Abstract
Objectives
All HIV/hepatitis C virus (HCV)-coinfected patients with chronic HCV infection and ≥ F2 fibrosis should be considered for HCV therapy. This study aimed to determine the rate of HCV treatment uptake among coinfected patients in Europe.
Methods
EuroSIDA patients with viraemic HCV infection were included in the study. Poisson regression was used to identify temporal changes and regional differences in HCV treatment uptake.
Results
A total of 1984 patients were included in the study, with a median follow-up time of 168 months [interquartile range (IQR) 121–204 months]. To date, 501 (25.3%) HIV/HCV-coinfected patients have received HCV therapy. Treatment incidence rose from 0.33 [95% confidence interval (CI) 0.16–0.50] per 100 person-years of follow-up (PYFU) in 1998 to 5.93 (95% CI 4.49–7.38) in 2007, falling to 3.78 (95% CI 2.50–5.07) in 2009. After adjustment, CD4 cell count > 350 cells/μL [incidence rate ratio (IRR) 1.33 (95% CI 1.06–1.67) vs. CD4 count 200−350 cells/μL] and ≥F2 liver fibrosis [IRR 1.60 (95% CI 1.14–2.25; P = 0.0065) vs. < F2 fibrosis] were predictors of anti-HCV treatment initiation. However, 22% of patients who remain untreated for HCV, with fibrosis data available, had ≥F2 fibrosis and should have been considered for treatment, while only 36% of treated patients had ≥F2 fibrosis.
Conclusions
Although treatment incidence for HCV has increased, there remain a large proportion of patients indicated for treatment who have yet to be treated.
Keywords: EuroSIDA, HIV/HCV coinfection, PEG-interferon, ribavirin, treatment completion
Introduction
The substantial declines in HIV-related mortality, as a consequence of the introduction of combination antiretroviral therapy (cART), have seen liver-related mortality assume increasing importance among HIV-positive individuals 1–3. Progression of liver disease is common with hepatitis C virus (HCV) infection and known to be accelerated in the presence of HIV 4,5. According to current guidelines, all coinfected patients with chronic HCV infection and significant fibrosis (≥F2) should be considered for HCV therapy given their increased risk of death from liver disease 6,7. HCV treatment should preferentially be offered to patients with controlled HIV infection, and therefore it is recommended when CD4 cell counts are above 350 cells/μL and often deferred when counts are below 200 cells/μL 7. However, patients with CD4 cell counts below 200 cells/μL have been shown to respond well to HCV treatment when HIV replication is suppressed 8. Despite these recommendations, the extent to which HIV/HCV-coinfected patients start HCV therapy is not well documented in Europe. Previous studies from 2003–2006, including a EuroSIDA study, have shown that a low proportion of patients are initiating anti-HCV therapy, typically less than 10% 9–12.
This study includes data up to 2010, when the gold standard treatment for HCV infection was a combination of pegylated interferon (peg-IFN) and ribavirin 6. However, HCV treatment is not generally well tolerated and response-guided therapy is used to determine whether treatment should continue for the full duration 7.
The aims of this study were to describe the temporal changes and regional differences in the uptake of anti-HCV therapy and to identify factors associated with treatment across Europe, documenting the rate of completion of full treatment duration and factors that predict completing therapy. In a subset of patients with available data, we also aimed to identify whether patients with significant fibrosis, in most urgent need of treatment, were selected for treatment.
Study participants
The EuroSIDA study is a large prospective, observational cohort study of 18 295 HIV-positive individuals in 105 centres across Europe, Israel and Argentina. The study has been described in detail previously 13. HCV antibody (HCVAb) status has been collected since 1997; persons who died or were lost to follow-up before this date did not routinely have HCVAb status collected. Centres that have determined HCV genotype or measured HCV RNA are requested to provide that information to the coordinating centre. Information on the collection of HCVAb, HCV RNA and genotype data has been given in detail elsewhere 14,15.
Data on alanine transaminase (ALT), aspartate transaminase (AST) and platelet counts have been collected since 1999 and 2005, respectively, while data on liver biopsy and Fibroscan® (Echosens S.A.S.U, Paris, France) 16 have been collected since 2010, with sites requested to list all previous test results where liver biopsy was graded using the METAVIR scoring system 17 and return the histological report for internal validation. Plasma hyaluronic acid (HA) has been measured in all HCVAb-positive patients with stored plasma samples; HA data collection is described elsewhere 18.
Statistical methods
All HCVAb-positive individuals positive for quantitative HCV RNA were included in these analyses. Baseline was defined as the date of first HCVAb-positive test result or recruitment to EuroSIDA, whichever occurred later. In analyses with the outcome of initiating HCV therapy, patients were followed up to their last visit, death or the date of starting HCV therapy, defined as treatment with at least interferon-α (peg-IFN) plus or minus ribavirin.
Trends over time in starting HCV therapy were described using Poisson regression models. Factors associated with HCV treatment uptake were investigated in univariable analyses and those that were significant with P < 0.1 were included in multivariable models. Investigated factors included age, gender, ethnic group, region of Europe, HIV transmission risk group, prior AIDS diagnosis, time-updated CD4 cell count, time-updated HIV RNA, time-updated HCV RNA, HCV genotype, time-updated hepatitis B virus surface antigen (HBsAg) status, starting cART, calendar year and time-updated alanine transaminase (ALT) levels.
Fibrosis levels among treated and untreated patients were summarized. As fibrosis progression rates have been shown to be slow in this population 19, fibrosis markers up to 2 years prior to initiation of HCV treatment were included, along with the last available measurement in those who remained untreated. The proportions of treated and untreated patients with significant fibrosis were compared using a combined definition of ≥F2 fibrosis from biopsy and Fibroscan, HA > 100 ng/mL and an AST to platelet ratio index (APRI 20) > 1.5, which have been shown to be accurate markers of significant fibrosis 21,22. A Fibroscan reading of > 7.6 kPa was used to identify ≥F2 fibrosis, in accordance with a recent review of the subject 23.
Completion of a full course of HCV therapy is defined dependent on HCV genotype, with a ‘full course’ considered to have been completed after a minimum of 48 weeks for genotype 1 or 4 and after a minimum of 24 weeks for genotype 2 or 3 6. However, because patients may have shorter treatment duration in the clinical setting, we also considered patients who completed at least 80% of the expected minimum treatment duration to have completed therapy, in line with previous studies concerning HCV treatment duration (i.e. 38.4 weeks for genotype 1 or 4 and 19.2 weeks for genotype 2 or 3) 24,25. The median length of treatment duration by HCV genotype, along with the percentage of patients completing a full course of treatment, were determined in patients with known HCV genotype and sufficient follow-up to have completed 80% of treatment.
Predictors of completing a full course of HCV therapy were determined using logistic regression models adjusted for age, gender, ethnic group, HIV transmission group, region of Europe, baseline cART use, prior AIDS diagnoses, HCV genotype, CD4 cell count at treatment initiation, HIV viral load at treatment initiation, HCV viral load at treatment initiation and calendar year of treatment initiation.
All analyses were performed using sas (version 9.2; SAS Institute, Cary, NC).
Results
Of the 4224 HCVAb-positive patients in EuroSIDA, 2633 had available HCV RNA measurements, of whom 2008 (76.3%) were HCV RNA positive and 1984 either never received HCV therapy or did so after baseline and were eligible for inclusion in this analysis. Median follow-up time was 168 months [interquartile range (IQR) 121–204 months]. In multivariable logistic regression, HCVAb-positive patients without HCV RNA data available compared to those with HCV RNA data available had lower CD4 cell counts at baseline [adjusted odds ratio (aOR) 0.91 (95% confidence interval (CI) 0.87–0.96; P = 0.0002) per doubling], were more likely to come from Eastern Europe [aOR 6.45 (95% CI 4.63–8.99; P < 0.0001) compared with Southern Europe] and were recruited to EuroSIDA later [aOR 1.09 (95% CI 1.07–1.12; P < 0.0001) per year later].
Table 1 shows the patient characteristics at baseline and at the date of HCV treatment initiation or last follow-up according to whether treated for HCV infection. A higher proportion of treated patients resided in Southern Europe (41.1% vs. 33.0% for untreated patients; P = 0.0039) and belonged to the men who have sex with men (MSM) HIV transmission group (12.1% vs. 8.4%, respectively), while a lower proportion of treated patients belonged to the injecting drug use (IDU) HIV transmission group (68.9% vs. 74.5%, respectively; global P = 0.09). At the time of anti-HCV treatment initiation or last follow-up for those not yet treated, ALT levels were higher in treated patients [median 76 (IQR 49−120) vs. 44 (37–49) U/L for untreated patients; P < 0.0001], as were CD4 cell counts [median 479 (IQR 349– 650) vs. 391 (227–614) cells/μL, respectively; P < 0.0001].
Table 1.
Patient characteristics at baseline and date of last follow-up or hepatitis C virus (HCV) treatment
| At baseline | At last follow-up or treatment | ||||||
|---|---|---|---|---|---|---|---|
| Untreated (n = 1483) | Treated (n = 501) | P-value* | Untreated (n = 1483) | Treated (n = 501) | P-value* | ||
| Age (years) [median (IQR)] | 33 (28−38) | 32 (28–37) | 0.29 | 44 (37–49) | 41 (35–46) | < 0.0001 | |
| Male (%) | 67.9 | 72.6 | 0.048 | ||||
| White (%) | 91.5 | 93.1 | 0.27 | ||||
| Region of Europe (%) | South | 33 | 41.1 | 0.0039 | |||
| West Central | 21.6 | 18.1 | |||||
| North | 17.5 | 12.9 | |||||
| East Central | 16.2 | 17.7 | |||||
| East | 11.7 | 10.3 | |||||
| HIV transmission group (%) | MSM | 8.4 | 12.1 | 0.09 | |||
| IDU | 74.5 | 68.9 | |||||
| Haemophiliac | 3.6 | 4.2 | |||||
| Heterosexual | 10.4 | 11.7 | |||||
| Other | 3.1 | 3.2 | |||||
| HCV genotype (%) | 1 | 45.2 | 41.2 | 0.084 | |||
| 2 | 2.8 | 2.2 | |||||
| 3 | 23.4 | 28.8 | |||||
| 4 | 11.9 | 13.5 | |||||
| Unknown | 16.7 | 14.5 | |||||
| HBsAg status (%) | Negative | 60.8 | 65.1 | 0.21 | 85 | 88.7 | 0.12 |
| Positive | 4.3 | 4.2 | 8.3 | 6.2 | |||
| Unknown | 34.9 | 30.8 | 6.7 | 5.2 | |||
| Started cART (%) | 21.1 | 26.4 | 0.014 | 86.7 | 86.3 | 0.85 | |
| ALT [units/litre (U/L)] | n (%) | 418 (28.2) | 38 (7.6) | 1308 (88.2) | 409 (81.6) | ||
| Median (IQR) | 51 (26–83) | 49.5 (30–106) | 0.44 | 44 (37–49) | 76 (49–120) | < 0.0001 | |
| CD4 count (cells/μL) [median (IQR)] | 268.5 (145–400) | 290 (158.5–429) | 0.017 | 391 (227–614) | 479 (349–650) | < 0.0001 | |
| HIV RNA < 500 copies/mL [% (95% CI)] | 26.8 (24.5–29.1) | 34.3 (30.2–38.5) | 0.0013 | 71.2 (68.8–73.5) | 80.3 (76.7–83.9) | < 0.0001 | |
| HCV RNA < 800 000 IU/mL [% (95% CI)] | 57.8 (55.3–60.3) | 59.7 (55.4–64.0) | 0.45 | 55.9 (53.4–58.5) | 57.9 (53.2–62.6) | 0.46 | |
ALT, alanine transaminase; cART, combination antiretroviral therapy; CI, confidence interval; HBsAg, hepatitis B virus surface antigen; IDU, injecting drug use; IQR, interquartile range; MSM, men who have sex with men.
*P-values for comparison of proportions or medians.
Uptake of HCV therapy
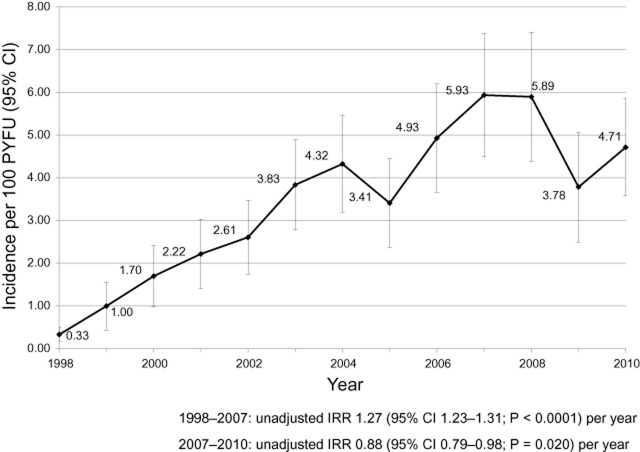
In total, 501 of 1984 patients (25.3%) have so far received HCV therapy in 18 303 person-years of follow-up (PYFU), giving an overall incidence of treatment of 2.74 per 100 PYFU (95% CI 2.50–2.97). Figure 1 shows how the incidence of treatment has changed over time. The overall incidence of treatment increased from 0.33 per 100 PYFU (0.16–0.50) in 1998 to 5.93 (4.49–7.38) in 2007 before falling to 3.78 (2.50–5.07) in 2009. In univariable Poisson regression models, the incidence of treatment increased by 27% per year (95% CI 23–31%; P < 0.0001) between 1998 and 2007, and fell by 12% per year (95% CI 2−21%; P = 0.020) between 2007 and 2010.
Figure 1.
Temporal change in the incidence of uptake of hepatitis C virus (HCV) treatment. The incidence rate ratio (IRR) was calculated from univariable Poisson regression. CI, confidence interval; PYFU, person-years of follow-up.
Table 2 shows univariable and multivariable Poisson regression parameter estimates for predictors of HCV treatment. In multivariable models, patients selected for treatment were more likely than untreated patients to reside in Southern Europe [incidence rate ratio (IRR) 1.38 (95% CI 1.06–1.82; P = 0.019) compared with Western Europe] and belong to the MSM HIV transmission group [IRR 1.36 (95% CI 1.00–1.83; P = 0.046) compared with IDU]. Treated patients were also more likely to have current CD4 cell counts > 350 cells/μL [IRR 1.33 (95% CI 1.06–1.67; P = 0.013) compared with CD4 counts between 200 and 350 cells/μL] and less likely to have CD4 cell counts < 200 cells/μL [IRR 0.42 (0.27–0.65; P = 0.0001) compared with CD4 counts between 200 and 350 cells/μL].
Table 2.
Univariable and multivariable Poisson parameter estimates for factors associated with anti-hepatitis C virus (HCV) treatment initiation
| Variable | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| Estimate | 95% confidence interval | P-value | Estimate | 95% confidence interval | P-value | ||
| Calendar year: 1998 | vs. 2003–2004 | 0.07 | (0.04–0.12) | < 0.0001 | 0.11 | (0.06–0.21) | < 0.0001 |
| 1999–2000 | vs. 2003–2004 | 0.48 | (0.34–0.68) | < 0.0001 | 0.43 | (0.29–0.65) | 0.0001 |
| 2001–2002 | vs. 2003–2004 | 0.89 | (0.68–1.16) | 0.39 | 0.65 | (0.47–0.89) | 0.0074 |
| 2005–2006 | vs. 2003–2004 | 1.72 | (1.37–2.15) | < 0.0001 | 0.97 | (0.73–1.29) | 0.85 |
| 2007–2008 | vs. 2003–2004 | 2.65 | (2.15–3.27) | < 0.0001 | 1.28 | (0.98–1.68) | 0.074 |
| 2009–2010 | vs. 2003–2004 | 1.68 | (1.33–2.12) | < 0.0001 | 0.82 | (0.61–1.11) | 0.20 |
| South | vs. West Central Europe | 1.17 | (0.98–0.40) | 0.081 | 1.38 | (1.06–1.82) | 0.019 |
| North | vs. West Central Europe | 0.74 | (0.57–0.95) | 0.021 | 1.02 | (0.73–1.44) | 0.89 |
| East Central | vs. West Central Europe | 1.20 | (0.96–1.51) | 0.12 | 1.03 | (0.74–1.43) | 0.85 |
| East | vs. West Central Europe | 1.38 | (1.03–1.84) | 0.030 | 1.18 | (0.78–1.78) | 0.45 |
| Started cART* | 3.29 | (2.54–4.26) | < 0.0001 | 1.33 | (0.93–1.90) | 0.12 | |
| CD4 count < 200 cells/μL* | vs. CD4 count 200−350 cells/μL | 0.21 | (0.14–0.32) | < 0.0001 | 0.42 | (0.27–0.65) | 0.0001 |
| CD4 > 350 cells/μL* | vs. CD4 count 200−350 cells/μL | 2.86 | (2.35–3.48) | < 0.0001 | 1.33 | (1.06–1.67) | 0.013 |
| HIV RNA < 500 copies/mL* | vs. ≥ 500 copies/mL | 3.08 | (2.51–3.78) | < 0.0001 | 1.39 | (1.07–1.80) | 0.012 |
| HCV RNA > 800 000 IU/mL* | vs. HCV RNA 616 to 800 000 IU/mL | 1.90 | (1.59–2.29) | < 0.0001 | 1.21 | (1.00–1.47) | 0.049 |
| MSM | vs. IDU | 1.48 | (1.13–1.94) | 0.0042 | 1.36 | (1.00–1.83) | 0.046 |
| Heterosexual | vs. IDU | 1.17 | (0.89–1.53) | 0.27 | 1.19 | (0.90–1.59) | 0.22 |
| Other | vs. IDU | 1.07 | (0.76–1.51) | 0.68 | 1.25 | (0.88–1.78) | 0.21 |
| HCV genotype 2 | vs. HCV genotype 1 | 0.75 | (0.41–1.36) | 0.35 | 1.05 | (0.56–1.94) | 0.89 |
| HCV genotype 3 | vs. HCV genotype 1 | 1.26 | (1.04–1.53) | 0.018 | 1.20 | (0.96–1.49) | 0.11 |
| HCV genotype 4 | vs. HCV genotype 1 | 1.09 | (0.84–1.41) | 0.51 | 1.20 | (0.90–1.59) | 0.21 |
| HCV genotype unknown | vs. HCV genotype 1 | 0.92 | (0.72–1.19) | 0.53 | 1.02 | (0.77–1.34) | 0.91 |
| NR < ALT < 3 times NR | vs. upper limit NR | 2.93 | (2.46–3.49) | < 0.0001 | 2.33 | (1.83–2.96) | < 0.0001 |
| ALT > 3 times NR | vs. upper limit NR | 3.39 | (2.65–4.34) | < 0.0001 | 3.56 | (2.61–4.86) | < 0.0001 |
| ALT unknown | vs. upper limit NR | 0.25 | (0.20–0.31) | < 0.0001 | 1.62 | (1.17–2.25) | 0.0039 |
The model was also adjusted for age, gender, race and hepatitis B virus surface antigen (HBsAg) status.
ALT, alanine transaminase; cART, combination antiretroviral therapy; IDU, injecting drug use; MSM, men who have sex with men; NR, normal range for ALT, defined as < 50 U/L for men and < 40 U/L for women; 3 times NR, 3 times the normal range.
*Time-updated variable.
Compared with untreated patients, treated patients more often had current HIV RNA levels below 500 HIV-1 RNA copies/mL [IRR 1.39 (95% CI 1.07–1.80; P = 0.012) compared with HIV RNA > 500 copies/mL], indicating well-controlled HIV infection with cART. At the time of treatment, treated patients were also more likely than untreated patients to have HCV RNA > 800 000 IU/mL [IRR 1.21 (95% CI 1.00–1.47; P = 0.049) compared with HCV RNA between 616 and 800 000 IU/mL] and raised ALT levels [upper normal range (uNR) < ALT < 3 times uNR, IRR 2.33 (95% CI 1.83–2.96; P < 0.0001); ALT > 3 times uNR, IRR 3.56 (95% CI 2.61–4.86; P < 0.0001) compared with ALT within the normal range]. In the multivariable model, the calendar year effect was similar to that shown in Figure 1, with the incidence of treatment increasing until the years 2007–2008 before falling in 2009–2010. However, in the multivariable model, taking changes in patient characteristics into account, changes in the incidence of treatment from 2003/2004 onwards were not significant [2005/2006, IRR 0.97 (95% CI 0.73–1.29; P = 0.85); 2007/2008, IRR 1.28 (95% CI 0.98–1.68; P = 0.074), and 2009/2010, IRR 0.82 (95% CI 0.61–1.11; P = 0.20) compared with 2003/2004].
The proportion of patients treated for HCV infection did not differ greatly for HCV genotypes 1 to 4 (23.6, 21.2, 29.5 and 27.8%, respectively; P = 0.084). Removing patients who contracted HIV infection via the MSM route, on the assumption that they were acutely infected with HCV, did not change the proportions treated (23.2, 20.9, 28.0 and 26.2%, respectively; P = 0.29), and in the multivariable model HCV genotype was not significantly associated with treatment initiation (Table 2).
Interactions between calendar year and region of Europe (P = 0.48) and calendar year and HIV transmission group (P = 0.80) were both nonsignificant, indicating that there were no significant differences in the increasing incidence of HCV treatment uptake across regions of Europe and HIV transmission groups, although the power to detect such differences was limited.
Liver fibrosis levels
Fibrosis markers were available in 800 of 1984 patients (40.3%) included in this study and are summarized in Table 3. Patients without fibrosis data were more likely to be MSM [odds ratio (OR) 1.53 (95% CI 1.07–2.18; P = 0.019) vs. IDU], reside in Northern Europe [OR 1.54 (95% CI 1.12–2.13; P = 0.0007) vs. Western Europe] and have lower CD4 cell counts at treatment or last follow-up [OR 0.72 (95% CI 0.67–0.79; P < 0.0001) per doubling]. Similar proportions of treated and untreated patients with biopsy/Fibroscan data available had ≥F2 fibrosis (43.9% vs. 40.8%, respectively; P = 0.65). Median HA levels were higher among treated patients (41.2 vs. 28.4 ng/mL, respectively; P = 0.015) and a higher proportion also had HA > 100 ng/mL (25.5% vs. 12.3%, respectively; P = 0.011). There was no statistical difference between the median APRI score of treated patients and that of untreated patients (0.78 vs. 0.94, respectively; P = 0.63), or the proportion of patients with APRI scores > 1.5 (24.1% vs. 29.0%, respectively; P = 0.55).
Table 3.
Fibrosis marker information prior to hepatitis C virus (HCV) treatment and at last available measurement for those untreated for HCV infection
| Fibrosis marker | Treated (n = 501) | Untreated† (n = 1154) | P-value | |
|---|---|---|---|---|
| Fibroscan/biopsy | n (%) | 66 (13.2) | 184 (15.9) | |
| < F2 | 37 (56.1) | 109 (59.2) | 0.65 | |
| ≥F2 | 29 (43.9) | 75 (40.8) | ||
| Hyaluronic acid | n (%) | 47 (9.4) | 488 (42.3) | |
| Median (IQR) (ng/mL) | 41.2 (23.4–106.9) | 28.4 (15.2–59.9) | 0.015 | |
| n (%) > 100 ng/mL | 12 (25.5) | 60 (12.3) | 0.011 | |
| APRI | n (%) | 54 (10.8) | 62 (5.4) | |
| Median (IQR) | 0.78 (0.48–1.47) | 0.94 (0.46–1.72) | 0.63 | |
| n (%) > 1.5 | 13 (24.1) | 18 (29.0) | 0.55 | |
| Any marker | n (%) | 150 (29.9) | 650 (56.3) | |
| Significant fibrosis* | 54 (36.0) | 143 (22.0) | 0.0003 | |
| Distribution of mismatched patients (treated without significant fibrosis and untreated with significant fibrosis) | n (%) | 96 | 143 | |
| South | 40 (41.7) | 66 (46.2) | ||
| West | 14 (14.6) | 27 (18.9) | ||
| North | 7 (7.3) | 28 (19.6) | ||
| East | 27 (28.1) | 13 (9.1) | ||
| East Central | 8 (8.3) | 9 (6.3) | ||
| Time prior to treatment/last follow-up that fibrosis measurement was taken | Median (IQR) (months) | 5.7 (2.7–11.9) | 57.1 (12.2–110.9) | < 0.0001 |
For treated patients, fibrosis data were included up to 2 years prior to treatment initiation; for untreated patients, the last available measurement was used.
APRI, aspartate transaminase to platelet ratio index; IQR, interquartile range.
*Significant fibrosis defined using a combined definition of any of ≥F2 fibrosis from Fibroscan/biopsy, HA > 100 ng/mL or APRI > 1.5.
Untreated patients who were alive at last follow-up.
Using a combined definition including all available fibrosis data, a higher proportion of patients treated for HCV infection were found to have significant fibrosis compared with those yet to be treated (41.3% vs. 31.7%, respectively; P = 0.031). In a sensitivity analysis additionally adjusting the model in Table 2 for the time-updated level of fibrosis, patients with ≥F2 fibrosis were 60% more likely to be treated [IRR 1.60 (95% CI 1.14–2.25; P = 0.0065) vs. < F2 fibrosis]. Among those with data, only 36.0% of patients who were treated for HCV infection had significant fibrosis indicating treatment, while 22.0% of patients untreated for HCV had significant fibrosis (Table 3). Both the majority of patients treated for HCV infection without significant fibrosis and the majority of patients with significant fibrosis untreated for HCV infection resided in Southern Europe (41.7% and 46.2%, respectively; Table 3), not unexpectedly, as the majority of patients included in the study were from Southern Europe. However, after additionally adjusting for the interaction between the level of fibrosis and region of Europe in the model in Table 2, we found that patients with ≥F2 fibrosis were less likely to be treated in Southern Europe [IRR 0.37 (95% CI 0.17–0.82; P = 0.014)] and Northern Europe [IRR 0.32 (95% CI 0.11–0.97; P = 0.044)] compared with Western Europe. Although IDU was not found to be associated with treatment in this model, 77% of patients with significant fibrosis who were not treated were IDU.
Completion of full HCV treatment duration
Figure 2 shows the median treatment duration in weeks and the percentage of patients who completed at least 80% of full treatment duration by HCV genotype. After removing those with unknown HCV genotype, 271 of 416 patients (65.1%) completed HCV treatment. In multivariable logistic regression assessing factors associated with completing at least 80% of full HCV treatment duration, patients who were younger [aOR 0.70 (95% CI 0.50–0.98; P = 0.038) per 10 years], with HCV genotype 2/3 [aOR 3.31 (95% CI 1.92–5.70; P < 0.0001) vs. HCV genotype 1] and HIV RNA < 500 copies/mL at treatment initiation [aOR 2.25 (95% CI 1.22–4.17; P = 0.0095) compared with HIV RNA ≥ 500 copies/mL], were more likely to complete a full course of HCV therapy. There was also borderline evidence to suggest that patients with CD4 cell counts < 200 cells/μL at treatment initiation were more likely to complete therapy [aOR 3.24 (95% CI 0.99–10.59; P = 0.051) vs. CD4 count between 200 and 350 cells/μL].
Figure 2.
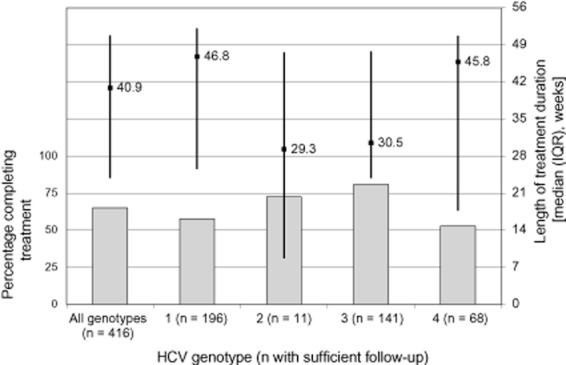
Percentage of patients completing hepatitis C virus (HCV) therapy and the median length of treatment duration by HCV genotype. A total of 416 of 429 patients (97.0%) treated for HCV infection with known HCV genotype had sufficient follow-up to determine whether they achieved the minimum treatment duration. Completion of therapy was defined as completing at least 80% of the minimum expected treatment duration; 38.4 weeks for genotypes 1 and 4, and 19.2 weeks for genotypes 2 and 3. IQR, interquartile range.
Forty-four of 416 patients (10.6%) discontinued treatment before completing 12 weeks of therapy. In a multivariable logistic regression model with the endpoint of discontinuing HCV treatment before 12 weeks of therapy, baseline cART use was associated with a 74% reduction in early HCV treatment termination [aOR 0.26 (95% CI 0.09–0.74; P = 0.012)].
Discussion
Twenty-five per cent of HCV viraemic patients were treated for HCV infection in EuroSIDA between 1998 and 2010. This is a higher proportion than has been reported in other studies where treatment uptake was low 9,10,12,25,26. One of the strengths of this study is that we were able to characterize HCV status using HCVAb and HCV RNA in a large number of patients over a long period of time. In the absence of HCV RNA data, the proportion of patients selected for treatment is likely to be underestimated as the denominator will include aviraemic HCVAb-positive patients. This, along with the length of follow-up (median 168 months) in the current study, explains the increase in treatment uptake observed compared with a previous EuroSIDA study (7.6%), which categorized patients as HCV positive based solely on HCV antibody testing 11, and another previous European study (12%) with a median follow-up of 72 months 26.
In univariable models the incidence of anti-HCV treatment uptake increased by 27% per year between 1998 and 2007 before falling by 12% per year between 2007 and 2010, while in multivariable analysis the changes after 2003/2004 were not statistically significant. The increasing uptake of anti-HCV therapy probably reflects the introduction of peg-IFN, with cure rates of approximately 70% for HCV genotype 2 or 3 and 35% for genotype 1 or 4 8,27–29. The trend of decreasing treatment uptake seen after a peak in 2007 is explained by different patient characteristics and possibly treatment saturation of the easy-to-treat patients eligible for interferon therapy, with patients and physicians choosing to wait for the first generation of directly acting agents for HCV. With positive predictors of HCV treatment success including younger age, HCV RNA < 800 000 IU/mL and non-HCV genotype 1, clinicians and their patients with low fibrosis stages, high HCV RNA or HCV genotype 1 may feel that they should wait for more efficacious HCV therapy on the horizon 30, potentially avoiding the unpleasant side effects of treatment with interferon 31.
Patients initiating HCV therapy had CD4 cell counts > 350 cells/μL, ALT values above the normal range and HIV RNA levels < 500 copies/mL, in line with current treatment guidelines 7. HCV RNA levels > 800 000 IU/mL were also associated with treatment uptake, even though high HCV viral loads have been associated with a poor response to treatment 32. Interestingly, patients residing in Southern compared with Western Europe and those belonging to the MSM HIV transmission group compared with IDU were also more likely to be treated. This does not seem to be explained by the HCV genotype distribution, as a similar proportion of patients were treated in each of the HCV genotype groups and the distribution of genotypes was similar across all regions (results not shown). The higher rate of treatment in Southern Europe could have a variety of explanations. There may be greater clinician experience of dealing with HIV/HCV coinfection in Southern Europe because of the higher prevalence of HCV infection in that region, but also differences in national guidelines and local traditions. That Eastern Europe was not found to have lower rates of treatment is probably attributable to the inclusion criteria in this study; in particular, many patients from that region were excluded as they did not have available HCV RNA data. The observation that MSM were more likely to be treated than IDU can be explained by recent outbreaks of acute HCV infection in this patient population 33,34, with treatment of acute HCV infection associated with much better cure rates than treatment of chronic HCV infection 35,36, while MSM are also considered to be more adherent to treatment, with IDU often a contraindication of therapy.
We found that a higher proportion of patients treated for HCV infection had significant fibrosis prior to treatment, as evaluated using liver biopsy, Fibroscan, HA and APRI data, compared with those yet to be treated at last follow-up. In sensitivity analysis, significant fibrosis was associated with a 60% increased incidence of treatment initiation; however, only one-third of patients treated for HCV infection were known to have ≥F2 fibrosis, while 22% of untreated patients with fibrosis data had ≥F2 fibrosis and should have been considered for treatment. We allowed a 2-year window prior to treatment for the fibrosis data and used the last available measurement in patients yet to be treated, so it is possible that some patients could have progressed in this time prior to initiating therapy or last follow-up. However, the median time prior to treatment for fibrosis measurements was 5.7 months in treated patients and progression rates have been shown to be slow 19. The median time prior to last follow-up for untreated patients was 57 months and it is more likely that progression would have taken place in these patients, meaning that it is possible that we are underestimating the level of fibrosis in untreated patients. We also found that patients with significant fibrosis were less likely to be treated in Southern and Northern Europe compared with those with significant fibrosis in Western Europe. Although fibrosis data were only available in a subset of the study population, this finding is likely to be at least partially explained by unmeasured confounding where there is contraindication to treatment, with 77% of untreated patients with ≥F2 fibrosis being IDU. However, there is a need for focus on correctly identifying eligible patients for therapy.
The median duration of HCV treatment was close to the guideline minimum duration of therapy for HCV genotype 1 or 4 and surpassed it for genotype 2 or 3. However, one-third of all patients started on therapy discontinued before completing the full treatment duration, with the lowest rates of completion for genotypes 1 and 4. The lower proportion of patients completing 80% of full treatment duration among patients with HCV genotype 1 or 4 reflects the heightened difficulty in treating these patients, with lower response rates and treatment termination expected with response-guided therapy 7.
In adjusted logistic regression models, we found that younger age, HCV genotype 2 or 3 and HIV RNA < 500 copies/mL at HCV treatment initiation were associated with HCV treatment completion. Younger age and HCV genotype 2 or 3 are well-known predictors of treatment success 30, while controlling HIV infection with cART, lowering HIV RNA and raising CD4 cell counts prior to initiating HCV therapy is best practise to ensure the highest chance of completing the full treatment duration, in line with current HCV treatment guidelines 6,7. We also found that initiating cART early during clinical care was associated with a lower rate of early HCV treatment discontinuation.
A limitation to this study is that in practice many patients may stop HCV treatment early because of adverse events, on which we have no data, while others may not be treated because of contraindication. Further, we have described the rate of significant fibrosis where biopsy and biomarker data are available. Although the biomarkers considered are well described, they will be less precise than biopsy at determining significant fibrosis and should be interpreted accordingly.
In conclusion, we have reported an increase in the incidence of treatment for HCV infection in EuroSIDA, with those selected for treatment mostly aligned with current treatment guidelines. However, there were marked regional differences and important deviations from the guidelines, as two-thirds of treated patients were without significant fibrosis. We have also shown that younger age, HCV genotypes 2 and 3 and undetectable HIV RNA at HCV treatment initiation are all associated with completing HCV therapy. Although 25% of those with documented viraemic HCV infection have undergone anti-HCV treatment, there remain patients, including some with significant fibrosis, who have not been exposed to anti-HCV treatment. Future studies of the reasons for this are warranted.
Acknowledgments
Conflicts of interest: OK has received honorarium, consultancy and/or lecture fees from Abbott, Gilead, GSK, Janssen, Merck, Tibotec and Viiv. AM has received honorarium, consultancy or guest speaker fees from Pfizer, Merck, Gilead, BI and BMS. JKR has received consultancy or lecture fees from Bionor, BMS, BI, GSK, ViiV, Abbott, Gilead, Pfizer, Merck, Tibotec and Janssen. MB has received honoraria for consultancy from BMS, BI, ViiV, Abbott, Gilead, Pfizer, Merck, Tibotec and Janssen. All other authors have no conflicts of interest to report.
Author contributions
DG led the study with equal supporting contributions from LP, OK and AM. DG and AM performed the statistical analyses. DG, JDL, JKR, OK and AM designed the study and DG drafted the manuscript. All co-authors contributed to redrafting and refinement of the manuscript. AM supervised the study.
Statement of funding
Primary support for EuroSIDA is provided by the European Commission BIOMED 1 (CT94-1637), BIOMED 2 (CT97-2713), the 5th Framework (QLK2-2000-00773), the 6th Framework (LSHP-CT-2006-018632), and the 7th Framework (FP7/2007-2013, EuroCoord n° 260694) programmes. Current support also includes unrestricted grants from Gilead, Pfizer, BMS, and Merck and Co. The participation of centres from Switzerland was supported by The Swiss National Science Foundation (Grant 108787).
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
The EuroSIDA study group.
References
- Gill J, May M, Lewden C, et al. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocroft A, Reiss P, Gasiorowski J, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. Jaids-J Acquir Immune Defic Syndr. 2010;55:262–270. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- Smit C, van den Berg C, Geskus R, Berkhout B, Coutinho R, Prins M. Risk of hepatitis-related mortality increased among hepatitis C virus/HIV-coinfected drug users compared with drug users infected only with hepatitis C virus – a 20-year prospective study. Jaids-J Acquir Immune Defic Syndr. 2008;47:221–225. doi: 10.1097/QAI.0b013e31815d2f59. [DOI] [PubMed] [Google Scholar]
- Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–88. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Opravil M, Sasadeusz J, Cooper DA, et al. Effect of baseline CD4 cell count on the efficacy and safety of peginterferon Alfa-2a (40KD) plus ribavirin in patients with HIV/hepatitis C virus coinfection. J Acquir Immune Defic Syndr. 2008;47:36–49. doi: 10.1097/QAI.0b013e31815ac47d. [DOI] [PubMed] [Google Scholar]
- Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97–100. doi: 10.1086/344907. [DOI] [PubMed] [Google Scholar]
- Fultz SL, Justice AC, Butt AA, Rabeneck L, Weissman S, Rodriguez-Barradas M. Testing, referral, and treatment patterns for hepatitis C virus coinfection in a cohort of veterans with human immunodeficiency virus infection. Clin Infect Dis. 36:1039–1046. doi: 10.1086/374049. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Rockstroh J, Soriano V, et al. Limited but increasing use of treatment for hepatitis C across Europe in patients coinfected with HIV and hepatitis C. Scand J Infect Dis. 2003;38:1092–1097. doi: 10.1080/00365540600786515. 2006. [DOI] [PubMed] [Google Scholar]
- Rauch A, Egger M, Reichen J, Furrer H. Chronic hepatitis C in HIV-infected patients: low eligibility and applicability of therapy with pegylated interferon-alpha plus ribavirin. Jaids-J Acquir Immune Defic Syndr. 2005;38:238–240. doi: 10.1097/01.qai.0000148535.97081.72. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- Grint D, Peters L, Reekie J, et al. Stability of hepatitis C virus (HCV) RNA levels among interferon-na+»ve HIV/HCV-coinfected individuals treated with combination antiretroviral therapy. HIV Med. 2013 doi: 10.1111/hiv.12033. [DOI] [PubMed] [Google Scholar]
- Soriano V, Mocroft A, Rockstroh J, et al. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008;198:1337–1344. doi: 10.1086/592171. [DOI] [PubMed] [Google Scholar]
- Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- Peters L, Mocroft A, Soriano V, et al. Hyaluronic acid levels predict risk of hepatic encephalopathy and liver-related death in HIV/viral hepatitis coinfected patients. Plos One. 2013;8:e64283. doi: 10.1371/journal.pone.0064283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. Aids. 2008;22:1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- Peters L, Rockstroh JK. Biomarkers of fibrosis and impaired liver function in chronic hepatitis C: how well do they predict clinical outcomes? Curr opin HIV AIDS. 2010;5:517–523. doi: 10.1097/COH.0b013e32833e3ee6. [DOI] [PubMed] [Google Scholar]
- Resino S, Bellon J, Asensio C, et al. Can serum hyaluronic acid replace simple non-invasive indexes to predict liver fibrosis in HIV/Hepatitis C coinfected patients? BMC Infect Dis. 2010;10:244. doi: 10.1186/1471-2334-10-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resino S, S+ínchez-Conde M, Berenguer J. Coinfection by human immunodeficiency virus and hepatitis C virus: noninvasive assessment and staging of fibrosis. Curr Opin Infect Dis. 2012;25:564–569. doi: 10.1097/QCO.0b013e32835635df. [DOI] [PubMed] [Google Scholar]
- Beste LA, Ioannou GN, Larson MS, Chapko M, Dominitz JA. Predictors of early treatment discontinuation among patients with genotype 1 hepatitis C and implications for viral eradication. Clin Gastroenterol Hepatol. 2010;8:972–978. doi: 10.1016/j.cgh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–325. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Effect of hepatitis C treatment on CD4+ T-cell counts and the risk of death in HIV-HCV-coinfected patients: the COHERE collaboration. Antivir Ther. 2012;17:1541–1550. doi: 10.3851/IMP2263. [DOI] [PubMed] [Google Scholar]
- Carrat F. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA (Chicago, Ill) 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguno M. Randomized trial comparing pegylated interferon α-2b versus pegylated interferon α-2a, both plus ribavirin, to treat chronic hepatitis C in human immunodeficiency virus patients. Hepatology. 2009;49:22–31. doi: 10.1002/hep.22598. [DOI] [PubMed] [Google Scholar]
- Ingiliz P. HIV-HCV co-infection facing HCV protease inhibitor licensing: implications for clinicians. Liver Int. 2012;32:1194–1199. doi: 10.1111/j.1478-3231.2012.02796.x. [DOI] [PubMed] [Google Scholar]
- Naggie S. Management of patients coinfected with HCV and HIV: a close look at the role for direct-acting antivirals. Gastroenterology. 142:1324–1334. doi: 10.1053/j.gastro.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2012;139:120–U178. doi: 10.1053/j.gastro.2010.04.013. 2010. [DOI] [PubMed] [Google Scholar]
- Rauch A, Martin M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV cohort study. Clin Infect Dis. 2005;41:395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–238. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- Deterding K. Early versus delayed treatment of acute hepatitis C: final results of the randomized controlled German hep-net acute HCV-III study. J Hepatol. 2012;56:S21. [Google Scholar]
- Gerlach JT. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. doi: 10.1016/s0016-5085(03)00668-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The EuroSIDA study group.