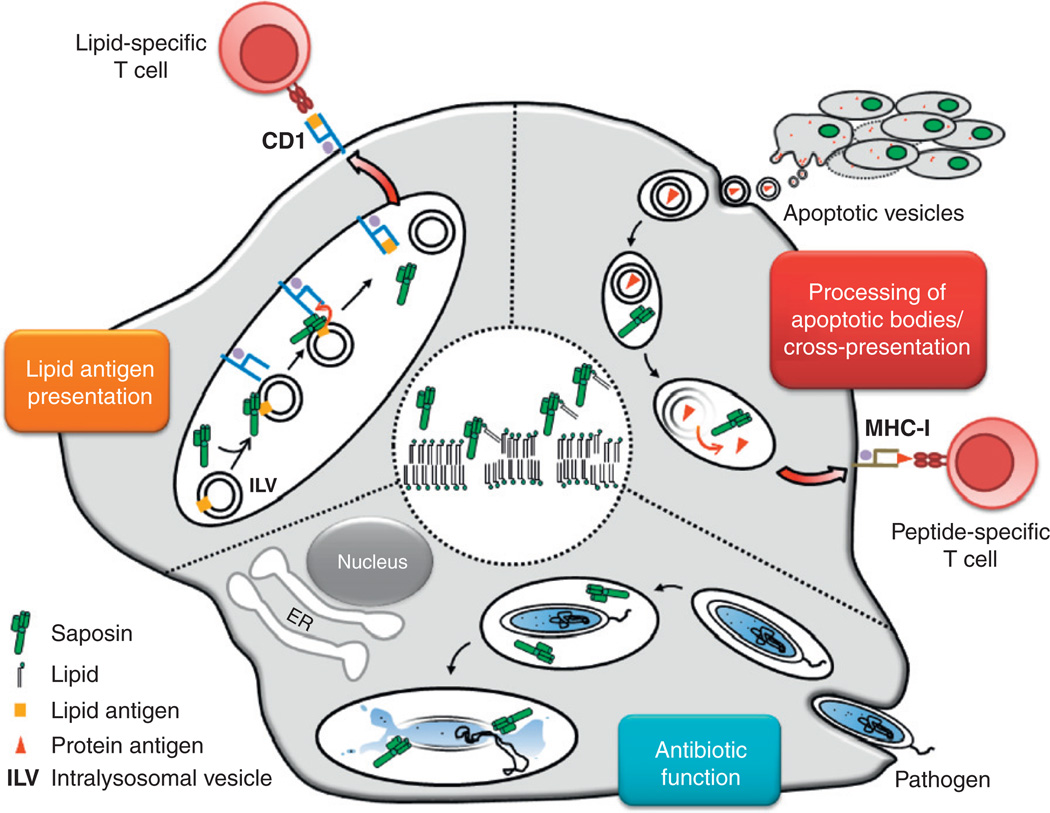
Immunological functions of saposins. The central circle demonstrates the universal mechanism of saposin action on lipid bilayers. Accordingly, saposin molecules insert into lysosomal membranes to induce bilayer disintegration and lipid extraction. Further, the graph represents the three immunological consequences of this mechanism. (1) Lipid antigen presentation: In lysosomes of APCs, saposins interact with intralysosomal vesicles (ILVs) that contain lipid antigens. Subsequently, SAPs facilitate the loading of CD1 molecules with lipid antigens to activate CD1-restricted lipid-reactive T cells. (2) Processing of apoptotic bodies / cross-presentation: Apoptotic bodies derived from tumor or infected cells are engulfed by phagocytes such as DCs or macrophages. Protein antigens contained in apoptotic vesicles are liberated in lysosomes upon membrane disintegration induced by saposins. This leads to antigen delivery from apoptotic bodies for subsequent cross-presentation of antigens to MHC-I-restricted CD8+ T cells. (3) Antibiotic function: Pathogens like intracellular bacteria are phagocytosed by macrophages or DCs. At the acidic pH of lysosomes, saposins unfold their antimicrobial effects through direct attack on the membranes of pathogens.
3.1. Characteristics of antigen-presenting CD1 molecules
The organization of the CD1 complex is similar to MHC class I. Each CD1 gene contains three exons encoding separate extracellular domains (α1, α2, α3) that noncovalently associate with β2-microglobulin (β2m) to form a stable transmembrane heterodimer of approximately 50 kDa (Brigl and Brenner, 2004). Five separate genes (CD1A, CD1B, CD1C, CD1D, and CD1E) are mapping to chromosome 3 in humans. In mice, CD1 maps to chromosome 1 and consists of two genes (CD1D1 and CD1D2). On the basis of sequence homology, CD1 proteins fall into one of two groups: group 1 CD1 molecules (CD1a, CD1b, and CD1c) and group 2 CD1 molecules (CD1d), with CD1e representing an intermediate. While CD1a-d proteins are mainly expressed on the surface of antigen-presenting cells (APCs), CD1e is exclusively found as a soluble molecule in lysosomes, and therefore cannot present antigens to CD1-restricted T cells (Angenieux et al., 2005). The subdivision of CD1 molecules correlates with functional differences. Accordingly, group 1 molecules present lipid antigens to T lymphocytes, while group 2 molecules display antigens to Natural Killer T (NKT) cells. Structurally, CD1 molecules contain a main hydrophobic groove that can be prolonged by further pockets (Moody et al., 2005). Group 1 CD1 molecules are mainly expressed by cortical thymocytes and myeloid cells, such as dendritic cells (DCs) and Langerhans cells (Dougan et al., 2007a). In addition, CD1c proteins are also expressed by marginal zone B cells and a fraction of peripheral blood B lymphocytes (Delia et al., 1988). In humans, group 2 CD1d molecules are found on cells of the myeloid lineage as well as on few nonlymphoid cells. However, cortical thymocytes and mantle zone B cells in lymph nodes exhibit the highest levels of CD1d expression (Exley et al., 2000). In mice, CD1d expression depends on the CD1D1 gene. CD1d molecules are mainly expressed by APCs, including hepatic stellate cells (Winau et al., 2007). In addition, cortical thymocytes as well as some activated T cells express CD1d proteins (Dougan et al., 2007a). Expression of CD1d on the surface of APCs is enhanced by proinflammatory cytokines, such as interferon-β (IFN-β), IFN-γ, and TNF-α, as well as by toll-like receptor (TLR)-2 and TLR-4 ligands (Skold et al., 2005). The peroxisome proliferator-activated receptor-γ (PPAR-γ) controls CD1d expression by triggering retinoic acid synthesis in human DCs (Szatmari et al., 2006). Furthermore, a number of pathogens modulate the extent of CD1d expression. Viruses such as Kaposi’s sarcoma-associated herpes virus (KSHV) and HIV downregulate the expression of CD1d on the plasma membrane (Andre et al., 2005; Sanchez et al., 2005). Finally, herpes simplex virus-1 (HSV-1) and vesicular stomatitis virus (VSV) can cause downmodulation of CD1d by suppressing CD1d recycling (Raftery et al., 2006; Yuan et al., 2006).
3.2. Intracellular trafficking of CD1 molecules
In analogy to MHC-I molecules, CD1 heavy chains are folded and associated with β2m within the lumen of the ER, in a process that involves calnexin and calreticulin, but excludes transporters associated with antigen processing (TAP) (Kang and Cresswell, 2002; Sugita et al., 2007). At this stage, the pocket formed by the α1 and α2 helices of the heavy chain can be loaded with hydrophobic spacers such as neutral phospholipids to stabilize the nascent CD1 molecule (Gadola et al., 2002). Accordingly, ER-resident lipid exchange proteins such as the microsomal triglyceride transfer protein (MTP) could fulfill this loading function (Dougan et al., 2005, 2007b). From the ER, CD1 molecules transit along the secretory pathway until they reach the plasma membrane, where they follow a major pathway of internalization subdivided into distinct intracellular trafficking routes. The type of endocytic compartment through which CD1 molecules transit largely depends on the presence of the amino acid sequence Y-X-X-Z (tyrosine-X-X-bulky hydrophobic residue) in the cytoplasmic moiety of the heavy chain (Chiu et al., 2002). At the plasma membrane, this motif mediates recruitment of the adaptor molecule AP-2 by CD1b, CD1c, and CD1d molecules, and promotes their subsequent internalization through clathrin-coated pits (Sever, 2003). Following their traffic to endosomes, CD1b and CD1d molecules are further sorted to late acidic endocytic compartments by the recruitment of AP-3 through the same cytoplasmic motif (Sugita et al., 2002). CD1b is almost exclusively located in lysosomes, whereas CD1c that does not bind AP-3 shows a broad localization throughout the endosomal pathway (Sugita et al., 2007). Notably, there is no evidence for targeting motifs in the cytoplasmic tail of CD1a proteins. Nevertheless, they are found in recycling endosomes and traffic back to the plasma membrane in an ARF6-dependent manner (Sugita et al., 1999). Finally, CD1e displays unique features in comparison to other CD1 molecules. Upon assembly, CD1e molecules are targeted to lysosomes without reaching the plasma membrane, where they exist in a cleaved soluble form and participate in the processing of microbial glycolipid antigens (de la Salle et al., 2005). Ubiquitination of the cytoplasmic tail is a prerequisite for CD1e proteins to target lysosomes (Maitre et al., 2008). In the endocytic system, CD1 molecules exchange lipids acquired in the secretory pathway with self or foreign lipids, and, except for CD1e, traffic to the plasma membrane where they display their lipid cargo to CD1-restricted T cells. Hence, the divergent trafficking routes of CD1 molecules may reflect an evolutionary adaptation to face the diversity of intracellular pathogen lifestyles through detection by the immune system.
3.3. CD1-restricted T cells
Michael Brenner and colleagues provided the first evidence that human CD1 molecules present lipid antigens to induce T cell responses (Beckman et al., 1994). The cognate antigen presented in the context of CD1b proved to be mycolic acid, a lipid contained in the cell wall of Mycobacterium tuberculosis. Intriguingly, T lymphocytes restricted by group 1 CD1 molecules demonstrate favored reactivity with lipid antigens originating from the cell wall of mycobacteria. M. tuberculosis is the etiologic agent of tuberculosis, one of the most ancient and life-threatening infectious diseases worldwide (Kaufmann, 2006). Since humans represent the principal reservoir of M. tuberculosis, it is tempting to speculate that group 1 CD1-restricted T cells might have developed as a result of coevolution of host and pathogen to provide specific protection against M. tuberculosis infection. Both hydrophobic peptides and lipids or glycolipids from mycobacteria can be presented by CD1 molecules. Accordingly, didehydroxymycobactin (DDM), a lipopeptide structurally related to siderophores, is presented to T cells in the context of CD1a (Moody et al., 2004). Mannosylated phosphatidylinositides (PIMs), including lipoarabinomannan (LAM), and diacylated sulfoglycolipids (Ac2SGLs) activate T cells when presented through CD1b (Gilleron et al., 2004; Sieling et al., 1995). Further, glucose monomycolate (GMM), produced upon interaction of biosynthetic pathways of host and pathogen, activates T cells restricted by CD1b (Moody et al., 2000a). Finally, hexosyl-1-phosphoisoprenoids stimulate CD1c-dependent T cells (Beckman et al., 1996; Moody et al., 2000b). Lymphocytes restricted by group 1 CD1 molecules are found in all the major phenotypic subsets of T cells, including single-positive CD4+ and CD8+ T cells, as well as double-negative (DN) CD4− CD8− T lymphocytes (Porcelli et al., 1992; Rosat et al., 1999; Sieling et al., 2000). Upon activation, CD1-restricted T cell clones develop TH1 effector functions dominated by the production of IFN-γ and TNF-α (Rosat et al., 1999; Sieling et al., 1999). In addition, DN and CD8+ CD1-restricted T cells exert potent cytotoxic functions toward M. tuberculosis-infected macrophages through Fas–Fas ligand interactions or the release of granulysin, respectively (Stenger et al., 1997). Using these mechanisms, CD1b-restricted T cells effectively kill M. tuberculosis-infected macrophages in a CD1b-dependent manner (Stenger et al., 1997). A feature shared by all CD1-restricted T cells is the basal recognition of CD1 molecules in the absence of foreign lipids (Porcelli et al., 1989). Autoreactive responses by T cells can stimulate the maturation of DCs toward a proinflammatory phenotype, which may play a critical role with regard to the ensuing generation of adaptive immune responses (Spada et al., 2000; Vincent et al., 2002). Recognition of sulfatide or GM1 by CD1-restricted T cells could form the basis of this autoreactivity (Shamshiev et al., 2000, 2002). In contrast to CD1b molecules, CD1a and CD1c are largely excluded from acidic subcellular compartments, and their antigen-presenting functions are not affected upon inhibition of endosomal acidification (Briken et al., 2000; Porcelli et al., 1992; Sieling et al., 1995). Optimal binding of PIMs, GMM, and LAM require an acidic environment in which the α-helices of CD1b can partially unfold (Ernst et al., 1998). Although these findings highlight an interesting mechanism that allows access of lipid antigens to the CD1b groove only upon their trafficking to the proper compartment, they cannot account for a model in which CD1b itself extracts lipids from membranes and thus, chaperoning helper molecules have to fill this gap. In this context, SAP-C has been identified as the critical saposin required for recognition of microbial lipid antigens by CD1b-restricted T cells (Winau et al., 2004b). Accordingly, human pSAP-deficient fibroblasts expressing CD1b failed to present mycolic acid, GMM, and LAM for activation of antigen-specific CD1b-restricted T cell clones. Moreover, T cell responses could be restored upon fibroblast reconstitution with SAP-C but not other SAPs (Winau et al., 2004b). The underlying mechanism involved SAP-C-mediated extraction of LAM from membranes and subsequent transfer to CD1b (Fig. 2.2), as indicated by coprecipitation experiments identifying a direct interaction between SAP-C and CD1b (Winau et al., 2004b). These findings demonstrated saposins as a missing link in antigen presentation of lipids to group 1 CD1-restricted T cells, and suggest that SAP-C dysfunctions potentially have adverse consequences concerning T cell immunity in infectious diseases like tuberculosis.
3.4. CD1d-restricted natural killer T cells
In contrast to humans, mice lack genes encoding group 1 CD1 molecules. Therefore, their repertoire of lipid-specific T cells is solely represented by lymphocytes restricted to CD1d molecules, namely NKT cells. Unique features of NKT cells include usage of an invariantly rearranged T cell receptor (TCR) a chain (Vα14-Jα18 in mice, Vα24-Jα18 in humans) paired with a limited set of TCR βchains, expression of diverse surface receptors characteristic for NK cells, and functional autoreactivity toward CD1d-expressing APCs in vitro (Bendelac et al., 1995; Budd et al., 1987; Dellabona et al., 1994; Fowlkes et al., 1987; Porcelli et al., 1993). Further, NKT cells express intermediate levels of TCR at the cell surface and a phenotype of activated/memory T cells in naive and germ-free mice, as well as in human cord blood, which may reflect the consequence of continuous basal TCR stimulation with self antigens (D’Andrea et al., 2000; Park et al., 2000; van Der Vliet et al., 2000). NKT cells are a heterogeneous population and dominated by a subset that reacts with the marine sponge-derived GSL antigen α-galactosylceramide (α-GalCer) presented by CD1d proteins (Chen et al., 1997; Kawano et al., 1997). By definition, NKT cells that respond to α-GalCer are referred to as invariant NKT (iNKT) cells. iNKT cells show exclusive usage of the TCR rearrangement Vα14-Jα18 coupled to Vβ8, Vβ7, or Vβ2 in mice, or the rearrangement Vα24-Jα18 associated with Vβ11 in humans, and promptly produce IFN-γ and interleukin-4 (IL-4) upon activation (Bendelac et al., 2007). By contrast, NKT cells that fail to respond to α-GalCer stimulation are referred to as noninvariant NKT cells, or type II NKT cells. Notably, type II NKT cells use diverse TCRs, and owing to the lack of specific markers to track them, their biology remains poorly understood.
3.4.1. Invariant NKT cells
The relevance of iNKT cells to diseases such as cancer, infection, or autoimmunity, has been extensively reviewed elsewhere (Bendelac et al., 2007; Godfrey et al., 2004). One of the most exciting features in the biology of iNKT cells pertains to the modes of their activation. In the face of infection, it has become evident that the host can use several pathways to activate iNKT cells. Firstly, activation may result from direct, TCR-mediated recognition of microbial lipid antigens presented in the context of CD1d-expressing APCs. Known antigens include α-glucuronosylceramides and α-galacturonosylceramides from Sphingomonas spp., diacylglycerols from Borrelia burgdorferi, the causative agent of Lyme disease, and phosphatidylinositol tetramannoside from M. tuberculosis (Fischer et al., 2004; Kinjo et al., 2005, 2006; Mattner et al., 2005; Sriram et al., 2005). Following a second pathway predominantly triggered by infection with Gram-negative lipopolysaccharide (LPS)-positive bacteria, such as Salmonella typhimurium, iNKT cells become activated upon recognition of self antigens presented by LPS-exposed DCs in an IL-12-dependent manner (Brigl et al., 2003). In addition to TLR-4-mediated activation of iNKT cells triggered by LPS, several other axes of DC sensitization through TLR ligation have been identified (De Libero et al., 2005). Accordingly, stimulation of DCs through the nucleic acid sensor TLR-9 results in the subsequent activation of iNKT cells (Paget et al., 2007). In the latter case, neosynthesized β-linked self GSLs and type I interferons provided by DCs were strictly required. Accordingly, stimulation of DCs through TLR-4, TLR-7, or TLR-9, could influence the expression of various glycosyltransferases involved in the biogenesis of GSLs (Paget et al., 2007; Salio et al., 2007). Importantly, blocking the de novo generation of GSLs abrogated the responses by iNKT cells. Finally, increased expression of CD1d/GSL complexes, representing ligands for the iNKT cell invariant TCR, could be visualized at the surface of APCs stimulated with LPS or a TLR-8 agonist (Salio et al., 2007). Thus, microbe-exposed APCs remodel the repertoire of self GSLs to produce dominant species that can be recognized by iNKT cells. How signals relayed through pattern recognition receptors can lead to selective induction of GSL antigens without compromising cellular lipid homeostasis remains to be clarified. Lastly, several recent studies indicate that iNKT cells could become activated in a pure cytokine-driven fashion without requirement for TCR tickling by CD1d–self-lipid complexes (Montoya et al., 2006; Nagarajan and Kronenberg, 2007). Accordingly, MCMV-infected DCs could activate iNKT cells in an IL-12-dependent, but CD1d-independent manner (Tyznik et al., 2008). In analogy to human CD1b, murine CD1d molecules primarily localize to lysosome-associated membrane protein 1 (LAMP-1)-positive organelles, indicating a trafficking route that includes late acidic compartments for acquisition of self lipids (Chiu et al., 2002). Of note, deletion of the AP-3-binding motif in the cytoplasmic tail of CD1d (CD1-TD) depletes the molecule from late endosomal and lysosomal compartments, while surface expression of the mutant molecule is slightly increased (Chiu et al., 1999). This tail modification leads to severe functional consequences specifically affecting iNKT cells. Accordingly, CD1-TD-expressing APCs fail to present self lipids and exogenous α-GalCer to iNKT cell hybridoma, and CD1-TD knock-in mice show impaired production of iNKT cells in the thymus, which results in profound defects of iNKT cells in peripheral organs (Chiu et al., 2002). Taken together, these findings identified late endocytic compartments as primary sites where CD1d molecules acquire self lipids or exchange self lipids with foreign antigens. Further, the endosomal route proved to be essential in the generation of CD1d–self-lipid complexes that are recognized by thymocytes, which ultimately facilitates iNKT cell development. Finally, these findings also suggested that lipid exchanges between CD1d and membranes might require helper molecules located in late endosomal and lysosomal compartments. First evidence supporting this hypothesis derived from studies performed with pSAP−/− mice, which are devoid of all four SAPs and selectively lack iNKT cells (Zhou et al., 2004a). In vitro, DCs and thymocytes from pSAP−/− mice failed to stimulate iNKT cell hybridoma, but showed intact functions in the presentation of self lipids to noninvariant NKT cell hybridoma, as well as a normal capacity to process exogenous proteins for activation of various antigen-specific MHC-IIrestricted T cells. In cell-free assays, recombinant SAP-A and SAP-C showed the highest efficiency in the exchange of trisialoganglioside GT1 loaded onto CD1d molecules with PS or sulfatide contained in liposomes, using an experimental pH that corresponded to lysosomes. However, SAPs alone could not extract GT1 bound to CD1d. By contrast, GM2AP extracted the ganglioside from CD1d but did not replace it (Zhou et al., 2004a). Using murine pSAP-deficient cell lines transduced with human CD1d as APCs, another study concluded that recognition of α-GalCer by a human iNKT cell line could be enhanced by reintroduction of human pSAP. However, pSAP did not increase autoreactive responses by the iNKT cell line (Kang and Cresswell, 2004). Finally, reintroduction of mutant pSAP constructs, each lacking one of the four saposins, revealed that SAP-B-expressing APCs most efficiently enhanced α-GalCer presentation to NKT cells (Yuan et al., 2007). However, no individual SAP proved to be absolutely essential in that process. In humans and mice, SAP-B seems to play a dominant role in the exchange of CD1d-bound self lipids acquired in the secretory pathway with self or foreign lipids present in lysosomes.
3.4.2. Noninvariant NKT cells
Similar to iNKT cells, type II NKT cells show reactivity to CD1d molecules expressed by APCs. In contrast to iNKT cells, type II NKT cells recognize CD1d-bound lipids that are loaded along the secretory pathway (Chiu et al., 1999). The myelin-derived self GSL sulfatide, which previously has been identified as a self antigen comparably presented by CD1a, CD1b, and CD1c molecules, is specifically recognized by a subset of type II NKT cells (Jahng et al., 2004; Shamshiev et al., 2002). Identification of this subpopulation using sulfatide-loaded CD1d tetramers revealed its specific enrichment in the central nervous system during experimental autoimmune encephalomyelitis (EAE). Interestingly, sulfatide treatment prevented antigen-induced EAE in wild type, but not in CD1d−/− mice. The underlying mechanism involved sulfatide-reactive type II NKT cells that prevented the production of IFN-γ and IL-4 by pathogenic myelin oligodendrocyte glycoprotein (MOG)-reactive T cells (Jahng et al., 2004). More recent studies explored the requirements for type II NKT cell hybridoma recognition of sulfogalactosylsphingosine (lysosulfatide), a sulfatide derivative lacking the fatty acid constituent (Roy et al., 2008). In this context and according to findings that type II NKT cells specialize in the recognition of antigens acquired along the secretory pathway, pSAP deficiency had no impact on the recognition of lysosulfatide by type II NKT cell hybridoma. However, at acidic pH, SAP-C enhanced the recognition of plate-bound CD1d molecules loaded with lysosulfatide by noninvariant NKT cells (Roy et al., 2008).
4. SAPS STIMULATE THE PROCESSING OF LIPID ANTIGENS BY LYSOSOMAL GLYCOSIDASES
The lysosomal system is of considerable biomedical importance since its alterations are associated with numerous human diseases. To date, more than 50 monogenic human diseases that are primarily associated with lysosomal dysfunction have been identified, and the majority of these conditions are classified as lysosomal storage disorders (LSDs). LSDs are caused by deficiencies in membrane proteins that transport degradation products out of the lysosome, or they are due to defects in molecules involved in the processing or trafficking of lysosomal proteins and GSLs (Fig. 2.1). Pathways of GSL production and degradation have recently attracted the attention of lipid immunologists since analyses of mouse models of human LSDs have uncovered unexpected antigen-processing defects with major impact on CD1-restricted T cell responses.
4.1. Hexosaminidase B
The mouse model of Sandhoff disease that lacks the β-subunit of hexosaminidase A and hexosaminidase B (Hexb−/− mice) has been instrumental in the elucidation of an endogenous antigen recognized by iNKT cells, namely, the GSL isoglobotriaosylceramide (iGb3) (Zhou et al., 2004b). In vivo analyses revealed a specific lack of iNKT cells in Hexb−/− mice, which suggested that functions of hexosaminidases in the catabolism of GSLs are required for the production of iNKT cell agonists in the thymus. In line with this postulate, APCs from Hexb−/− mice expressing CD1d molecules failed to stimulate autoreactive responses by iNKT cell hybridoma, whereas responses to α-GalCer were preserved. By testing the potential antigenicity of several GSL species belonging to the globo-, isoglobo-, and neolacto-series, which are produced in lysosomes upon the action of hexosaminidases (Fig. 2.1), only iGb3 could stimulate murine and human iNKT cells (Zhou et al., 2004b). Hence, by removing the terminal N-acetyl-β-d-galactosamine residue from iGb4, Hex-B produces iGb3 that is recognized by iNKT cells. Possibly due to missing self on CD1d molecules, either by lack of generation (Hexb−/−) or by deficient CD1d loading (pSAP−/−) of endogenous antigens, both knock-out strains fail to develop iNKT cells. Therefore, potential iGb3–SAP–CD1d interactions have been proposed. Accordingly, SAP-B could exchange CD1dbound GT1 with free iGb3 or iGb4 (Zhou et al., 2004b). However, while immunological and novel biochemical evidence points toward iGb3 as the natural self antigen, its physiological role remains vividly challenged by several studies in humans and mice (Christiansen et al., 2008; Gadola et al., 2006; Li et al., 2009; Porubsky et al., 2007; Speak et al., 2007). Recently, SAP-B−/− mice were described to accumulate Gb3 in various tissues, which is in agreement with previous findings that SAP-B is required to activate the degradation of Gb3 by 903B1;-galactosidase A (Sun et al., 2008). Since globosides and isoglobosides use the same degradation pathway, SAP-B−/− mice could provide an interesting model to test the possible accumulation of iGb3 or other potential endogenous antigens for iNKT cells.
4.2. α-Galactosidase A
First evidence uncovering the antigen-processing component of α-galactosidase A (α-Gal-A) derived from a study in which APCs from a-Gal-A−/− mice (Fabry disease) failed to present the synthetic disaccharide antigen Gala(1→2) α-GalCer to iNKT cell hybridoma (Prigozy et al., 2001). Accordingly, removal of the terminal galactose residue by lysosomal α-Gal-Awas required to expose the α-GalCer epitope to the invariant TCR. In the catabolism of globosides, α-Gal-A functions downstream of Hex-B to produce LacCer from Gb3 (Fig. 2.1). In addition, α-Gal-A degrades the galactolipid Ga2Cer to β-GalCer (Ohshima et al., 1997). Interestingly, α-Gal-A shows a broader substrate specificity than previously expected, since it can also remove terminal galactose residues bound in the a(1→3) configuration. Therefore, iGb3 that contains a terminal a(1→3)-branched galactose could represent a physiological substrate for α-Gal-A. Consequently, enzyme deficiency in α-Gal-A activity could lead to iGb3 accumulation. Unexpectedly, α-Gal-A−/− mice demonstrated a lack of iNKT cells (Prigozy et al., 2001; Zhou et al., 2004b). In contrast to Hexb−/− mice, this deficit was only partial and specific for iNKT cells located in peripheral organs. Since Fabry and Sandhoff diseases have different etiologies and display diverse patterns of GSL storage, a generalized defect of iNKT cell selection has been proposed in LSD mice. Analyses of knock-out mouse models of Tay–Sachs disease in which hexosaminidase A is lacking (HexA−/− mice), Sandhoff disease (Hexb−/− mice), GM1 gangliosidosis (β-Gal−/− mice), and mice deficient for the endosomal transmembrane protein NPC1 involved in cholesterol homeostasis, revealed reduced frequencies as well as functional defects of iNKT cells (Gadola et al., 2006; Schumann et al., 2007). The hypothesis has been proposed that the degree of iNKT cell deficiency in each mouse model could be related to the extent of lipid stored, irrespective of specific lipid entities. Therefore, lipid storage itself could exert a nonspecific negative impact on the selection of thymic iNKT cell precursors. Upon immunological analysis of α-Gal-A−/− mice, we found specific loss of peripheral iNKT cells in accordance with previous reports (Zhou et al., 2004b). These defects were the direct consequence of iNKT cells chronically exposed to self GSLs (unpublished observations). Moreover, DCs from a-Gal-A−/− mice inducedCD1d-dependent production of IFN-γ and IL-4 by iNKT cells in the absence of exogenous antigen. Additionally, wild-type DCs treated with an inhibitor of α-Gal-A elicited NKT cell activation. Further, reconstitution of α-Gal-A-deficient DCs with recombinant enzyme, or iGb3 blocking in Fabry DCs, abrogated iNKT cell responses. In a more recent study analyzing iNKT cells in diverse animal models of LSDs, in which GSLs, glycosaminoglycans, or both accumulate, defective iNKT cell development could only be observed in mice affected by combined deficiency in sulfatase activity. However, these defects were generalized to other T cell subsets. By contrast, mice with single lysosomal enzyme deficiencies showed normal iNKT cell development (Plati et al., 2009). In conclusion, constitutive or induced deficiency in α-Gal-A activity leads to accumulation of endogenous self antigens such as iGb3 for subsequent activation of iNKT cells.
4.3. α-Mannosidase
A decisive function of α-mannosidase in the processing of carbohydrate antigens has been clarified in the human system. Accordingly, DCs from a patient with congenital deficiency in α-mannosidase failed to present mycobacterial hexamannosylated phosphatidyl-myo-inositides (PIM6) to a CD1b-restricted T cell line (de la Salle et al., 2005). In detail, the enzyme is required for the stepwise degradation of PIM6 to PIM species that contain fewer mannose residues, including the stimulating antigen PIM2. Importantly, the generation of stimulating PIM2 species required assistance by soluble CD1e molecules (de la Salle et al., 2005). In contrast to SAPs, which are ubiquitously expressed and primarily required in the catabolism of GSLs, CD1e is mainly expressed in immune cells, and could therefore specifically act as an immunological lipid transfer protein (Angenieux et al., 2005). In analogy to saposins, whether CD1e uses a “solubilizer” or “liftase” mode of action remains to be clarified.
5. SAPOSINS DISRUPT VESICLES RELEASED BY APOPTOTIC CELLS
5.1. Implications for apoptosis
Upon hypoxia, trauma, or the effect of noxious substances, cells die by necrosis, which involves depletion of the intracellular ATP stores associated with cell swelling and rupture of cellular organelles (Winau et al., 2005). Ultimately, necrotic cells burst and release their organellar and cytosolic content into the surrounding tissue, which subsequently causes inflammation. In sharp contrast to necrosis, apoptosis represents a regulated form of cell death that prevents inflammatory responses under physiological conditions (Winau et al., 2005). Accordingly, apoptotic cells shrink, condense their DNA and organelles prior to fragmentation, release membrane blebs, and finally disintegrate into apoptotic bodies. Thus, apoptosis avoids cell leakage and secondary harmful inflammation, and represents a “silent” way of death that cells undergo during development and tissue homeostasis (Ravichandran and Lorenz, 2007). However, the silencing feature of apoptosis can be overridden in the context of infection or cancer, when apoptotic bodies become vehicles for antigens and tumor- or pathogen-associated molecular patterns that trigger immune responses (Winau et al., 2004a). A hallmark of apoptosis is the exposure of PS, which is normally confined to the inner leaflet of the cytoplasmic membrane in living cells, on the surface of apoptotic cells (Savill et al., 2002). Further, apoptotic cells release high amounts of chemoattractant nucleotides and lysophosphatidylcholine (LPC), which subsequently recruit phagocytes (Elliott et al., 2009). Capture and subsequent internalization of apoptotic bodies involves specific recognition of externalized PS by diverse phagocytic receptors, including the scavenger receptor CD36, brain angiogenesis inhibitor 1 (BAI1), T cell immunoglobulin and mucin domain-containing molecule 4 (TIM-4), Mer tyrosine kinase, and stabilin-2 (Ravichandran and Lorenz, 2007). Finally, apoptotic bodies are incorporated into phagosomes, which subsequently mature, and eventually fuse with lysosomes for terminal degradation. Elucidation of these clearance pathways is of great interest since removal of apoptotic cells by DCs bears important implications for the establishment of immune tolerance (Albert et al., 1998a, 2001; Kawane et al., 2006). While rapid progress has been made toward the understanding of molecular processes inherent to the delivery, recognition, and engulfment of apoptotic bodies, the mechanism of the critical final processing step, describing their lysosomal disintegration, remains largely unexplored. Ultrastructural examination of apoptotic vesicles by electron microscopy reveals similar features to ILVs. Therefore, we anticipated that the special mode of action of saposins on intralysosomal membranes could be used to disrupt apoptotic vesicles located in lysosomes, following phagocytosis by macrophages or DCs (Fig. 2.2). Of note, high content of anionic phospholipids in ILVs favors their solubilization upon functional interaction with specific SAPs in lysosomes (Ciaffoni et al., 2001). We propose that PS on apoptotic bodies deploys its actual specific function inside the phagocytes, namely, as molecular target for saposins in lysosomes to facilitate disintegration of apoptotic vesicles (Fig. 2.2, unpublished observations).
5.2. Saposins facilitate antigen cross-presentation
Presentation of peptide antigens by MHC molecules to T lymphocytes classically comprises two major pathways. Following the MHC-I pathway, endogenous proteins that are synthesized inside the cells, such as antigens produced by viruses, are primarily located in the cytosol. Subsequently, the multienzyme complex of the proteasome degrades the proteins into peptide fragments, which are translocated into the ER through TAP (Goldberg and Rock, 1992; Shepherd et al., 1993). After loading of MHC-I molecules in the ER assisted by the peptide-loading complex, consisting of tapasin, calreticulin, and Erp57, MHC-I-peptide complexes are transported to the cell surface of the APC for specific recognition by CD8+ T cells (Degen et al., 1992; York and Rock, 1996). By contrast, exogenous antigens derived from pathogenic bacteria, for example, are endocytosed by APCs for subsequent degradation by cathepsins in late endosomal / lysosomal compartments, prior to loading onto MHC-II molecules. Subsequently, CD4+ T cells recognize complexes of MHC-II and peptide exposed on the APC surface. However, exogenous antigens can also be presented by MHC-I molecules in a process termed cross-presentation (Vyas et al., 2008). Accordingly, the respective antigen crosses from the endosomal route to the MHC-I pathway. The activation of CD8+ T cells by cross-presented antigens is referred to as cross-priming, and DCs are APCs uniquely equipped for cross-presentation (Bevan, 1976; Guermonprez et al., 2002). To date, multiple mechanisms for the cellular pathway of cross-presentation have been proposed, which are likely not mutually exclusive (Vyas et al., 2008). Moreover, several antigen vehicles have been described to have cross-priming abilities, including proteins, peptides, and heat-shock proteins (HSP) chaperoning peptides (Srivastava, 2002). In addition, apoptotic cells represent a potent device to deliver antigens to the cross-presentation pathway. Notably, apoptotic bodies derived from tumors or host cells infected with viruses induce vigorous CD8+ T cell responses (Albert et al., 1998b). Further, macrophages infected with mycobacteria release apoptotic vesicles that are engulfed by uninfected bystander DCs for cross-priming of CD8+ T cells (Schaible et al., 2003; Winau et al., 2006). Ultimately, immunization with apoptotic vesicles released by mycobacteria-infected macrophages elicits CD8+ T cell responses and protects against tuberculosis (Winau et al., 2006). Therefore, apoptotic bodies as mediators of antigen cross-presentation are part of a unique detour pathway that promotes T cell responses in tumor and infection immunity (Winau et al., 2004a). However, it remains unclear as to how antigens enclosed in apoptotic bodies become accessible for cross-presentation to CD8+ T cells. Our previous findings suggested that successful cross-priming requires pSAP-dependent processing of apoptotic vesicles in recipient DCs (Winau et al., 2006). Thus, we propose that saposins unseal apoptotic bodies for antigen delivery in DCs and subsequent CD8+ T cell responses (Fig. 2.2).
6. SAP-LIKE PROTEINS IN ANTIMICROBIAL DEFENSE
The family of SAPLIPs comprehends heterogeneous and functionally divergent proteins that share a conserved motif of six cysteine residues associated by three disulfide bonds (Munford et al., 1995). This motif forms the characteristic “saposin fold” that allows SAPLIPs to interact with lipids (Bruhn, 2005). The SAPLIP domain can be regarded as an ancestral molecule since it is present both in humans and in one of the most primitive eukaryotes, namely amoebozoans. Entamoeba histolytica is a prototypical pathogenic amoebozoan that produces amoebapores. These molecules belong to the family of SAPLIPs and exert cytolytic activities against bacteria and human cells, by forming pores in the target cell membrane as a killing principle (Leippe et al., 1994a, b). E. histolyticais the etiologic agent of human amoebiasis, and recent evidence suggests that amoebapores could be responsible for the tissue destruction upon infection (Bracha et al., 2003). To date, 19 genes encoding SAPLIPs have been identified in E. histolytica. Considering that the primary function of amoebapores is the destruction of phagocytosed bacteria for nutritional purposes, a diversity of SAPLIPs might be required for subsequent digestive steps. Interestingly, SAPLIPs with similar lytic functions have been described in humans and other mammals. These include human granulysin and porcine NK-lysin that share the highest degree of homology among members of the SAPLIP family. Similar to amoebapores, granulysin and NK-lysin show a broad spectrum of antimicrobial activity, killing parasites, bacteria, and fungi (Ernst et al., 2000; Leippe, 1995; Pena et al., 1997; Stenger et al., 1998). In combination with perforin, granulysin released by T cells kills M. tuberculosisin macrophages by affecting the integrity of its cell wall, leading to subsequent osmotic bacterial lysis (Stenger et al., 1998). NK-lysin is predominantly stored by T cells and NK cells in cytosolic granules, and is released upon activation (Andersson et al., 1995). In addition, NK-lysin was found to be lytic against YAC-1 tumor targets, and especially potent at killing tumor cell lines that had increased surface levels of PS (Andersson et al., 1995; Schroder-Bormet al., 2005). By contrast, granulysin has been more extensively studied with regard to its tumorolytic functions against human T cell lymphoma of the Jurkat type (Kaspar et al., 2001). Moreover, several studies could unequivocally demonstrate the importance of granulysin and antimicrobial proteins in various infectious diseases (Heusel et al., 1994; Kagi et al., 1994; Lowin et al., 1994; Stenger et al., 1998). To date, the structures of six SAPLIPs have been resolved, starting with the first crystal of NK-lysin (Liepinsh et al., 1997). They all show the same α-helical fold of five helices connected by three disulfide bonds. Surprisingly, the predicted modes of action by lytic SAPLIPs suggest extremely diverse mechanisms used to achieve membrane permeabilization. In its active state, the pore-forming amoebapore A is a dimer stabilized by electrostatic interactions that involve a unique, centrally positioned histidine residue (Andra and Leippe, 1994). One side of the dimer is exclusively hydrophobic, which allows its insertion into membranes. Once docked, the protein oligomerizes to create ring-like pores that resemble channels (Gutsmann et al., 2003). The histidine residue functions as a pH-dependent switch. Activation of this switch occurs at low pH and allows the dimerization that is crucial for the cytolytic function (Andra and Leippe, 1994). Considering its function, it is therefore not surprising that this residue is highly conserved among all amoebapore isoforms. By contrast, NK-lysin and granulysin permeabilize membranes in a monomeric state through an electrostatic process termed electroporation (Miteva et al., 1999). Concerning both SAPLIPs, membrane recruitment occurs through interactions of positively charged residues in the SAPLIP and negatively charged phospholipids in the target membrane. Subsequent conformational changes allow the two halves of the molecules to slit the membrane in a scissor-like fashion, ultimately causing osmotic lysis (Anderson et al., 2003). In Naegleria fowleri, the SAPLIP naegleriapores are encoded in larger multipeptide precursor structures, each potentially giving rise to multiple glycosylated naegleriapore-like molecules (Herbst et al., 2004). Finally, functional characterization of a novel SAPLIP in E. histolytica, namely SAPLIP 3, revealed fusogenic activities similar to SAP-C in mammalians (Winkelmann et al., 2006). Accordingly, we highlight the possibility that saposins might possess conserved evolutionary traits of ancient weapons which potentially mediate direct antimicrobial functions (Fig. 2.2).
7. CONCLUSIONS
Decades ago, saposins have been considered as negligible test tube activators of lysosomal glycosidases. Today, saposins emerge as critical components of membrane homeostasis and unexpected actors on the scene of immunology. In addition to their well-established capacities in sphingolipid degradation and membrane digestion, saposins also fulfill important immunological functions. Based on their universal principle to interact with membrane bilayers in lysosomes, SAPs exert versatile helper functions further defined by their respective interaction partners. In this context, saposins can mobilize lipids from membranes and associate with lipid-degrading enzymes, in order to degrade or generate antigenic epitopes. Further, saposins interact with antigen-presenting molecules to facilitate the loading of lipid antigens onto CD1 proteins for subsequent activation of lipid-reactive T lymphocytes. Additionally, the membrane-perturbing properties of SAPs can mediate the disintegration of apoptotic bodies for antigen delivery in APCs and subsequent cross-presentation. Finally, saposins could have antimicrobial functions through direct membrane attacks on pathogens.
REFERENCES
- Ahn VE, Faull KF, Whitelegge JP, Fluharty AL, Prive GG. Crystal structure of saposin B reveals a dimeric shell for lipid binding. Proc. Natl. Acad. Sci. USA. 2003;100:38–43. doi: 10.1073/pnas.0136947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn VE, Leyko P, Alattia JR, Chen L, Prive GG. Crystal structures of saposins A and C. Protein Sci. 2006;15:1849–1857. doi: 10.1110/ps.062256606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphav-beta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998a;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998b;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Sawaya MR, Cascio D, Ernst W, Modlin R, Krensky A, Eisenberg D. Granulysin crystal structure and a structure-derived lytic mechanism. J. Mol. Biol. 2003;325:355–365. doi: 10.1016/s0022-2836(02)01234-2. [DOI] [PubMed] [Google Scholar]
- Andersson M, Gunne H, Agerberth B, Boman A, Bergman T, Sillard R, Jornvall H, Mutt V, Olsson B, Wigzell H, et al. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 1995;14:1615–1625. doi: 10.1002/j.1460-2075.1995.tb07150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra J, Leippe M. Pore-forming peptide of Entamoeba histolytica. Significance of positively charged amino acid residues for its mode of action. FEBS Lett. 1994;354:97–102. doi: 10.1016/0014-5793(94)01103-6. [DOI] [PubMed] [Google Scholar]
- Andre P, Perlemuter G, Budkowska A, Brechot C, Lotteau V. Hepatitis C virus particles and lipoprotein metabolism. Semin. Liver Dis. 2005;25:93–104. doi: 10.1055/s-2005-864785. [DOI] [PubMed] [Google Scholar]
- Angenieux C, Fraisier V, Maitre B, Racine V, van der Wel N, Fricker D, Proamer F, Sachse M, Cazenave JP, Peters P, et al. The cellular pathway of CD1e in immature and maturing dendritic cells. Traffic. 2005;6:286–302. doi: 10.1111/j.1600-0854.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Beckman EM, Melian A, Behar SM, Sieling PA, Chatterjee D, Furlong ST, Matsumoto R, Rosat JP, Modlin RL, Porcelli SA. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J. Immunol. 1996;157:2795–2803. [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Berent SL, Radin NS. Mechanism of activation of glucocerebrosidase by co-beta-glucosidase (glucosidase activator protein) Biochim. Biophys. Acta. 1981;664:572–582. doi: 10.1016/0005-2760(81)90134-x. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha R, Nuchamowitz Y, Mirelman D. Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: Molecular analysis and effect on pathogenicity. Eukaryot. Cell. 2003;2:295–305. doi: 10.1128/EC.2.2.295-305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradova V, Smid F, Ulrich-Bott B, Roggendorf W, Paton BC, Harzer K. Prosaposin deficiency: Further characterization of the sphingolipid activator protein-deficient sibs. Multiple glycolipid elevations (including lactosylceramidosis), partial enzyme deficiencies and ultrastructure of the skin in this generalized sphingolipid storage disease. Hum. Genet. 1993;92:143–152. doi: 10.1007/BF00219682. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu. Rev. Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- Briken V, Jackman RM, Watts GF, Rogers RA, Porcelli SA. Human CD1b and CD1c isoforms survey different intracellular compartments for the presentation of microbial lipid antigens. J. Exp. Med. 2000;192:281–288. doi: 10.1084/jem.192.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn H. A short guided tour through functional and structural features of saposin-like proteins. Biochem. J. 2005;389:249–257. doi: 10.1042/BJ20050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd RC, Miescher GC, Howe RC, Lees RK, Bron C, MacDonald HR. Developmentally regulated expression of T cell receptor beta chain variable domains in immature thymocytes. J. Exp. Med. 1987;166:577–582. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH, Dautry-Varsat A, Bendelac A. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 1999;189:103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Park SH, Benlagha K, Forestier C, Jayawardena-Wolf J, Savage PB, Teyton L, Bendelac A. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d. Nat. Immunol. 2002;3:55–60. doi: 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, Godfrey DI, Sandrin MS. Humans lack iGb3 due to the absence of functional iGb3-synthase: Implications for NKT cell development and transplantation. PLoS Biol. 2008;6:e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christomanou H, Chabas A, Pampols T, Guardiola A. Activator protein deficient Gaucher’s disease. A second patient with the newly identified lipid storage disorder. Klin. Wochenschr. 1989;67:999–1003. doi: 10.1007/BF01716064. [DOI] [PubMed] [Google Scholar]
- Chu Z, Witte DP, Qi X. Saposin C–LBPA interaction in late-endosomes/lysosomes. Exp. Cell Res. 2005;303:300–307. doi: 10.1016/j.yexcr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Ciaffoni F, Salvioli R, Tatti M, Arancia G, Crateri P, Vaccaro AM. Saposin D solubilizes anionic phospholipid-containing membranes. J. Biol. Chem. 2001;276:31583–31589. doi: 10.1074/jbc.M102736200. [DOI] [PubMed] [Google Scholar]
- Ciaffoni F, Tatti M, Salvioli R, Vaccaro AM. Interaction of saposin D with membranes: Effect of anionic phospholipids and sphingolipids. Biochem. J. 2003;373:785–792. doi: 10.1042/BJ20030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaffoni F, Tatti M, Boe A, Salvioli R, Fluharty A, Sonnino S, Vaccaro AM. Saposin B binds and transfers phospholipids. J. Lipid Res. 2006;47:1045–1053. doi: 10.1194/jlr.M500547-JLR200. [DOI] [PubMed] [Google Scholar]
- Conzelmann E, Sandhoff K. AB variant of infantile GM2 gangliosidosis: Deficiency of a factor necessary for stimulation of hexosaminidase A-catalyzed degradation of ganglioside GM2 and glycolipid GA2. Proc. Natl. Acad. Sci. USA. 1978;75:3979–3983. doi: 10.1073/pnas.75.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann E, Sandhoff K. Purification and characterization of an activator protein for the degradation of glycolipids GM2 and GA2 by hexosaminidase A. Hoppe Seylers Z. Physiol. Chem. 1979;360:1837–1849. doi: 10.1515/bchm2.1979.360.2.1837. [DOI] [PubMed] [Google Scholar]
- Conzelmann E, Burg J, Stephan G, Sandhoff K. Complexing of glycolipids and their transfer between membranes by the activator protein for degradation of lysosomal ganglioside GM2. Eur. J. Biochem. 1982;123:455–464. doi: 10.1111/j.1432-1033.1982.tb19789.x. [DOI] [PubMed] [Google Scholar]
- D’Andrea A, Goux D, De Lalla C, Koezuka Y, Montagna D, Moretta A, Dellabona P, Casorati G, Abrignani S. Neonatal invariant Valpha24+ NKT lymphocytes are activated memory cells. Eur. J. Immunol. 2000;30:1544–1550. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Maitre B, Mourey L, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- De Libero G, Moran AP, Gober HJ, Rossy E, Shamshiev A, Chelnokova O, Mazorra Z, Vendetti S, Sacchi A, Prendergast MM, et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. 2005;22:763–772. doi: 10.1016/j.immuni.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Degen E, Cohen-Doyle MF, Williams DB. Efficient dissociation of the p88 chaperone from major histocompatibility complex class I molecules requires both beta 2-microglobulin and peptide. J. Exp. Med. 1992;175:1653–1661. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delia D, Cattoretti G, Polli N, Fontanella E, Aiello A, Giardini R, Rilke F, Della Porta G. CD1c but neither CD1a nor CD1b molecules are expressed on normal, activated, and malignant human B cells: Identification of a new B-cell subset. Blood. 1988;72:241–247. [PubMed] [Google Scholar]
- Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4–8– T cells. J. Exp. Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J. Exp. Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. Curr. Top. Microbiol. Immunol. 2007a;314:113–141. doi: 10.1007/978-3-540-69511-0_5. [DOI] [PubMed] [Google Scholar]
- Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J. Exp. Med. 2007b;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst WA, Maher J, Cho S, Niazi KR, Chatterjee D, Moody DB, Besra GS, Watanabe Y, Jensen PE, Porcelli SA, et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity. 1998;8:331–340. doi: 10.1016/s1074-7613(00)80538-5. [DOI] [PubMed] [Google Scholar]
- Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, Clayberger C, Krensky AM, Leippe M, Bloom BR, Ganz T, et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J. Immunol. 2000;165:7102–7108. doi: 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, Tahir SM, Patton KT, Blumberg RS, Porcelli S, Chott A, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SH, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes BJ, Kruisbeek AM, Ton-That H, Weston MA, Coligan JE, Schwartz RH, Pardoll DM. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987;329:251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann–Pick type C2 disease. Proc. Natl. Acad. Sci. USA. 2003;100:2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Suzuki K, Vanier MT, Popko B, Maeda N, Klein A, Henseler M, Sandhoff K, Nakayasu H. Targeted disruption of the mouse sphingolipid activator protein gene: A complex phenotype, including severe leukodystrophy and wide-spread storage of multiple sphingolipids. Hum. Mol. Genet. 1996;5:711–725. doi: 10.1093/hmg/5.6.711. [DOI] [PubMed] [Google Scholar]
- Furst W, Sandhoff K. Activator proteins and topology of lysosomal sphingolipid catabolism. Biochim. Biophys. Acta. 1992;1126:1–16. doi: 10.1016/0005-2760(92)90210-m. [DOI] [PubMed] [Google Scholar]
- Furst W, Machleidt W, Sandhoff K. The precursor of sulfatide activator protein is processed to three different proteins. Biol. Chem. Hoppe Seyler. 1988;369:317–328. doi: 10.1515/bchm3.1988.369.1.317. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Collinson LM, Backer JM, Hopkins CR. Human VPS34 is required for internal vesicle formation within multivesicular endosomes. J. Cell Biol. 2001;155:1251–1264. doi: 10.1083/jcb.200108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, Schmidt RR, Jones EY, Cerundolo V. Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat. Immunol. 2002;3:721–726. doi: 10.1038/ni821. [DOI] [PubMed] [Google Scholar]
- Gadola SD, Silk JD, Jeans A, Illarionov PA, Salio M, Besra GS, Dwek R, Butters TD, Platt FM, Cerundolo V. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J. Exp. Med. 2006;203:2293–2303. doi: 10.1084/jem.20060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Bohmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: What’s in a name? Nat. Rev. Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Goldberg AL, Rock KL. Proteolysis, proteasomes and antigen presentation. Nature. 1992;357:375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Gutsmann T, Riekens B, Bruhn H, Wiese A, Seydel U, Leippe M. Interaction of amoebapores and NK-lysin with symmetric phospholipid and asymmetric lipopolysaccharide / phospholipid bilayers. Biochemistry. 2003;42:9804–9812. doi: 10.1021/bi034686u. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Harzer K, Paton BC, Poulos A, Kustermann-Kuhn B, Roggendorf W, Grisar T, Popp M. Sphingolipid activator protein deficiency in a 16-week-old atypical Gaucher disease patient and his fetal sibling: Biochemical signs of combined sphingolipidoses. Eur. J. Pediatr. 1989;149:31–39. doi: 10.1007/BF02024331. [DOI] [PubMed] [Google Scholar]
- Harzer K, Paton BC, Christomanou H, Chatelut M, Levade T, Hiraiwa M, O’Brien JS. Saposins (sap) A and C activate the degradation of galactosylcer-amide in living cells. FEBS Lett. 1997;417:270–274. doi: 10.1016/s0014-5793(97)01302-1. [DOI] [PubMed] [Google Scholar]
- Hawkins CA, de Alba E, Tjandra N. Solution structure of human saposin C in a detergent environment. J. Mol. Biol. 2005;346:1381–1392. doi: 10.1016/j.jmb.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Herbst R, Marciano-Cabral F, Leippe M. Antimicrobial and pore-forming peptides of free-living and potentially highly pathogenic Naegleria fowleri are released from the same precursor molecule. J. Biol. Chem. 2004;279:25955–25958. doi: 10.1074/jbc.M401965200. [DOI] [PubMed] [Google Scholar]
- Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Huttler S, Rohlmann A, Schneider W, Sandhoff K, Herz J. Cellular uptake of saposin (SAP) precursor and lysosomal delivery by the low density lipoprotein receptor-related protein (LRP) EMBO J. 1998;17:4617–4625. doi: 10.1093/emboj/17.16.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa M, Soeda S, Kishimoto Y, O’Brien JS. Binding and transport of gangliosides by prosaposin. Proc. Natl. Acad. Sci. USA. 1992;89:11254–11258. doi: 10.1073/pnas.89.23.11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa M, O’Brien JS, Kishimoto Y, Galdzicka M, Fluharty AL, Ginns EI, Martin BM. Isolation, characterization, and proteolysis of human prosaposin, the precursor of saposins (sphingolipid activator proteins) Arch. Biochem. Biophys. 1993;304:110–116. doi: 10.1006/abbi.1993.1328. [DOI] [PubMed] [Google Scholar]
- Hiraiwa M, Martin BM, Kishimoto Y, Conner GE, Tsuji S, O’Brien JS. Lysosomal proteolysis of prosaposin, the precursor of saposins (sphingolipid activator proteins): Its mechanism and inhibition by ganglioside. Arch. Biochem. Biophys. 1997;341:17–24. doi: 10.1006/abbi.1997.9958. [DOI] [PubMed] [Google Scholar]
- Ho MW, O’Brien JS. Gaucher’s disease: Deficiency of ‘cid’ β-glucosidase and reconstitution of enzyme activity in vitro. Proc. Natl. Acad. Sci. USA. 1971;68:2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooghe-Peters EL, Fowlkes BJ, Hooghe RJ. A new neuronal marker identified by phosphorylcholine-binding myeloma proteins. Nature. 1979;281:376–378. doi: 10.1038/281376a0. [DOI] [PubMed] [Google Scholar]
- Hornick CA, Hamilton RL, Spaziani E, Enders GH, Havel RJ. Isolation and characterization of multivesicular bodies from rat hepatocytes: An organelle distinct from secretory vesicles of the Golgi apparatus. J. Cell Biol. 1985;100:1558–1569. doi: 10.1083/jcb.100.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkova H, Cervenkova M, Ledvinova J, Tochackova M, Hrebicek M, Poupetova H, Befekadu A, Berna L, Paton BC, Harzer K, et al. A novel mutation in the coding region of the prosaposin gene leads to a complete deficiency of prosaposin and saposins, and is associated with a complex sphingolipidosis dominated by lactosylceramide accumulation. Hum. Mol. Genet. 2001;10:927–940. doi: 10.1093/hmg/10.9.927. [DOI] [PubMed] [Google Scholar]
- Hullin-Matsuda F, Luquain-Costaz C, Bouvier J, Delton-Vandenbroucke I. Bis(monoacylglycero)phosphate, a peculiar phospholipid to control the fate of cholesterol: Implications in pathology. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:313–324. doi: 10.1016/j.plefa.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Hirabayashi Y. Glucosylceramide synthase and glycosphingolipid synthesis. Trends Cell Biol. 1998;8:198–202. doi: 10.1016/s0962-8924(98)01249-5. [DOI] [PubMed] [Google Scholar]
- Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Cresswell P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J. Biol. Chem. 2002;277:44838–44844. doi: 10.1074/jbc.M207831200. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat. Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- Karlsson KA. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, Hanson DA, Kluck RM, Hitoshi Y, Johnson DE, et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J. Immunol. 2001;167:350–356. doi: 10.4049/jimmunol.167.1.350. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Tuberculosis: Back on the immunologists’ agenda. Immunity. 2006;24:351–357. doi: 10.1016/j.immuni.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Hiraiwa M, O’Brien JS. Saposins: Structure, function, distribution, and molecular genetics. J. Lipid Res. 1992;33:1255–1267. [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Gruenberg J. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: Stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- Kondoh K, Sano A, Kakimoto Y, Matsuda S, Sakanaka M. Distribution of prosaposin-like immunoreactivity in rat brain. J. Comp. Neurol. 1993;334:590–602. doi: 10.1002/cne.903340407. [DOI] [PubMed] [Google Scholar]
- Kretz KA, Carson GS, Morimoto S, Kishimoto Y, Fluharty AL, O’Brien JS. Characterization of a mutation in a family with saposin B deficiency: A glycosylation site defect. Proc. Natl. Acad. Sci. USA. 1990;87:2541–2544. doi: 10.1073/pnas.87.7.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois S, Zeng J, Hassan AJ, Canuel M, Morales CR. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 2003;22:6430–6437. doi: 10.1093/emboj/cdg629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leippe M. Ancient weapons: NK-lysin, is a mammalian homolog to pore-forming peptides of a protozoan parasite. Cell. 1995;83:17–18. doi: 10.1016/0092-8674(95)90229-5. [DOI] [PubMed] [Google Scholar]
- Leippe M, Andra J, Muller-Eberhard HJ. Cytolytic and antibacterial activity of synthetic peptides derived from amoebapore, the pore-forming peptide of Entamoeba histolytica. Proc. Natl. Acad. Sci. USA. 1994a;91:2602–2606. doi: 10.1073/pnas.91.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leippe M, Andra J, Nickel R, Tannich E, Muller-Eberhard HJ. Amoebapores, a family of membranolytic peptides from cytoplasmic granules of Entamoeba histolytica: Isolation, primary structure, and pore formation in bacterial cytoplasmic membranes. Mol. Microbiol. 1994b;14:895–904. doi: 10.1111/j.1365-2958.1994.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Li SC, Kihara H, Serizawa S, Li YT, Fluharty AL, Mayes JS, Shapiro LJ. Activator protein required for the enzymatic hydrolysis of cerebroside sulfate. Deficiency in urine of patients affected with cerebroside sulfatase activator deficiency and identity with activators for the enzymatic hydrolysis of GM1 ganglioside and globotriaosylceramide. J. Biol. Chem. 1985;260:1867–1871. [PubMed] [Google Scholar]
- Li SC, Sonnino S, Tettamanti G, Li YT. Characterization of a nonspecific activator protein for the enzymatic hydrolysis of glycolipids. J. Biol. Chem. 1988;263:6588–6591. [PubMed] [Google Scholar]
- Li CM, Park JH, Simonaro CM, He X, Gordon RE, Friedman AH, Ehleiter D, Paris F, Manova K, Hepbildikler S, et al. Insertional mutagenesis of the mouse acid ceramidase gene leads to early embryonic lethality in homozygotes and progressive lipid storage disease in heterozygotes. Genomics. 2002;79:218–224. doi: 10.1006/geno.2002.6686. [DOI] [PubMed] [Google Scholar]
- Li Y, Thapa P, Hawke D, Kondo Y, Furukawa K, Hsu FF, Adlercreutz D, Weadge J, Palcic MM, Wang PG, et al. Immunologic glycosphingolipidomics and NKT cell development in mouse thymus. J. Proteome Res. 2009;8:2740–2751. doi: 10.1021/pr801040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh E, Andersson M, Ruysschaert JM, Otting G. Saposin fold revealed by the NMR structure of NK-lysin. Nat. Struct. Biol. 1997;4:793–795. doi: 10.1038/nsb1097-793. [DOI] [PubMed] [Google Scholar]
- Locatelli-Hoops S, Remmel N, Klingenstein R, Breiden B, Rossocha M, Schoeniger M, Koenigs C, Saenger W, Sandhoff K. Saposin A mobilizes lipids from low cholesterol and high bis(monoacylglycerol)phosphate-containing membranes: Patient variant Saposin A lacks lipid extraction capacity. J. Biol. Chem. 2006;281:32451–32460. doi: 10.1074/jbc.M607281200. [DOI] [PubMed] [Google Scholar]
- Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Maitre B, Angenieux C, Salamero J, Hanau D, Fricker D, Signorino F, Proamer F, Cazenave JP, Goud B, Tourne S, et al. Control of the intracellular pathway of CD1e. Traffic. 2008;9:431–445. doi: 10.1111/j.1600-0854.2008.00707.x. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Vanier MT, Saito Y, Tohyama J, Suzuki K. A mutation in the saposin A domain of the sphingolipid activator protein (prosaposin) gene results in a late-onset, chronic form of globoid cell leukodystrophy in the mouse. Hum. Mol. Genet. 2001;10:1191–1199. doi: 10.1093/hmg/10.11.1191. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Kido M, Tadano-Aritomi K, Ishizuka I, Tominaga K, Toida K, Takeda E, Suzuki K, Kuroda Y. Mutation in saposin D domain of sphingolipid activator protein gene causes urinary system defects and cerebellar Purkinje cell degeneration with accumulation of hydroxy fatty acid-containing ceramide in mouse. Hum. Mol. Genet. 2004;13:2709–2723. doi: 10.1093/hmg/ddh281. [DOI] [PubMed] [Google Scholar]
- Matsuda J, Yoneshige A, Suzuki K. The function of sphingolipids in the nervous system: Lessons learnt from mouse models of specific sphingolipid activator protein deficiencies. J. Neurochem. 2007;103(Suppl 1):32–38. doi: 10.1111/j.1471-4159.2007.04709.x. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Mehl E, Jatzkewitz H. A cerebrosidesulfatase from swine kidney. Hoppe Seylers Z. Physiol. Chem. 1964;339:260–276. [PubMed] [Google Scholar]
- Miteva M, Andersson M, Karshikoff A, Otting G. Molecular electroporation: A unifying concept for the description of membrane pore formation by antibacterial peptides, exemplified with NK-lysin. FEBS Lett. 1999;462:155–158. doi: 10.1016/s0014-5793(99)01520-3. [DOI] [PubMed] [Google Scholar]
- Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Montoya CJ, Jie HB, Al-Harthi L, Mulder C, Patino PJ, Rugeles MT, Krieg AM, Landay AL, Wilson SB. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J. Immunol. 2006;177:1028–1039. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- Moody DB, Guy MR, Grant E, Cheng TY, Brenner MB, Besra GS, Porcelli SA. CD1b-mediated T cell recognition of a glycolipid antigen generated from mycobacterial lipid and host carbohydrate during infection. J. Exp. Med. 2000a;192:965–976. doi: 10.1084/jem.192.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000b;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- Moody DB, Young DC, Cheng TY, Rosat JP, Roura-Mir C, O’Connor PB, Zajonc DM, Walz A, Miller MJ, Levery SB, et al. T cell activation by lipopeptide antigens. Science. 2004;303:527–531. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- Moody DB, Zajonc DM, Wilson IA. Anatomy of CD1-lipid antigen complexes. Nat. Rev. Immunol. 2005;5:387–399. doi: 10.1038/nri1605. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Martin BM, Yamamoto Y, Kretz KA, O’Brien JS, Kishimoto Y. Saposin A: Second cerebrosidase activator protein. Proc. Natl. Acad. Sci. USA. 1989;86:3389–3393. doi: 10.1073/pnas.86.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford RS, Sheppard PO, O’Hara PJ. Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. J. Lipid Res. 1995;36:1653–1663. [PubMed] [Google Scholar]
- Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- O’Brien JS, Kishimoto Y. Saposin proteins: Structure, function, and role in human lysosomal storage disorders. FASEB J. 1991;5:301–308. doi: 10.1096/fasebj.5.3.2001789. [DOI] [PubMed] [Google Scholar]
- O’Brien JS, Kretz KA, Dewji N, Wenger DA, Esch F, Fluharty AL. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988;241:1098–1101. doi: 10.1126/science.2842863. [DOI] [PubMed] [Google Scholar]
- O’Brien JS, Carson GS, Seo HC, Hiraiwa M, Kishimoto Y. Identification of prosaposin as a neurotrophic factor. Proc. Natl. Acad. Sci. USA. 1994;91:9593–9596. doi: 10.1073/pnas.91.20.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JS, Carson GS, Seo HC, Hiraiwa M, Weiler S, Tomich JM, Barranger JA, Kahn M, Azuma N, Kishimoto Y. Identification of the neurotrophic factor sequence of prosaposin. FASEB J. 1995;9:681–685. doi: 10.1096/fasebj.9.8.7768361. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, Sugimoto Y, Pastan I, Gottesman MM, Brady RO, et al. alpha-Galactosidase A deficient mice: A model of Fabry disease. Proc. Natl. Acad. Sci. USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Park SH, Benlagha K, Lee D, Balish E, Bendelac A. Unaltered phenotype, tissue distribution and function of Valpha14(+) NKT cells in germ-free mice. Eur. J. Immunol. 2000;30:620–625. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J. Immunol. 1997;158:2680–2688. [PubMed] [Google Scholar]
- Piper RC, Luzio JP. Late endosomes: Sorting and partitioning in multi-vesicular bodies. Traffic. 2001;2:612–621. doi: 10.1034/j.1600-0854.2001.20904.x. [DOI] [PubMed] [Google Scholar]
- Plati T, Visigalli I, Capotondo A, Buono M, Naldini L, Cosma MP, Biffi A. Development and maturation of invariant NKT cells in the presence of lysosomal engulfment. Eur. J. Immunol. 2009;39:2748–2754. doi: 10.1002/eji.200939639. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4–8– T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8– alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J. Exp. Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl. Acad. Sci. USA. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- Qi X, Chu Z. Fusogenic domain and lysines in saposin C. Arch. Biochem. Biophys. 2004;424:210–218. doi: 10.1016/j.abb.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Raftery MJ, Winau F, Kaufmann SH, Schaible UE, Schonrich G. CD1 antigen presentation by human dendritic cells as a target for herpes simplex virus immune evasion. J. Immunol. 2006;177:6207–6214. doi: 10.4049/jimmunol.177.9.6207. [DOI] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: Signals for a good meal. Nat. Rev. Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Remmel N, Locatelli-Hoops S, Breiden B, Schwarzmann G, Sandhoff K. Saposin B mobilizes lipids from cholesterol-poor and bis(monoacylglycero)phosphaterich membranes at acidic pH. Unglycosylated patient variant saposin B lacks lipid-extraction capacity. FEBS J. 2007;274:3405–3420. doi: 10.1111/j.1742-4658.2007.05873.x. [DOI] [PubMed] [Google Scholar]
- Rigat B, Wang W, Leung A, Mahuran DJ. Two mechanisms for the recapture of extracellular GM2 activator protein: Evidence for a major secretory form of the protein. Biochemistry. 1997;36:8325–8331. doi: 10.1021/bi970571c. [DOI] [PubMed] [Google Scholar]
- Rorman EG, Scheinker V, Grabowski GA. Structure and evolution of the human prosaposin chromosomal gene. Genomics. 1992;13:312–318. doi: 10.1016/0888-7543(92)90247-p. [DOI] [PubMed] [Google Scholar]
- Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling PA, Frederique D, Modlin RL, Porcelli SA, Furlong ST, Brenner MB. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. J. Immunol. 1999;162:366–371. [PubMed] [Google Scholar]
- Rossmann M, Schultz-Heienbrok R, Behlke J, Remmel N, Alings C, Sandhoff K, Saenger W, Maier T. Crystal structures of human saposins C andD: Implications for lipid recognition and membrane interactions. Structure. 2008;16:809–817. doi: 10.1016/j.str.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Roy KC, Maricic I, Khurana A, Smith TR, Halder RC, Kumar V. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J. Immunol. 2008;180:2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, Besra GS, Platt FM, Cerundolo V. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl. Acad. Sci. USA. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J. Clin. Invest. 2005;115:1369–1378. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K, Kolter T. Topology of glycosphingolipid degradation. Trends Cell Biol. 1996;6:98–103. doi: 10.1016/0962-8924(96)80999-8. [DOI] [PubMed] [Google Scholar]
- Sano A, Hineno T, Mizuno T, Kondoh K, Ueno S, Kakimoto Y, Inui K. Sphingolipid hydrolase activator proteins and their precursors. Biochem. Biophys. Res. Commun. 1989;165:1191–1197. doi: 10.1016/0006-291x(89)92728-9. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 2003;9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- Schlote W, Harzer K, Christomanou H, Paton BC, Kustermann-Kuhn B, Schmid B, Seeger J, Beudt U, Schuster I, Langenbeck U. Sphingolipid activator protein 1 deficiency in metachromatic leucodystrophy with normal arylsulphatase A activity. A clinical, morphological, biochemical, and immunological study. Eur. J. Pediatr. 1991;150:584–591. doi: 10.1007/BF02072213. [DOI] [PubMed] [Google Scholar]
- Schnabel D, Schroder M, Sandhoff K. Mutation in the sphingolipid activator protein 2 in a patient with a variant of Gaucher disease. FEBS Lett. 1991;284:57–59. doi: 10.1016/0014-5793(91)80760-z. [DOI] [PubMed] [Google Scholar]
- Schroder-Borm H, Bakalova R, Andra J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005;579:6128–6134. doi: 10.1016/j.febslet.2005.09.084. [DOI] [PubMed] [Google Scholar]
- Schumann J, Facciotti F, Panza L, Michieletti M, Compostella F, Collmann A, Mori L, De Libero G. Differential alteration of lipid antigen presentation to NKT cells due to imbalances in lipid metabolism. Eur. J. Immunol. 2007;37:1431–1441. doi: 10.1002/eji.200737160. [DOI] [PubMed] [Google Scholar]
- Sever S. AP-2 makes room for rivals. Dev. Cell. 2003;5:530–532. doi: 10.1016/s1534-5807(03)00304-6. [DOI] [PubMed] [Google Scholar]
- Shamshiev A, Donda A, Prigozy TI, Mori L, Chigorno V, Benedict CA, Kappos L, Sonnino S, Kronenberg M, De Libero G. The alphabeta T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 2000;13:255–264. doi: 10.1016/s1074-7613(00)00025-x. [DOI] [PubMed] [Google Scholar]
- Shamshiev A, Gober HJ, Donda A, Mazorra Z, Mori L, De Libero G. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 2002;195:1013–1021. doi: 10.1084/jem.20011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JC, Schumacher TN, Ashton-Rickardt PG, Imaeda S, Ploegh HL, Janeway CA, Jr, Tonegawa S. TAP1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell. 1993;74:577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ, et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- Sieling PA, Jullien D, Dahlem M, Tedder TF, Rea TH, Modlin RL, Porcelli SA. CD1 expression by dendritic cells in human leprosy lesions: Correlation with effective host immunity. J. Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- Sieling PA, Ochoa MT, Jullien D, Leslie DS, Sabet S, Rosat JP, Burdick AE, Rea TH, Brenner MB, Porcelli SA, et al. Evidence for human CD4+ T cells in the CD1-restricted repertoire: Derivation of mycobacteria-reactive T cells from leprosy lesions. J. Immunol. 2000;164:4790–4796. doi: 10.4049/jimmunol.164.9.4790. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J. Immunol. 2005;175:3584–3593. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, et al. Self-recognition of CD1 by gamma/delta T cells: Implications for innate immunity. J. Exp. Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speak AO, Salio M, Neville DC, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc. Natl. Acad. Sci. USA. 2007;104:5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel R, Bach G, Sury V, Mengistu G, Meidan B, Shalev S, Shneor Y, Mandel H, Zeigler M. A mutation in the saposin A coding region of the prosaposin gene in an infant presenting as Krabbe disease: First report of saposin A deficiency in humans. Mol. Genet. Metab. 2005;84:160–166. doi: 10.1016/j.ymgme.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: Chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- Sugita M, Grant EP, van Donselaar E, Hsu VW, Rogers RA, Peters PJ, Brenner MB. Separate pathways for antigen presentation by CD1 molecules. Immunity. 1999;11:743–752. doi: 10.1016/s1074-7613(00)80148-x. [DOI] [PubMed] [Google Scholar]
- Sugita M, Cao X, Watts GF, Rogers RA, Bonifacino JS, Brenner MB. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity. 2002;16:697–706. doi: 10.1016/s1074-7613(02)00311-4. [DOI] [PubMed] [Google Scholar]
- Sugita M, Barral DC, Brenner MB. Pathways of CD1 and lipid antigen delivery, trafficking, processing, loading, and presentation. Curr. Top. Microbiol. Immunol. 2007;314:143–164. doi: 10.1007/978-3-540-69511-0_6. [DOI] [PubMed] [Google Scholar]
- Sun Y, Witte DP, Ran H, Zamzow M, Barnes S, Cheng H, Han X, Williams MT, Skelton MR, Vorhees CV, et al. Neurological deficits and glycosphingolipid accumulation in saposin B deficient mice. Hum. Mol. Genet. 2008;17:2345–2356. doi: 10.1093/hmg/ddn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I, Pap A, Ruhl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J. Exp. Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatti M, Salvioli R, Ciaffoni F, Pucci P, Andolfo A, Amoresano A, Vaccaro AM. Structural and membrane-binding properties of saposin D. Eur. J. Biochem. 1999;263:486–494. doi: 10.1046/j.1432-1327.1999.00521.x. [DOI] [PubMed] [Google Scholar]
- Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: The mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro AM, Tatti M, Ciaffoni F, Salvioli R, Maras B, Barca A. Function of saposin C in the reconstitution of glucosylceramidase by phosphatidylserine liposomes. FEBS Lett. 1993;336:159–162. doi: 10.1016/0014-5793(93)81631-9. [DOI] [PubMed] [Google Scholar]
- Vaccaro AM, Tatti M, Ciaffoni F, Salvioli R, Serafino A, Barca A. Saposin C induces pH-dependent destabilization and fusion of phosphatidylserine-containing vesicles. FEBS Lett. 1994;349:181–186. doi: 10.1016/0014-5793(94)00659-8. [DOI] [PubMed] [Google Scholar]
- Vaccaro AM, Ciaffoni F, Tatti M, Salvioli R, Barca A, Tognozzi D, Scerch C. pH-dependent conformational properties of saposins and their interactions with phospholipid membranes. J. Biol. Chem. 1995;270:30576–30580. doi: 10.1074/jbc.270.51.30576. [DOI] [PubMed] [Google Scholar]
- van Der Vliet HJ, Nishi N, de Gruijl TD, von Blomberg BM, van den Eertwegh AJ, Pinedo HM, Giaccone G, Scheper RJ. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood. 2000;95:2440–2442. [PubMed] [Google Scholar]
- Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat. Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 2008;8:607–618. doi: 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Grabowski GA, Qi X. Phospholipid vesicle fusion induced by saposin C. Arch. Biochem. Biophys. 2003;415:43–53. doi: 10.1016/s0003-9861(03)00219-4. [DOI] [PubMed] [Google Scholar]
- Wenger DA, DeGala G, Williams C, Taylor HA, Stevenson RE, Pruitt JR, Miller J, Garen PD, Balentine JD. Clinical, pathological, and biochemical studies on an infantile case of sulfatide/GM1 activator protein deficiency. Am. J. Med. Genet. 1989;33:255–265. doi: 10.1002/ajmg.1320330223. [DOI] [PubMed] [Google Scholar]
- Wilkening G, Linke T, Uhlhorn-Dierks G, Sandhoff K. Degradation of membrane-bound ganglioside GM1. Stimulation by bis(monoacylglycero)phosphate and the activator proteins SAP-B and GM2-AP. J. Biol. Chem. 2000;275:35814–35819. doi: 10.1074/jbc.M006568200. [DOI] [PubMed] [Google Scholar]
- Winau F, Kaufmann SH, Schaible UE. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell. Microbiol. 2004a;6:599–607. doi: 10.1111/j.1462-5822.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- Winau F, Schwierzeck V, Hurwitz R, Remmel N, Sieling PA, Modlin RL, Porcelli SA, Brinkmann V, Sugita M, Sandhoff K, et al. Saposin C is required for lipid presentation by human CD1b. Nat. Immunol. 2004b;5:169–174. doi: 10.1038/ni1035. [DOI] [PubMed] [Google Scholar]
- Winau F, Hegasy G, Kaufmann SH, Schaible UE. No life without death— Apoptosis as prerequisite for T cell activation. Apoptosis. 2005;10:707–715. doi: 10.1007/s10495-005-2940-6. [DOI] [PubMed] [Google Scholar]
- Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles cross-prime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Winkelmann J, Leippe M, Bruhn H. A novel saposin-like protein of Entamoeba histolytica with membrane-fusogenic activity. Mol. Biochem. Parasitol. 2006;147:85–94. doi: 10.1016/j.molbiopara.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Wright CS, Li SC, Rastinejad F. Crystal structure of human GM2-activator protein with a novel beta-cup topology. J. Mol. Biol. 2000;304:411–422. doi: 10.1006/jmbi.2000.4225. [DOI] [PubMed] [Google Scholar]
- Wright CS, Zhao Q, Rastinejad F. Structural analysis of lipid complexes of GM2-activator protein. J. Mol. Biol. 2003;331:951–964. doi: 10.1016/s0022-2836(03)00794-0. [DOI] [PubMed] [Google Scholar]
- York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu. Rev. Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- Yuan W, Dasgupta A, Cresswell P. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat. Immunol. 2006;7:835–842. doi: 10.1038/ni1364. [DOI] [PubMed] [Google Scholar]
- Yuan W, Qi X, Tsang P, Kang SJ, Illarionov PA, Besra GS, Gumperz J, Cresswell P. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc. Natl. Acad. Sci. USA. 2007;104:5551–5556. doi: 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004a;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004b;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]