Abstract
The internal microenvironment in peripheral nerves is highly regulated in order to maintain normal axonal impulse transmission to or from the central nervous system. In humans, this regulation is facilitated by specialized tight junction (TJ)-forming endoneurial microvascular endothelial cells, and perineurial myofibroblasts that form multiple concentric layers around nerve fascicles. The endoneurial endothelial cells come in direct contact with circulating blood, and thus, can be considered the blood-nerve barrier (BNB). Studies on the molecular and biophysical properties of the human BNB in vivo or in situ are limited. Owing to the recent isolation of primary human endoneurial endothelial cells (pHEndECs) and the development of simian virus 40 large T-antigen immortalized cell lines, data are emerging on the structural and functional characteristics of these cells. These data aim to increase our understanding of how solutes, macromolecules, nutrients and hematogenous leukocytes gain access into or are restricted from the endoneurium of peripheral nerves. These concepts have clinical relevance in understanding normal peripheral nerve homeostasis, the response of peripheral nerves to external insult and stresses such as drugs and toxins and the pathogenesis of peripheral neuropathies. This review discusses current knowledge in this nascent and exciting field of microvascular biology.
Keywords: blood-nerve barrier, human, in vitro models, leukocyte trafficking, microvascular repair, mitogens, peripheral nerve, solute permeability, transendothelial electrical resistance
Introduction
Peripheral nerves are responsible for the impulse transmission from the periphery to the central nervous system (CNS) for processing and transmit output impulses from the CNS back to the periphery to facilitate effector functions such as locomotion. These impulses are generated and directed via the sequential process of axonal depolarization and subsequent repolarization, a process dependent on sodium and potassium channel flux. This process is crucial for normal human function, thus, the ionic gradients within peripheral nerves must be tightly regulated to prevent variations in sodium and potassium concentration from altering signal transmission [1,2]. Furthermore, solute and macromolecules that could influence the ionic balance within peripheral nerves require regulated control.
Human peripheral nerves have a multilayered structural organization with an anastomosis of blood vessels that supply these layers. Peripheral nerves are divided into an outermost epineurium, inner perineurium and innermost endoneurium (Figure 1A). The endoneurium is completely surrounded by the perineurium (forming a nerve fascicle), with several nerve fascicles embedded within the epineurium. The endoneurium is of critical importance to the primary function of peripheral nerves as it contains the axons and their supporting Schwann cells, which either myelinate segments of single axons or envelope clusters of small axons without myelination [1–4].
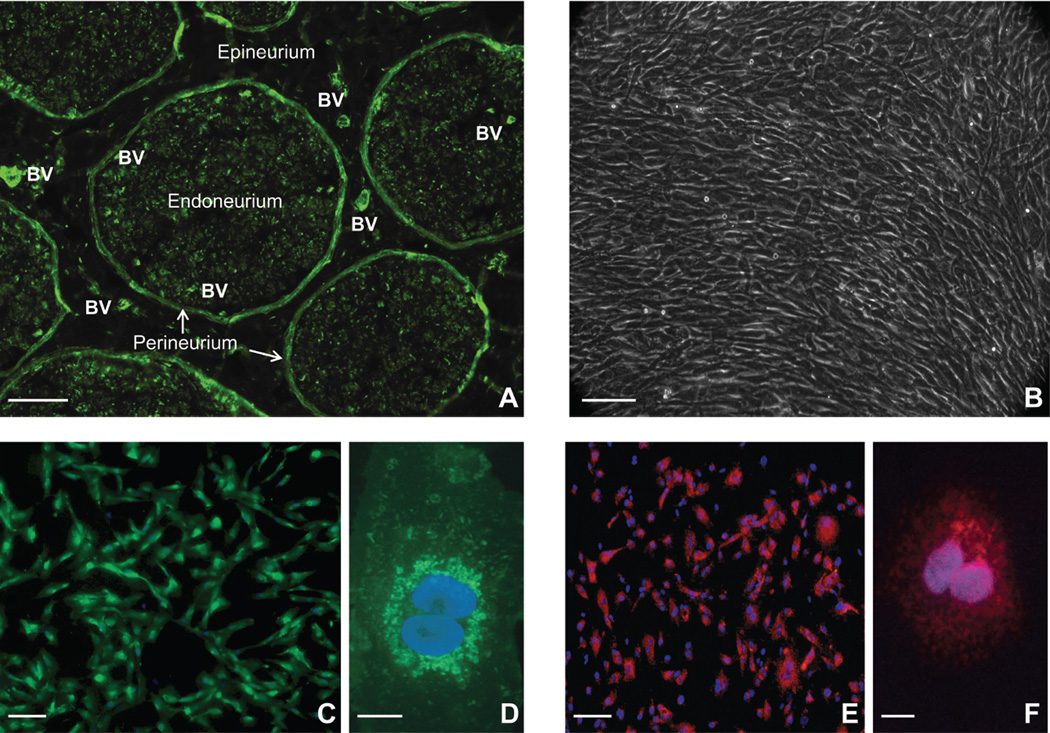
Figure 1. Peripheral nerve anatomy and characteristics of primary endoneurial endothelial cells.
A digital photomicrograph of an axial cryostat section of human sciatic nerve stained with fluoresceinated UEA-1 demonstrates the multilayered anatomical organization of peripheral nerves, with blood vessels (BV) within the epineurium and endoneurium indicated (A). A digital phase contrast photomicrograph demonstrates confluent, spindle-shaped pHEndECs six days after being cultured on rat tail collagen-coated CellBIND® tissue culture plates at the onset of a flow-dependent leukocyte trafficking assay (B). Proliferating pHEndECs strongly bind UEA-1, as expected for human vascular endothelial cells (C) with more intense perinuclear staining seen at higher magnification (D), as shown by these direct immunocytochemistry digital photomicrographs of fixed cells cultured on rat-tail collagen coated glass coverslips. Intracellular vWF expression, another marker of vascular endothelial cells, is demonstrated on fixed, permeabilized proliferating pHEndEC cultures grown on rat-tail collagen coated glass coverslips (E), with intracellular perinuclear expression verified at higher magnification (F), based on these digital indirect immunocytochemistry photomicrographs. Scale bars 100 µm for 1A–C and 1E, 5 µm for 1D and 1F.
The peripheral nerve vascular supply is derived from radial branches of nearby arteries, forming the vasa nervosum that runs in the longitudinal axis of the nerve in the outer epineurium. These vessels generate branches that transverse deeper into the epineurium, forming small arteries and arterioles, eventually resulting in pre-capillary arterioles that run on the external surface of the perineurium. These vessels subsequently produce branches that penetrate the concentric multilayered perineurium to enter the endoneurium, forming capillaries. These capillaries may drain into larger post-capillary venules within the endoneurium, with venules emerging through the perineurium. Venules and small veins run longitudinally within the epineurium, eventually joining to form the larger veins that drain the peripheral nerve into nearby veins via radial branches [1,2,5].
Ultrastructural examination of the human peripheral nerve vascular supply demonstrates electron-dense rich intercellular TJs between endothelial cells within the endoneurium, with small 50–100 nm intracellular pinocytic vesicles. These endothelial cells lack fenestrations. This is in contrast to endothelial cells within the epineurium and perineurium that contain numerous fenestrations and lack TJs [3,4]. These observational data imply that endoneurial microvascular cells are specialized endothelial cells, similar to endothelial cells that form the restrictive blood-brain and blood-testis barriers. These ultrastructural studies also demonstrate intercellular TJs between adjacent perineurial myofibroblast cells, particularly within the inner concentric layers [3,4]. These observations indicate that specialized interfaces within peripheral nerves occur, with expected restrictive barrier-like properties.
The lack of TJs between macrovascular endothelial cells within the epineurium as well as the presence of fenestrations between these cells suggests that solutes, macromolecules and other blood-borne substances may “leak” into the epineurium and constitute its interstitial fluid [6]. Due to the fact that the epineurium structurally consists of longitudinal arrays of collagen fibers (needed to maintain the structural integrity of the peripheral nerve), these substances can freely diffuse within this layer. Thus, the perineurium serves to protect the innermost layer of peripheral nerves, the endoneurium, from this passive diffusion of epineurial interstitial fluid components [1–4]. The specialized endoneurial microvascular endothelial cells come in direct contact with substances in circulating blood, and can be considered the blood-nerve barrier (BNB).
Compared to other microvascular endothelial cells, very little is known about human endoneurial endothelial cells. Understanding the biology of these endothelial cells can provide insights into how solutes and macromolecules gain access into or are expelled from the endoneurium, providing cues to the nutritional requirements of peripheral nerves and the mechanisms of regulated removal of metabolic end-products. Resident macrophages and mast cells are commonly observed in normal peripheral nerves. These cells could act as primary responders to endoneurial injury and participate in the innate immune response to foreign pathogens. Rare T-cells may be seen in normal nerves, hypothesized to be involved in normal immune surveillance. However, recruitment of hematogenous monocytes and T-cells is commonly seen following peripheral nerve injury or in immune-mediated peripheral neuropathies such as Guillain-Barré syndrome, implying that functional and structural modifications occur at the human BNB [1,2]. In situ observational studies in human and rodent peripheral nerves imply progressive BNB maturation during fetal and post-natal development [7,8]. Endoneurial endothelial cell proliferation and restoration of restrictive barrier function is intuitively expected during peripheral nerve recovery from injury [9,10]. It is important to know whether endogenous or exogenous mitogens participate in these processes, and their cellular sources.
Knowledge of the human BNB had been limited for many years by difficulties isolating and culturing endoneurial endothelial cells. Due to functional and phenotypic differences between microvascular endothelial cells from different tissues and species [11–13], this work requires human endoneurial endothelial cells. Recent success within the last 4 years has resulted in the initial characterization of primary human endoneurial endothelial cells (pHEndECs), also known as peripheral nerve microvascular endothelial cells (PnMECs), and the development of simian virus 40 large T-antigen (SV40 LTA) immortalized cell lines, with emerging data on the molecular and biophysical characteristics of the human BNB in vitro, and responses to different substances under normal physiological and pathophysiological conditions [14–19].
Molecular characterization of human endoneurial endothelial cells
pHEndECs have been successfully isolated and purified from the sciatic nerves of recently decedent individuals. The sciatic nerve is the largest nerve in the human body and provides sufficient material to isolate endoneurial endothelial cells (constitute <0.1% of the cellular components of peripheral nerves) using endoneurial stripping, enzymatic digestion and density gradient centrifugation. These cells have been successfully cultured and expanded for up to 10 passages in vitro, as well as immortalized via stable transfection with plasmids containing SV40 LTA , as well as retrovirus vectors containing temperature-sensitive SV40 LTA and telomerase [14–19]. These endothelial cells when grown to confluence, form the framework for in vitro models of the human BNB (Figure 1B). Immunocytochemistry, flow cytometry, polymerase chain reaction (PCR) and western blot techniques have been employed to determine the molecular characteristics of these human BNB-forming primary and immortalized cell lines.
Vascular endothelial cell markers
Primary and immortalized human endoneurial endothelial cells bind Ulex Europaeus Agglutinin-1 (UEA-1), the most sensitive marker of vascular endothelial cells in vitro and in situ (Figure 1C, D). This indicates α-L-fucose expression by the BNB-forming cells in vitro, as expected in vivo [14,15,20]. Furthermore, these cells intensely uptake fluorescently-labeled acetylated low density lipoproteins, indicative of the expression of specialized scavenger receptors, as expected of microvascular endothelial cells [14,15,17,19]. Von Willebrand factor (also known as factor VIII related antigen), a large multimeric glycoprotein that is important for hemostasis and constitutively produced in intracellular Weibel-Palade bodies [21], is reliably expressed by pHEndECs [14,17] (Figure 1E, F) with reported loss of expression in plasmid-transfected immortalized cells [15] and retained expression with retroviral transfected cells [19]. Loss of Weibel-Palade bodies has been described in immortalized human endothelial cell lines [22], implying that these cell lines may be less useful for studying mechanisms of hemostasis relevant to endoneurial microvascular repair in peripheral nerves.
Growth factor ligands/ receptors
pHEndECs reliably proliferate in vitro for at least 8 passages with a doubling of cell numbers every 48 hours during the early logarithmic growth phase, followed by contact inhibition [14]. Immortalized endoneurial endothelial cells have been successfully expanded to 27 passages (>45 population doublings and >80 days in continuous culture) with variable population doubling time from approximately 28 to 53 hours without contact inhibition in regular growth medium [15]. Temperature-sensitive SV40 LTA immortalized cell lines cultured at 33°C have been successfully expanded to 30 passages with a doubling time of about 3 days (varied between approximately 48 to 96 hours), with loss of proliferative capacity associated with senescence observed when cultured at 37°C [19]. The full repertoire of mitogens, co-factors and growth factor receptors required for endoneurial endothelial cell proliferation are currently unknown.
Culture on surfaces coated with type I rat tail collagen seems to be necessary for in vitro proliferation and the formation of confluent BNB monolayers. Several mitogens, including vascular endothelial growth factor (VEGF) are included in specialized media or growth supplements described to culture primary and immortalized human endoneurial endothelial cells. PnMECs have been shown to also express VEGF, but not angiopoeitin, basic fibroblast growth factor (bFGF) or transforming growth factor (TGF)-β1 by western blot [17]. Endogenous VEGF expression implies a possible autocrine effect in cellular proliferation. In the embryonic rodent brain, high levels of VEGF receptors, VEGFR1 and VEGFR2 have been described during angiogenesis, with low levels observed in the adult blood-brain barrier endothelium [23]. VEGF is also widely known to modulate mammalian microvascular permeability; with the formation of adrenal cortex capillary endothelial fenestrations in vitro [24]. VEGF has also been implicated in altering TJ protein expression by PnMECs following exposure to advanced glycation end-products (mimicking diabetic conditions) in vitro [25]. The dose-dependent effects of VEGF and the expression profile of its signaling receptors by human BNB-forming endothelial cells in vitro under different experimental conditions require further investigation.
Glial cell-derived neutrophic factor (GDNF) and nerve growth factor (NGF) were not expressed by PnMECs in vitro by PCR or western blot, while messenger RNA (mRNA) for brain-derived neurotrophic factor (BDNF) without protein expression by western blot has been described [17]. Interestingly, these primary cells express the glycosylphosphatidylinositol (GPI)-anchored membrane protein receptor for GDNF, GFRα1, suggesting responsiveness to exogenous GDNF [18,26]. In support of this, GDNF upregulated pHEndEC GFRα1 expression as a response to diffuse endothelial injury induced by serum withdrawal in vitro, suggesting a positive feedback loop [26].
Adherens and tight junction proteins
Intercellular adherens junctions (AJs) and TJs, and their associated binding, signaling/regulatory proteins are essential characteristics of restrictive barrier-forming microvascular endothelium [27–30]. Primary and immortalized human endoneurial endothelial cells have been shown to express mRNA transcripts or protein for claudin-1, claudin-2, the endothelial specific claudin-5, claudin-12 and claudin-19, as well as occludin, zona occludens (ZO)-1, ZO-2, junctional adhesion molecule-A (JAM-A), vascular endothelial (VE)-cadherin and β-catenin [14,17–19,26] (Table 1). However, mRNA or protein expression does not necessarily imply localization at sites of intercellular contacts required for AJ and TJ formation. Similarly, contamination of purified human endoneurial endothelial cell cultures by myelinating Schwann cells (known to form autotypic TJs in non-compacted myelin components between adjacent membrane lamellae of the same Schwann cell in vivo and in situ) could confound these assays [31,32]. Immunocytochemistry of confluent cultures has demonstrated expression of AJ protein VECadherin, and TJ proteins claudin-5 and occludin and TJ-associated proteins ZO-1 and ZO-2 at sites of cell-to-cell contact [14,19,26]. Interestingly, pHEndECs demonstrated nuclear expression of claudin-1, with perinuclear claudin-2 and diffuse cytoplasmic JAM-A expression in vitro [14], suggesting that these molecules may not actively participate in human BNB function under normal conditions.
Table 1.
Human endoneurial endothelial cell adherens and tight junction protein expression.
| PROTEIN | CELL LINE | mRNA | Protein (WB) | ICC | Cellular Localization |
|---|---|---|---|---|---|
| β-Catenin | pHEndEC (26) | + | + | N/A | N/A |
| VE-Cadherin | pHEndEC (26) | + | + | + | Intercellular Membrane |
| ZO-1 | pHEndEC (14, 26) | + | + | + | Intercellular Membrane |
| FH-BNB (19) | + | N/A | + | Intercellular Membrane | |
| ZO-2 | FH-BNB (19) | + | N/A | + | Intercellular Membrane |
| Occludin | pHEndEC (14, 26) | + | + | + | Intercellular Membrane |
| PnMEC (17, 18) | + | + | N/A | N/A | |
| FH-BNB (19) | + | N/A | + | Intercellular Membrane | |
| Claudin-5 | pHEndEC (14, 26) | + | + | + | Intercellular Membrane |
| PnMEC (16, 17, 18) | + | + | N/A | N/A | |
| FH-BNB (19) | + | + | + | Intercellular Membrane | |
| Claudin-1 | pHEndEC (14) | + | N/A | + | Nuclear |
| FH-BNB (19) | + | N/A | N/A | N/A | |
| Claudin-2 | pHEndEC (14) | + | N/A | + | Perinuclear |
| Claudin-12 | FH-BNB (19) | + | N/A | N/A | N/A |
| Claudin-19 | FH-BNB (19) | + | N/A | N/A | N/A |
| JAM-A | pHEndEC (14, 26) | + | + | + | Cytoplasmic |
| FH-BNB (19) | + | N/A | N/A | N/A | |
Key: pHEndEC: primary human endoneurial endothelial cells, PnMEC: peripheral nerve microvascular endothelial cells, FH-BNB: conditionally immortalized human peripheral nerve microvascular endothelial cells, mRNA: messenger RNA, WB: western blot, ICC: immunocytochemistry, +: detected, N/A: not assessed. Parentheses represent reference numbers.
Conditioned medium from immortalized human peripheral nerve pericyte lines, as well as bFGF, hydrocortisone and GDNF, has been reported to induce claudin-5 protein levels (~50% of untreated levels) within 24–48 hours of plating primary or immortalized BNB-forming endothelial cells at high density without significant changes in occludin [16–18]. Thus, it has been postulated that claudin 5 expression at the BNB is primarily responsible for its TJ barrier characteristics. However, immunocytochemical evidence demonstrating increased claudin-5 expression at intercellular contacts is lacking in these reports. In a model of diffuse endothelial injury of confluent pHEndEC cultures, GDNF at low nanomolar concentrations that maximally restored barrier function failed to induce mRNA transcripts or protein expression for β-catenin, VE-Cadherin, ZO-1 or occludin relative to basal untreated conditions. There was a small increase in total claudin-5 protein (~30% of basal levels), without significant differences in mRNA expression, or claudin-5 tyrosine phosphorylation [26].
Observational in situ immunohistological data from developing and adult human sciatic nerves demonstrated equivalent endoneurial microvessel claudin-5 expression during neonatal development (when the BNB is immature and permissive based on rodent work) when compared to adult nerves [7,8]. Published in vitro immunocytochemistry data has shown that the GDNF-induced improvement in human BNB TJ barrier function following serum withdrawal was dependent on “rearranged during transfection” (RET)-tyrosine kinase signaling pathways (RET is the receptor for members of the GDNF family of extracellular signaling molecules) associated with F-actin cytoskeletal filament relocation towards cytoplasmic membranes, resulting in more continuous intercellular AJs and TJs with fewer non-continuous spaces between adjacent cells [26]. This is congruent with studies in rodent embryonic brains and neonatal rat sciatic nerves that also demonstrate that TJ barrier maturation is associated with cytoskeletal rearrangements and disappearance of clefts between endothelial cells [8,28,33].
Work is needed to deduce the full repertoire of AJ and TJ proteins expressed at the human BNB, as well as the temporal relationship between protein expression and translocation to intercellular junctions with resultant restrictive barrier function. Differences in the repertoire and distribution of AJ and TJ proteins between human and mouse myelinating Schwann cells in adult peripheral nerves [32] further emphasizes the need to perform expression profile studies using human pHEndECs for in situ testing on human endoneurial microvessels in peripheral nerve biopsies. These studies could provide insights into normal human BNB development and restoration of barrier function following injury such as hypoxia, trauma or toxic insult. Figure 2 depicts the known and hypothesized AJ and TJ proteins, and growth factor receptors expressed at the human BNB, and the proposed relationship between GDNF and actin cytoskeletal reorganization during recovery from diffuse endothelial injury.
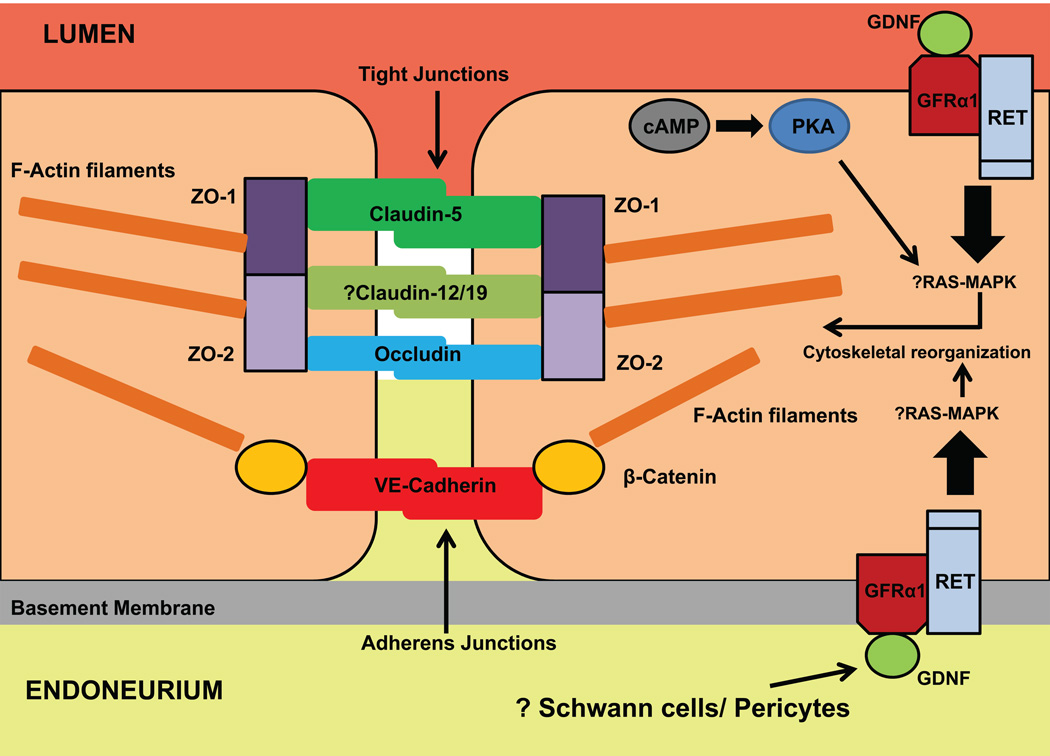
Figure 2. Human BNB intercellular junctional complex.
This figure illustrates the known AJ and TJ proteins expressed by the human BNB in vitro, and their accessory or regulatory proteins and their membrane localization guided by knowledge of other restrictive barrier forming endothelial cells. There is immunocytochemistry evidence for ZO-1, ZO-2, claudin-5, occludin and VE-Cadherin expression at intercellular contact sites, without direct evidence for claudin-12 and claudin-19. pHEndEC β-catenin expression has been demonstrated by PCR and western blot. GFRα1, the receptor for GDNF, complexed with RET, is expressed by pHEndECs, and mediates recovery of restrictive barrier characteristics at the human BNB in vitro following serum withdrawal, dependent on RET-tyrosine kinase mediated signaling. There is preliminary evidence that the Ras-MAPK signaling pathway acts downstream of RET, with the net effect being cytoskeletal reorganization of F-actin filaments, with relocation towards the cytoplasmic membrane and intercellular contact sites, providing the scaffold for more continuous AJ and TJ and fewer intercellular clefts. Cyclic adenosine monophosphate (cAMP), acting via protein kinase A (PKA) has been shown to play a minor role in this process, independent of GDNF, with a mild additive effect observed when multiple mitogens are administered to induce pHEndEC recovery from diffuse endothelial injury. Schwann cells and pericytes are potential cellular sources for GDNF in peripheral nerves.
Transporters
As a restrictive microvascular barrier necessary to maintain the internal homeostatic microenvironment in peripheral nerves, the human BNB is expected to possess specialized transporters on its luminal and abluminal surfaces to facilitate directional influx of solutes, nutrients and macromolecules and efflux of waste products of metabolism or xenobiotics. Current knowledge on BNB transporters has been guided by expectations based on other restrictive microvascular barriers, such as the blood-brain barrier (BBB), and observations in the peripheral nerves of other species [34–47]. In vivo human data, in situ data from human peripheral nerve biopsies and data from non-cultured human BNB-forming endothelial cells are generally lacking. Hypothesized BNB transporters based on in vitro data from cultured human endoneurial endothelial cells can be classified as ionic, nutrient and xenobiotic transporters (Table 2).
Table 2.
Human blood-nerve barrier transporter expression in vitro.
| PROTEIN | CELL LINE | mRNA | Protein (WB/FACS) |
ICC |
|---|---|---|---|---|
| Alkaline Phosphatase | pHEndEC (14) | + | + | + |
| THEndEC (15) | + | + | N/A | |
| GLUT-1 | pHEndEC (14) | + | + | + |
| THEndEC (15) | + | N/A | N/A | |
| FH-BNB (19) | + | + | N/A | |
| MCT-1 | pHEndEC (14) | + | N/A | N/A |
| THEndEC (15) | + | + | N/A | |
| CRT | pHEndEC (14) | + | N/A | N/A |
| THEndEC (15) | + | + | N/A | |
| LAT-1 | pHEndEC (14) | + | N/A | N/A |
| THEndEC (15) | + | + | N/A | |
| γ-glutamyl transpeptidase | pHEndEC (14) | + | + | + |
| THEndEC (15) | + | + | N/A | |
| P-glycoprotein/ MDR1a | pHEndEC (14) | + | + | + |
| THEndEC (15) | + | + | N/A | |
| FH-BNB (19) | + | + | N/A | |
| MRP-1 | FH-BNB (19) | + | N/A | N/A |
| OATP-C | THEndEC (15) | N/A | + | N/A |
| OAT-3 | THEndEC (15) | N/A | + | N/A |
Key: pHEndEC: primary human endoneurial endothelial cells, THEndEC: SV40 large T-antigen immortalized human endoneurial endothelial cells, FH-BNB: conditionally immortalized human peripheral nerve microvascular endothelial cells, mRNA: messenger RNA, WB: western blot, FACS: fluorescent activated cell sorting, ICC: immunocytochemistry, +: detected, N/A: not assessed. Parentheses represent reference numbers.
Alkaline phosphate (AP) is an enzyme that can be considered a capillary endothelium ionic transporter, as it can transfer phosphate groups, as well as serve as a membrane pump or cell adhesion molecule, in addition to the hydrolysis of phosphate esters [48–51]. AP is specifically expressed on capillary endothelial cells in several tissues and in mammalian peripheral nerves, it has been shown to discriminate between endoneurial and epineurial vessels [5]. AP expression has been demonstrated on primary and immortalized human endoneurial endothelial cells in vitro. Loss of AP expression was observed with immortalized human endoneurial endothelial cells at higher passages [14,15]. It is hypothesized that other ionic transporters that may maintain ionic concentrations within the endoneurium are expressed at the BNB. The putative monovalent and divalent adenosine triphosphate (ATP)-dependent and independent transporters present on the BNB are currently unknown.
Nutrient transporters are presumed to facilitate the transport of glucose, amino acids and lipids from the blood circulation into the endoneurium. Glucose transporter-1 (GLUT-1), the major glucose transporter in the body, is highly expressed by human endoneurial endothelial cells, including serially passaged immortalized cells [14,15]. GLUT-1 has been described in situ on adult and developing sciatic nerve endoneurial capillaries by indirect immunofluorescence [52].This implies an important role for facilitated transport of D-glucose into the endoneurium as a source of energy under normal conditions [35]. Monocarboxylate transporter-1 (MCT-1), a major transporter for monocarboxylic acids such as L-lactate, is similarly expressed by primary and immortalized human endoneurial endothelial cells [14,15], implying a role as an influx transporter, bringing in L-lactate from the bloodstream for energy utilization during periods of starvation or as an efflux transporter, removing lactate from the endoneurium as an aerobic metabolic by-product or following anaerobic metabolism following axonal or Schwann cell injury [53]. Creatine transporter (CRT) transports creatine (required for the temporary storage of high energy phosphate groups) across vascular endothelium. Creatine provides a high energy phosphate reservoir needed for ATP generation during periods of high metabolic activity [40]. CRT expression has been demonstrated on human primary and immortalized BNB-forming endothelial cells [14,15], implying that creatine transport into the endoneurium may be necessary for normal peripheral nerve function.
The Na+-independent L-type amino acid transporter 1 (LAT-1), which forms a heterodimer with 4F2 cell-surface antigen heavy chain is known to preferentially transport neutral and aromatic amino acids, such as L-phenylalanine and L-tryptophan [47,54]. LAT-1 is expressed by primary and immortalized human endoneurial endothelial cells [14,15], implying an important role as an influx transporter for this class of amino acids for energy utilization within peripheral nerves. γ-glutamyl transpeptidase, a known transporter of amino acids across restrictive barrier-forming endothelium [36,41,51,55], that also functions as an enzyme that catalyzes the transfer of the γ-glutamyl moiety of glutathione to an acceptor (amino acid, peptide, or water), is expressed by these cells [14,15], providing another means by which amino acids can be transported across the BNB. Its catalytic function could also provide a pathway for drug and xenobiotic detoxification in peripheral nerves.
Xenobiotic transporters demonstrated thus far to be expressed by primary or immortalized human endoneurial cells include members of the ATP-binding cassette (ABC) or multi-drug resistance and organic anion transporter families. ABC transporters include P-glycoprotein (also known as multi-drug resistance gene 1a; MDR1a), a major efflux transporter for chemotherapeutic drugs such as vincristine, and multi-drug resistance protein-1 (MRP1), an efflux transporter with significant substrate overlap with P-gp [14,15,19]. MRP-1 may transport a broader range of xenobiotics used as antineoplastic or therapeutic agents including folate-based antimetabolites, anthracyclines, vinca alkaloids, anti-androgens, and numerous glutathione (GSH) and glucuronide conjugates of these compounds as well as organic anions and heavy metals. MRP1 also transports diverse physiological substrates such as folates, GSH and GSH disulfide (GSSG), as well as sulphate-, GSH- and glucuronide-conjugates of steroids, leukotrienes and prostaglandins [47,56,57]. The activities of P-gp and MRP1 may be important for the protection of peripheral nerves from toxic insult as well as in normal cellular processes such as export of endogenous metabolic intermediates [47,56–58].
Immortalized human endoneurial endothelial cells express organic anion transporters organic anion transporting polypeptide-C (OATP-C) and organic anion transporter 3 (OAT-3) at similar levels to immortalized brain microvascular endothelial cells that form the BBB [15]. OATP-C is a member of a family of solute carriers that function as Na+ and ATP-independent transporters for amphipathic organic compounds such as steroids, thyroid hormones, anionic peptides, drugs (such as 3-hydroxy-3-methyl-glutarylcoenzyme A reductase inhibitors) and xenobiotics [47,59,60]. OAT-3 involved in the transport and excretion of drugs such as benzyl penicillin, methotrexate, ciprofloxacin, indomethacin and cimetidine, and toxic organic ions. OAT-3 also functions as an organic anion exchanger, coupling the uptake of one organic anion molecule with the efflux of one endogenous dicarboxylic acid molecule [47,61,62]. It is speculated that these organic anion transporters serve as efflux transporters may protect axons and Schwann cells within the endoneurium from the toxic effects of these organic molecules and compounds.
Further work is needed to determine the full repertoire of transporters expressed at the human BNB. Studies may be focused on elucidating their cellular membrane localization (luminal or abluminal, or both) and influx and efflux kinetic characteristics for a variety of substrates and decipher what adaptations may occur to maintain normal substrate transport. This information should result in detailed comprehension of the metabolic requirements of peripheral nerves in health, during starvation and at times of stress, as well as provide insights into targeted drug delivery into peripheral nerves and the prevention of drug-induced toxicities. Figure 3 illustrates the currently known transporters expressed at the human BNB in vitro and their hypothesized cellular localization.
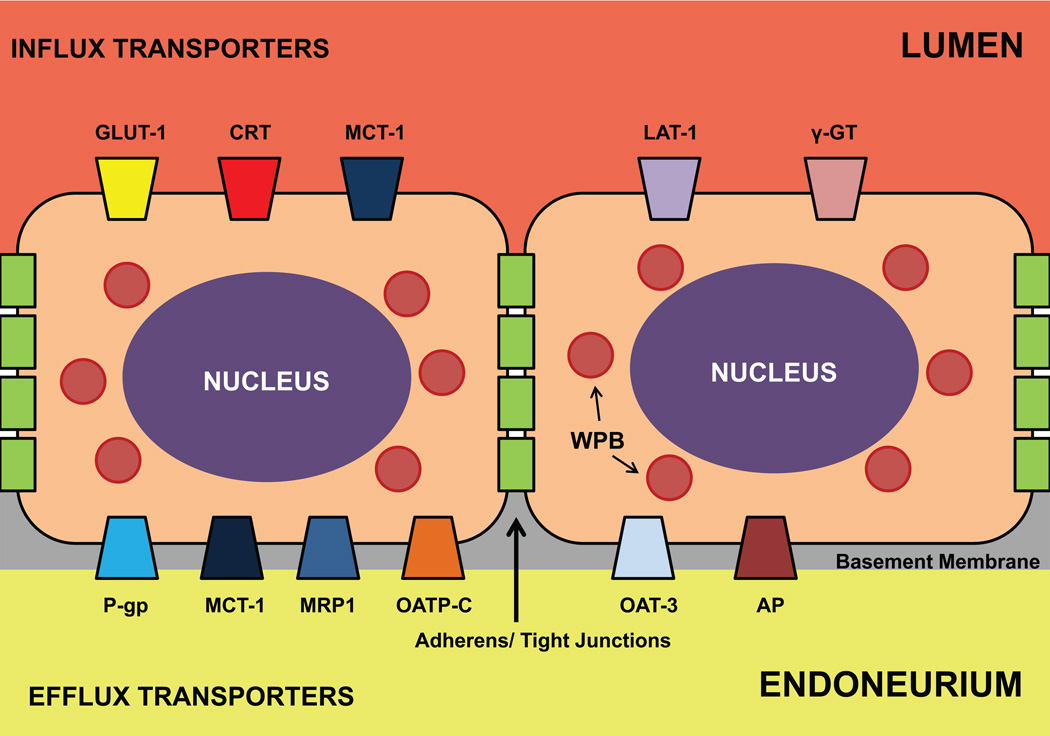
Figure 3. Human BNB transporters.
This figure depicts the known transporters and their putative cellular localization and actions at the BNB based on PCR, western blot, flow cytometry and immunocytochemical studies performed with primary and immortalized human endoneurial endothelial cells in vitro. The influx transporters are shown on the luminal side, while efflux transporters are shown on the abluminal side. It is hypothesized that MCT-1 may a dual role as an influx and efflux transporter dependent on endoneurial energy requirements and the presence of toxic metabolites. Adherens and tight junctions should prevent paracellular transport of small polar molecules from the bloodstream into the endoneurium. Weibel-Palade bodies (WPB), known to contain vWF and P-selectin, are also shown.
Cellular adhesion molecules and chemokines
Hematogenous leukocyte trafficking across microvascular endothelium is an evolutionally conserved characteristic required for normal tissue immunosurveillance, as well as response to injury, inflammation or infection, proving an essential link between the innate and adaptive immune response. Aberrant leukocyte trafficking has been described as a pathological hallmark of autoimmune neuropathies. Leukocyte trafficking is a coordinated, sequential process that involves the interaction of selectins and their carbohydrate counterligands with properties similar to C-type lectins (e.g. sialyl Lewis x), chemokines and other chemoattractant molecules and their receptors, integrins interacting with specific cell adhesion molecules (CAMs) and matrix metalloproteinases that facilitate extravasation across endothelial basement membranes [63–66].
pHEndECs express E- and P-selectin at early passages (<8) in vitro [14,67], as well as CD34, a transmembrane sialomucin glycoprotein that is expressed on vascular endothelial cells, particularly capillaries, and is known to mediate L-selectin dependent T-cell homing into high endothelial venules in lymph nodes [14,68,69]. These cells also express intercellular adhesion molecule-1 (ICAM-1: interacts with αLβ2 and αMβ2 integrins), JAM-A (interacts with αLβ2, and implicated in leukocyte adhesion and migration in some models of inflammation) [70], vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced fibronectin variant called fibronectin connecting segment-1 (FN CS-1), which contains the conserved LDV peptide sequence required for integrin binding [14,67]. The latter two CAMs interact with α4β1 integrin. Platelet endothelial cell adhesion molecule (PECAM-1), also known as CD31, a major component of endothelial cell intercellular junctions that may participate in angiogenesis, integrin activation and leukocyte migration [70,71], has been demonstrated on immortalized PnMECs [19].
Following physiological pro-inflammatory cytokine treatment of confluent cultures for up to 48 hours in vitro, there was a time-dependent increase in E-selectin, P-selectin, ICAM-1, VCAM-1 and FN-CS1 without change in JAM-A expression, reaching maximum levels by 24 hours [67]. These data imply a role for these adhesion molecules in the recruitment of hematogenous leukocytes under normal and inflammatory conditions. In support of this, αMβ2 integrin-ICAM-1 interactions have been shown to mediate the adhesion and transmigration of pathogenic mononuclear leukocytes derived from patients with Guillain-Barré syndrome at the human BNB in vitro using a flow-dependent model that mimics capillary hemodynamics [67].
Chemokines, or chemotactic cytokines are small 8–14 kDa molecules that attract leukocytes across concentration and haptotactic gradients in vitro and in vivo, signaling via G-protein coupled receptors. Chemokines are classified into four subfamilies based on the organization of two positionally conserved cysteine residues near the N-terminus: the CXC (α), CC (β), CX3C (δ), C (γ) subfamilies. Chemokines are also involved in embryogenesis, normal neuronal-glial interactions, neuronal protection from toxins, and synaptic transmission, reflecting their importance in development and homeostasis in addition to inflammation [72–74]. Using an in vitro chemokine antibody array, confluent pHEndECs were shown to constitutively express CXCL1-3, CXCL5, CXCL7, CXCL8, CXCL10, CCL4, CCL5, CCL22, CCL23, CCL24, and CCL26, suggesting homeostatic roles for these chemokines (e.g. immune surveillance) at the human BNB [67].
Physiological doses of pro-inflammatory cytokines induced de novo cytoplasmic expression of CCL2, CCL20, CCL27, CXCL9 and CXCL11 at the BNB in vitro, as well as a 5.8-fold increase in CXCL2-3, 4.4-fold increase in CXCL8 and 2.5-fold increase in CXCL10 expression. Less than 2- fold increases in CCL4, CCL5, CCL23, CXCL5 and CXCL7 were also observed. CCL26 was reduced to 0.9-times its basal levels, without significant change in CCL22 and CCL24 expression. These observations suggest potential activation of the innate (i.e. neutrophil dependent: CXCL2-3, CXCL8 interacting with chemokine receptors CXCR1 and CXCR2; monocyte-dependent: CCL2 interacting with CCR2) and adaptive (i.e. T-cell-dependent: CXCL9, 10 and 11 interacting with CXCR3 on CD4+ T-helper 1 (Th1) cells, CCL20 interacting with CCR6 on CD4+ T-helper17 (Th17) cells and CCL27 interacting with CCR10 on activated Tcells) immune signaling pathways at the human BNB at the early stages of inflammation [67].
Further work is needed to decipher which chemokines are most relevant for specific leukocyte subpopulation recruitment into the peripheral nerve endoneurium across the BNB during immunosurveillance and in inflammatory states. It is currently unknown which chemokine receptors are expressed by endoneurial endothelial cells, as this may shed further light into directional angiogenesis during peripheral nerve development and recovery from injury. Figure 4 demonstrates the currently known array of cell adhesion molecules expressed by the human BNB and the effect of physiological pro-inflammatory cytokine exposure in vitro.
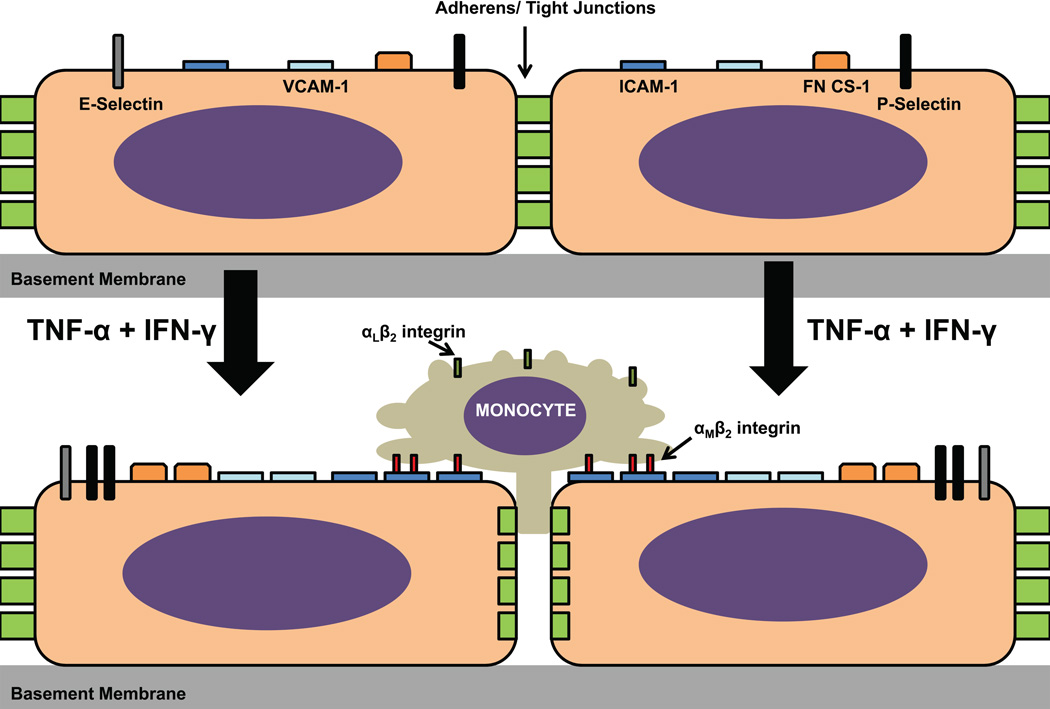
Figure 4. Hunan endoneurial endothelial cell adhesion molecules and effect of proinflammatory cytokines.
The constitutive expression of CAMs by the human BNB in vitro is depicted in the upper image, with a time-dependent increase in cytoplasmic protein expression observed following exogenous treatment of confluent cultures with 10 U/mL TNF-α and 20 U/mL IFN-γ over 48 hours. CD31 and CD34 expression have been described, but the effect of cytokine treatment on their expression has not been evaluated. Cytoplasmic JAM-A expression by pHEndECs has been described in vitro, but its expression was not regulated by proinflammatory cytokine treatment (not shown in figure). Using a flow-dependent in vitro BNB-leukocyte trafficking model, ICAM-1 significantly contributed to pathogenic peripheral blood mononuclear cell adhesion and transmigration, dependent on interactions with αMβ2 integrin, rather than its more ubiquitously expressed counterligand, αLβ2 integrin. Video microscopy shows that these mononuclear cells firmly arrest and congregate at contact sites between confluent endothelial cells, with a subset of leukocytes slowly migrating across the BNB via the paracellular route without significant retraction of neighboring endothelial cells from each other during the course of a 30-minute assay with estimated in vivo capillary flow rates based on red blood cell velocities (refer to published supplementary data for reference [61] for videos). Further studies are needed to determine whether transcellular trafficking occurs at the human BNB in vitro and in vivo, as observed in other microvascular transmigration model systems.
Biophysical characterization of the in vitro human blood-nerve barrier
Based on mammalian in vivo studies, the BNB is second only to the BBB in terms of restrictive endothelial barrier properties in the body [1,6,42,75–78]. Electron-dense intercellular contacts, consistent with TJs, have been demonstrated between human endoneurial endothelial cells in situ and in vitro [3,4,14]. Primary and immortalized human endoneurial endothelial cells have been cultured to confluence in transwell systems or specialized tissue culture plates to ascertain their biophysical properties as TJ-forming barriers in vitro. Endothelial biophysical properties may be divided into transendothelial electrical resistance (TEER), solute permeability and hydraulic conductivity.
Transendothelial electrical resistance
The human BNB TEER in vivo is currently unknown, but estimated to be similar to the BBB. Using transwell systems, published mean in vitro TEER values vary between ~20–35 Ω.cm2 [16–19] and ~110–180 Ω.cm2 [14,15,26,67] depending on the experimental conditions, and whether primary or immortalized endothelial cells were studied. Using continuous electrical cell impedance sensing, TEER values > 250 Ω have been observed (unpublished observations). Several factors have been shown to influence human BNB TEER in vitro, including seeding density at the time of initial plating, constitution of the extracellular matrix used to culture endothelial cells, co-culture with astrocyte and immortalized human brain and peripheral nerve pericyte-conditioned media and several mitogens including GDNF, bFGF, TGF-β1 and hydrocortisone (in a concentration-dependent manner), cyclic adenosine monophosphate (cAMP), and time after initial plating [14–19,26]. Based on observations with pHEndECs, TEER may peak and plateau 5–12 days after initial plating [14,15,26]. The significant discrepancy between published in vitro human BNB TEER values may reflect differences in initial seeding density and the time when TEER was measured after initial plating on transwell systems (> 3 million cells/cm2 and 1–2 days associated with lower TEER values [16–19], compared to 250,000 cells/cm2 and 5–12 days with higher TEER values [14,15,26]), as shown in Table 3.
Table 3.
Biophysical properties of the human in vitro blood-nerve barrier
| Blood-nerve barrier Endothelial cell line |
Culture conditions | Assay time (after plating, in days) |
TEER (Ω .cm2) |
Solute Permeability (Tracer/ value) |
|---|---|---|---|---|
| pHEndEC (14, 26, 67) | 250,000 cells/cm2, Rat tail collagen-coated transwell inserts, 3 µm pore size | 5–7 10–12 |
~110–130a ~140–160 |
70 KDa Dextran-FITC (<5% input @ 15 min) 70 KDa Dextran-FITC (0.7% input @ 15 min) |
| THEndEC (15) | 250,000 cells/cm2, Glutaraldehyde-crosslinked Rat tail collagen-coated transwell inserts, 3 µm pore size | 7 | ~180 | Sodium-FITC (4.84% input @ 15 min) 70 KDa Dextran-FITC (0.39% input @ 15 min) |
| PnMEC (17,18) | 3,000,000 cells/cm2, Rat tail collagen-coated transwell inserts, 0.4 µm pore size | 2 | ~20–35b | [Carboxyl-14C] inulin (10–15 µL @ 20 min) |
| DH-BNB/ FH-BNB (16) | 3,000,000 cells/cm2, Rat tail collagen-coated transwell inserts, 0.4 µm pore size | 1–2 | ~20–30c | N/A |
| DH-BNB/ FH-BNB (19) | 30,000 cells/ cm2, Rat tail collagen-coated transwell inserts, 0.4 µm pore size | 1–2 | ~30–40 | Sodium-FITC (0.60 × 10−3 cm/min over 1 h) 4 KDa Dextran-FITC (0.34 × 10−3 cm/min over 1 h) |
Key: pHEndEC: primary human endoneurial endothelial cells, THEndEC: SV40 large T-antigen immortalized human endoneurial endothelial cells, PnMEC: peripheral nerve microvascular endothelial cells, DH-BNB/ FH-BNB: conditionally immortalized human peripheral nerve microvascular endothelial cells,
Enhanced by GDNF>TGFβ1>bFGF>HC>cAMP/PKA following diffuse endothelial injury. Not affected by physiological doses of TNF-α and IFN-γ;
Enhanced by co-culture with astrocyte, immortalized brain and peripheral nerve pericyte-conditioned media, and GDNF;
Enhanced by hydrocortisone, N/A: not assessed. Parentheses represent reference numbers.
The human BNB is unlike the BBB which has a glia limitans surrounding its microvascular endothelial cells. The glia limitans is formed by astrocyte and microglia foot processes that contribute to barrier function [27,28,36,79,80]. Due to ultrastructural changes in intercellular junctions observed in peripheral nerve microvessels once they cross the inner perineurial layer into the endoneurium [3], it is hypothesized that cellular or molecular components restricted to the endoneurium are important for maintaining the specialized BNB characteristics. It is unknown whether peripheral nerve pericytes, which share a common basement membrane with endoneurial endothelial cells directly contribute to barrier function in vivo or secrete mitogens as described by immortalized cells in vitro [17,18].
Following diffuse endothelial injury mediated by serum withdrawal in vitro, GDNF potently induced human BNB TEER recovery within 48 hours at low nanomolar concentrations (0.03 nM), dependent on RET-tyrosine kinase signaling pathways, independent of maximal concentrations of other mitogens (TGF-β1, bFGF and hydrocortisone) that were capable of restoring TEER, albeit less efficiently and at higher molar concentrations within the nanomolar range (suggesting redundancy that may be essential for normal biologic function in vivo) [26]. Schwann cells are glial cells present in the peripheral nervous system [81] that are restricted to the endoneurium and are known to secrete GDNF in vitro [82]. Increased Schwann cell GDNF expression has also been described following rat sciatic nerve injury and human nerve root avulsion in vivo [83,84]. The potential role of Schwann cells in BNB development during embryogenesis and post-natal maturation, maintenance of BNB function and BNB recovery following injury has yet to be elucidated.
Increased leukocyte trafficking has been described in inflamed nerves, implying compromise or “breakdown” to the BNB. Claudin-5 downregulation and altered ZO-1 localization has been described in endoneurial microvessels from peripheral nerves obtained from patients with chronic inflammatory neuropathies [85]. Thus, it is feasible that reduced TEER as a consequence of BNB compromise may occur. However, physiological pro-inflammatory cytokine treatment of confluent pHEndEC cultures grown on transwell inserts for 24 hours did not reduce TEER compared to untreated cultures [67], supporting the hypothesis that leukocyte trafficking at the BNB is an active rather than passive process, as described with the multi-step paradigm. Based on initial in vitro flow-dependent studies, leukocyte trafficking at the human BNB predominantly occurs via the paracellular route [67], suggesting that real-time alterations in TEER occur during inflammation. The effect of shear forces and the dynamic changes that may occur during leukocyte extravasation and their effects on BNB TEER are yet to be established.
Solute permeability
Studies evaluating the permeability of the human BNB to some solutes and macromolecules (as a measure of barrier function) have been performed in vitro [14,15,17]. The permeability for any given solute or macromolecule across a restrictive microvascular barrier will depend on its size, polarity, lipophilicity, and ability to undergo facilitated receptor-mediated transcytosis [30,47]. It can be hypothesized that smaller, non-polar, highly lipophilic molecules with specific influx transporters may demonstrate the highest permeability across the BNB. Permeability coefficients (PC) are commonly used to quantify solute permeability in vitro [30]. PC is dependent on the ratio of the concentration of the solute that is transported to the input concentration, the volume of the receiving well that collects the solute following transport, the endothelial surface area through which the solute undergoes transport and the assay time [86].
Mean PC values for the in vitro human BNB under resting conditions reported or calculated for sodium fluorescein (molecular weight 376 Da), radioactive inulin (molecular weight ~4.5 kDa) and high molecular weight fluoresceinated dextran (molecular weight 70 kDa) are ~20 × 10−3 cm/min, ~10 × 10−3 cm/min and ~1.6–2.8 × 10−3 cm/min respectively [14,15,17], demonstrating an inverse relationship between molecular weight and PC that is reportedly more pronounced at lower molecular weights [30]. A recent study using temperature-sensitive SV40 LTA-immortalized PnMECs surprisingly demonstrated a mean lower PC for low molecular weight fluorescently labeled dextran (molecular weight 4 kDa: 0.34 × 10−3 cm/min) than sodium fluorescein (0.60 × 10−3 cm/min) [19]. Technical differences in endothelial cell plating and assay times, model transwell systems, solute input concentrations and sampling of transported solute at pre-defined time points may account for these differences (Table 3).
It is unknown how these PC values compare to the human BNB in vivo. Converting the mean PC of high molecular weight fluoresceinated dextran to a PC-surface area product would yield a value of approximately 3–6-fold higher than the measured PC-surface area product for transferrin (molecular weight 80 kDa) in rat sciatic nerves [42]. The effects of capillary shear forces and the extracellular matrix, as well as Schwann cells, perineurial cells or pericytes (or combination thereof) in enhancing BNB function in vivo are currently unknown, although it is hypothesized that shear forces could enhance TEER and reduce permeability to multiple solutes in vitro as observed with a flow-dependent in vitro BBB model [80,87].
Kinetic studies of solute, drug and macromolecular permeability using human in vitro BNB models should provide some insights into the regulation of internal microenvironment of peripheral nerves and adaptations during stress or injury. How these substances are restricted from, permeate into or retained within the endoneurium following interactions with the BNB could enhance therapeutic strategies for peripheral neuropathies and neuropathic pain, or to prevent toxic neuropathies, e.g. secondary to chemotherapeutic drugs such as vincristine or organic molecules such as alcohol and toluene.
Hydraulic conductivity
Hydraulic conductivity (or transendothelial volume flow) can be described as a measure of the permissivity of water transport across a vascular barrier [30,88]. The specialized microenvironment of peripheral nerves is maintained by regulated blood-nerve fluid exchange directly across the endoneurial vascular endothelium and, in some instances, indirectly across the multilayered perineurium and turnover of endoneurial fluid by proximo-distal convective fluid flow [2]. This fluid exchange is vital for the maintenance of endoneurial physiological parameters including blood flow, oxygen tension, pH, oncotic pressure, hydrostatic pressure, and ion concentrations [2]. Endoneurial edema is a well known consequence of peripheral nerve injury and inflammation in vivo, implying dysfunction of, or limitations in the adaptive capacity of the BNB to increased endoneurial fluid production in these disorders. There is currently no information on the hydraulic conductivity of the human BNB in vitro. Evaluating the determinants and mechanisms by which endoneurial endothelial cells transport water should provide further insights relevant to understanding fluid homeostasis within peripheral nerves and adaptations that may occur in peripheral nerve disease, particularly inflammatory neuropathies.
Conclusions
This review summarizes current knowledge of the human BNB, driven predominantly by in vitro observations of primary and immortalized endoneurial endothelial cells. The relative dearth in healthy human peripheral nerve specimens and difficulties in performing functional, mechanismdriven assays to evaluate BNB function in healthy individuals could limit the confirmation of these initial observations with the BNB in situ and in vivo. Nonetheless, these cell lines provide an avenue to increase our understanding of the molecular and biophysical characteristics of the human BNB, elucidate the determinants and signaling pathways relevant to BNB formation, maturation and adaptation under normal physiological and pathophysiological states and initiate the process of enhancing or restricting drug delivery into peripheral nerves. Scientific investigation with these human cell lines should foster the growth of this nascent and exciting field of microvascular biology.
Acknowledgments
Special thanks to past and current members of the Neuromuscular Immunopathology Research Laboratory and collaborators who have assisted with the isolation, characterization and propagation of primary and immortalized human endoneurial endothelial cells, and the development of the transwell and flow-dependent human in vitro BNB model systems. Research in the Neuromuscular Immunopathology Research Laboratory is currently supported by National Institutes of Health (NIH) grants R21 NS073702 (2011–2013), R21 NS078226 (2012–2014), R01 NS075212 (2012–2017) and a subaward P30 AI27767 (2012–2014) to E.E.U. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
References
- 1.Olsson Y. Microenvironment of the peripheral nervous system under normal andpathological conditions. Crit Rev Neurobiol. 1990;5:265–311. [PubMed] [Google Scholar]
- 2.Mizisin AP, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironmentduring development, aging and in response to trauma, disease and toxic insult. ActaNeuropathol. 2011;121:291–312. doi: 10.1007/s00401-010-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reina M, López A, Villanueva M, de Andrés J, León G. [morphology of peripheralnerves, their sheaths, and their vascularization] Rev Esp Anestesiol Reanim. 2000;47:464–475. [PubMed] [Google Scholar]
- 4.Reina M, López A, Villanueva M, De Andrés J, Machés F. [the blood-nerve barrier inperipheral nerves] Rev Esp Anestesiol Reanim. 2003;50:80–86. [PubMed] [Google Scholar]
- 5.Bell M, Weddell A. A descriptive study of the blood vessels of the sciatic nerve in therat, man and other mammals. Brain. 1984;107 ( Pt 3):871–898. doi: 10.1093/brain/107.3.871. [DOI] [PubMed] [Google Scholar]
- 6.Malmgren L, Olsson Y. Differences between the peripheral and the central nervoussystem in permeability to sodium fluorescein. J Comp Neurol. 1980;191:103–107. doi: 10.1002/cne.901910106. [DOI] [PubMed] [Google Scholar]
- 7.Pummi K, Heape A, Grénman R, Peltonen J, Peltonen S. Tight junction proteins zo-1,occludin, and claudins in developing and adult human perineurium. J Histochem Cytochem. 2004;52:1037–1046. doi: 10.1369/jhc.3A6217.2004. [DOI] [PubMed] [Google Scholar]
- 8.Smith C, Atchabahian A, Mackinnon S, Hunter D. Development of the blood-nervebarrier in neonatal rats. Microsurgery. 2001;21:290–297. doi: 10.1002/micr.1055. [DOI] [PubMed] [Google Scholar]
- 9.Hirakawa H, Okajima S, Nagaoka T, Takamatsu T, Oyamada M. Loss and recovery ofthe blood-nerve barrier in the rat sciatic nerve after crush injury are associated with expressionof intercellular junctional proteins. Exp Cell Res. 2003;284:196–210. doi: 10.1016/s0014-4827(02)00035-6. [DOI] [PubMed] [Google Scholar]
- 10.Bush MS, Reid AR, Allt G. Blood-nerve barrier: Ultrastructural and endothelial surfacecharge alterations following nerve crush. Neuropathol Appl Neurobiol. 1993;19:31–40. doi: 10.1111/j.1365-2990.1993.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 11.Aird W. Phenotypic heterogeneity of the endothelium:I. Structure, function, andmechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 12.Aird W. Phenotypic heterogeneity of the endothelium:Ii. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 13.Yano K, Gale D, Massberg S, Cheruvu P, Monahan-Earley R, Morgan E, Haig D, vonAndrian U, Dvorak A, Aird W. Phenotypic heterogeneity is an evolutionarily conserved feature ofthe endothelium. Blood. 2007;109:613–615. doi: 10.1182/blood-2006-05-026401. [DOI] [PubMed] [Google Scholar]
- 14.Yosef N, Xia R, Ubogu E. Development and characterization of a novel human in vitroblood-nerve barrier model using primary endoneurial endothelial cells. J Neuropathol ExpNeurol. 2010;69:82–97. doi: 10.1097/NEN.0b013e3181c84a9a. [DOI] [PubMed] [Google Scholar]
- 15.Yosef N, Ubogu EE. An immortalized human blood-nerve barrier endothelial cell line forin vitro permeability studies. Cell Mol Neurobiol. 2013;33:175–86. doi: 10.1007/s10571-012-9882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashiwamura Y, Sano Y, Abe M, Shimizu F, Haruki H, Maeda T, Kawai M, Kanda T. Hydrocortisone enhances the function of the blood-nerve barrier through the up-regulation ofclaudin-5. Neurochem Res. 2011;36:849–855. doi: 10.1007/s11064-011-0413-6. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu F, Sano Y, Abe MA, Maeda T, Ohtsuki S, Terasaki T, Kanda T. Peripheralnerve pericytes modify the blood-nerve barrier function and tight junctional molecules throughthe secretion of various soluble factors. J Cell Physiol. 2011;226:255–266. doi: 10.1002/jcp.22337. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu F, Sano Y, Saito K, Abe MA, Maeda T, Haruki H, Kanda T. Pericyte-derivedglial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brainbarrier and the blood-nerve barrier. Neurochem Res. 2012;37:401–409. doi: 10.1007/s11064-011-0626-8. [DOI] [PubMed] [Google Scholar]
- 19.Abe M, Sano Y, Maeda T, Shimizu F, Kashiwamura Y, Haruki H, Saito K, Tasaki A, Kawai M, Terasaki T, Kanda T. Establishment and characterization of human peripheral nervemicrovascular endothelial cell lines: A new in vitro blood-nerve barrier (bnb) model. Cell StructFunct. 2012;37:89–100. doi: 10.1247/csf.11042. [DOI] [PubMed] [Google Scholar]
- 20.Holthöfer H, Virtanen I, Kariniemi A, Hormia M, Linder E, Miettinen A. Ulex europaeus ilectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982;47:60–66. [PubMed] [Google Scholar]
- 21.Rondaij M, Bierings R, Kragt A, van Mourik J, Voorberg J. Dynamics and plasticity ofweibel-palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Sawasaki Y, Hata J, Mukai K, Goto T. Spontaneous transformation andimmortalization of human endothelial cells. In Vitro Cell Dev Biol. 1990;26:265–274. doi: 10.1007/BF02624456. [DOI] [PubMed] [Google Scholar]
- 23.Risau W, Esser S, Engelhardt B. Differentiation of blood-brain barrier endothelial cells. Pathol Biol (Paris) 1998;46:171–175. [PubMed] [Google Scholar]
- 24.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelialgrowth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu F, Sano Y, Haruki H, Kanda T. Advanced glycation end-products inducebasement membrane hypertrophy in endoneurial microvessels and disrupt the blood-nervebarrier by stimulating the release of tgf-beta and vascular endothelial growth factor (vegf) bypericytes. Diabetologia. 2011;54:1517–1526. doi: 10.1007/s00125-011-2107-7. [DOI] [PubMed] [Google Scholar]
- 26.Yosef N, Ubogu EE. Gdnf restores human blood-nerve barrier function via ret tyrosinekinase-mediated cytoskeletal reorganization. Microvasc Res. 2012;83:298–310. doi: 10.1016/j.mvr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Abbott N, Patabendige A, Dolman D, Yusof S, Begley D. Structure and function of theblood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 28.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: Development,composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 29.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation ofepithelial differentiation and proliferation. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. PhysiolRev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, Nakayama K, Okamura Y, Sasaki H, Miyachi Y, Furuse M, Tsukita S. Tight junctions in schwann cells ofperipheral myelinated axons: A lesson from claudin-19-deficient mice. J Cell Biol. 2005;169:527–538. doi: 10.1083/jcb.200501154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alanne MH, Pummi K, Heape AM, Grenman R, Peltonen J, Peltonen S. Tight junctionproteins in human schwann cell autotypic junctions. J Histochem Cytochem. 2009;57:523–529. doi: 10.1369/jhc.2009.951681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulze C, Firth JA. Interendothelial junctions during blood-brain barrier development inthe rat: Morphological changes at the level of individual tight junctional contacts. Brain Res DevBrain Res. 1992;69:85–95. doi: 10.1016/0165-3806(92)90125-g. [DOI] [PubMed] [Google Scholar]
- 34.Allt G, Lawrenson J. The blood-nerve barrier: Enzymes, transporters and receptors--acomparison with the blood-brain barrier. Brain Res Bull. 2000;52:1–12. doi: 10.1016/s0361-9230(00)00230-6. [DOI] [PubMed] [Google Scholar]
- 35.Froehner S, Davies A, Baldwin S, Lienhard G. The blood-nerve barrier is rich in glucosetransporter. J Neurocytol. 1988;17:173–178. doi: 10.1007/BF01674204. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins R, O'Kane R, Simpson I, Viña J. Structure of the blood-brain barrier and its rolein the transport of amino acids. J Nutr. 2006;136:218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 37.Kanda T, Iwasaki T, Yamawaki M, Ikeda K. Isolation and culture of bovine endothelialcells of endoneurial origin. J Neurosci Res. 1997;49:769–777. doi: 10.1002/(SICI)1097-4547(19970915)49:6<769::AID-JNR11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 38.Kido Y, Tamai I, Okamoto M, Suzuki F, Tsuji A. Functional clarification of mct1-mediatedtransport of monocarboxylic acids at the blood-brain barrier using in vitro cultured cells and invivo bui studies. Pharm Res. 2000;17:55–62. doi: 10.1023/a:1007518525161. [DOI] [PubMed] [Google Scholar]
- 39.Michel M, Shinowara N, Rapoport S. Presence of a blood-nerve barrier within bloodvessels of frog sciatic nerve. Brain Res. 1984;299:25–30. doi: 10.1016/0006-8993(84)90784-4. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsuki S, Tachikawa M, Takanaga H, Shimizu H, Watanabe M, Hosoya K, Terasaki T. The blood-brain barrier creatine transporter is a major pathway for supplying creatine to thebrain. J Cereb Blood Flow Metab. 2002;22:1327–1335. doi: 10.1097/01.WCB.0000033966.83623.7D. [DOI] [PubMed] [Google Scholar]
- 41.Orte C, Lawrenson J, Finn T, Reid A, Allt G. A comparison of blood-brain barrier andblood-nerve barrier endothelial cell markers. Anat Embryol (Berl) 1999;199:509–517. doi: 10.1007/s004290050248. [DOI] [PubMed] [Google Scholar]
- 42.Poduslo J, Curran G, Berg C. Macromolecular permeability across the blood-nerve andblood-brain barriers. Proc Natl Acad Sci U S A. 1994;91:5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rechthand E, Smith Q, Rapoport S. Facilitated transport of glucose from blood intoperipheral nerve. J Neurochem. 1985;45:957–964. doi: 10.1111/j.1471-4159.1985.tb04087.x. [DOI] [PubMed] [Google Scholar]
- 44.Saito T, Zhang Z, Ohtsubo T, Noda I, Shibamori Y, Yamamoto T, Saito H. Homozygousdisruption of the mdrla p-glycoprotein gene affects blood-nerve barrier function in miceadministered with neurotoxic drugs. Acta Otolaryngol. 2001;121:735–742. doi: 10.1080/00016480152583683. [DOI] [PubMed] [Google Scholar]
- 45.Sano Y, Shimizu F, Nakayama H, Abe M, Maeda T, Ohtsuki S, Terasaki T, Obinata M, Ueda M, Takahashi R, Kanda T. Endothelial cells constituting blood-nerve barrier have highlyspecialized characteristics as barrier-forming cells. Cell Struct Funct. 2007;32:139–147. doi: 10.1247/csf.07015. [DOI] [PubMed] [Google Scholar]
- 46.Wadhwani K, Smith Q, Rapoport S. Facilitated transport of l-phenylalanine across bloodnervebarrier of rat peripheral nerve. Am J Physiol. 1990;258:R1436–R1444. doi: 10.1152/ajpregu.1990.258.6.R1436. [DOI] [PubMed] [Google Scholar]
- 47.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brainbarrier as a supporting and protecting interface for the brain;importance for cns drugdiscovery and development. Pharm Res. 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 48.Gallo R, Dorschner R, Takashima S, Klagsbrun M, Eriksson E, Bernfield M. Endothelialcell surface alkaline phosphatase activity is induced by il-6 released during wound repair. JInvest Dermatol. 1997;109:597–603. doi: 10.1111/1523-1747.ep12337529. [DOI] [PubMed] [Google Scholar]
- 49.Latker C, Shinowara N, Miller J, Rapoport S. Differential localization of alkalinephosphatase in barrier tissues of the frog and rat nervous systems: A cytochemical andbiochemical study. J Comp Neurol. 1987;264:291–302. doi: 10.1002/cne.902640302. [DOI] [PubMed] [Google Scholar]
- 50.Nakano Y, Beertsen W, van den Bos T, Kawamoto T, Oda K, Takano Y. Site-specificlocalization of two distinct phosphatases along the osteoblast plasma membrane: Tissue nonspecificalkaline phosphatase and plasma membrane calcium atpase. Bone. 2004;35:1077–1085. doi: 10.1016/j.bone.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Ng K, Schallenkemp J. Biochemical characteristics of a primary blood-brain barrier cellculture system as a function of the activity of the proteases used in tissue disaggregation. JNeurosci Methods. 1996;68:49–53. doi: 10.1016/0165-0270(96)00064-7. [DOI] [PubMed] [Google Scholar]
- 52.Muona P, Jaakkola S, Salonen V, Peltonen J. Expression of glucose transporter 1 inadult and developing human peripheral nerve. Diabetologia. 1993;36:133–140. doi: 10.1007/BF00400694. [DOI] [PubMed] [Google Scholar]
- 53.Véga C, Poitry-Yamate C, Jirounek P, Tsacopoulos M, Coles J. Lactate is released andtaken up by isolated rabbit vagus nerve during aerobic metabolism. J Neurochem. 1998;71:330–337. doi: 10.1046/j.1471-4159.1998.71010330.x. [DOI] [PubMed] [Google Scholar]
- 54.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloningand characterization of a transporter for large neutral amino acids activated by the heavy chainof 4f2 antigen (cd98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 55.Muruganandam A, Herx L, Monette R, Durkin J, Stanimirovic D. Development ofimmortalized human cerebromicrovascular endothelial cell line as an in vitro model of thehuman blood-brain barrier. FASEB J. 1997;11:1187–1197. doi: 10.1096/fasebj.11.13.9367354. [DOI] [PubMed] [Google Scholar]
- 56.Pajic M, Norris MD, Cohn SL, Haber M. The role of the multidrug resistance-associatedprotein 1 gene in neuroblastoma biology and clinical outcome. Cancer Lett. 2005;228:241–246. doi: 10.1016/j.canlet.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 57.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobioticsby mammalian atp-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 58.Sharom FJ. The p-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161–178. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 59.Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. A novelhuman hepatic organic anion transporting polypeptide (oatp2). Identification of a liver-specifichuman organic anion transporting polypeptide and identification of rat and humanhydroxymethylglutaryl-coa reductase inhibitor transporters. J Biol Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- 60.Konig J, Cui Y, Nies AT, Keppler D. A novel human organic anion transportingpolypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest LiverPhysiol. 2000;278:G156–G164. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- 61.Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organicanion transporters (oats) in drug therapy. Handb Exp Pharmacol. 2011:29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]
- 62.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: Discovery,pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010;31:1–71. doi: 10.1002/bdd.693. [DOI] [PubMed] [Google Scholar]
- 63.Man S, Ubogu E, Ransohoff R. Inflammatory cell migration into the central nervoussystem: A few new twists on an old tale. Brain Pathol. 2007;17:243–250. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alon R, Ley K. Cells on the run: Shear-regulated integrin activation in leukocyte rollingand arrest on endothelial cells. Curr Opin Cell Biol. 2008;20:525–532. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitmentduring inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review:Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successfulimmune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 67.Yosef N, Ubogu EE. Alpha(m)beta(2)-integrin-intercellular adhesion molecule-1 interactions drive the flow-dependent trafficking of guillain-barre syndrome patient derivedmononuclear leukocytes at the blood-nerve barrier in vitro. J Cell Physiol. 2012;227:3857–3875. doi: 10.1002/jcp.24100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fina L, Molgaard H, Robertson D, Bradley N, Monaghan P, Delia D, Sutherland D, Baker M, Greaves M. Expression of the cd34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 69.Puri K, Finger E, Gaudernack G, Springer T. Sialomucin cd34 is the major l-selectinligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nourshargh S, Krombach F, Dejana E. The role of jam-a and pecam-1 in modulatingleukocyte infiltration in inflamed and ischemic tissues. J Leukoc Biol. 2006;80:714–718. doi: 10.1189/jlb.1105645. [DOI] [PubMed] [Google Scholar]
- 71.Newman PJ. The biology of pecam-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ubogu E, Cossoy M, Ransohoff R. The expression and function of chemokines involvedin cns inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 73.Ubogu EE. Chemokine receptors as specific anti-inflammatory targets in peripheralnerves. Endocr Metab Immune Disord Drug Targets. 2011;11:141–153. doi: 10.2174/187153011795564124. [DOI] [PubMed] [Google Scholar]
- 74.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hultström D, Malmgren L, Gilstring D, Olsson Y. Fitc-dextrans as tracers formacromolecular movements in the nervous system. A freeze-drying method for dextrans ofvarious molecular sizes injected into normal animals. Acta Neuropathol. 1983;59:53–62. doi: 10.1007/BF00690317. [DOI] [PubMed] [Google Scholar]
- 76.Olsson Y. Studies on vascular permeability in peripheral nerves.I. Distribution ofcirculating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. ActaNeuropathol. 1966;7:1–15. doi: 10.1007/BF00686605. [DOI] [PubMed] [Google Scholar]
- 77.Olsson Y. Topographical differences in the vascular permeability of the peripheralnervous system. Acta Neuropathol. 1968;10:26–33. doi: 10.1007/BF00690507. [DOI] [PubMed] [Google Scholar]
- 78.Olsson Y. Studies on vascular permeability in peripheral nerves.Iv. Distribution ofintravenously injected protein tracers in the peripheral nervous system of various species. ActaNeuropathol. 1971;17:114–126. doi: 10.1007/BF00687487. [DOI] [PubMed] [Google Scholar]
- 79.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brainbarrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 80.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned mediumand shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. NatRev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 82.Springer JE, Seeburger JL, He J, Gabrea A, Blankenhorn EP, Bergman LW. Cdnasequence and differential mrna regulation of two forms of glial cell line-derived neurotrophicfactor in schwann cells and rat skeletal muscle. Exp Neurol. 1995;131:47–52. doi: 10.1016/0014-4886(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 83.Bar KJ, Saldanha GJ, Kennedy AJ, Facer P, Birch R, Carlstedt T, Anand P. Gdnf and itsreceptor component ret in injured human nerves and dorsal root ganglia. Neuroreport. 1998;9:43–47. doi: 10.1097/00001756-199801050-00009. [DOI] [PubMed] [Google Scholar]
- 84.Hammarberg H, Piehl F, Cullheim S, Fjell J, Hökfelt T, Fried K. Gdnf mrna in schwanncells and drg satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- 85.Kanda T, Numata Y, Mizusawa H. Chronic inflammatory demyelinating polyneuropathy:Decreased claudin-5 and relocated zo-1. J Neurol Neurosurg Psychiatry. 2004;75:765–769. doi: 10.1136/jnnp.2003.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song L, Ge S, Pachter J. Caveolin-1 regulates expression of junction-associatedproteins in brain microvascular endothelial cells. Blood. 2007;109:1515–1523. doi: 10.1182/blood-2006-07-034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress inblood-brain barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rumbaut RE, Wang J, Huxley VH. Differential effects of l-name on rat venular hydraulicconductivity. Am J Physiol Heart Circ Physiol. 2000;279:H2017–H2023. doi: 10.1152/ajpheart.2000.279.4.H2017. [DOI] [PubMed] [Google Scholar]