Abstract
There is a need to implement a vaccine to protect against Chlamydia trachomatis infections. To test a new vaccine mice were immunized with the C. muridarum native major outer membrane protein (nMOMP) solubilized with either amphipol A8-35 or the detergent Z3-14. Ovalbumin was used as a negative control and mice inoculated intranasally with C. muridarum as positive controls. Animals vaccinated with nMOMP mounted strong Chlamydia-specific humoral and cell mediated immune responses. Mice vaccinated with nMOMP/A8-35 had a higher ratio of antibodies to denatured EB over live EB, recognized more synthetic MOMP peptides and had higher neutralizing titers than sera from mice immunized with nMOMP/Z3-14. T-cell lymphoproliferative responses and levels of IFN-γ were also higher in mice vaccinated with nMOMP/A8-35 than with nMOMP/Z3-14. Following immunization animals were challenged intravaginally with C. muridarum. Based on the number of mice with positive vaginal cultures, length of vaginal shedding, total number of positive vaginal cultures and number of Chlamydia inclusion forming units recovered, nMOMP/A8-35 elicited a more robust protection than nMOMP/Z3-14. By depleting T-cells with antibodies we determined that CD4+ and not CD8+ T-cells, mediated the protection elicited by nMOMP/A8-35. Mice were subsequently mated and based on the number of pregnant mice and number of embryos, animals vaccinated with nMOMP/A8-35 or nMOMP/Z3-14 had fertility rates equivalent to the positive control group immunized with live EB and the fertility controls. In conclusion, increased accessibility of epitopes in the nMOMP/A8-35 preparation may account for the very robust protection against infection and disease elicited by this vaccine.
Keywords: Amphipols, detergents, Chlamydia, thermal stability, major outer membrane protein, vaccine protection
INTRODUCTION
Chlamydia trachomatis is worldwide the leading cause of bacterial sexually transmitted diseases and can also produce ocular, gastrointestinal and respiratory infections (1, 2). Annually, up to 4–5 million new genital C. trachomatis infections are reported in the United States (1, 3). Although effective antimicrobial therapy is available, over 50% of the chlamydial infections are asymptomatic and even in symptomatic cases treatment failures can occur (4). Furthermore, countries that have established screening programs, followed by antibiotic therapy, have observed an increase in the prevalence of the infection (5). This increase is thought to be due to a block in the development of natural immunity as a result of the antibiotic therapy (5, 6). Therefore, the implementation of a vaccine appears to be the best approach to control and eradicate these diseases (7–10).
In the 1960’s various investigators tested live and inactivated whole-organism vaccines in humans and non-human primates to protect against trachoma (2, 11–13). Several vaccine formulations were reported to be effective. However, the protection was found to be short-lived and serovar or serogroup specific. Furthermore, some groups of immunized individuals suffered a hypersensitivity reaction upon reexposure to Chlamydia (2, 13–15). Although the cause of this hypersensitivity reaction is still under investigation, it is thought to be secondary to exposure to one of the antigenic components present in the whole bacterium (16). Thus, there is a need to formulate a subunit vaccine.
When the sequence of the C. trachomatis major outer membrane protein (MOMP) was analyzed, it was found to have regions of DNA unique to each serovar (17, 18). Therefore, the likelihood that the protection elicited by the trachoma vaccine was due to MOMP was considered (7, 8, 19). Unfortunately, attempts to elicit protection in several animal models using recombinant MOMP, MOMP peptides and DNA MOMP-based vaccines, yielded disappointing results (20–22). The possibility that the native conformation of MOMP, or post-translational modification of the protein, were necessary for protection instigated the search for preparations of the native MOMP. Using detergents a trimeric form of MOMP, considered to correspond to its native structure, was isolated. Mice vaccinated with this preparation mounted a strong immune response that was protective against genital and respiratory challenges (23–27). Subsequently, the same type of preparation was found to elicit protection in non-human primates against an ocular infection (28). Detergents however, can have toxic effects at high concentrations and tend to destabilize membrane proteins accelerating their denaturation (29–33). Thus, detergents are not considered ideal vehicles to formulate vaccines.
In the 1990’s, Tribet et al. developed amphipathic polymers, termed amphipols (APols), designed to keep membrane proteins soluble in water in the absence of detergent (34). This group of investigators showed that several integral membrane proteins, including Escherichia coli OmpF, a protein similar in structure to MOMP, were kept soluble in their native conformation by APols (35). Based on these findings, the Chlamydia nMOMP was extracted with detergents and transferred to APols. Immunization of mice with this preparation was found to elicit a more robust protection against an intranasal chlamydial challenge than the detergent-based formulation (31). Here, to assess the feasibility of eliciting protection against a vaginal challenge with Chlamydia and to characterize the immune mechanisms involved in protection, we tested in parallel nMOMP preparations formulated in detergents and in APols. Our results show that nMOMP formulated with APols induces very robust protection against a vaginal infection and preserves fertility. The protection is dependent on CD4+ T-cells and may result from the increased accessibility to the immune system of epitopes in the nMOMP/A8-35 formulation when compared to the nMOMP/A8-35 preparation.
MATERIALS AND METHODS
Stocks of C. muridarum
The C. muridarum (Cm) strain Nigg II (previously called Chlamydia trachomatis mouse pneumonitis (MoPn) biovar, strain Nig II; obtained from the American Type Culture Collection, ATCC; Manassas, VA) was grown in McCoy cells (36). Elementary bodies (Cm-EBs) were purified and stored in SPG (0.2 M sucrose, 20 mM sodium phosphate pH 7.2 and 5 mM glutamic acid) as described (37).
Purification and preparation of C. muridarum nMOMP
The nMOMP was extracted and purified as described (25, 26). Briefly, Cm was grown in McCoy cells, harvested and washed with PBS. Following digestion of the pellet with DNAse the nMOMP was extracted by incubating twice with [3-[(3-Cholamidopropyl)-dimethylammonio]-1-propane sulfonate] (CHAPS; Anatrace; Maumee, OH) and once with n-Tetradecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (Anzergent® Z3-14; Anatrace). The supernatant was transfer to a hydroxyapatite column and the MOMP trimer was eluted using a phosphate buffer gradient (37).
The purity of the nMOMP preparation was assessed by several methods. Using the limulus amoebocyte assay (BioWhittaker, Inc., Walkersville, MD) the nMOMP was found to have less than 0.05 EU of LPS/mg of protein (24). Mass spectrometry analyses and N-terminal sequencing of nMOMP both revealed a purity of >99% (38, 39). The apparent MW was determined by SDS-PAGE (40).
To prepare the nMOMP/Z3-14 for immunization the protein was concentrated and fixed with 2% glutaraldehyde (Sigma-Aldrich; St. Louis, MO) at room temperature for 2 min as previously described. Glycine (Bio-Rad Laboratories; Hercules, CA) was added to stop the reaction. The nMOMP/Z3-14 was dialyzed against PBS (pH 7.4) with 0.05% Z3-14 before immunization. To formulate the nMOMP/A8-35 the protein from the hydroxyapatite column was incubated at room temperature for 2 h with Amphipol A8-35 (Anatrace) at a weight ratio of 2/1 (31). To remove the Z3-14 rehydrated BioBeads SM- 2 Adsorbent (Bio-Rad; Hercules, CA) were added at a weight ratio of 1/2.5×103 (Z3-14/Biobeads), and the mixture incubated at 4°C for 16 h when the beads were removed by centrifugation.
Characterization of the nMOMP/A8-35 and nMOMP/Z3-14 preparations by nuclear magnetic resonance (NMR)
To label the nMOMP with 15N, following infection with Cm, the McCoy monolayers where cultured with BioExpress® 2000 (U-15N) insect cell media (Cambridge Isotopes Laboratories, Inc; Andover, MA) supplemented with 1 mg/ml of glucose, 50 μg/ml of gentamicin sulfate and 1 μg/ml of cycloheximide. The nMOMP was extracted as described above and the trimer structure confirmed by SDS-PAGE. The NMR samples were prepared in a volume of 300 μL with 10% D2O, 100 mM sodium phosphate, pH 7.4; 300 μM nMOMP in 25 mM dodecylphosphocholine (DPC; Anatrace and 300 μM nMOMP with 24 mg/ml APol A8-35, as described above. The NMR 15N HSQC data were collected on a Varian Inova 800 MHz spectrometer (160 scans, 1,024 points, 48 increments). Data were processed using NMR pipeline (41).
Immunization protocols
To elicit protection before the mice reached sexual maturity, three-weeks-old female BALB/c (H-2d) mice (Charles River Laboratories; Wilmington, MA), were vaccinated with the nMOMP, or ovalbumin (OVA; Sigma-Aldrich; Saint Louis, MO), twice, two-weeks apart, by the colonic route (col.) (10 μg protein/mouse/immunization), followed by two times by the intramuscular (i.m.) (7 μg protein/mouse/immunization) plus the subcutaneous (s.c.) (3 μg protein/mouse/immunization) routes. Mucosal, followed by systemic immunization, has been found to be the most effective vaccination schedule for inducing protection against a chlamydial genital challenge (42). CpG-1826 (10 μg/mouse/dose; 5′-TCCATGACGTTCCTGACGTT-3′; Trilink Biotechnologies Inc., San Diego, CA) and Montanide ISA 720 VG (Seppic Inc, Fairfield, NJ; at a 30:70 volume ratio of nMOMP plus CpG to Montanide) were used as adjuvants (24, 42, 43). Montanide was only delivered by systemic routes. For col. immunization, mice were kept without food overnight and the vaccinations were performed using a 4.5 Fg dosing catheter (Harvard Apparatus, Holliston, MA) (44). OVA was solubilized in PBS, mixed with Z3-14 or APols like MOMP and used as a negative control. Another negative control group was inoculated intranasally (i.n.) with MEM-0. Positive control mice were immunized i.n. once with 1×104 inclusion forming units (IFU) of Cm (45). All experiments were repeated twice. The animal protocols were approved by, the University of California Irvine, Animal Care and Use Committee.
Immunoassays
Blood was collected from the periorbital region and Cm-specific antibody titers in sera were determined by an enzyme-linked immunosorbent assay (ELISA) as previously described (23, 45). Briefly, multiwell plates (Corning Glass Works, Corning, NY) were coated overnight with 1 μg of purified native, or boiled (for 30 m in the presence of 1μg/μl of β-mercaptoethanol) Cm-EB per well and serial dilutions of serum were added. The antigen-antibody reactions were detected with horseradish peroxidase-conjugated goat anti-mouse antibodies. The following antibodies were utilized: IgG (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD), IgG1, IgG2a and IgA (Southern Biotechnology Associates, Inc., Birmingham, AL). The substrate, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic) acid was used for color development. The plates were scanned at 405 nm in an ELISA reader (Labsystem Multiscan; Helsinki, Finland). Titers were calculated using as a background the OD of preimmunization sera ±2SD and reported as geometric mean titers (GMT).
To detect antibodies elicited by vaccination to B-cell specific linear epitopes, overlapping 25-mers corresponding to the mature Cm MOMP amino acid sequence were chemically synthesized (SynBioSci Corp.; Livermore, CA) (46). Peptide 25 (p25) overlaps the N- and C-terminus of MOMP. The peptides were adsorbed onto high binding affinity ELISA plates (10 μg/ml; 100 μl/well of a 96 well plate) and the antibody binding was determined in triplicates as described above using a 1:200 dilution of serum and a 1/10,000 dilution of anti-mouse IgG (47).
In vitro neutralization assays were performed as published (48). 1×104 IFU of Cm were added to serial dilutions of mouse sera made with Ca2+ and Mg2+ free PBS, pH 7.2, and supplemented with 5% guinea pig serum. After incubation for 45 min at 37°C, the mixture was inoculated into HeLa-229 cells grown on shell vials by centrifugation. After 30 h of incubation at 37°C the monolayers were fixed and stained with a pool of monoclonal antibodies (mAb) to MOMP, the 60-kDa cysteine-rich protein (crp), the 150-kDa putative outer membrane protein and the LPS, prepared in our laboratory (45). The titer of a sample was the dilution that yielded 50% neutralization relative to the negative control serum from MEM-0 immunized mice.
A T cell lymphoproliferative assay (LPA) was performed using splenocytes as previously described (45). In short, splenic T-cells were collected and UV inactivated Cm-EB, or nMOMP/A8-35, were added at a concentration of 1 EB, or 20 μg of nMOMP/A8-35, to 1 antigen presenting cell (APC) which were prepared by irradiating splenocytes with 3,300 rads of 137Cs. Negative control wells received medium (RPMI-1640+10%FBS) alone and positive controls wells ConA (5 μg/ml). At 96 hrs of incubation cell proliferation was measured by addition of 1 μCi of (methyl 3H) thymidine (47 Ci/mmol; Amersham, Arlington Heights, IL) per well. The mean count was obtained from triplicate cultures. The LPA was also used to assess the toxicity of the nMOMP/Z3-14 and nMOMP/A8-35 preparations using splenocytes from naive mice and ConA as a non-specific stimulant.
Levels of IFN-γ and IL-4 were determined using commercial kits (BD Pharmingen, San Diego, CA) in supernatants from splenic T cells stimulated as described above (31).
T cell depletion
Anti-CD4 rat IgG2b (GK1.5 mCD4) and anti-CD8 rat IgG2a (53-6.72 mCD8) antibodies (BioXCell; West Lebanon, NH) were administered intra-peritoneally. Animals received 500 μg/mouse of each antibody, at days (−5) and (−1) before the challenge, followed by 250 μg/mouse of each antibody, twice a week for five weeks after challenge (49, 50).
Flow cytometry analysis was used to verify in vivo T-cell depletion before the genital challenge. Four mice were included in each monoclonal treated group and four animals, inoculated with PBS, were used as controls. Mice were euthanized and whole spleen cell populations (2 × 106 total cells; 95% viable as determined by trypan blue exclusion) were stained for 45 min at 4°C with anti-CD4-PE, anti-CD8a-PerCP or fluorescent conjugated rat (IgG2a and IgG2b) isotype control antibodies (5 ug/106 splenocytes; BD Biosciences; San Diego, CA). Cells were washed twice, analyzed by flow cytometry (BD FASCcalibur) for at least 10,000 events/sample and gated for lymphocytes. Dead cells and monocytes were excluded using forward and side scattering gating.
Intravaginal challenge
Mice were treated subcutaneously four days before the challenge with 1 mg/mouse of medroxy progesterone acetate (Greenstone Ltd, Peapack, NJ) (51). At four weeks after the last immunization, mice were inoculated intravaginally (i.vag.), under xylazine/ketamine anesthesia, with 104 IFU of Cm in 20 μl of SPG (45, 52). To confirm that all mice were on diestrus, the vaginal cytology was checked before the challenge.
Genital cultures
Vaginal swabs were cultured twice weekly for the first two weeks and then at 7-days interval for an additional four weeks following the genital challenge (45). HeLa-229 cells grown in 48-well tissue culture plates were inoculated with 10-fold dilutions of the vaginal swabs and incubated for 30 h at 37 °C. The monolayers were fixed with methanol and chlamydial inclusions were stained using a pool of mAb described above. The limit of detection was 2 IFU/culture.
Fertility Studies
At six weeks following the challenge, female mice were housed with proven breeder male mouse for 18 days and then repeated once if necessary (45). Fertility is defined as at least one embryo/mouse.
Statistics
The two-tailed unpaired Student’s t test, the Mann-Whitney Rank Sum test, the Chi-square test and the Fisher’s exact test were used for statistical analysis with the program SigmaStat version 3.5. Differences were considered significant for values of P<0.05.
RESULTS
Antibody responses in serum following immunization
BALB/c mice were vaccinated with nMOMP/A8-35 or nMOMP/Z3-14 using CpG-and Montanide as adjuvants and the negative control groups with OVA instead of nMOMP. As a positive control mice were immunized with live Cm-EB and as a non-immunization control animals received MEM-0. Serum samples were collected the day before the i.vag. challenge with Cm. As shown in Table I, animals vaccinated with nMOMP/A8-35 had similar Cm-specific IgG GMT, using native EB as the antigen, when compared with mice immunized with nMOMP/Z3-14 (51,200 versus 62,413; P>0.05). The control group immunized with 104 IFU of Cm-EB had an IgG GMT of 8,063. Mice immunized with OVA/Z3-14, or OVA/A8-35, had IgG antibody titers below the limit of detection. To determine if immunization with the different vaccine preparations elicited antibodies to epitopes not accessible in the native EB, IgG levels were also determined using boiled EB. Higher increases in antibody levels to boiled EB were observed in mice vaccinated with nMOMP/A8-35 (304,332/51,200; ratio = 6.86), or nMOMP/Z3-14 (226,118/62,413; = 4.00), than in animals immunized with EB (10,159/8,063 = 1.33) (P<0.05).
Table I.
Serum antibody GMT (range) the day before the intravaginal challenge with C. muridarum.
| Experimental groups | Total IgG
|
IgG1 | IgG2a | IgG2a / IgG1 | IgA | Neutralizing titer | ||
|---|---|---|---|---|---|---|---|---|
| Native EB | Boiled EB | Ratio | ||||||
|
|
|
|
|
|||||
| nMOMP/Z3–14 | 62,413a (51,200–102,400) | 226,118a (102,400–409,600) | 4.00a | 21,527a (12,800–51,200) | 344,431a (204,800–409,600) | 16 | 336a (200–400) | 250 |
| nMOMP/A8–35 | 51,200a,b (51,200–51,200) | 304,332a,b (204,800–819,200) | 6.86a | 51,200a,b (12,800–204,800) | 487,099a,b (204,800–1,638,400) | 10 | 238a,b (100–400) | 1250c |
| OVA/Z3-14 | <100 (<100-<100) | <100 (<100-<100) | - | <100 (<100-<100) | <100 (<100-<100) | - | <100 (<100-<100) | <50 |
| OVA/A8-35 | <100 (<100-<100) | <100 (<100-<100) | - | <100 (<100-<100) | <100 (<100-<100) | - | <100 (<100-<100) | <50 |
| Cm-EB | 8,063 (6,400–12,800) | 10,159 (6,400–12,800) | 1.33 | 6,400 (6,400–6,400) | 60,887 (51,200–102,400) | 10 | 1,131 (800–1,600) | 1250 |
P<0.05 by Mann-Whitney Rank Sum Test compared to Cm-EB immunized group.
P>0.05 by Mann-Whitney Rank Sum Test compared to nMOMP/Z3-14 immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to nMOMP/Z3-14 immunized group.
To assess if a Th1 or a Th2 type response was induced, the titers of IgG1 and IgG2a were measured (Table I). In the two groups of mice immunized with nMOMP the ratios of IgG2a to IgG1 were 16:1 for the nMOMP/Z3-14 and 10:1 for nMOMP/A8-35 groups, indicating a strong Th1-biased response. This ratio was 10:1 for the Cm-EB control group. The Th1-biased response was also supported by the IFN-γ and IL-4 data (Table II).
Table II.
T-cell proliferative responses of immunized mice from the day before the intravaginal challenge with C. muridarum.
| Experimental groups | Stimulated with Cm-EB
|
Stimulated with nMOMP/A8-35
|
||||
|---|---|---|---|---|---|---|
| T-cell proliferationa,b (Δcpm) × 103 | SIb | IFN-γb (pg/ml) × 103 | IL-4b (pg/ml) | T-cell proliferationa,b (Δcpm) × 103 | SIb | |
|
|
|
|
|
|
|
|
| nMOMP/Z3-14 | 4.67 ± 3.46c,d | 20.13 ± 10.60c,d | 15.43 ± 8.74c,d | <4 ± <4 | 2.79 ± 1.92c,d | 9.98 ± 6.87c,d |
| nMOMP/A8-35 | 14.29 ± 1.59e,f,g | 31.10 ± 20.30c,f | 20.09 ± 3.63c,f,h | 7.56 ± 3.19c,f,g | 10.28 ± 4.50c,e,f | 19.97 ± 9.63c,f |
| OVA/Z3-14 | 0.49 ± 0.12 | 2.10 ± 0.30 | 1.12 ± 1.10 | <4 ± <4 | 0.63 ± 0.21 | 2.51 ± 0.53 |
| OVA/A8-35 | 0.42 ± 0.17 | 2.40 ± 0.90 | 1.10 ± 1.24 | <4 ± <4 | 0.43 ± 0.28 | 2.25 ± 0.78 |
| Cm-EB | 14.57 ± 5.15 | 47.90 ± 17.83 | 33.59 ± 0.91 | 5.36 ± 1.10 | 1.57 ± 0.35 | 4.38 ± 1.10 |
The ratio of UV-inactivated Cm-EB to antigen presenting cells was 1:1.
The values are means ± 1 SD of four different cultures.
P<0.05 by Mann-Whitney Rank Sum Test compared to Cm-EB immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to OVA/Z3-14 immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to nMOMP/Z3-14 immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to OVA/A8-35 immunized group.
P>0.05 by Mann-Whitney Rank Sum Test compared to Cm-EB immunized group.
P>0.05 by Mann-Whitney Rank Sum Test compared to nMOMP/Z3-14 immunized group.
The IgA GMT in serum were low in mice immunized with nMOMP/Z3-14 (336) or nMOMP/A8-35 (238) and not statically different from each other. In the positive control group inoculated with Cm-EB, the IgA GMT was 1,131. Higher titers of neutralizing antibodies were found in sera from mice vaccinated with nMOMP/A8-35, or Cm-EB (1,250), when compared to nMOMP/Z3-14 (250; P<0.05).
The IgG GMT in vaginal washes were not statistically different between the nMOMP/A8-35 (381; range 160–1,280), the nMOMP/Z3-14 (160; 40–320) and the Cm-EB (25; 10–80) immunized groups. Low levels of IgA were detected in the vaginal washes of mice immunized with both nMOMP/A8-35 (20; 10–40) and the nMOMP/Z3-14 (16; 10–20) preparations as well as with Cm-EB (32; 20–40).
Mapping of MOMP B-cell epitopes
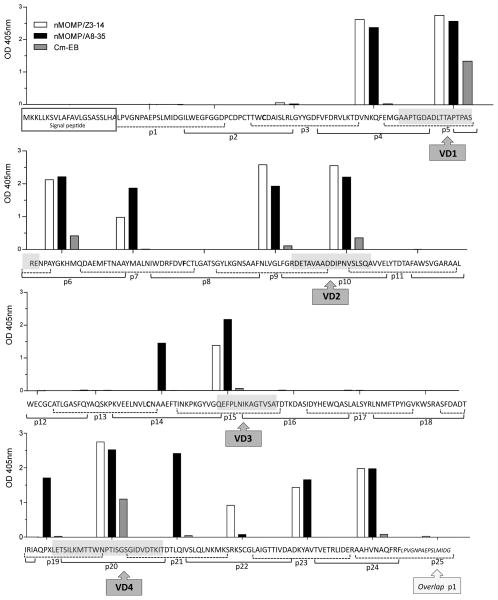
To determine the B-cell epitopes recognized by antibodies elicited by immunization, ELISA plates coated with 25-mers MOMP overlapping peptides were probed with serum samples collected the day before the i.n. challenge (Fig. 1). Sera from mice immunized with live EB bound exclusively to peptides corresponding to the VD, preferentially VD1 (p5, p6), VD2 (p10) and VD4 (p20). Sera from mice vaccinated with nMOMP/A8-35, or nMOMP/Z3-14, also reacted with all four VD and, in addition bound to peptides that included domains of VD1 (p4) and VD2 (p9). CD2 (p7) and CD5 (p23, p24) were also recognized by antibodies from these two groups of nMOMP-immunized animals. Only sera from mice vaccinated with nMOMP/A8-35 bound to p14 (CD3), p19 (that includes regions of CD4 and VD4) and p21 (that overlaps VD4 and CD5). In contrast, p22 in CD5 was only recognized by antibodies from mice immunized with nMOMP/Z3-14. Ten peptides (p1, p2 and p3 in CD1; p8 in CD2; p11, p12 and p13 in CD3 and p16, p17 and p18 in CD4), were not recognized by any sera from the three groups of immunized mice.
Figure 1. Binding of serum antibodies to synthetic C. muridarum MOMP peptides.
Serum samples from mice immunized with nMOMP/A8-35, nMOMP/Z3-14, or EB, were collected the day before the intravaginal challenge and their reactivities to 25-mer peptides corresponding to the Cm mature MOMP were analyzed by ELISA.
Evaluation of the toxicity of the nMOMP/A8-35 and nMOMP/Z3-14 preparations
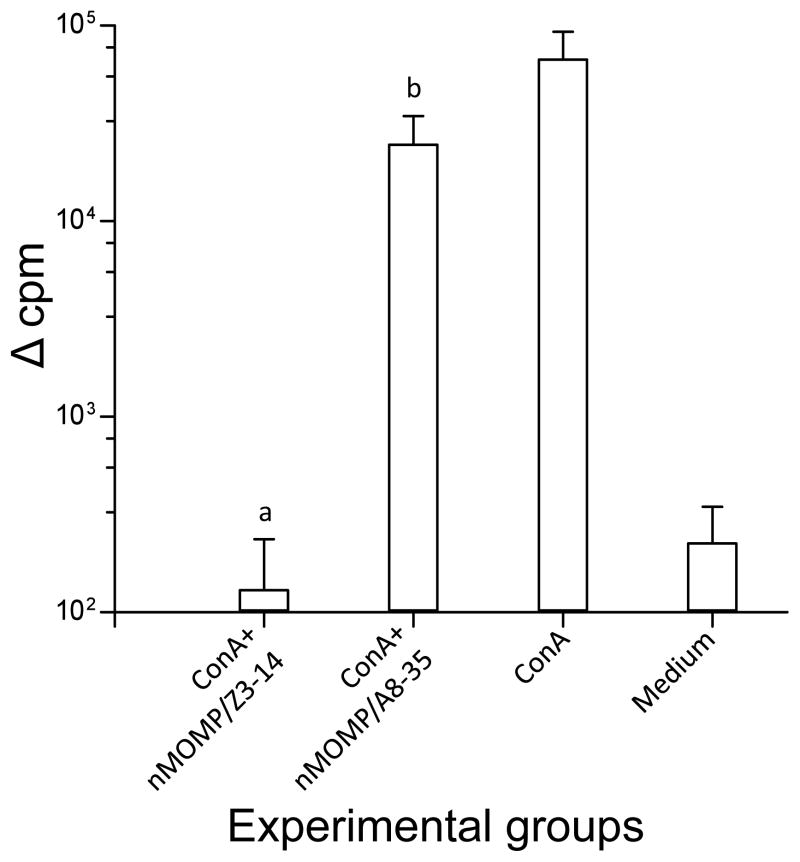
To assess the potential cell-toxicity of the nMOMP/A8-35 and nMOMP/Z3-14 preparations, splenic T-cells from naïve mice were stimulated non-specifically in vitro with ConA. While T-cells stimulated with ConA in the presence of nMOMP/A8-35 proliferated to levels similar to those stimulated only with ConA (24,627 ± 9,909 versus 67,226 ± 25,971 Δcpm; P>0.05), cells stimulated with ConA in the presence of nMOMP/Z3-14 (130 ± 107 Δcpm) did not, indicative of the toxicity of the detergent (Fig. 2). Therefore, the T-cell responses in immunized mice were evaluated using only EB and nMOMP/A8-35 as antigens.
Figure 2. T-cell proliferative responses in the presence of nMOMP/A8-35 or nMOMP/Z3-14.
To evaluate the possible toxic effects of nMOMP/A8-35 and nMOMP/Z3-14, T-cells from naive mice were stimulated in vitro with ConA in the presence of the two nMOMP preparations. T-cell proliferative responses as determined by the increase in counts per minute (cpm). The values are means ± 1 SD of four different experiments.
a, P<0.05 by Mann-Whitney Rank Sum Test compared to ConA, or ConA+nMOMP/A8-35, stimulated groups and P>0.05 compared to Medium stimulated group.
b, P<0.05 by Mann-Whitney Rank Sum Test compared to Medium stimulated group and P>0.05 compared to ConA stimulated group.
T-cell responses of immunized mice
To determine the T-cell responses elicited by vaccination mice from each group were euthanized the day before the i.vag. challenge and their spleens collected. T-cells were separated and stimulated with Cm-EBs, or nMOMP/A8-35 as antigens, medium as a negative control or ConA as a positive non-specific control. Proliferation was determined from the incorporation of [3H] thymidine. As shown in Table II, animals vaccinated with nMOMP/A8-35 had a significantly (P<0.05) higher proliferative T-cell immune response (14.29 × 103 Δcpm; SI=31.10), compared to nMOMP/Z3-14 (4.67 × 103 Δcpm; SI=20.13) and OVA/A8-35 immunized groups (0.42 × 103 Δcpm; SI=2.40). The LPA of nMOMP/A8-35 vaccinated mice was comparable to Cm-EB inoculated control (14.57 × 103 Δcpm; SI=47.90; P=1).
When T-cells were stimulated with nMOMP/A8-35, the proliferative response in the group vaccinated with nMOMP/A8-35 (10.28 × 103 Δcpm; SI=19.97) was significantly higher than in the nMOMP/Z3-14 (2.79 × 103 Δcpm; SI=9.98), OVA/A8-35 (0.43 × 103 Δcpm; SI=2.25) or Cm-EB (1.57 × 103 Δcpm; SI=4.38) immunized groups (P<0.05).
Levels of IFN-γ and IL-4 were determined in the supernatants from splenocytes stimulated with Cm-EBs (Table II). Significant higher levels of IFN-γ were found in the groups vaccinated with the nMOMP/A8-35 (20.09 ± 3.63 ng/ml) and nMOMP/Z3-14 (15.43 ± 8.74 ng/ml) preparations when compared to their respective OVA immunized groups (1.10 ± 1.24 and 1.12 ± 1.10; P<0.05). These levels however, were significantly lower than those found in the animals immunized with Cm-EB (33.59 ± 0.91 ng/ml; P<0.05) but not different between them (P=0.062). IL-4 was only detected in the nMOMP/A8-35 (7.56 ± 3.19 pg/ml) and Cm-EB (5.36 ± 1.10 pg/ml) immunized groups.
Characterization of nMOMP/A8-35 and nMOMP/detergent by NMR
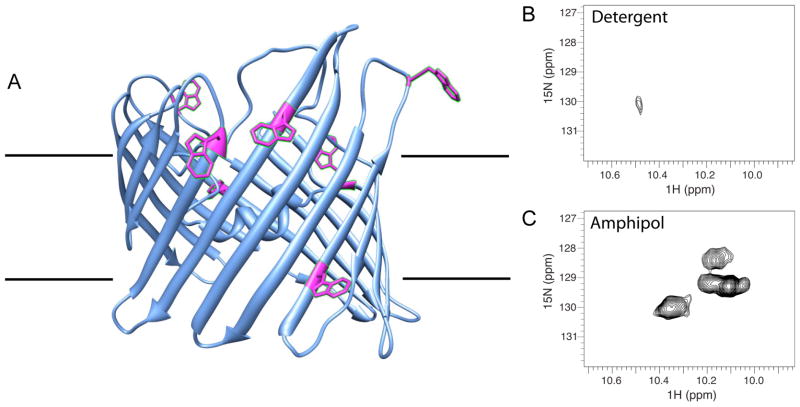
We prepared a fully 15N-labeled nMOMP sample for NMR studies and collected a one-dimensional proton NMR spectrum in Z3-14 or A8-35. Using a band-selective pulse centered on the aromatic region, our experiment was designed to observe aromatic and amide signals only without interference of the large detergent signals in the aliphatic range (53). Unfortunately, NMR signals of nMOMP were too broad to detect in the Z3-14 environment (data not shown). This was not unexpected since the average Z3-14 micelle particle alone is >30 kDa and when added to a membrane protein, this contributes significantly to the total size of the particle. In fact, Z3-14 is rarely used in NMR studies because large particles give broad lines (often too broad to detect). DPC micelles have an average mass of 17 kDa and are considered a superior membrane substitute since protein-DPC particles are smaller and tumble faster. In addition, the phosphocholine head group of DPC is a natural feature of cellular membranes. Since we could not get valid NMR data in Z3-14, we used DPC. This sample was split and transferred into DPC or A8-35. NMR 15N-HSQC spectra of these samples are shown in Figure 3. Trp signals appear in a distinct region of the spectrum and therefore, their solvent accessibility can be assessed. Also, Trp side chains allowed to rotate freely in solution give the sharpest NMR signals. nMOMP contains eight Trp residues; seven of these are positioned in proximity to the membrane surface depicted in a model of MOMP (Fig. 3A). Panels 3B and 3C show regions where Trp indole protons typically resonate. In the nMOMP/DPC spectrum, we find only one weak signal. The lack of Trp signals in Figure 3B is consistent with these side chains being restricted and occluded by detergents (DPC). In contrast, we find five strong Trp signals in the spectrum of nMOMP/A8-35 (Fig. 3C). Because the nMOMP/A8-35 particle size is still large, we can only detect signals corresponding to flexible, solvent accessible regions. The presence of Trp signals in the nMOMP/A8-35 NMR spectrum indicates these groups have increased exposure compared to Trp rings in the nMOMP/DPC sample.
Figure 3. NMR studies: tryptophan side chains are more exposed when nMOMP is in complex with A8-35 compared to detergent.
A) Model of nMOMP (73) showing positions of seven of the eight Trp side chains at the interface of the lipid bilayer. One Trp occurs in VD4, which is not shown in this structural model. The 800 MHz NMR 15N HSQC spectra of 0.3mM nMOMP B) solubilized in DPC, or C) trapped with A8-35, in 90% H2O/10% D2O, 100 mM sodium phosphate, pH 7.4. Only Trp indole signals resonate in the 1H region from 10–10.6. NMR data of 15N-labeled MOMP in the detergent DPC shows only one, very weak Trp signal (B). In contrast, at least five strong Trp signals are seen when the protein is in APol A8-35 (C).
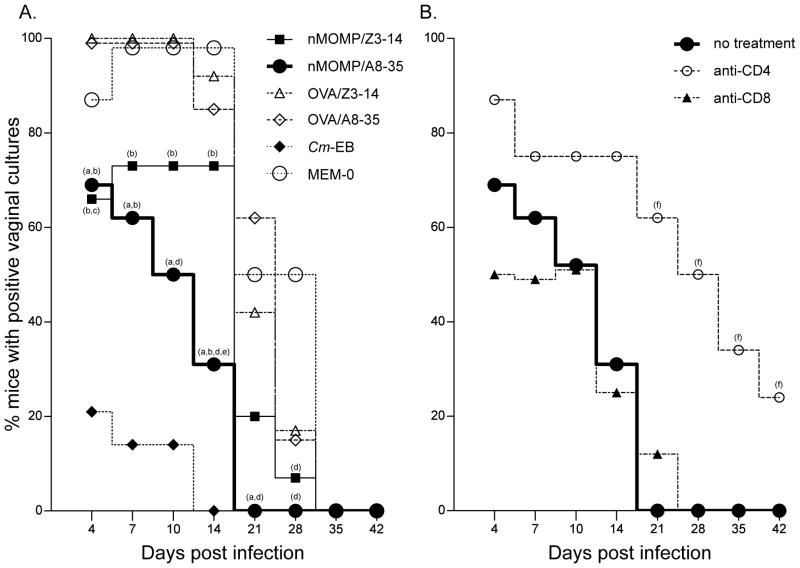
Recovery of C. muridarum from vaginal cultures
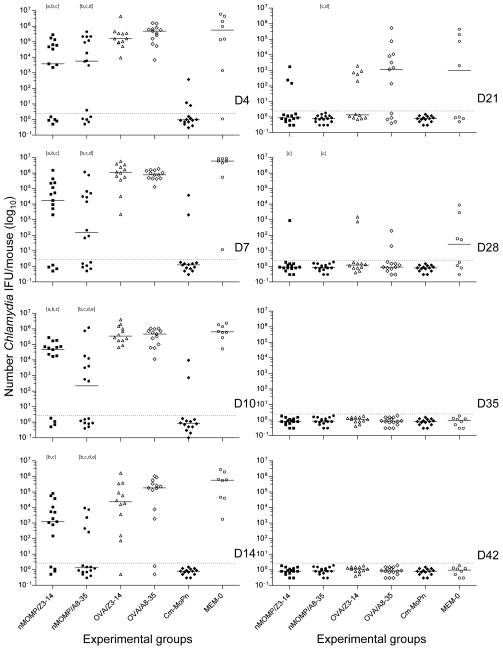
The course of the infection was assessed twice a week for the first two weeks post-challenge and once a week for four additional weeks using vaginal cultures (Figs. 4A & 5; Table III). The number of mice vaccinated with nMOMP/A8-35, or nMOMP/Z3-14, that shed over the course of the experiment were 69% (11/16) and 80% (12/15), respectively (P>0.05; Table III). The nMOMP/A8-35 group was statistically different from its corresponding OVA-immunized controls (100%; P<0.05) and not statistically different from Cm-EB (29%; 4/14) immunized control animals.
Figure 4. Percentage of mice with positive vaginal cultures. A) Percent immunized BALB/c mice with positive C. muridarum vaginal cultures. B) Percent nMOMP/A8-35 vaccinated BALB/c mice, depleted and non-depleted of CD4+ or CD8+ T-cells, with positive C. muridarum vaginal cultures.
Following immunization mice were challenged intravaginally and vaginal cultures were collected over a six weeks period.
a P<0.05 by the Fisher’s Exact test compared to the OVA/A8-35 immunized group;
b P<0.05 by the Fisher’s Exact test compared to the Cm-EB immunized group;
c P<0.05 by the Fisher’s Exact test compared to the OVA/Z3-14 immunized group;
d P<0.05 by the Fisher’s Exact test compared to the MEM-0 immunized group;
e P<0.05 by the Fisher’s Exact test compared to the nMOMP/Z3-14 immunized group;
f P<0.05 by the Fisher’s Exact test compared to the nMOMP/A8-35 no treatment group.
Figure 5. Number of C. muridarum IFU recovered from the vagina following the intravaginal challenge.
Following the intravaginal challenge the number of C. muridarum IFU were quantitated for each individual culture. Dots represent individual animals and the horizontal bars correspond to the medians.
a P<0.05 by the Mann-Whitney Rank Sum Test compared to the OVA/Z3-14 immunized group.
b P<0.05 by the Mann-Whitney Rank Sum Test compared to the Cm-EB immunized group.
c P<0.05 by the Mann-Whitney Rank Sum Test compared to the MEM-0 immunized group.
d P<0.05 by the Mann-Whitney Rank Sum Test compared to the OVA/A8-35 immunized group.
e P<0.05 by the Mann-Whitney Rank Sum Test compared to the nMOMP/Z3-14 immunized group.
Table III.
Results of vaginal cultures and fertility studies.
| Experimental groups | # of mice shed/ Total # mice (% +) | Total # IFU shed/mouse median (range) | Length of shedding Median days (range) | # of fertile mice/ Total # mice (% +) | mouse (mean ± 1SD) |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| nMOMP/Z3-14 | 12/15a (80) | 147,031c,d,e (<2–1,921,070) | 21d,e (4–35) | 12/15h,i (80) | 4.87±3.56c,k,l |
| nMOMP/A8-35 | 11/16b,h (69) | 14,125d,e,f (<2–2,817,998) | 12d,e,f,g (4–21) | 12/16b,h ,i(75) | 3.75±2.86f,k ,l |
| OVA/Z3-14 | 12/12 (100) | 2,719,510 (272,555–9,862,992) | 25 (14–35) | 7/12a (58) | 2.00±1.91d,m |
| OVA/A8-35 | 13/13 (100) | 1,886,340 (1,159,330–4,800,672) | 28 (14–35) | 4/13a,j (31) | 1.31±2.18d,m |
| MEM-0 | 8/8 (100) | 9,059,562 (894,734–13,003,396) | 32 (21–35) | 4/7 (57) | 1.86±2.04 |
| Cm-EB | 4/14 (29) | <2 (<2–38,315) | 4 (4–14) | 14/14 (100) | 6.07±2.16 |
| Fertility control | - | - | 14/16 (88) | 4.44±2.76 |
P<0.05 by the Fisher’s Exact test compared to the Cm-EB immunized group.
P<0.05 by the Fisher’s Exact test compared to the OVA/A8-35 immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to OVA/Z3-14 immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to Cm-EB immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to MEM-0 immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to OVA/A8-35 immunized group.
P<0.05 by Mann-Whitney Rank Sum Test compared to nMOMP/Z3-14 immunized group.
P>0.05 by the Fisher’s Exact test compared to the Cm-EB immunized group.
P>0.05 by the Fisher’s Exact test compared to the Fertility control group.
P<0.05 by the Fisher’s Exact test compared to the Fertility control group.
P>0.05 by the Mann-Whitney Rank Sum Test compared to the Cm-EB immunized group.
P>0.05 by the Mann-Whitney Rank Sum Test compared to the Fertility control group.
P<0.05 by the Mann-Whitney Rank Sum Test compared to the Fertility control group.
As shown in Fig 4A, the percentage of mice that shed in the nMOMP/A8-35 vaccinated group was significantly (P<0.05) lower than in the OVA/A8-35 immunized group at D4 (69% vs 100%), D7 (62% vs 100%), D10 (50% vs 100%), D14 (31% vs 85%) and D21 (0% vs 42%). At D4 p.c. significantly fewer animals had positive vaginal cultures in the group vaccinated with nMOMP/Z3-14 when compared to the OVA/Z3-14 immunized mice (66% vs 100%; P<0.05). Further, significantly fewer mice shed in the nMOMP/A8-35 vaccinated than in the nMOMP/Z3-14 immunized animals at D14, 31% vs 73% (P<0.05).
A significant decrease (57%) in the length of time of shedding was observed in the nMOMP/A8-35 vaccinated animals (median days 12; range 4–21) when compared to the nMOMP/Z3-14 immunized mice (21; 4–35) or the OVA/A8-35 immunized mice (28; 14–35 (P<0.05) (Table III). The negative control groups OVA/Z3-14 (25; 14–35), OVA/A8-35 (28; 14–35), or MEM-0 (32; 21–35), shed longer than the nMOMP vaccinated animals, while the positive control Cm-EB immunized group had the shortest shedding time (4; 4–14).
There was also a statistically significant difference between the total number of positive vaginal cultures during the six weeks of the experiment when comparing the nMOMP/A8-35 versus the nMOMP/Z3-14 vaccinated animals (Figs. 4A and 5). A total of 26.6% (34/128) of the vaginal cultures collected from the nMOMP/A8-35 vaccinated animals were positive, while 39.2% (47/120) of the cultures from the mice immunized with nMOMP/Z3-14 were positive (P<0.05). These values were statistically different from their respective negative control groups: OVA/A8-35 (57.7%; 60/104) and OVA/Z3-14 (56.3%; 54/96) or the MEM-0 (60.9%; 39/64). The positive Cm-EB immunized control group (6.3%; 7/112) was statistically significantly lower than any other group (P<0.05).
A significant reduction in the total number of Cm IFUs/mouse recovered over the course of the experiment was observed in the groups vaccinated with nMOMP/Z3-14 (median: 147,031; range: <2–1,921,070), or nMOMP/A8-35 (14,125; <2–2,817,998), when compared to their respective negative controls immunized with OVA/Z3-14, (2,719,510; 272,555–9,862,992), or OVA/A8-35 (1,886,340; 1,159,330–4,800,672), respectively (P<0.05) (Table III). No statistically significant difference was obtained when comparing the two nMOMP-vaccinated groups. The control Cm-EB immunized animals had the lowest total number of IFU/mouse (<2; <2–38,315) and that was statistically significant different from any other group (P<0.05).
The nMOMP/A8-35 vaccinated group shed significantly less IFU compared to the OVA/A8-35 immunized mice from D4 (median number of IFU = 5,647 vs 486,324) thru D21 (<2 vs 1,146) (P<0.05) (Fig. 5). The quantity of Cm IFU shed by the group vaccinated with nMOMP/Z3-14 differed significantly from the OVA/Z3-14 immunized mice at D4 (3,835 vs 160,234), D7 (17,390 vs 1,110,582), and D10 (46,168 vs 324,819) (P<0.05). Furthermore, the nMOMP/A8-35 vaccinated animals shed less than the nMOMP/Z3-14 immunized group on D10 (217 vs 46,168) and D14 (<2 vs 1,236) (P<0.05). The median number of IFU shed by nMOMP/A8-35 vaccinated mice was below the limit of detection starting on D14 and all mice in this group had negative cultures by D21, two weeks earlier than the nMOMP/Z3-14 immunized animals.
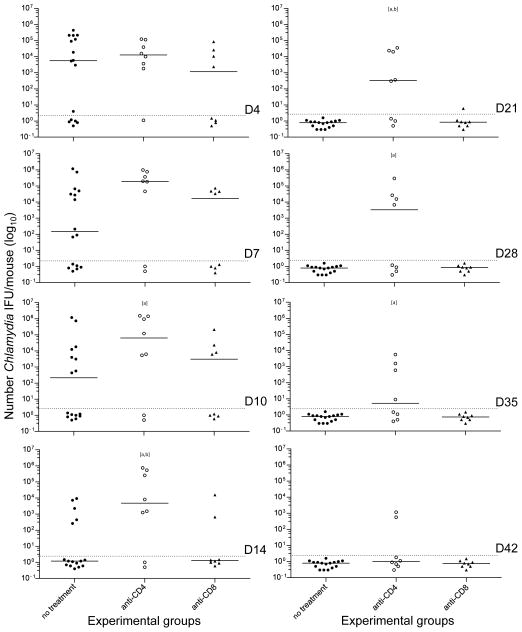
T-cell depletion experiments
To determine what type of T-cell effects the protection elicited by the nMOMP/A8-35 vaccine, immunized mice were depleted before and after the vaginal challenge of CD4+ or CD8+ T-cells using mAb (Figs. 4B & 6). Before challenge, mice treated with the anti-CD8+ mAb, had an 84.70% (1.86/12.5) ±2.61SD decrease in CD8 cells while animals treated with the anti-CD4+ mAb had an 86.29% (3.5/25.54) ± 4.02 decrease in CD4+ cells, when compared to the PBS control inoculated group. Significantly more mice shed in the anti-CD4+ treated group when compared to non-treated group at D21 (62% vs 0%) and onward (D28: 50%, D35: 37%, D42: 25% vs 0%) (P<0.05). In the CD4+ T-cell depleted group there was an increase in length of days of shedding (median days 31.5; range 4–49) versus the non-treated (12; 4–21) and the CD8+ T-cell depleted (9; 4–28) groups (P<0.05). Also, during the six weeks of the experiment, there is a statistically significant difference (P<0.05) between the total number of positive vaginal cultures in the CD4+ T-cell depleted group (60.9%; 39/64), versus the non-treated (26.6%; 34/128) and the CD8+ T-cell depleted (23.4%; 15/64) groups. In the CD8+ T-cell depleted and the non-treated groups, the total number of positive cultures was similar (P>0.05). In addition, the nMOMP/A8-35 vaccinated group depleted of CD4+ T-cells shed significantly higher numbers of IFU compared to either the non-depleted nMOMP/A8-35 vaccinated group at D10 (median = 63,813 vs 217), D14 (4,832 vs <2), D21 (333 vs <2), D28 (3,365 vs <2) and D35 (<2 vs <2) or with the anti-CD8 treatment group, at D14 (4,832 vs <2) and D21 (333 vs <2) (P<0.05).
Figure 6. Number of C. muridarum IFU recovered from the vagina in CD4+ or CD8+ T-cell-depleted nMOMP/A8-35-immunized BALB/c mice.
Following immunization with nMOMP/A8-35 groups of mice were depleted of CD4+ or CD8+ T-cells. The animals were challenged intravaginally with C. muridarum and vaginal cultures were collected over a six weeks period.
Dots represent individual animals and the horizontal bars correspond to the medians.
a P<0.05 by the Mann-Whitney Rank Sum Test compared to the nMOMP/A8-35 not treated group.
b P<0.05 by Mann-Whitney Rank Sum Test compared to anti-CD8 treated group.
Fertility Studies
Six weeks after the intravaginal infection, mice were mated to determine the ability of the vaccine to protect against Chlamydia induced infertility (Table III). The nMOMP/A8-35 (12/16; 75%) and the nMOMP/Z3-14 (12/15; 80%) vaccinated groups had similar fertility rates when compared with the Cm-EB (14/14; 100%), or the fertility control groups (14/16; 88%), or among themselves (P>0.05). The nMOMP/A8-35 immunized group differed statistically from the OVA/A8-35 control (4/13; 31%; P<0.05). The animals immunized with OVA/A8-35 (4/13; 31%) and OVA/Z3-14 (7/12; 58%) had statistically significant lower fertility rates when compared to the Cm-EB group (P<0.05).
The mean total number of embryos in the nMOMP/A8-35 (3.75 ± 2.86), or the nMOMP/Z3-14 (4.87 ± 3.56) immunized groups, were no different than in Cm-EB (6.07 ± 2.16), or the fertility control groups (4.44 ± 2.76), or among themselves (P>0.05; Table III). The nMOMP/A8-35 immunized group had statistically significant more embryos than the OVA/A8-35 control (1.31 ± 2.18). Mice immunized with OVA/A8-35 or OVA/Z3-14 (2.00 ± 1.91) had statistically lower number of embryos when compared to Cm-EB immunized or the fertility control groups (P<0.05).
DISCUSSION
Here we tested the ability of a native MOMP preparation, formulated with a detergent (Z3-14) or an APol (A8-35), to induce in mice a protective immune response against an i.vag. challenge with C. muridarum. To elicit a Th1 response, CpG-1826 and Montanide ISA 720 VG were used as adjuvants and the vaccine was delivered by a combination of mucosal and systemic routes. Montanide was only used systemically. Based on the IgG2a/IgG1 antibody ratios in serum and IFN-γ levels in supernatants from stimulated splenocytes, both formulations induced robust Th1-biased responses. Overall, using native and heat-denatured EB as the antigen, the immune responses elicited by nMOMP/A8-35 were stronger than those achieved with nMOMP/Z3-14. Furthermore, using synthetic peptides corresponding to the amino acid sequence of MOMP, it was determined that more linear B-cell epitopes were recognized by antibodies elicited by vaccination with nMOMP/A8-35 than with nMOMP/Z3-14. Mice were challenged intravaginally with C. muridarum and based on number of mice with positive vaginal cultures, length of shedding, total number of positive vaginal cultures and number of IFU recovered, the protection obtained with nMOMP/A8-35 was more robust than that achieved with nMOMP/Z3-14. Treatment of nMOMP/A8-35 vaccinated mice with an anti-CD4 antibody, but not with an anti-CD8 antibody, abrogated the protective effect of the vaccine. Fertility rates in nMOMP/A8-35 and nMOMP/Z3-14 immunized mice were comparable to those observed in the fertility control groups. The enhanced protection elicited by nMOMP/A8-35 may be the result of better accessibility of protective epitopes to the immune system in the APol nMOMP preparation versus the detergent solubilized protein.
Most of our current vaccines are based on live or inactivated whole pathogens and therefore, maintain the native structure of their antigenic components (54, 55). As a result, upon exposure to a pathogen, the immune system of the vaccinated individual reacts with a well-directed response to the native antigens. New subunit vaccines, formulated with highly purified antigens that may lack the correct conformation, present new challenges (56–58). In order for a subunit vaccine to elicit a robust immune response the structure of the antigen and accessibility of protective domains must be optimized (59, 60).
Using vaccine formulations with nMOMP/Z3-14 protection has been elicited in mice against genital and respiratory C. muridarum challenges and in non-human primates against a C. trachomatis ocular infection (23, 26, 28). However, as shown here, the detergent present in the nMOMP/Z3-14 preparation has a profound in vitro toxic effect that could also be, at high concentrations, detrimental in vivo (32, 33). In addition, detergents bound to proteins are in a constant and rapid equilibrium with the solution (31). As a result, the antigen may change in conformation over time during storage and delivery of the vaccine and also when trafficking inside the body. For example, when membrane proteins are diluted under the critical micelle concentration of the detergent, as it would happen following injection into an individual, the protein aggregates and denaturation may occur (31, 35). Both processes can result in epitopes becoming inaccessible or unrecognizable. Membrane protein-APol complexes, on the other hand, are highly stable and do not dissociate following dilution thus, preventing protein aggregation (35, 61). Therefore, the nMOMP/APol formulation should make for a safer delivery of the antigen to target cells while better maintaining the protein structure than the nMOMP/Z3-14 preparation (62, 63). Furthermore, detergents and APols form around the transmembrane region of membrane proteins a belt into which hydrophobic protein surfaces are buried (64–68). Whether the belt is comprised of detergent, or APols, has been shown to modulate the structure and dynamics of extramembrane protein loops which can be expected to affect their antigenic properties (69). Finally, membrane proteins may be delivered in significantly different manners to cells of the immune system depending on whether they are surfactant-free, and possibly aggregated, as can be the case for detergent-based preparations, or kept soluble by the APol until delivered to a cell plasma or endocytic membrane (34, 35).
Membrane protein structures most often feature a ring of aromatic residues at the headgroup region of the lipid bilayer (70). In fact, it has been shown thermodynamically that aromatic rings partition preferentially to the phosphocholine headgroup region more favorably than any other amino acid group. Tryptophan residues, in particular, have the largest ΔG of association with the phosphocholine headgroups (71, 72). Similar to other membrane proteins, known porin structures display the aromatic ring distributions that favor headgroup interactions. Although no structure of MOMP currently exists, four topological models of MOMP, corresponding to Cm and the C. trachomatis serovars C, D and F, have been proposed (73–76). All of these models position the seven or eight Trp rings in proximity to the lipid headgroup region. One of these models is shown in Figure 3 (73). Based on NMR measurements, we find that five Trp signals are solvent accessible in the APols preparation, but only one very weak Trp is detectable in DPC.
Because Z3-14 contains zwitterionic groups similar to phosphocholine, it is reasonable to suggest that the Z3-14 headgroup might favorably interact with Trp aromatic rings. In addition, Lys and Arg residues within MOMP extracellular loops could interact via salt bridge with the Z3-14 sulfate anion or the DPC phosphate anion. Both of these stabilizing interactions would create a situation where either Z3-14 or DPC detergent could efficiently occlude protein groups. Since the APol used in this study does not contain a zwitterionic group, or any feature that mimics lipid headgroup distribution, it is not surprising that we find increased exposure for Trp residues normally buried at the headgroup interface. MOMP displayed on the surface of Chlamydia would be expected to have even more protein buried since outer membranes are substantially thicker than the diameter of Z3-14 micelles. Thus the accessible protein surface increases with the following order: Chlamydia-EB < nMOMP/Z3-14 < nMOMP/A8-35.
Farris et al., (27) have shown that the protection elicited by nMOMP/Z3-14 is dependent on CD4+ and antibodies but not on CD8+ T cells. Here we also showed that the protection elicited by the nMOMP/A8-85 is dependent on CD4+ but not on CD8+ T cells. The conformation of the antigen, and therefore accessibility to the immune system and processing, not only affects the epitopes that are recognized by antibodies but also influences T-cell responses. For example, Musson et al. (77) showed that the degree of antigen processing was dependent on the localization of the epitopes of the Yersinia pestis Caf1 protein. Epitopes located in the globular domains were presented by newly synthesized MHC class II, after low pH-dependent lysosomal processing, while epitopes from a flexible strand of the protein were presented by mature MHC class II, independent of low pH, and did not require proteolytic processing. Warren et al. (78) have also described an antigenic peptide recognized by CD8+ T lymphocytes consisting of two non-continuous peptide segments spliced in reverse order to that in the native protein. This type of modification could be affected by the conformation of the protein during proteasome processing. Furthermore, Tikhonova et al. (79) have shown that, T-cells not undergoing MHC-specific thymic selection, can express T-cell receptors that recognize conformational epitopes independently of MHC molecules.
In humans and mice T helper cell epitopes have been localized mainly to the constant domains (CD) of MOMP (46, 80). Here, to determine what domains of MOMP function as T helper cell antigens, we characterized MOMP peptides that elicited an IgG response following immunization with EB or the two MOMP preparations. Peptides corresponding to the four VD of MOMP were recognized by IgG antibodies present in the serum from mice immunized with Cm-EB and with nMOMP/A8-35 or nMOMP/Z3-14. Although antibodies from mice immunized with EB only recognized the VD, sera from animals vaccinated with the nMOMP preparations also bound to peptides corresponding to the CD. Specific MOMP peptides in CD3, CD4 and CD5 were exclusively recognized by antibodies from animals vaccinated with nMOMP/A8-35 and a different peptide in CD5 bound only to sera from mice immunized with nMOMP/Z3-14. The finding that, a broader set of peptides was recognized by sera from mice immunized with nMOMP/A8-35 versus nMOMP/Z3-14, suggests that the accessibility of certain domains of MOMP to the immune system is different between the two formulations a premise supported by our NMR data.
The increased accessibility of MOMP epitopes in the APols formulation to the humoral and cell mediated immune systems may therefore explain our findings. Overall, higher neutralizing antibody levels, T-cell proliferative responses and levels of IFN-γ, were observed in mice immunized with nMOMP/A8-35 versus nMOMP/Z3-14. These may account for the more robust protection observed in the nMOMP/A8-35 vaccinated group of animals. The best protection however, was achieved in mice immunized with live EB. The greater protection observed in these animals may be due to the presence in the EB of additional protective antigens other than MOMP and/or, to better presentation of conformational epitopes of MOMP. In addition, EB replication following i.n. inoculation with live organisms, results in dissemination of Cm to most organs, including long-term colonization of the gastrointestinal tract, could account for the most robust protection elicited in the control mice versus those vaccinated with nMOMP (81). However, implementation of a live Chlamydia vaccine is highly unlikely due to the potential induction of a hypersensitivity reaction upon exposure to this pathogen in EB-vaccinated individuals (2, 82). Furthermore, the complexities of growing and purifying large numbers of EB, safety concerns and the high cost of production are significant limitations of a whole-organism vaccine. Therefore, there is a need to optimize our current subunit vaccine candidates.
In conclusion, we showed that nMOMP formulated with APols induces a very robust protection although, like almost all of our current vaccines, does not elicit sterilizing immunity (55). Using a computer model, Chlamydia vaccines with these characteristics, have been shown to potentially have a major impact on the prevalence of these infections (83). Some new and under development subunit vaccines use integral membrane proteins that require components, such as detergents, to keep them in solution (59, 60). APols may be explored as an alternative to detergents to increase the safety and immunogenicity of these antigens.
Acknowledgments
This work was supported by Public Health Service grant AI067888 and AI092129 from the National Institute of Allergy and Infectious Diseases. JLP’s work was supported by the French Centre National de la Recherche Scientique and University Paris-7.
We would like to thank Professor Rommie E. Amaro for the permission to use her 3D model of MOMP (Fig. 3A).
Abbreviations used in this article
- nMOMP
native Major Outer Membrane Protein
- Z3-14
zwitterionic 3-14 detergent
- APol
amphipol
- Cm
Chlamydia muridarum
- EB
elementary bodies
- CD
constant domain
- VD
variable domain
- NMR
nuclear magnetic resonance
- GMT
geometric mean titer
- DPC
Dodecylphosphocholine
- IFU
inclusion forming units
- MEM-0
minimum essential media without serum
- i.n
intranasal
References
- 1.Chlamydia screening among sexually active young female enrollees of health plans--United States, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009;58:362–365. [PubMed] [Google Scholar]
- 2.Schachter J, Dawson CR. Human chlamydial infections. PSG Pub. Co; Littleton, Mass: 1978. [Google Scholar]
- 3.Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR. Prevalence of chlamydial and gonococcal infections among young adults in the United States. Jama. 2004;291:2229–2236. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 4.Stamm W. Chlamydia trachomatis infections of the adult. In: Holmes PSKK, Stamm WE, Piot P, Wasserheit JW, Corey L, Cohen MS, Watts DH, editors. Sexually transmitted diseases. McGrawHill Book Co; New York: 2008. pp. 575–593. [Google Scholar]
- 5.Gotz H, Lindback J, Ripa T, Arneborn M, Ramsted K, Ekdahl K. Is the increase in notifications of Chlamydia trachomatis infections in Sweden the result of changes in prevalence, sampling frequency or diagnostic methods? Scand J Infect Dis. 2002;34:28–34. doi: 10.1080/00365540110077001. [DOI] [PubMed] [Google Scholar]
- 6.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005;192:1836–1844. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 7.de la Maza LM, Peterson EM. Vaccines for Chlamydia trachomatis infections. Curr Opin Investig Drugs. 2002;3:980–986. [PubMed] [Google Scholar]
- 8.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 9.Rockey DD, Wang J, Lei L, Zhong G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev Vaccines. 2009;8:1365–1377. doi: 10.1586/erv.09.98. [DOI] [PubMed] [Google Scholar]
- 10.Farris CM, Morrison RP. Vaccination against Chlamydia Genital Infection Utilizing the Murine C. muridarum Model. Infect Immun. 2011;79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Grayston JT, Woolridge RL, Wang S. Trachoma vaccine studies on Taiwan. Ann N Y Acad Sci. 1962;98:352–367. doi: 10.1111/j.1749-6632.1962.tb30558.x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor HR. Trachoma: a blinding scourge from the Bronze Age to the twenty-first century. Haddington Press Pry Ltd; Victoria, Australia: 2008. [Google Scholar]
- 14.Dawson C, Wood TR, Rose L, Hanna L. Experimental inclusion conjunctivitis in man. 3. Keratitis and other complications. Arch Ophthalmol. 1967;78:341–349. doi: 10.1001/archopht.1967.00980030343015. [DOI] [PubMed] [Google Scholar]
- 15.Nichols RL, Bell SD, Jr, Haddad NA, Bobb AA. Studies on trachoma. VI. Microbiological observations in a field trial in Saudi Arabia of bivalent rachoma vaccine at three dosage levels. Am J Trop Med Hyg. 1969;18:723–730. [PubMed] [Google Scholar]
- 16.Morrison RP, Lyng K, Caldwell HD. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 18.Stephens RS, Sanchez-Pescador R, Wagar EA, Inouye C, Urdea MS. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987;169:3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal S, Barnhart KM, Wei Q, Abai AM, Peterson EM, de la Maza LM. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine. 1999;17:459–465. doi: 10.1016/s0264-410x(98)00219-9. [DOI] [PubMed] [Google Scholar]
- 21.Su H, Caldwell HD. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992;175:227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong-Ji Z, Yang X, Shen C, Lu H, Murdin A, Brunham RC. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect Immun. 2000;68:3074–3078. doi: 10.1128/iai.68.6.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;73:8153–8160. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun G, Pal S, Weiland J, Peterson EM, de la Maza LM. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine. 2009;27:5020–5025. doi: 10.1016/j.vaccine.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, Cocco MJ, Peterson EM, de la Maza LM. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol. 2007;189:6222–6235. doi: 10.1128/JB.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect Immun. 2002;70:4812–4817. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farris CM, Morrison SG, Morrison RP. CD4+ T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect Immun. 2010;78:4374–4383. doi: 10.1128/IAI.00622-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, Peterson EM, Pal S, de la Maza LM, Caldwell HD. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol. 2009;182:8063–8070. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blake MS, Wetzler LM. Vaccines for gonorrhea: where are we on the curve? Trends Microbiol. 1995;3:469–474. doi: 10.1016/s0966-842x(00)89012-5. [DOI] [PubMed] [Google Scholar]
- 30.Castro CA, Hogan JB, Benson KA, Shehata CW, Landauer MR. Behavioral effects of vehicles: DMSO, ethanol, Tween-20, Tween-80, and emulphor-620. Pharmacol Biochem Behav. 1995;50:521–526. doi: 10.1016/0091-3057(94)00331-9. [DOI] [PubMed] [Google Scholar]
- 31.Tifrea DF, Sun G, Pal S, Zardeneta G, Cocco MJ, Popot JL, de la Maza LM. Amphipols stabilize the Chlamydia major outer membrane protein and enhance its protective ability as a vaccine. Vaccine. 2011;29:4623–4631. doi: 10.1016/j.vaccine.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speijers GJ, Danse LH, Krajnc-Franken MA, van Leeuwen FX, Helleman PW, Beuvery EC, Vos JG, Avd Heijden C. Subacute toxicity of Zwittergent administered intramuscularly. Vaccine. 1989;7:364–368. doi: 10.1016/0264-410x(89)90203-x. [DOI] [PubMed] [Google Scholar]
- 33.Speijers GJ, Danse LH, Beuvery EC, Derks HJ, Vos JG. Local reactions of Zwittergent-containing meningococcal vaccine after intramuscular injection in rats: comparison with the effect of diphtheria-pertussis-tetanus-polio vaccine. Vaccine. 1988;6:419–422. doi: 10.1016/0264-410x(88)90142-9. [DOI] [PubMed] [Google Scholar]
- 34.Tribet C, Audebert R, Popot JL. Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci U S A. 1996;93:15047–15050. doi: 10.1073/pnas.93.26.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tribet C, Diab C, Dahmane T, Zoonens M, Popot JL, Winnik FM. Thermodynamic characterization of the exchange of detergents and amphipols at the surfaces of integral membrane proteins. Langmuir. 2009;25:12623–12634. doi: 10.1021/la9018772. [DOI] [PubMed] [Google Scholar]
- 36.Nigg C. An Unidentified Virus Which Produces Pneumonia and Systemic Infection in Mice. Science. 1942;95:49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- 37.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–3369. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yen TY, Pal S, de la Maza LM. Characterization of the disulfide bonds and free cysteine residues of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Biochemistry. 2005;44:6250–6256. doi: 10.1021/bi047775v. [DOI] [PubMed] [Google Scholar]
- 40.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 41.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 42.Carmichael JR, Pal S, Tifrea D, de la Maza LM. Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine. Vaccine. 2011;29:5276–5283. doi: 10.1016/j.vaccine.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ralli-Jain P, Tifrea D, Cheng C, Pal S, de la Maza LM. Enhancement of the protective efficacy of a Chlamydia trachomatis recombinant vaccine by combining systemic and mucosal routes for immunization. Vaccine. 2010;28:7659–7666. doi: 10.1016/j.vaccine.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amorij JP, Westra TA, Hinrichs WL, Huckriede A, Frijlink HW. Towards an oral influenza vaccine: comparison between intragastric and intracolonic delivery of influenza subunit vaccine in a murine model. Vaccine. 2007;26:67–76. doi: 10.1016/j.vaccine.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 45.Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–3362. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su H, Morrison RP, Watkins NG, Caldwell HD. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990;172:203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pal S, Cheng X, Peterson EM, de la Maza LM. Mapping of a surface-exposed B-cell epitope to the variable sequent 3 of the major outer-membrane protein of Chlamydia trachomatis. J Gen Microbiol. 1993;139:1565–1570. doi: 10.1099/00221287-139-7-1565. [DOI] [PubMed] [Google Scholar]
- 48.Peterson EM, Zhong GM, Carlson E, de la Maza LM. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988;56:885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol. 2005;175:7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Letters. 1981;12:111–115. [Google Scholar]
- 52.de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roumestand C, Mispelter J, Austruy C, Canet D. The Use of Band Filtering in Multidimensional NMR. Evaluation of Two “User-Friendly” Techniques. J Magn Reson B. 1995;109:153–163. [Google Scholar]
- 54.Quadros C. Vaccines. Pan American Health Organization; Washington: 2004. Preventing disease and protecting health. [Google Scholar]
- 55.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 56.Harandi AM, Davies G, Olesen OF. Vaccine adjuvants: scientific challenges and strategic initiatives. Expert Rev Vaccines. 2009;8:293–298. doi: 10.1586/14760584.8.3.293. [DOI] [PubMed] [Google Scholar]
- 57.Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21(Suppl 2):S89–95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 58.Hui GS, Hashimoto CN. Adjuvant formulations possess differing efficacy in the potentiation of antibody and cell mediated responses to a human malaria vaccine under selective immune genes knockout environment. Int Immunopharmacol. 2008;8:1012–1022. doi: 10.1016/j.intimp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ionescu-Matiu I, Kennedy RC, Sparrow JT, Culwell AR, Sanchez Y, Melnick JL, Dreesman GR. Epitopes associated with a synthetic hepatitis B surface antigen peptide. J Immunol. 1983;130:1947–1952. [PubMed] [Google Scholar]
- 61.Zoonens M, Giusti F, Zito F, Popot JL. Dynamics of membrane protein/amphipol association studied by Forster resonance energy transfer: implications for in vitro studies of amphipol-stabilized membrane proteins. Biochemistry. 2007;46:10392–10404. doi: 10.1021/bi7007596. [DOI] [PubMed] [Google Scholar]
- 62.Popot JL, Berry EA, Charvolin D, Creuzenet C, Ebel C, Engelman DM, Flotenmeyer M, Giusti F, Gohon Y, Hong Q, Lakey JH, Leonard K, Shuman HA, Timmins P, Warschawski DE, Zito F, Zoonens M, Pucci B, Tribet C. Amphipols: polymeric surfactants for membrane biology research. Cell Mol Life Sci : CMLS. 2003;60:1559–1574. doi: 10.1007/s00018-003-3169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Popot JL. Amphipols, nanodiscs, and fluorinated surfactants: three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu Rev Biochem. 2010;79:737–775. doi: 10.1146/annurev.biochem.052208.114057. [DOI] [PubMed] [Google Scholar]
- 64.Zoonens M, Catoire LJ, Giusti F, Popot JL. NMR study of a membrane protein in detergent-free aqueous solution. Proc Natl Acad Sci U S A. 2005;102:8893–8898. doi: 10.1073/pnas.0503750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gohon Y, Pavlov G, Timmins P, Tribet C, Popot JL, Ebel C. Partial specific volume and solvent interactions of amphipol A8-35. Anal Biochem. 2004;334:318–334. doi: 10.1016/j.ab.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 66.Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Popot JL, Guittet E. Inter- and intramolecular contacts in a membrane protein/surfactant complex observed by heteronuclear dipole-to-dipole cross-relaxation. J Magn Reson. 2009;197:91–95. doi: 10.1016/j.jmr.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Catoire LJ, Zoonens M, van Heijenoort C, Giusti F, Guittet E, Popot JL. Solution NMR mapping of water-accessible residues in the transmembrane beta-barrel of OmpX. Eur Biophys J. 2010;39:623–630. doi: 10.1007/s00249-009-0513-2. [DOI] [PubMed] [Google Scholar]
- 68.Althoff T, Mills DJ, Popot JL, Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. The EMBO J. 2011;30:4652–4664. doi: 10.1038/emboj.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Etzkorn M, Raschle T, Hagn F, Gelev V, Rice AJ, Walz T, Wagner G. Cell-free expressed bacteriorhodopsin in different soluble membrane mimetics: biophysical properties and NMR accessibility. Structure. 2013;21:394–401. doi: 10.1016/j.str.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nikaido H. Porins and specific diffusion channels in bacterial outer membranes. J Biol Chem. 1994;269:3905–3908. [PubMed] [Google Scholar]
- 71.White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 72.Yau WM, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–14718. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 73.Feher VA, Randall A, Baldi P, Bush RM, de la Maza LM, Amaro RE. A 3-dimensional trimeric beta-barrel model for Chlamydia MOMP contains conserved and novel elements of Gram-negative bacterial porins. PLoS One. 2013;8:e68934. doi: 10.1371/journal.pone.0068934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Findlay HE, McClafferty H, Ashley RH. Surface expression, single-channel analysis and membrane topology of recombinant Chlamydia trachomatis Major Outer Membrane Protein. BMC Microbiol. 2005;5:5. doi: 10.1186/1471-2180-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Maranon MJ, Bush RM, Peterson EM, Schirmer T, de la Maza LM. Prediction of the membrane-spanning beta-strands of the major outer membrane protein of Chlamydia. Protein Sci : a publication of the Protein Society. 2002;11:1854–1861. doi: 10.1110/ps.3650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Berg E, Feng X, Shen L, Smith T, Costello CE, Zhang YX. Identification of surface-exposed components of MOMP of Chlamydia trachomatis serovar F. Protein Sci. 2006;15:122–134. doi: 10.1110/ps.051616206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musson JA, Morton M, Walker N, Harper HM, McNeill HV, Williamson ED, Robinson JH. Sequential proteolytic processing of the capsular Caf1 antigen of Yersinia pestis for major histocompatibility complex class II-restricted presentation to T lymphocytes. J Biol Chem. 2006;281:26129–26135. doi: 10.1074/jbc.M605482200. [DOI] [PubMed] [Google Scholar]
- 78.Warren EH, Vigneron NJ, Gavin MA, Coulie PG, Stroobant V, Dalet A, Tykodi SS, Xuereb SM, Mito JK, Riddell SR, Van den Eynde BJ. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science. 2006;313:1444–1447. doi: 10.1126/science.1130660. [DOI] [PubMed] [Google Scholar]
- 79.Tikhonova AN, Van Laethem F, Hanada K, Lu J, Pobezinsky LA, Hong C, Guinter TI, Jeurling SK, Bernhardt G, Park JH, Yang JC, Sun PD, Singer A. alphabeta T cell receptors that do not undergo major histocompatibility complex-specific thymic selection possess antibody-like recognition specificities. Immunity. 2012;36:79–91. doi: 10.1016/j.immuni.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ortiz L, Demick KP, Petersen JW, Polka M, Rudersdorf RA, Van der Pol B, Jones R, Angevine M, DeMars R. Chlamydia trachomatis major outer membrane protein (MOMP) epitopes that activate HLA class II-restricted T cells from infected humans. J Immunol. 1996;157:4554–4567. [PubMed] [Google Scholar]
- 81.Igietseme JU, Portis JL, Perry LL. Inflammation and clearance of Chlamydia trachomatis in enteric and nonenteric mucosae. Infect Immun. 2001;69:1832–1840. doi: 10.1128/IAI.69.3.1832-1840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrison RP, Belland RJ, Lyng K, Caldwell HD. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989;170:1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–127. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]