Abstract
Objectives
To determine effect of gentle loads applied to the knee on mRNA expression of nerve growth factor, particularly, the active beta subunit (NGFβ) in cartilage and chondrocyte.
Methods
Cyclic compressive loads in vivo and fluid flow in vitro were used to determine the mRNA levels. Alteration of Rac1 GTPase as well as effect of salubrinal, a specific inhibitor of eIF2α phosphatase was assessed using fluorescence resonance energy transfer (FRET)-based Rac1 biosensor.
Results
Knee loading at 1 N reduced mRNA levels of NGFβ and its low affinity receptor, p75 in cartilage and subchondral bone. In cartilage, knee loading at 1 N reduced the phosphorylation level of p38 MAPK (p38-p) and activity of Rac1 GTPase. Consistent with in vivo results, fluid flow at 5 and 10 dyn/cm2 reduced mRNA levels of NGFβ and p75 in C28/I2 human chondrocytes. SB203580, which decreases p38-p, reduced the mRNA levels of NGFβ and p75. Silencing Rac1 by siRNA decreased the levels of p38-p and NGFβ mRNA but not p75. Furthermore, administration of salubrinal reduced FRET-based activity of Rac1 as well as the mRNA levels of NGFβ and p75.
Conclusions
These results provide evidence that mechanical stimulation and salubrinal may attenuate pain perception-linked NGFβ signaling through Rac1-mediated p38 MAPK.
Keywords: knee loading, fluid flow, cartilage, chondrocyte, NGF
Introduction
Mechanical stimuli play a critical role in homeostasis of musculoskeletal tissues including bone and articular cartilage. Physical exercises, for instance, enhance new bone formation, while gentle mobilization of synovial joints reduces activities of degenerative enzymes such as matrix metalloproteinases (MMPs).1, 2, 3 Knee loading is a modality that applies gentle, lateral loads to the knee, providing a model system for understanding loading effects on preventing bone loss and cartilage degradation.4, 5 Animal studies suggest that knee loading not only stimulates bone formation in the tibia and femur but also reduces activities of MMPs in articular cartilage.6,7 In this study, we employed knee loading to explore potential effects on neuronal signaling, in particular pain perception. Massage therapy is a unique form of mechanical stimuli for treating a variety of health conditions, including joint disorders as a regimen to relieve pain for those with osteoarthritis of the knee.8,9 Although a growing body of evidence supports the efficacy of massage therapy,10,11 little is known about the mechanism of its pain relieving action. As pointed out recently, the origin of chronic pain in osteoarthritic joint is less clear whether it is due primarily to damage of sensory nerves in bone and inflammation in synovium or due in part to innervation from mesenchyme into the aneural entity, cartilage.12
In this study, we sought a surrogate target to block for relieving pain in the articular cartilage of joints. We considered nerve growth factor (NGF), particularly, β-subunit (NGFβ), as well as its low affinity receptor, p75 as potential markers for pain perception in the joint.13 The level of NGFβ was reported to be low in normal chondrocytes, increased in mild osteoarthritic cartilage and further enhanced in severe osteoarthritic cartilage.14 Among three subunits, the active component of NGF protein is 118-amino-acid sequence of the β subunit. The most studied class of trophic factors that are involved in trophic function or survival of neuron is the neurotrophins. Other than NGF, three major neurotrophins have been isolated from mammals. Unlike tyrosine kinases receptors (Trk), each neurotrophin binds to p75 with similar affinity thus p75 is also called ‘a common receptor’. In particular, the activation of the p75 receptor has been shown to promote neuronal cell death thus suggested a therapeutic target for neuropsychiatric disease.15 The previous report also suggested that NGF and p75 causes the pathogenesis of discogenic pain in intervertebral discs.16
Although cartilage is not an innervated tissue, the expression of NGF and its receptors is developmentally regulated.17 A basal expression level of NGFβ is high in embryos undergoing skeletal morphogenesis and low in mature cartilage. It is reported that its expression is linked to pain perception and increased in arthritic joints.18 Herein we addressed a question: Does mechanical stimulation downregulate expression of NGFβ in cartilage and chondrocytes? Since load-driven downregulation of MMPs is in part mediated by p38 mitogen activated protein kinase (MAPK) signaling and GTPases, we hypothesized that gentle mechanical loading reduces the mRNA levels of NGFβ and p75 through p38 MAPK.
In testing our hypothesis, knee loading was applied to mice to determine a potential loading modality effective for downregulation of NGF and receptor genes in the cartilage. In vitro fluid flow experiments were also conducted using C28/I2 chondrocyte cells. We determined the mRNA levels of NGFβ and p75 using quantitative PCR and the phosphorylation level of p38. Focusing on the role of Rac1 GTPase, its expression and activity were determined using immunoprecipitation of an active form of Rac1, RNA interference with siRNA specific to Rac1, and a fluorescence resonance energy transfer (FRET) technique with a Rac1 biosensor. Since a synthetic chemical agent, salubrinal, is reported to protect against neurotoxicity in the central nervous system, we examined its effect on expression of NGF.
Materials and Methods
Animals
Experimental procedures were approved by the Indiana University Animal Care and Use Committee and were in compliance with the Guiding Principles in the Care and Use of Animals endorsed by the American Physiological Society. Three mice were housed per cage, and they were fed with mouse chow and water ad libitum. Two groups of C57/BL/6 female mice (~12 weeks, Harlan Laboratories) were used, in which the group I was for examining effects of time (1 h and 3 h), and the group II for testing effects of loading amplitude (1 N and 3 N). Six unloaded knees served as control.
Knee Loading
Cyclic compression was applied to the mouse right knee using a custom-made piezoelectric loading device.19 The mouse was mask-anesthetized using 2% isoflurane, and lateral loads to the knee were applied for 5 min at 5 Hz with a peak-to-peak force of 1 and 3 N. The left hindlimb was used as sham-loaded control, with the left knee placed under the loading rod in the same procedure without applying a voltage signal to the loader. The femoral articular cartilage and subchondral bone tissue were harvested from loaded and non-loaded mice 1 h after the loading bout.
In vitro experiments
Human chondrocytes, C28/I2 cells,20 were cultured in DMEM (Lonza) containing 10% FBS (Hyclone) and antibiotics (Life Technologies). For mechanical stimulation, cells were grown on glass slides treated with 1% rat-tail collagen (BD Biosciences). Twenty four hours before flow application, cells were incubated in DMEM containing 0.5% FBS. The slides were loaded into a Streamer Gold flow device chamber (Flexcell International). For inhibition of p38 phosphorylation, cells were incubated with 10, 20 and 40 μM SB203580 (Calbiochem) for 15 min. Salubrinal, an agent acting as an inhibitor of eIF2α phosphatase (Tocris Bioscience), was administered at 2, 10, and 50 μM.
Rac1 Activity Assay
The activity level of Rac1 was detected with a Rac1 activation assay kit (Millipore) using the procedure provided by the manufacturer. In brief, the protein sample was lysed in a magnesium lysis/wash buffer containing 10 βg/ml leupeptin and 10 βg/ml aprotinin. The cell lysate was incubated with a Rac1 assay reagent (agarose beads) to precipitate Rac1-GTP. The level of Rac1-GTP was detected with anti-Rac1 antibody using a standard Western blot procedure.
Silencing Rac1 GTPase using siRNA
To evaluate the role of Rac1 GTPase in regulation of p38 MAPK and NGF, cells were treated with siRNA specific to Rac1 GTPase (Invitrogen). The targeted Rac1 sequence was 5’-AGGGUCUAGCCAUGGCUAAGGAGAU-3’, while a negative siRNA (Stealth™ siRNA negative control High, Invitrogen) was used as a nonspecific control. C28/I2 cells were transiently transfected with Rac1 siRNA or control siRNA in Opti-MEM I medium with Lipofectamine RNAiMAX (Invitrogen) following the manufacturer's instructions. Six hours later, the medium was replaced by regular culture medium. The efficiency of silencing Rac1 was assessed with immunoblotting 48 h after the transfection.
Real-time PCR
The mRNA expressions of NGFβ and p75 were determined using quantitative real-time PCR with the following primers: human NGFβ-TCAGCATTCCCTTGACACTG (forward), TGCTCCTGTGAGTCCTGTTG (backward); human p75- GTGGGACAGAGTCTGGGTGT (forward), AAGGAGGGGAGGTGATAGGA (backward); human GAPDHGCACCGTCAAGGCT GAGAAC (forward), ATGGTGGTGAAGAC GCCAGT (backward); mouse NGFβ–A- CCAGTGAAATTAGGCTCCCTG (forward), CCTTGGCAAAACCTTTATTGG (backward); mouse NGFβ–BTGATCGGCGTACAGGCAGA (forward), GCTGAAGTTTAGTCCAGTGGG (backward); mouse p75- CTAGGGGTGTCCTTTGGAGGT (forward), CAGGGTTCACACACGGTCT (backward); mouse GAPDH- TGCACCACCAACTGCTTAG (forward), GGATGCAGAGAAGATGTTC (backward). The mouse cartilage was dissected, washed in the saline, and frozen at -80°C. The frozen tissue was cut into pieces, and homogenized in the mortar and pestle. RNA was extracted using a QIAzol lysis buffer followed by further extraction with an RNeasy Plus mini kit (Qiagen). Reverse transcription was performed, and real-time PCR was carried out using ABI 7500 with SYBR green PCR kits (Applied Biosystems). The mRNA level of GAPDH was used as an internal control. Data were further normalized with respect to unloaded controls in the in vivo and in vitro experiments.21
Western Blot Analysis
For Western blot analysis, the sample was washed in the cold saline and mechanically dissociated and homogenized using the mortar and pestle on ice. The sample was then centrifuged at 4°C and a supernatant was used for Western blotting. To reduce background staining, the amount of proteins for loading was reduced and antibody concentrations were lowered. Furthermore, a concentration of milk in the blocking buffer was increased to 5% and the membrane was blocked overnight at 4°C. After reactions with primary and secondary antibodies, the membrane was vigorously rinsed for an extended period. Samples isolated from C28/I2 chondrocytes were transferred to the RIPA lysis buffer containing inhibitors for proteases and phosphatases. Isolated proteins were fractionated using 10% SDS gels and electro-transferred to Immobilon-P membranes (Millipore). Immunoblots were carried out using antibodies specific to p38 MAPK, phospho p38 MAPK, and β-actin (Sigma). After incubation with secondary antibodies conjugated with HRP, signals were detected with ECL chemiluminescence. Images were captured using an image analyzer (LAS-3000, Fuji Photo Film) and analyzed using Multi Gauge V 3.0 software.
Fluorescence Resonance Energy Transfer (FRET)
To visualize Rac1 activity in response to salubrinal, FRET imaging was conducted using a cyan fluorescent protein (CFP)-yellow fluorescent protein (YFP) Rac1 biosensor. The filter sets (Semrock) were chosen for CFP excitation at 438 ± 24 nm (center wavelength ± bandwidth), CFP emission at 483 ± 32 nm, and YFP emission at 542 ± 27 nm. Time-lapse images were acquired at an interval of 5 min using a fluorescence microscope (Nikon). The level of Rac1 activity was determined by computing an emission ratio of YFP/CFP for individual cells using NIS-Elements software (Nikon).
Statistical Analysis
The in vivo experiment was conducted with n = 6 per group. The in vitro experiment was independently carried out three times. Statistical significance was calculated using independent t test for two group comparison, and one-way ANOVA followed by Dunnett's post hoc test for more than two groups. Data are reported with S.E., and the asterisks (*, **, and ***) denote p < 0.05, p < 0.01, and p < 0.001, respectively.
Results
Mechanical stimulation downregulates NGF signaling
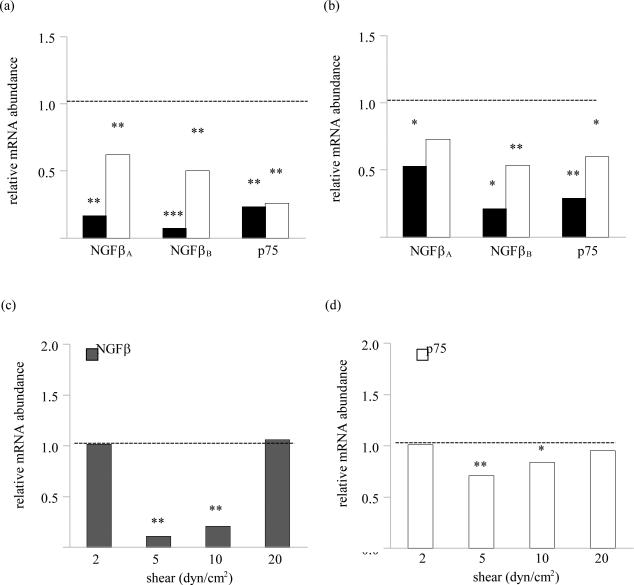
Compared to the non-loaded controls, knee loading at 1 N significantly reduced the mRNA levels of NGFβ and p75 in the cartilage and subchondral bone. Such load-induced downregulation of NGFβ and p75 mRNA persisted 3 h following the application of loading except for the mRNA level of NGFβ variant A in subchondral bone (figure 1a and 1b). Fluid shear at 5 and 10 dyn/cm2 also downregulated the mRNA levels of NGFβ and p75 in C28/I2 cells as compared to the no-flow controls (figure 1c and 1d).
Figure 1.
Effects of mechanical stimulation on the mRNA levels of NGFβ and p75. (A) Effects of knee loading on the mRNA levels of NGFβ–A, NGFβ–B, and p75 in the articular cartilage. (B) Effects of knee loading on the mRNA levels of NGFβ–A, NGFβ–B, and p75 in subchondral bone. (C) Effects of shear stress on the levels of NGFβ mRNA in C28/I2 cells. (D) Effects of shear stress on the levels of p75 mRNA in C28/I2 cells. The dash line represents the level of the control group.
Phosphorylation of p38 is involved in loading effects on NGF signaling
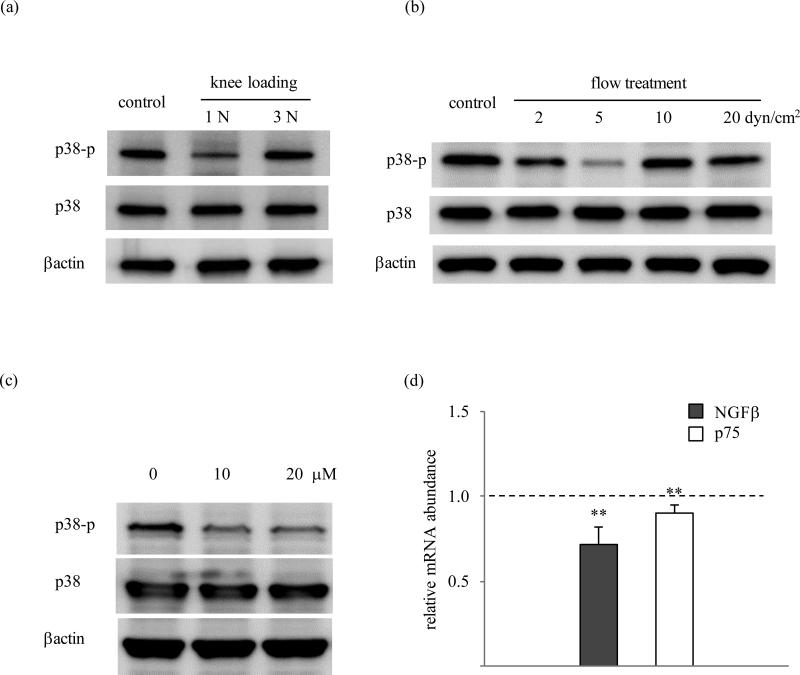
Knee loading at 1 N but not at 3 N decreased the phosphorylation level of p38 (p-p38) in the cartilage (figure 2a). Consistent with this reduction of p-p38 at relatively low amplitude in vivo, fluid flow at 5 dyn/cm2 in vitro also reduced p-p38. At 2, 10, and 20 dyn/cm2, however, flow-driven reduction of p-p38 was not observed, leading to biphasic response over different flow shear range of 0 to 20 dyn/cm2 (figure 2b). Administration of 10 and 20 μM SB203580 suppressed p38 phosphorylation within 15 min in C28/I2 cells (figure 2c). As compared to the controls, SB203580 (10 μM) significantly reduced the level of NGFβ mRNA (p=0.0014) and p75 mRNA (p=0.007) in 60 min in C28/I2 cells (figure 2d).
Figure 2.
p38 MAPK signaling. (A) Phosphorylation of p38 in mouse cartilage in response to knee loading. (B) Phosphorylation of p38 in C28/I2 cells in response to fluid flow at 2 – 20 dyn/cm2. (C) Effects of SB203580 on phosphorylation of p38 in C28/I2 cells. (D) Effects of SB203580 on the mRNA levels of NGFβ and p75 in C28/I2 cells.
Knee loading suppresses Rac1 and siRNA for Rac1 downregulates NGF
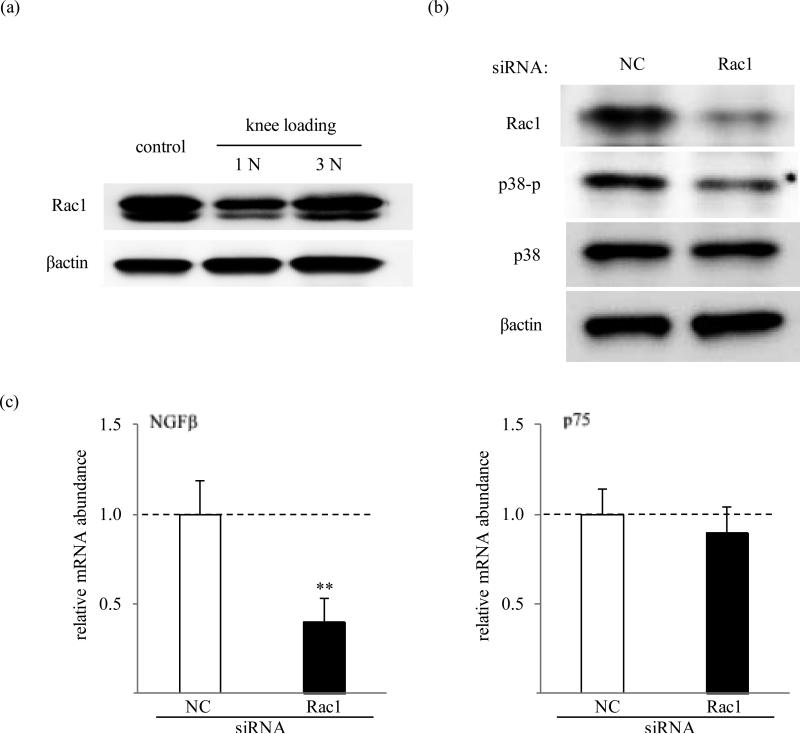
We further investigated whether Rac1 is involved in regulation of NGF mRNA through p38 MAPK. In the mouse cartilage, knee loading suppressed Rac1 GTPase at 1 N but not at 3 N (figure 3a). Silencing of Rac1 GTPase by siRNA abolished production of Rac1 protein as compared to the negative control siRNA. The cells transfected with Rac1 siRNA showed a reduced level of p38 phosphorylation (figure 5b). Subsequently, gene expression of NGFβ was significantly downregulated by 2-fold (p=0.0048) in human C28/I2 chondrocytes transfected with Rac1 siRNA, while mRNA level of p75 did not show statistical difference (figure 3c).
Figure 3.
Involvement of Rac1 GTPase. (A) Activity of Rac1 in mouse cartilage in response to knee loading. (B) Effects of RNA interference with Rac1 siRNA on phosphorylation of p38 in C28/I2 cells. (C) Effects of Rac1 siRNA on the mRNA levels of NGFβ and p75 in C28/I2 cells. The dash lines represent the level of the control group. NC: non-specific control siRNA.
Salubrinal induces attenuation of NGF mRNA expression and Rac1 in chondrocytes
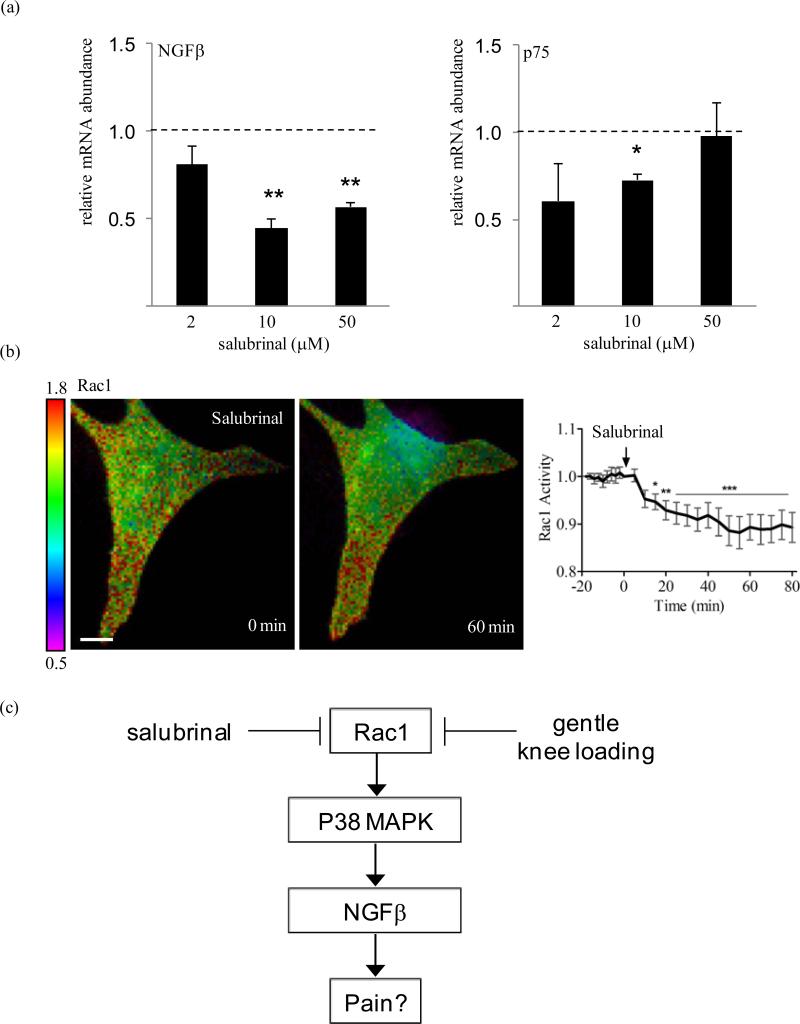
Salubrinal administration (10 μM) led to downregulation of the mRNA levels of NGFβ (p=0.002) and p75 (p=0.019) as compared to the vehicle control, 24 h following treatment to human C28/I2 cells. Salubrinal at 50 μM also gave rise to a reduction of mRNA levels for NGFβ (p=0.001) but not p75 (figure 4a). In the FRET-based assay, administration of salubrinal at 10 μM significantly decreased activity of Rac1 GTPase in C28/I2 cells within 15 min and sustained its decrease more than 60 min (figure 4b).
Figure 4.
Effects of salubrinal. (A) mRNA levels of NGFβ and p75 by 10 μM salubrinal in C28/I2 cells. n=6. (B) Reduction in Rac1 activity in C28/I2 cells by FRET. The arrow indicates the commencement time (t=0) for salubrinal administration at 10 μM to C28/I2 cells. The color bar represents emission ratio of YFP/CFP, an index of Rac1 activation. Ratio images were scaled according to the corresponding color bar. Scale bar = 10 μm. (C) Proposed mechanism of Rac1 mediated regulation of NGFβ through p38 MAPK. In osteoarthritic joints, patients accompanying chronic pain are manifested with an elevated level of NGFβ and p75.
Discussion
It is reported that the expression of NGFβ is elevated in osteoarthritic cartilage.14 In this study, we demonstrate that knee loading at 1 N reduces the mRNA levels of NGFβ and p75 in the articular cartilage and subchondral bone of the mice, and shear stress at 5 and 10 dyn/cm2 on C28/I2 chondrocytes downregulates their mRNA levels. Pain of the knee joint is multi-factorial and inflammatory synovium and subchondral bone collapse are suggested to play a role in osteoarthritic joints.14 Alternatively, it is hypothesized that the invasion of articular cartilage by vascularized mesenchymal tissues followed by the innervation of sensory nerves is associated with severity of injury as well as chronic pain.22 Nerves exist in trabecular bone of the epiphysis23, and they grow in response to NGFβ.24 Although healthy cartilage does not consist of vascular or neural tissues, arthritic cartilage loses its ability to remain aneural and avascular.25,26,27 It has been reported that dynamic loading to cartilage evokes stimulation of matrix synthesis,28 as well as regulation of enzymatic activities of matrix metalloproteinases.29 In addition to the reported regulatory role in matrix homeostasis, the result herein points out that mechanical stimuli at moderate amplitudes regulate transcription of NGFβ and its receptor in cartilage and chondrocytes.
Knee loading induces not only pressure alterations but also pressure driven fluid flow to chondrocytes. Unlike well-studied effects of normal stress on chondrocytes, it has been recently suggested that a consequence of compressive loading is production of hydrostatic pressure as well as fluid flow to cartilage.30 In osteoarthritis, chondrocytes are exposed to flow shear due primarily to synovial fluid and high amplitude of fluid flow reproduces the hallmarks of osteoarthritis in vitro.31 The frequency of 5 Hz might not be representative of massage to humans by hands but more pertinent to those by vibrator for foot massage. The levels of loading in vivo have been optimized to produce anabolic response in bone and cartilage (ref). In the current study, 1 N was able to suppress mRNA expression of NGF and p75 while 3 N was not. The culture model was derived from the previous report. 30-31 Unlike the exposure time applied in the previous in vitro model (ref), we found 0.5 h exposure at 5 and 10 dyn/cm2 led to comparable effects in cells as in the current in vivo compressive loading (1 N) from tissues.
The immunoblot results were limited to p38, showing biphasic effects, as the lower load/fluid shear levels applied were inhibitory. As shown in wider force range, however, the mRNA data on load- and shear-driven alteration of NGF and p75 were more convincingly demonstrated the same biphasic responses. A more thorough study is warranted to address load-driven suppression of NGF at protein level.
In this study, we focused on the role of Rac1 in load-driven downregulation of NGFβ. Rac1 has been shown to activate p38 MAPK,32 and laminar fluid shear has been reported to induce transient alteration of p-p38 and Rac1.33 Consistent with those previous studies, we observed that Rac1 siRNA in C28/I2 cells reduced p-p38. In our siRNA experiment, however, load-driven downregulation of p75 was not affected by RNA interference. It is thus possible that other GTPases such as RhoA and cdc42 are involved in the regulation of p75, and possibly of NGFβ. For instance, we have previously observed that the activity of RhoA alters in response to fluid flow in a flow-intensity dependent manner.34 Further analysis is necessary to determine a regulatory mechanism of p-p38 in cartilage and chondrocytes. Although TrkA is the high affinity receptor for NGFβ, our pilot data using real time qPCR indicated that the basal expression of TrkA in human C28/I2 cells is 6 fold lower than that of p75 (not shown). Thus, the current study was focused on p75 rather than Trk.
The in vitro result also reveals that salubrinal, a specific inhibitor for dephosphorylation of eukaryotic translation initiation factor 2α (eIF2α), can attenuate transcription of NGFβ and activity of Rac1. Our result suggests that suppression of both NGFβ and p75 mRNA expression is achievable at 10 μM but not necessarily at the higher dose at 50 μM. In the central nervous system, salubrinal has been shown to protect against excitotoxic neuronal injury induced by Kainic acid.35 Kainic acid-induced brain injury is a long-standing animal model of seizure and is known to stimulate NGF expression in the hippocampus.36 However, any effect of salubrinal on the peripheral nervous system remains undetermined. Our in vitro results suggest that both gentle mechanical loading and salubrinal share the Rac1-mediated signaling pathway for -mRNA expression of NGFβ (figure 4c). In myocardial remodeling, it is reported that deficiency of Rac1 reduces stress to the endoplasmic reticulum.37 Since the elevated phosphorylation level of eIF2α by salubrinal also suppresses stress to the endoplasmic reticulum, the observed linkage of salubrinal to Rac1 is consistent with downregulation of NGFβ.
In response to administration of 10 μM salubrinal, the response of the Rac1 biosensor in the FRET analysis presents variations among cells. Approximately 40% of the cells (7 out of 17 cells) exhibited a clear decrease in the activity of Rac1, while the others did not show a significant change. The observation clearly indicate that the result with PCR and Western blotting can only present the average response, and the degree of pain reception may not be necessarily represented by the response of a whole population of cells.
In summary, this study demonstrates that knee loading at 1 N and fluid flow at 5 dyn/cm2 can attenuate mRNA expression of NGFβ mediated by Rac1. The result suggests that gentle knee loading analogous to massage therapy is beneficial not only to enhancing bone formation and accelerating wound healing but also to preventing NGFβ-induced nerve growth and pain perception in cartilage. Further analysis targeted to the loading effects on pain perception may warrant our basic understanding of the role of mechanical stimulation and massage therapy in relieving pains associated with joint diseases such as osteoarthritis.
Acknowledgements
We appreciate M. Goldring for C28/I2 chondrocyte cells, M. Matsuda for a Rac1 biosensor, and M. Hamamura and L. Zhao for technical support. This study was supported by NIH R01AR052144. All authors state that they have no conflicts of interest.
Footnotes
Disclosure Statement
The authors declare that there are no financial conflicts of interest.
References
- 1.McAteer ME, Niziolek PJ, Ellis SN, et al. Mechanical stimulation and intermittent parathyroid hormone treatment induce disproportional osteogenic, geometric, and biomechanical effects in growing mouse bone. Calcif Tissue Int. 2010;86(5):389–96. doi: 10.1007/s00223-010-9348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Arnandis I, Guillén MI, Gomar F, et al. Control of cell migration and inflammatory mediators production by CORM-2 in osteoarthritic synoviocytes. PLoS One. 2011;6(9):e24591. doi: 10.1371/journal.pone.0024591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun HB, Yokota H. Altered mRNA level of matrix metalloproteinase-13 in MH7A synovial cells under mechanical loading and unloading. Bone. 2001;28(4):399–403. doi: 10.1016/s8756-3282(00)00459-2. [DOI] [PubMed] [Google Scholar]
- 4.Dodge T, Wanis M, Ayoub R, et al. Mechanical loading, damping, and load-driven bone formation in mouse tibiae. Bone. 2012 Oct;51(4):810–8. doi: 10.1016/j.bone.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang P, Hamamura K, Turner CH, et al. Lengthening of mouse hindlimbs with joint loading. J Bone Miner Metab. 2010;28(3):268–75. doi: 10.1007/s00774-009-0135-x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P, Su M, Liu Y, et al. Knee loading dynamically alters intramedullary pressure in mouse femora. Bone. 2007;40(2):538–43. doi: 10.1016/j.bone.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun HB, Zhao L, Tanaka S, et al. Moderate joint loading reduces degenerative actions of matrix metalloproteinases in the articular cartilage of mouse ulnae. Connect Tissue Res. 2012;53(2):180–6. doi: 10.3109/03008207.2011.628765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Luigi AJ. Complementary and alternative medicine in osteoarthritis. PM R. 2012;4(5 Suppl):S122–33. doi: 10.1016/j.pmrj.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Bronfort G, Haas M, Evans R, et al. Effectiveness of manual therapies: the UK evidence report. Chiropr Osteopat. 2010;18:25, 3. doi: 10.1186/1746-1340-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One. 2012;7(2):e30248. doi: 10.1371/journal.pone.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peter WF, Jansen MJ, Hurkmans EJ, Bloo H, Dekker J, Dilling RG, Hilberdink W, Kersten-Smit C, de Rooij M, Veenhof C, Vermeulen HM, de Vos RJ, Schoones JW, Vliet Vlieland TP. Guideline Steering Committee - Hip and Knee Osteoarthritis. Physiotherapy in hip and knee osteoarthritis: development of a practice guideline concerning initial assessment, treatment and evaluation. Acta Reumatol Port. 2011;36(3):268–81. [PubMed] [Google Scholar]
- 12.Schaible HG. Mechanisms of Chronic Pain in Osteoarthritis. Curr Rheumatol Rep. 2012;14(6):549–56. doi: 10.1007/s11926-012-0279-x. [DOI] [PubMed] [Google Scholar]
- 13.Iwakura N, Ohtori S, Orita S, et al. Role of low-affinity nerve growth factor receptor inhibitory antibody in reducing pain behavior and calcitonin gene-related Peptide expression in a rat model of wrist joint inflammatory pain. J Hand Surg Am. 2010;35(2):267–73. doi: 10.1016/j.jhsa.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Iannone F, De Bari C, Dell'Accio F, et al. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology (Oxford) 2002;41(12):1413–8. doi: 10.1093/rheumatology/41.12.1413. [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Kunugi H. p75NTR as a therapeutic target for neuropsychiatric diseases. Curr Mol Pharmacol. 2009;2(1):70–6. doi: 10.2174/1874467210902010070. [DOI] [PubMed] [Google Scholar]
- 16.Orita S, Ohtori S, Nagata M, et al. Inhibiting nerve growth factor or its receptors downregulates calcitonin gene-related peptide expression in rat lumbar dorsal root ganglia innervating injured intervertebral discs. J Orthop Res. 2010;28(12):1614–20. doi: 10.1002/jor.21170. [DOI] [PubMed] [Google Scholar]
- 17.Gigante A, Bevilacqua C, Pagnotta A, et al. Expression of NGF, Trka and p75 in human cartilage. Eur J Histochem. 2003;47(4):339–44. [PubMed] [Google Scholar]
- 18.Asaumi K, Nakanishi T, Asahara H, et al. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone. 2000;26(6):625–33. doi: 10.1016/s8756-3282(00)00281-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, Sun Q, Turner CH, et al. Knee loading accelerates bone healing in mice. J Bone Miner Res. 2007;22(12):1979–87. doi: 10.1359/jbmr.070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang ZJ, Zhuang H, Wang GX, et al. MiRNA-140 is a negative feedback regulator of MMP-13 in IL-1β-stimulated human articular chondrocyte C28/I2 cells. Inflamm Res. 2012;61(5):503–9. doi: 10.1007/s00011-012-0438-6. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011;63(9):2700–10. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- 23.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51(2):204–11. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390–8. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 25.Walsh DA, Bonnet CS, Turner EL, et al. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15(7):743–51. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Fransès RE, McWilliams DF, Mapp PI, et al. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthritis Cartilage. 2010 Apr;18(4):563–71. doi: 10.1016/j.joca.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh DA, McWilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 2010;49(10):1852–61. doi: 10.1093/rheumatology/keq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald JB, Jin M, Chai DH, et al. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 2008;283(11):6735–43. doi: 10.1074/jbc.M708670200. [DOI] [PubMed] [Google Scholar]
- 29.Thomas RS, Clarke AR, Duance VC, et al. Effects of Wnt3A and mechanical load on cartilage chondrocyte homeostasis. Arthritis Res Ther. 2011;13(6):R203. doi: 10.1186/ar3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Zhu F, Tong Z, et al. Response of chondrocytes to shear stress: antagonistic effects of the binding partners Toll-like receptor 4 and caveolin-1. FASEB J. 2011 Oct;25(10):3401–15. doi: 10.1096/fj.11-184861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu F, Wang P, Lee NH, et al. Prolonged application of high fluid shear to chondrocytes recapitulates gene expression profiles associated with osteoarthritis. PLoS One. 2010 Dec 29;5(12):e15174. doi: 10.1371/journal.pone.0015174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Ilasaca M. Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol. 1998 Aug 1;56(3):269–77. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 33.Avvisato CL, Yang X, Shah S, et al. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007 Aug 1;120(Pt 15):2672–82. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- 34.Hamamura K, Swarnkar G, Tanjung N, et al. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect Tissue Res. 2012;53(5):398–406. doi: 10.3109/03008207.2012.671398. [DOI] [PubMed] [Google Scholar]
- 35.Sokka AL, Putkonen N, Mudo G, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007 Jan 24;27(4):901–8. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gall C, Murray K, Isackson PJ. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 1991 Jan;9(1-2):113–23. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Zhu H, Shen E, et al. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59(8):2033–42. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]