Abstract
Background
While more than 50% of smokers make a serious quit attempt each year, less than 10% quit permanently. Evidence from studies of adolescent smoking and other substances of abuse suggest that alternative reinforcers, a construct of Behavioral Economic Theory, may contribute to the likelihood of smoking cessation in adults. This study examined the behavioral economics of smoking cessation within a smoking cessation clinical trial and evaluated how depressive symptoms and behavioral economic variables are associated with smoking cessation.
Methods
A sample of 469 smokers, enrolled in an effectiveness trial that provided counseling and 8 weeks of 21mg nicotine patches, was analyzed. Alternative reinforcers (substitute and complementary reinforcers) and depressive symptoms were examined in relation to 7-day point prevalence abstinence, verified with breath carbon monoxide, 8 weeks after the quit date.
Results
Controlling for covariates associated with cessation (nicotine dependence, age of smoking initiation, patch adherence), participants who were abstinent at week 8 showed significantly higher substitute reinforcers at all time-points, compared to those who were smoking (p’s < .05). Participants who were abstinent at week 8 showed lower complementary reinforcers and depressive symptoms at all time-points, compared to those who were smoking, but significant differences were confined to week 8 (p’s < .01). There was no significant interaction between alternative reinforcers and depressive symptoms across the eight weeks on week 8 abstinence.
Conclusions
These results support continued examination of Behavioral Economic Theory in understanding adult smoking cessation in order to inform future treatments and guidelines.
Keywords: smoking cessation, behavioral economics, alternative reinforcers, depression
1. INTRODUCTION
Despite widespread awareness of the harms of cigarette smoking, about 19% of the US population continues to smoke (Centers for Disease Control and Prevention (CDC), 2012). Approximately 70% of current smokers would like to quit smoking (CDC, 2002) and 52% make a serious quit attempt each year (CDC, 2011). However, approximately 80% of smokers who make an unassisted quit attempt relapse within the first month (Benowitz, 2009) and only about one-third of smokers who use medications to quit smoking are successful (Stead and Lancaster, 2012). Novel models of nicotine dependence treatment are needed to address the relatively stable national prevalence of cigarette smoking seen over the last seven years (CDC, 2012).
While nicotine is widely considered the primary reinforcing element of cigarette smoking, Behavioral Economic Theory posits that the choice to engage in a rewarding behavior, such as smoking, also depends on access to, and the availability of, alternative reinforcers, and that alternative reinforcers can enhance or reduce the reinforcing value of smoking (Green and Freed, 1993). Alternative reinforcers are other rewarding events, feelings, actions, and behaviors that people perceive as meaningful (Johnson and Bickel, 2003). There are two types of alternative reinforcers relevant to smoking behavior. Substitute reinforcers are behaviors or products that replace and decrease the likelihood of smoking. While substitute reinforcers are often not physically similar to smoking, these behaviors may have other characteristics that are shared with smoking (Green and Fisher, 2000). For example, physical activity and smoking are both used for weight management (Byrne and Byrne, 1993; Camp et al., 1993), indicating that physical activity may be a suitable substitute reinforcer for smoking. Complementary reinforcers are behaviors or stimuli that an individual associates with smoking, therefore increasing the likelihood of smoking. In the presence of complementary reinforcers, such as alcohol, coffee, or other smokers, the reinforcing value of smoking is increased, and the likelihood of smoking is subsequently increased. Thus, individuals trying to quit smoking may be more likely to succeed if they increase substitute reinforcers and decrease complementary reinforcers.
The influence of both types of alternative reinforcers is relevant for many different substances of abuse (Higgins et al., 2004). However, literature on the behavioral economics of cigarette smoking is limited. In a longitudinal study, Audrain-McGovern et al. (2004) found that substitute reinforcers decreased the odds of adolescent smoking uptake and progression nearly two-fold, while complementary reinforcers increased the odds of adolescent smoking progression by 14%. In a separate study, young adult smokers in a cessation program who had an increase in substitute reinforcers during the treatment period were nearly twice as likely to quit smoking at the end of treatment (Audrain-McGovern et al., 2009). While studies of adolescents, human laboratory studies (Epstein et al., 1991; Bickel et al., 1992, 1995; MacKillop et al., 2012; Acker and MacKillop, 2013), and studies using financial incentives to promote smoking abstinence (e.g., Volpp et al., 2009) have demonstrated the influence of alternative reinforcers on smoking behavior, to our knowledge, the relationship between self-reported, naturally occurring alternative reinforcers (vs. study-related financial incentives) and smoking cessation has yet to be studied within a clinical trial of adult treatment-seeking smokers.
Pre-treatment depressive symptoms and increases in depressive symptoms during treatment predict poor cessation outcomes (Covey et al., 1990; Zelman et al., 1992; Niaura et al., 2001; Burgess et al., 2002; Kahler et al., 2002; Berlin and Covey, 2006). However, few studies have assessed the relationship between depression and alternative reinforcers in the context of nicotine dependence. Depression, even at subclinical levels, is associated with a reduction in substitute reinforcers (Lewinsohn and MacPhillamy, 1974; Lewinsohn and Amenson, 1978; Lewinsohn et al., 1998; Audrain-McGovern et al., 2011), but limited research exists regarding this relationship in the context of smoking. This reduction in substitute reinforcers may increase the reinforcing value of smoking, as smoking may provide a pleasurable and stimulating experience that is otherwise lacking in an individual’s life (Green and Fisher, 2000; Perkins et al., 2000). Thus, the combination of depressive symptoms and a reduction in substitute reinforcers may decrease the likelihood of smoking cessation (Audrain-McGovern et al., 2009).
This study sought to: 1) determine whether changes in substitute and complementary reinforcers during a smoking cessation program were associated with smoking cessation outcomes, 2) determine whether changes in depressive symptoms during a smoking cessation program were associated with smoking cessation outcomes, and 3) evaluate the potential interaction of alternative reinforcers and depressive symptoms on smoking cessation outcomes. Based on previous research, it was expected that higher rates of cessation would be associated with an increase in substitute reinforcers, a decrease in complementary reinforcers, and a decrease in depressive symptoms during the cessation attempt. It was also expected that depressive symptoms and alternative reinforcers might interact to determine cessation outcomes, such that the cessation rate would be the highest among participants who showed a decrease in depressive symptoms in addition to either an increase in substitute reinforcers or a decrease in complementary reinforcers. The results of this investigation may guide future studies in the use of Behavioral Economic Theory to further understand adult smoking cessation.
2. METHODS
2.1 Participants
Participants were recruited via advertisements for a free smoking cessation study at two urban academic settings in major Northeast and Midwest U.S. cities (ClinicalTrials.gov Identifier: NCT01047527). At each site, interested individuals called a central telephone number to inquire about the study, and an evaluation of study interest and initial eligibility was completed by phone.
Eligible participants were at least 18 years of age, reported smoking at least 10 cigarettes per day, and were interested in smoking cessation treatment. Participants were excluded if they had a current medical problem for which transdermal nicotine is contraindicated (e.g., uncontrolled hypertension, allergy to latex), had a heart attack in the past 6 months, a lifetime DSM-IV diagnosis of psychosis, bipolar disorder, or current suicidality as identified by the Mini International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 1998), or were unable to communicate in English. Female participants were excluded if they were pregnant, planning a pregnancy, or lactating. Participants underwent a medical history and physical exam to confirm eligibility. The University of Pennsylvania and Northwestern University Institutional Review Boards approved the study procedures.
2.2 Study Procedures
The trial was designed as an effectiveness study, meaning that inclusion and exclusion criteria were limited, no placebo was used, and, overall, the emphasis in the design was on external validity. Participants received 48 weeks of behavioral counseling and were randomized to receive 8, 24, or 52 weeks of transdermal nicotine, concurrent with the counseling. For the present analysis, data were restricted to the first 8 weeks of transdermal nicotine treatment. Thus, all participants received 8 weeks of open-label 21mg transdermal NRT (Nicoderm CQ; GlaxoSmithKline, Research Triangle Park, NC) and behavioral counseling. Participants received an in-person pre-quit counseling session at week-2 (baseline), which focused on preparing for cessation, and set a quit date for week 0, at which time participants were to start using NRT. At weeks 0, 4, and 8, participants received behavioral counseling over the telephone. These counseling sessions, based on standard smoking cessation behavioral treatment (Fiore et al., 2008), focused on managing urges and triggers to smoking and developing strategies to avoid relapse. Substitute and complementary reinforcers were not a targeted area in this counseling program, as this is not currently recommended formally in the aforementioned guidelines. Assessments were conducted at baseline (in-person) and at weeks 0, 4, and 8 (by telephone). Week 8 report of smoking cessation was biochemically confirmed, using breath carbon monoxide (CO).
2.3 Measures
2.3.1 Covariates
At baseline, participants completed self-report measures of demographics (e.g., age, race, sex) and smoking history and behavior (e.g., cigarettes per day, number of previous quit attempts). The Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) was administered to assess participants’ level of nicotine dependence. FTND scores range from 0 to 10, with higher scores reflecting greater dependence.
2.3.2 Pleasant Events Schedule
The Pleasant Events Schedule (PES; MacPhillamy and Lewinsohn, 1982) is a self-report inventory of the frequency and enjoyability of common rewarding activities and events an individual has engaged in over the past 30 days. For this study, the 320-item PES was adapted to measure alternative reinforcers that occur in an individual’s natural environment. Items were collapsed into content classes by a single author (e.g. “artwork” and “photography” were collapsed into “arts and hobbies”), which resulted in 45 items. This version of the PES was based on an adaptation to the PES used previously to evaluate smoking behavior among adolescents (Audrain-McGovern et al., 2009, 2011). The cross product of the frequency score (0=has not happened to 2=happened often) and enjoyability score (0=not pleasurable to 2=very pleasurable) for each item provided a measure of an individual’s reinforcement from that activity, or the “obtained pleasure” rating. Individuals were also asked whether they associate each activity with smoking or the urge to smoke. If the individual associated the activity with smoking, it was considered a complementary reinforcer for that individual (e.g., drinking coffee, drinking alcohol). If the individual did not associate the activity with smoking, it was considered a substitute reinforcer for that individual (e.g., exercise, playing individual sports). The cross products of the substitute reinforcers were summed to provide an individual’s overall index of substitute reinforcers and the cross products of the complementary reinforcers were summed to provide an individual’s overall index of complementary reinforcers. Substitute and complementary reinforcer scores on this measure range from 0 to 180. This coding scheme has been used previously to examine adolescent smoking (Audrain-McGovern et al., 2009; 2011) and this measure has been used to assess rewarding events among substance abusers (Van Etten et al., 1998), the relationship between pleasant activities and mood, and in a variety of studies on the treatment of depression and drug addiction (MacPhillamy and Lewinsohn, 1982). Missing data on the frequency or enjoyability of an item was replaced by the item mean. If participants had ≥25% missing data on the frequency or enjoyability of items designated as substitute or complementary reinforcers, data for the respective scale were considered “missing”. An item was not included in either scale if the designation of the activity as a substitute or complementary reinforcer was not made. In the present study, the Cronbach’s alphas for the PES subscales of frequency, enjoyability, and association with smoking were: .87, .88, and .98, respectively.
2.3.3 Inventory of Depressive Symptomatology
The Inventory of Depressive Symptomatology (IDS; Rush et al., 1986, 1996) is a 30-item self-report measure used to assess the severity and frequency of depressive symptomatology during the 7-day period prior to assessment. The IDS includes all of the criterion symptom domains designated by the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders – 4th Edition (DSM-IV; American Psychiatric Association, 1994) to diagnose a major depressive episode. Each item or symptom is interval scaled from 0 (not present) to 3 (very severe) and the sum of 28 of the 30 item scores creates the total score. IDS scores range from 0 to 84, with higher scores reflecting greater severity of depressive symptoms. The IDS has been used in a variety of research and clinical settings (Rush et al., 1996; Yonkers et al., 2001; Ninan et al., 2002; Rush et al., 2005). In the present study, Cronbach’s alpha for the IDS was .80.
2.3.4 Transdermal Nicotine (Patch) Adherence
Daily patch use from weeks 0 to 8 was assessed at each session using a timeline follow-back measure (Brown et al., 1998). This method has been utilized in other studies to assess retrospective reports of daily patch use (Schnoll et al., 2009). Weekly adherence, defined as wearing the patch for at least 6 days, was utilized in the present analysis, as done previously (Schnoll et al., 2010).
2.3.5 Smoking Behavior
Self-reports of smoking were obtained at each session using the timeline follow-back measure for smoking, which has been shown to have good reliability and validity for assessing retrospective reports of daily smoking (Brown et al., 1998). This procedure uses a standardized methodology to allow for the collection of smoking rate each day between designated assessment time-points. At week 8, all participants were asked to provide a breath sample for biochemical verification of smoking status. Levels of breath carbon monoxide (CO) were measured in parts per million (ppm). Consistent with established guidelines (SRNT Subcommittee on Biochemical Verification, 2002), participants were considered abstinent at week 8 if they self-reported abstinence for 7 days prior to the assessment and provided a breath sample with CO ≤ 10ppm. Participants who withdrew from the study, failed to provide a sample, or provided a CO breath sample greater than 10ppm were considered smokers.
2.4 Statistical Analysis
This is an intent-to-treat analysis, which included all participants who completed the first counseling treatment session (baseline). Sample characteristics, including demographics and smoking history, were examined using descriptive statistics (e.g., mean, standard deviation, proportions). Associations of background variables with abstinence rates were assessed using chi-square tests (for categorical variables) or ANOVA (for continuous variables).
Generalized Estimating Equations (GEE) were used to determine whether changes in substitute and complementary reinforcers from baseline to week 8 were associated with smoking abstinence at week 8. Main and interaction effects for the repeated measures independent variable (i.e., time; baseline, week 0, week 4, week 8) and the between-group independent variable (i.e., smoking status at week 8; smoking or abstinent) were assessed. Similar models were conducted to determine whether changes in severity of depression symptoms from baseline to week 8 were associated with abstinence at week 8. All models included covariates.
To evaluate the interaction between changes in alternative reinforcers and changes in depressive symptoms on abstinence, multivariate logistic regression models were conducted. For this analysis, change in substitute reinforcers, change in complementary reinforcers, and change in depressive symptoms were defined as the difference between baseline and week 8 scores for each variable. All variables were standardized (to have a standard deviation equal to 1) to assist with interpretation.
Multivariate time-to-event models assessed whether changes in substitute and complementary reinforcers between baseline and week 4 were associated with a transition from abstinence to lapse between week 4 and week 8 (Wileyto et al., 2005). This type of alternating state multivariate data consists of times to transition between runs of abstinent days (≥ one day) and runs of smoking days (≥ one day; Hougaard, 2000). Up to 8 cycles of lapse events were evaluated and participants could cycle through multiple events; smoking data were self-report. In these models, Cox regression was used to assess the association between reinforcers and relapse, controlling for covariates, and standard errors were adjusted for repeated measures using the cluster-correlated robust variance estimate (Williams, 2000).
All statistical analyses were completed using SPSS (Version 20.0, IBM Corp., Armonk, New York) and STATA (Version 13.1, StataCorp, College Station, Texas).
3. RESULTS
3.1 Sample Characteristics and Covariates
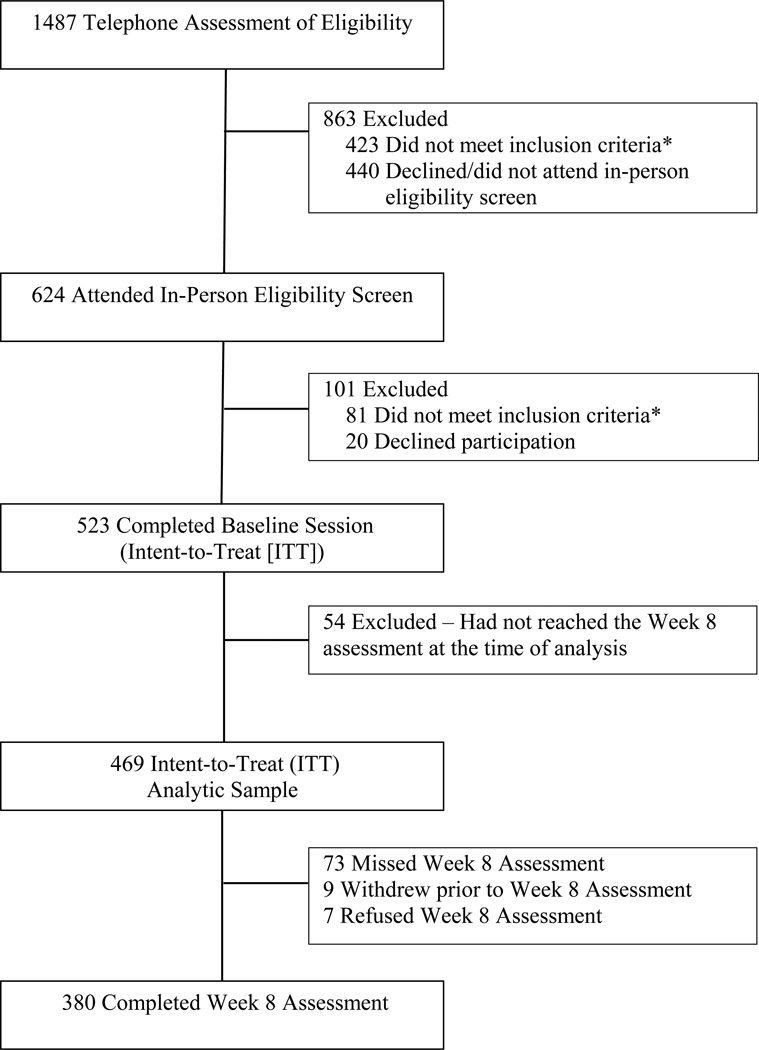
The flow of participants in the study is shown in Figure 1; sample characteristics are shown in Table 1. The overall abstinence rate at week 8 was 30.7%. The mean (SD) CO level for week 8 smokers was 13.4ppm (9.8); the mean (SD) CO level for week 8 abstainers was 3.6ppm (2.6). Two smoking history variables measured at baseline were associated with week 8 smoking behavior. Participants who were smoking at week 8 exhibited higher FTND scores (F[1,469] = 5.31, p < .05) and started smoking at an earlier age (F[1,469] = 15.92, p < .01), compared to participants who were abstinent at week 8. Patch adherence during the entire 8-week treatment period was significantly associated with week 8 abstinence rates (see Table 2). In modeling analyses, patch adherence, defined as the mean number of days the patch was worn, FTND score, and age of smoking initiation were treated as covariates.
Figure 1.
Participant Flow
Note. *A list of the reasons for participant ineligibility can be provided by the authors upon request.
Table 1.
Participant Characteristics (N = 469)
| Characteristic | Week 8 Smokers | Week 8 Abstainers | Total |
|---|---|---|---|
| (n = 325) | (n = 144) | (n = 469) | |
| Sex (% male) | 49.8 | 50.7 | 50.1 |
| Age (mean, SD) | 45.5 (11.6) | 46.7 (12.8) | 45.9 (12.0) |
| Race (% Caucasian) | 53.1 | 42.4 | 49.7 |
| Marital status (% married) | 31.4 | 31.2 | 31.3 |
| Sexual orientation (% heterosexual) | 92.9 | 89.0 | 81.4 |
| Education (% ≤ GED) | 32.3 | 26.4 | 30.5 |
| Income (% ≤ 35,000, annually) | 59.8 | 55.6 | 58.2 |
| FTND (mean, SD)* | 5.3 (2.0) | 4.8 (1.8) | 5.2 (2.0) |
| Cigarettes per day (mean, SD) | 17.9 (8.1) | 15.4 (6.5) | 17.2 (7.7) |
| Age of smoking initiation (mean, SD)** | 15.9 (4.8) | 17.7 (6.7) | 16.5 (5.5) |
| Years of smoking (mean, SD) | 28.4 (12.4) | 28.2 (13.1) | 28.4 (12.6) |
| Longest duration of previous abstinence from cigarettes (days; mean, SD) | 266.3 (788.4) | 361.6 (993.5) | 296.2 (857.7) |
| Longest duration of previous abstinence from cigarettes (median; mode) | 21.0; 0 | 37.0; 0 | 29.0; 0 |
Note. FTND = Fagerström Test for Nicotine Dependence (possible range: 0 [low dependence] to 10 [high dependence]); differences between Week 8 Smokers and Abstainers:
p < .05,
p < .01; recruitment sites differed significantly by age, age of smoking initiation, gender, race, education, and income (p < .05) but only age of smoking initiation was related to week 8 smoking status and thus was included in models as a covariate.
Table 2.
Rate of Week 8 Abstinence by Weekly Patch Adherence
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | |
|---|---|---|---|---|---|---|---|---|
| n = 433 | n =433 | n =433 | n =428 | n =407 | n =405 | n =405 | n =404 | |
| Patch Non-Adherent | 19.5 | 16.7 | 13.8 | 8.5 | 10.8 | 14.6 | 18.9 | 16.7 |
| Patch Adherent | 34.7 | 36.2 | 37.7 | 38.7 | 39.8 | 40.6 | 40.3 | 41.7 |
| χ2 | 4.19 | 10.67 | 18.99 | 29.19 | 23.60 | 21.50 | 15.66 | 22.83 |
| χ2 significance | .041 | .001 | < .001 | < .001 | < .001 | < .001 | < .001 | < .001 |
Note. Number listed indicates percentage of participants abstinent at week 8. Patch adherent defined as using the patch ≥ 6 days/week.
Df = 1
The correlations between substitute reinforcers and depressive symptoms were r = −.19 (p < .001) at baseline, r = −.22 (p < .001) at week 0, r = −.25 (p < .001) at week 4, and r = −.22 (p < .001) at week 8. The correlations between complementary reinforcers and depressive symptoms were r = .01 (p > .05) at baseline, r = .02 (p > .05) at week 0, r = .14 (p < .005) at week 4, and r = .09 (p > .05) at week 8.
3.2 Alternative Reinforcers
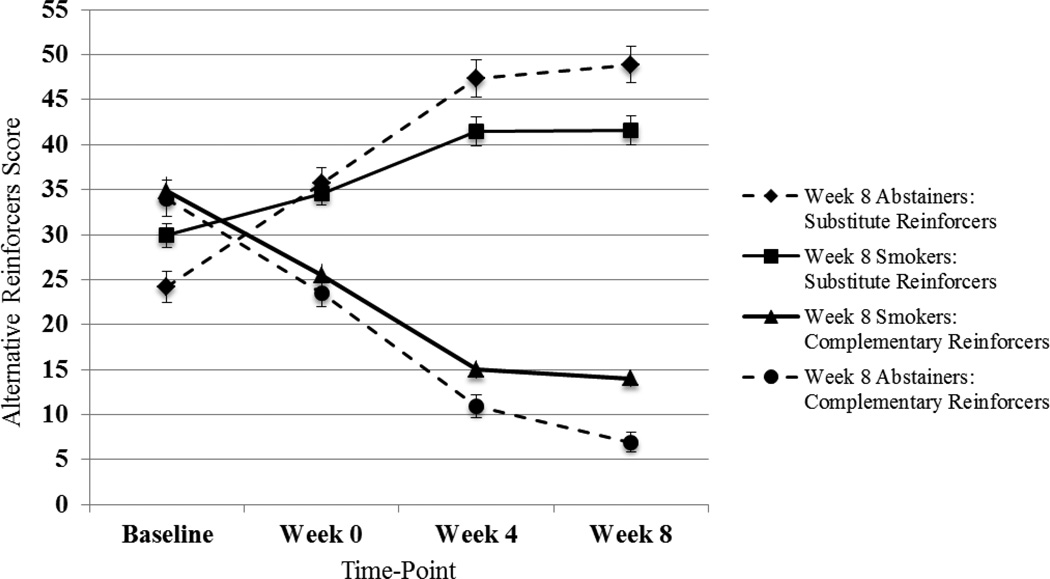
The regression analysis (GEE) showed that level of substitute reinforcers from baseline to week 8 increased across time from baseline, but increased more for those demonstrating week 8 abstinence (three-way interaction, χ2 [3] = 34.72, p < .001; Figure 2). As shown in Table 3, controlling for covariates, week 8 abstainers reported significantly higher levels of substitute reinforcers at weeks 0, 4, and 8, relative to week 8 smokers (p’s < .05).
Figure 2.
Change in Substitute and Complementary Reinforcers among Week 8 Smokers and Abstainers
Note. FTND score, age of smoking initiation, and patch adherence were controlled for in GEE analyses; FTND score was significantly associated with week 8 abstinence in both models; patch adherence was significantly associated with week 8 abstinence in the substitute reinforcers model. Substitute reinforcer score ranges: Baseline (0-112); Week 0 (0-120); Week 4 (0-126); Week 8 (0-132). Complementary reinforcer score ranges: Baseline (0-154); Week 0 (0-103); Week 4 (0-90); Week 8 (0-78).
Table 3.
GEE Analyses of Relationships between Alternative Reinforcers, Depressive Symptoms, and Week 8 Smoking Abstinence, over Time
| Model 1: Substitute Reinforcers | β | 95% CI | p |
|---|---|---|---|
| Constant | 33.65 | 26.05 to 41.24 | < .001 |
| FTND | −1.86 | −2.64 to −1.07 | < .001 |
| Age of smoking initiation | −0.04 | −0.32 to 0.23 | .77 |
| Patch adherence | 0.13 | 0.03 to 0.23 | .009 |
| Baseline (Week -2) Substitute Reinforcers | Ref. | ||
| Week 0 Substitute Reinforcers | 5.37 | 2.93 to 7.82 | < .001 |
| Week 4 Substitute Reinforcers | 11.91 | 9.36 to 14.46 | < .001 |
| Week 8 Substitute Reinforcers | 12.04 | 9.37 to 14.70 | < .001 |
| W8 Smoking Status (baseline adjustment) | −5.76 | −10.21 to −1.31 | .011 |
| Week 0 × W8 Smoking Status | 5.47 | 1.11 to 9.83 | .01 |
| Week 4 × W8 Smoking Status | 10.48 | 6.06 to 14.89 | < .001 |
| Week 8 × W8 Smoking Status | 12.05 | 7.59 to 16.50 | < .001 |
| Model 2: Complementary Reinforcers | β | 95% CI | p |
| Constant | 32.79 | 26.93 to 38.64 | < .001 |
| FTND | 0.94 | 0.34 to 1.53 | .002 |
| Age of smoking initiation | 0.06 | −0.15 to 0.27 | .57 |
| Patch adherence | −0.07 | −0.15 to 0.00 | .05 |
| Baseline (Week -2) Complementary Reinforcers | Ref. | ||
| Week 0 Complementary Reinforcers | −9.70 | −12.09 to −7.32 | < .001 |
| Week 4 Complementary Reinforcers | −20.70 | −23.19 to −18.21 | < .001 |
| Week 8 Complementary Reinforcers | −20.90 | −23.50 to −18.30 | < .001 |
| W8 Smoking Status (baseline adjustment) | −0.45 | −4.19 to 3.30 | .81 |
| Week 0 × W8 Smoking Status | −0.50 | −4.76 to 3.77 | .82 |
| Week 4 × W8 Smoking Status | −1.91 | −6.23 to 2.41 | .39 |
| Week 8 × W8 Smoking Status | −6.04 | −10.40 to −1.68 | .007 |
| Model 3: Depressive Symptoms | β | 95% CI | p |
| Constant | 8.59 | 5.63 to 11.55 | < .001 |
| FTND | 0.70 | 0.40 to 1.01 | < .001 |
| Age of smoking initiation | 0.01 | −0.10 to 0.12 | .91 |
| Patch adherence | −0.03 | −0.07 to 0.00 | .09 |
| Baseline (Week -2) Depressive Symptoms | Ref. | ||
| Week 0 Depressive Symptoms | −1.50 | −2.37 to −0.63 | .001 |
| Week 4 Depressive Symptoms | −1.21 | −2.11 to −0.30 | .009 |
| Week 8 Depressive Symptoms | −1.55 | −2.50 to −0.60 | .001 |
| W8 Smoking Status (baseline adjustment) | 1.07 | −0.62 to 2.76 | .22 |
| Week 0 × W8 Smoking Status | −1.52 | −3.07 to 0.04 | .06 |
| Week 4 × W8 Smoking Status | −1.20 | −2.78 to 0.37 | .13 |
| Week 8 × W8 Smoking Status | −2.40 | −3.98 to −0.81 | .003 |
Likewise, the level of complementary reinforcers decreased from baseline to week 8 for smokers, and decreased significantly more for week 8 abstainers (three-way interaction: χ2 [3] = 8.89, p < .03; Figure 2). As shown in Table 3, controlling for covariates, week 8 abstainers reported significantly lower levels of complementary reinforcers only at week 8, relative to week 8 smokers (p < .01).
3.3 Depressive Symptoms
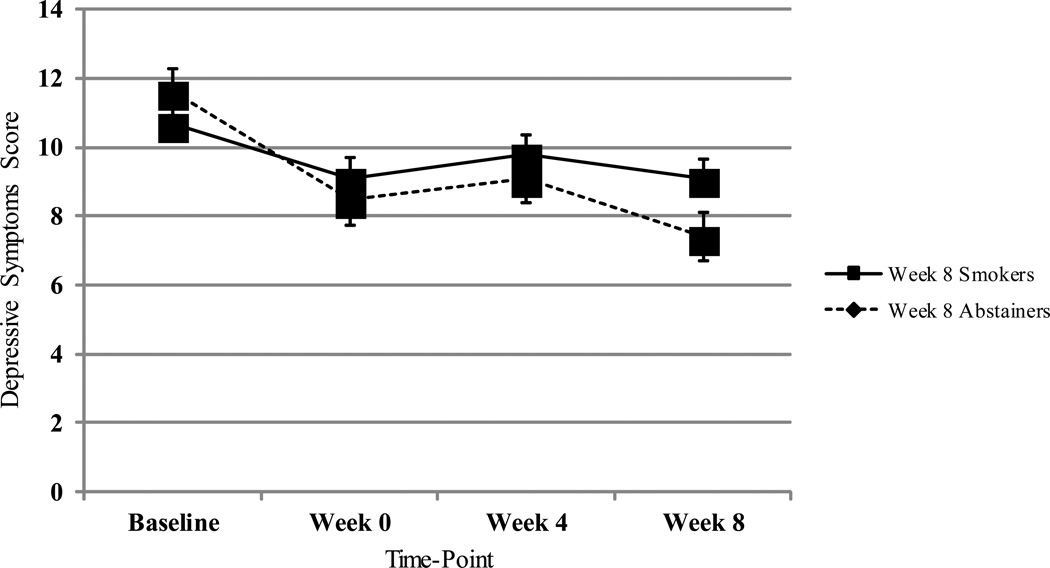
Depressive symptoms decreased from baseline to week 8 for both week 8 smokers and abstainers, but decreased significantly more for week 8 abstainers (three-way interaction: χ2 [3] = 9.09, p < .03; Figure 3). As shown in Table 3, controlling for covariates, week 8 abstainers reported significantly lower levels of depressive symptoms only at week 8, compared to week 8 smokers (p < .01); the difference between week 8 abstainers and smokers in self-reported depressive symptoms at week 0 approached significance (p = .06).
Figure 3.
Change in Depressive Symptoms among Week 8 Smokers and Abstainers
Note. FTND score, age of smoking initiation, and patch adherence were controlled for in GEE analysis; FTND score was significantly associated with week 8 abstinence in this model. Depressive symptoms score ranges: Baseline (0-48); Week 0 (0-55); Week 4 (0-48); Week 8 (0- 49).
3.4 Alternative Reinforcers and Depressive Symptoms
Consistent with the GEE analysis, the multivariate logistic regression models for week 8 abstinence indicated that participants were significantly more likely to be abstinent at week 8 if they demonstrated a greater increase in substitute reinforcers from baseline to week 8 (OR = 1.57 [95% CI: 1.23-2.01], p < .001). In this model, controlling for covariates, change in depressive symptoms and the interaction between substitute reinforcers and depressive symptoms were not significantly associated with week 8 abstinence (see Model 1, Table 4).
Table 4.
Regression Models of CO-confirmed 7-day Point-Prevalence Abstinence at 8 weeks.
| Model 1: Substitute Reinforcers | OR | 95% CI | p |
|---|---|---|---|
| FTND | .84 | .75 – .95 | .004 |
| Age of smoking initiation | 1.06 | 1.02 – 1.10 | .007 |
| Change in substitute reinforcers | 1.57 | 1.23 – 2.01 | < .001 |
| Change in depressive symptoms | .83 | .65 – 1.06 | .13 |
| Nicotine patch adherence | 1.06 | 1.03 – 1.09 | < .001 |
| Change in substitute reinforcers × Change in depressive symptoms | .90 | .71 – 1.14 | .38 |
| Model 2: Complementary Reinforcers | OR | 95% CI | p |
| FTND | .86 | .77 – .97 | .012 |
| Age of smoking initiation | 1.06 | 1.02 – 1.10 | .005 |
| Change in complementary reinforcers | .80 | .63 – 1.01 | .059 |
| Change in depressive symptoms | .77 | .61 – .97 | .028 |
| Nicotine patch adherence | 1.06 | 1.03 – 1.09 | < .001 |
| Change in complementary reinforcers × Change in depressive symptoms | .99 | .79 – 1.25 | .94 |
Note. Smoking coded as reference group.
Unlike the GEE analysis, the multivariate logistic regression models for week 8 abstinence indicated that a greater decrease in complementary reinforcers from baseline to week 8 was only marginally associated with week 8 abstinence (OR = .80 [95% CI: .63-1.01], p = .06). In this model, a greater decrease in depressive symptoms was significantly associated with week 8 abstinence rates (OR = .77 [95% CI: .61-.97], p = .03). Additionally, there was no significant interaction between change in complementary reinforcers and change in depressive symptoms on week 8 abstinence rates (see Model 2, Table 4).
3.5 Models of Relapse
Finally, Cox regression models of relapse between week 4 and week 8, based on measures of substitute and complementary reinforcers between baseline and week 4, indicated that, controlling for covariates (FTND, age of smoking initiation, and patch adherence), a greater increase in substitute reinforcers between baseline and week 4 was associated with a significant reduction in the likelihood of relapse between week 4 and week 8 (HR = .99 [95% CI: .98-.99], p = .04). The change in the use of complementary reinforcers between baseline and week 4, however, was not associated with risk for relapse between week 4 and week 8 (HR = 1.00 [95% CI: .99-1.01], p = .55).
4. DISCUSSION
This study, to our knowledge, is the first to examine the relationship between behavioral economic variables and smoking cessation within the context of an adult smoking cessation randomized clinical trial and to evaluate how depression interacts with behavioral economic variables in relation to smoking cessation. The primary goals of the investigation were to evaluate the relationships between changes in alternative reinforcers and depressive symptoms during a smoking cessation treatment program and smoking abstinence rates following smoking cessation treatment. The results suggest that changes in alternative reinforcers and depressive symptoms are important concomitant factors with successful smoking cessation. Additional notable results are discussed below.
Individuals who successfully quit smoking following eight weeks of smoking cessation treatment demonstrated a greater increase in engagement in rewarding activities they did not associate with smoking (substitute reinforcers) during the treatment period compared to individuals who were smoking following eight weeks of treatment. Additionally, while all participants demonstrated a decrease in activities they did associate with smoking (complementary reinforcers) during the treatment period, only at week 8 did individuals who successfully quit smoking have fewer complementary reinforcers than individuals who were smoking following eight weeks of treatment. Previous epidemiological studies of this relationship have demonstrated that alternative reinforcers contribute to the uptake of, and progression to, regular smoking, as well as the likelihood of successful smoking cessation (Audrain-McGovern et al., 2004, 2009). However, this is the first study to evaluate how changes in activities an individual finds rewarding are associated with short-term cessation within the context of a cessation intervention trial with adults.
Further, although these results demonstrate an association between changes in alternative reinforcers and smoking cessation outcomes, and not the influence of alternative reinforcers on smoking cessation outcomes, the relapse model analysis indicates that a greater increase in substitute reinforcers during the first four weeks of treatment was associated with a significantly greater likelihood for abstinence between week 4 and week 8. This relationship was not found for complementary reinforcers, however. In addition, these models used self-report smoking data between weeks 4 and 8.
The results of this study suggest a need for future trials testing whether smoking cessation counseling should incorporate an assessment of alternative reinforcers into treatment planning. These trials should assess whether cessation outcomes improve when counselors: 1) help individuals identify their substitute and complementary reinforcers, and 2) assist them in the development of strategies to increase the range and frequency of enjoyable activities not associated with smoking and decrease the range and frequency of activities associated with smoking. Such an approach has been utilized in the context of treatment for opiate and cocaine dependence (Higgins et al., 2003; Abbott, 2009) and could be adopted for nicotine dependence treatment and compared to standard behavioral models. The formal application of the Community Reinforcement Approach involves a functional analysis to identify alternative reinforcers and therapeutic strategies to build substitute reinforcers and decrease complementary reinforcers. In our view, this is considerably different than the traditional cognitive-behavioral counseling models or use of financial incentives evaluated previously for treating nicotine dependence. At the time of this manuscript, a randomized clinical trial to formally evaluate the Community Reinforcement Approach for treating nicotine dependence, using the methods proposed by Smith et al. (2001) and applied by Higgins et al. (2003), had yet to be conducted.
The results of the present investigation also provide additional support for the role of depressive symptoms during a smoking cessation attempt. While all participants demonstrated a decrease in depressive symptoms during the treatment period, only at week 8 did individuals who successfully quit smoking have fewer depressive symptoms than individuals who were smoking following eight weeks of treatment. These results are somewhat consistent with previous studies (Covey et al., 1990; Zelman et al., 1992; Niaura et al., 2001; Burgess et al., 2002; Kahler et al., 2002; Berlin and Covey, 2006), which showed that depressive symptoms preceding smoking cessation treatment and increases in depressive symptoms during treatment also predict poor cessation outcomes. Again, due to the inability to infer causality in this investigation, it is possible that individuals who were abstinent at week 8 showed this greater decrease in depressive symptoms due to their success in smoking cessation. However, regulation of negative moods has been found to be an important determinant of smoking cessation and maintenance of abstinence (Carmody et al., 2007), indicating that changes in depressive symptoms following a quit attempt are important phenomena that warrant continued investigation and attention in practice (Burgess et al., 2002).
Given previous findings that depressive symptoms were associated with reductions in substitute reinforcers (Lewinsohn and MacPhillamy, 1974; Lewinsohn and Amenson, 1978; Lewinsohn et al., 1998; Audrain-McGovern et al., 2011), it was hypothesized that changes in alternative reinforcers and depressive symptoms would interact in their association with week 8 abstinence rates. The lack of a significant interaction suggests that these two variables may have independent relationships with smoking cessation outcomes. Alternatively, given that the two constructs were strongly correlated, collinearity in the model may account for the non-significant interaction between the two variables. Future studies should further examine the relationship between these two variables and their potential discrete associations with smoking cessation outcomes.
The present results should be considered within the context of limitations. First, as stated previously, a causal relationship between changes in alternative reinforcers, changes in depressive symptoms, and cessation cannot be inferred from these results. Although these variables temporally preceded the week 8 assessment of smoking behavior, all potential confounding variables were not controlled for in this study, and participants were not randomized to different forms of behavioral counseling that targeted participants’ alternative reinforcers or depressive symptoms. Second, the present study does not report on long-term treatment outcomes to assess whether these variables are related to sustained cessation. Third, the Pleasant Events Schedule used in this trial was modified from the original scale by a single author (see Audrain-McGovern et al., 2009, 2011), potentially limiting the generalizability of the present results. Lastly, although the study was designed as an effectiveness study to maximize external validity, the sample was composed of adult treatment-seeking smokers, limiting the generalizability of the results to adults receiving assistance with smoking cessation.
Nevertheless, the results of this investigation provide support for the further examination of Behavioral Economic Theory in understanding adult smoking cessation, which could eventually inform future smoking cessation treatments and guidelines. Nicotine addiction, which manifests itself differently in each smoker, requires interventions that are tailored to differences in the reinforcement individuals derive from smoking and other activities (Benowitz, 2009). Additional studies are required to further understand the relationships between alternative reinforcers, depressive symptoms, and smoking cessation, particularly in relation to long-term treatment outcomes. However, this study suggests that use of Behavioral Economic Theory can guide the way in which researchers and practitioners conceptualize adult smoking cessation, and may contribute to the formulation of novel treatment modalities for smoking cessation.
Acknowledgments
Role of Funding Source
This study was supported by grants from the National Institutes of Health (DA025078, DA033681, and CA143187).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors contributed to this study and the preparation of this manuscript. All authors approve of the manuscript. Ms. Goelz led the analysis and manuscript preparation and oversaw the study implementation at Penn; Dr. Schnoll and Dr. Hitsman designed the overall study and served as site lead investigators; Dr. Audrain-McGovern advised on the inclusion of measures and manuscript preparation; Mr. Rivera and Mr. D’Avanzo oversaw data collection at Penn; Ms. Veluz-Wilkins oversaw data collection at Northwestern; Dr. Jepson and Dr. Wileyto assisted with data analysis; Dr. Leone assisted with data collection and manuscript preparation.
Conflicts of Interest
Dr. Schnoll receives medication and placebo free of charge from Pfizer and provides consultation to Pfizer and GlaxoSmithKline.
REFERENCES
- Abbott PJ. A review of the community reinforcement approach in the treatment of opioid dependence. J. Psychoactive Drugs. 2009;41:379–385. doi: 10.1080/02791072.2009.10399776. [DOI] [PubMed] [Google Scholar]
- Acker J, MacKillop J. Behavioral economic analysis of cue-elicited craving for tobacco: a virtual reality study. Nicotine Tob. Res. 2013;15:1409–1416. doi: 10.1093/ntr/nts341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Rodgers K, Cuevas J, Wileyto EP. Young adult smoking: what factors differentiate ex-smokers, smoking cessation treatment seekers and nontreatment seekers? Addict. Behav. 2009;34:1036–1041. doi: 10.1016/j.addbeh.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011;106:178–187. doi: 10.1111/j.1360-0443.2010.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP. Applying a behavioral economic framework to understanding adolescent smoking. Psychol. Addict. Behav. 2004;18:64–73. doi: 10.1037/0893-164X.18.1.64. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: the role of coping and personality traits. Addiction. 2006;101:1814–1821. doi: 10.1111/j.1360-0443.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology (Berl.) 1995;118:250–259. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Hughes JR, DeGrandpre RJ, Higgins ST, Rizzuto P. Behavioral economics of drug self-administration. IV. The effects of response requirement on the consumption of and interaction between concurrently available coffee and cigarettes. Psychopharmacology (Berl.) 1992;107:211–216. doi: 10.1007/BF02245139. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav. 1998;12:101–112. [Google Scholar]
- Burgess ES, Brown RA, Kahler CW, Niaura R, Abrams DB, Goldstein MG, Miller IW. Patterns of change in depressive symptoms during smoking cessation: who's at risk for relapse? J. Consult. Clin. Psychol. 2002;70:356–361. doi: 10.1037//0022-006X.70.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: a review. J. Psychosom. Res. 1993;37:565–574. doi: 10.1016/0022-3999(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Camp DE, Klesges RC, Relyea G. The relationship between body weight concerns and adolescent smoking. Health Psychol. 1993;12:24–32. doi: 10.1037//0278-6133.12.1.24. [DOI] [PubMed] [Google Scholar]
- Carmody TP, Vieten C, Astin JA. Negative affect, emotional acceptance, and smoking cessation. J. Psychoactive Drugs. 2007;39:499–508. doi: 10.1080/02791072.2007.10399889. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults--United States, 2000. MMWR. 2002;51:642–645. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Current cigarette smoking among adults - United States, 2011. MMWR. 2012;61:889–894. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Quitting smoking among adults - United States, 2001–2010. MMWR. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Compr. Psychiatry. 1990;31:350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Bulik CM, Perkins KA, Caggiula AR, Rodefer J. Behavioral economic analysis of smoking: money and food as alternatives. Pharmacol. Biochem. Behav. 1991;38:715–721. doi: 10.1016/0091-3057(91)90232-q. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME, Murray EW, Bennett G, Heishman S, Husten C, Morgan G, Williams C, Christiansen BA, Piper ME, Hasselblad V, Fraser D, Theobald W, Connell M, Leitzke C 2008 PHS Guideline Update Panel, 2008 PHS Guideline Update Liaisons, 2008 PHS Guideline Update Staff. Treating tobacco use and dependence: 2008 update US Public Health Service Clinical Practice Guideline executive summary. Respir. Care. 2008;53:1217–1222. [PubMed] [Google Scholar]
- Green L, Fisher EBJ. Economic substitutability: some implications for health behavior. In: Bickel WK, Vuchinich RE, editors. Reframing Health Behavior Change with Behavior Economics. Mahwah, NJ: Erlbaum; 2000. pp. 115–144. [Google Scholar]
- Green L, Freed DE. The substitutability of reinforcers. J. Exp. Anal. Behav. 1993;60:141–158. doi: 10.1901/jeab.1993.60-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence - a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu. Rev. Psychol. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Higgins S, Sigmon S, Wong C, Heil S, Badger G, Donham R, Dantona R, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Arch. Gen. Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Hougaard P. Analysis of Multivariate Survival Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- Johnson MW, Bickel WK. The behavioral economics of cigarette smoking: the concurrent presence of a substitute and an independent reinforce. Behav. Pharmacol. 2003;14:137–144. doi: 10.1097/00008877-200303000-00005. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, Mueller TI, Miller IW. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. J. Abnorm. Psychol. 2002;111:670–675. doi: 10.1037//0021-843x.111.4.670. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Amenson CS. Some relations between pleasant and unpleasant mood-related events and depression. J. Abnorm. Psychol. 1978;87:644–654. doi: 10.1037//0021-843x.87.6.644. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, MacPhillamy DJ. The relationship between age and engagement in pleasant activities. J. Gerontol. 1974;29:290–294. doi: 10.1093/geronj/29.3.290. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin. Psychol. Rev. 1998;18:765–794. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Brown CL, Stojek MK, Murphy CM, Sweet L, Niaura RS. Behavioral economic analysis of withdrawal- and cue-elicited craving for tobacco: an initial investigation. Nicotine Tob. Res. 2012;14:1426–1434. doi: 10.1093/ntr/nts006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhillamy DJ, Lewinsohn PM. The Pleasant Events Schedule - studies on reliability, validity, and scale intercorrelation. J. Consult. Clin. Psychol. 1982;50:363–380. [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol. Addict. Behav. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Ninan PT, Rush J, Crits-Christoph P, Kornstein SG, Manber R, Thase ME, Trivedi MH, Rothbaum BO, Zajecka J, Borian FE, Keller MB. Symptomatic and syndromal anxiety in chronic forms of major depression: effect of nefazodone, cognitive behavioral analysis system of psychotherapy, and their combination. J. Clin. Psychiatry. 2002;63:434–441. doi: 10.4088/jcp.v63n0510. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Hickcox ME, Grobe JE. Behavioral economics of tobacco smoking. In: Bickel WK, Vuchinich RE, editors. Reframing Health Behavior Change with Behavior Economics. Mahwah, NJ: Lawrence Erlbaum Associates, Inc.; 2000. pp. 267–294. [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory of Depressive Symptomatology (IDS) - preliminary findings. Psychopharmacol. Bull. 1986;22:985–990. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol. Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Carmody TJ, Ibrahim HM, Markowitz JC, Keitner GI, Kornstein SG, Arnow B, Klein DN, Manber R, Dunner DL, Gelenberg AJ, Kocsis JH, Nemeroff CB, Fawcett J, Thase ME, Russell JM, Jody DN, Borian FE, Keller MB. Self-reported depressive symptom measures: sensitivity to detecting change in a randomized, controlled trial of chronically depressed, nonpsychotic outpatients. Neuropsychopharmacology. 2005;30:405–416. doi: 10.1038/sj.npp.1300614. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol. Biochem. Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, Lerman C. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann. Intern. Med. 2010;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Smith JE, Meyers RJ, Miller WR. The community reinforcement approach to the treatment of substance use disorders. Am. J. Addict. 2001;10(Suppl.):51–59. doi: 10.1080/10550490150504137. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stead L, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. J. Evid. Based Med. 2012;5:242. doi: 10.1002/14651858.CD008286.pub2. [DOI] [PubMed] [Google Scholar]
- Van Etten ML, Higgins ST, Budney AJ, Badger GJ. Comparison of the frequency and enjoyability of pleasant events in cocaine abusers vs. non-abusers using a standardized behavioral inventory. Addiction. 1998;93:1669–1680. doi: 10.1046/j.1360-0443.1998.931116695.x. [DOI] [PubMed] [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, Galvin R, Zhu J, Wan F, DeGuzman J, Corbett E, Weiner J, Audrain-McGovern J. A randomized, controlled trial of financial incentives for smoking cessation. N. Engl. J. Med. 2009;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J. Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine Tob. Res. 2005;7:257–268. doi: 10.1080/14622200500055673. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Ramin SM, Rush AJ, Navarrete CA, Carmody T, March D, Heartwell SF, Leveno KJ. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. Am. J. Psychiatry. 2001;158:1856–1863. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. J. Consult. Clin. Psychol. 1992;60:943–952. doi: 10.1037//0022-006x.60.6.943. [DOI] [PubMed] [Google Scholar]