Abstract
Embryonic stem cells (ESCs) have previously been reported to reprogram somatic cells following fusion. The resulting ES–somatic cell hybrids have been shown to adopt the transcriptional profile of ESCs, suggesting that the pluripotent program is dominant. ES–somatic cell hybrids have most characteristics of pluripotent cells in vitro; however, it remains unclear whether the somatic genome is an active partner in the hybrid cells or simply retained predominately as silent cargo. Furthermore, the functional properties of ES–somatic cell hybrids in vivo have been limited to studies on their contribution to teratomas and developing embryos/chimeras. The extent of their pluripotency remains largely unclear. Here we determined that the somatic genome is actively transcribed by generating ES–somatic cell hybrids using Rag2-deficient ESCs fused to autologous wild-type somatic cells. Rag2 expression was detected during in vitro differentiation, suggesting that the somatic genome follows the correct temporal cues during differentiation. Furthermore, ES–somatic cell hybrids maintain their tetraploid state following 4 weeks of differentiation in vivo and are immune tolerated when transferred into matched individuals. The ES–somatic cell hybrids can efficiently differentiate into hematopoietic precursors in both myeloid and lymphoid lineages in vitro, suggesting that the somatic genome is actively transcribed following cell fusion based reprogramming. However, the ES–somatic cell hybrids showed an altered hematopoietic potential following in vitro differentiation and were unable to show hematopoietic engraftment in a mouse model.
Introduction
Embryonic stem cells (ESCs) are isolated from the inner cell mass (ICM) of a blastocyst. They have the ability to self-renew indefinitely in vitro while retaining pluripotentiality. The reprogramming of somatic cells can be achieved by dedifferentiating somatic cells to an embryonic state to obtain pluripotency. Somatic cells can be reprogrammed to a pluripotent state by a number of methods, including somatic cell nuclear transfer (SCNT), cell fusion to ESCs, and induction of pluripotency by defined factors, giving rise to induced pluripotent stem cells (iPSCs). The functional signatures in the resulting PSCs that are generated using these different reprogramming methods show considerable variability, and the interrogation of the different techniques has aided in the elucidation of both the differentiation and dedifferentiation process.
SCNT involves the transfer of a somatic cell nucleus to an enucleated oocyte, followed by embryonic activation. This process restores totipotency to the somatic cell nucleus. The mechanism of reprogramming by SCNT involves adenosine triphosphate (ATP)-dependent chromatin remodeling, followed by the establishment of the totipotent epigenetic signature. The functional assessment and therapeutic application of ESCs isolated from SCNT embryos, termed ntESCs, was first reported in a proof-of-principle study using donor cells from immune-deficient Rag2−/− mice (Rideout et al., 2002). These mutant mice lack mature B and T lymphocytes and do not produce antibodies in their serum. Targeted homologous recombination was used to correct the defective Rag2 recombinase gene in mutant ntESCs, and differentiated in vitro into hematopoietic stem cells (HSCs) for transplantation (Kyba et al., 2002; Rideout et al., 2002). The ntESC-derived HSCs were engrafted into the donor mice, and they reconstituted the hematopoietic system, including the formation of B and T lymphocytes (Rideout et al., 2002). These findings further demonstrate that cells reprogrammed by SCNT and their derivatives are the functional equivalent to ESCs.
Pluripotency can be induced in somatic cells through the ectopic expression of Oct4, Sox2, c-Myc, and Klf-4, giving rise to iPSCs (Takahashi and Yamanaka, 2006). The functional properties of iPSCs have been shown by their germ-line contribution to chimeras (Okita et al., 2007) and the generation of offspring from tetraploid aggregated embryos (Boland et al., 2009; Zhao et al., 2009). Furthermore, derivatives of iPSCs have been shown to be functionally equivalent to ESCs in differentiation and transplantation studies in rodent disease models (Hanna et al., 2007; Wernig et al., 2008). The induction of pluripotency is a stepwise process that involves the switching off of differentiation markers followed by the activation of genes that control the pluripotency network in ESCs (Brambrink et al., 2008; Maherali and Hochedlinger, 2008).
Reprogramming by cell fusion involves the hybridization of somatic cells with ESCs, resulting in an ES–somatic cell hybrid. Both mouse (Tada et al., 2001) and human ESCs (Cowan et al., 2005; Yu et al., 2006) have been shown to reprogram somatic cells by cell fusion. ES–somatic cell hybrids have most properties of ESCs in vitro, including gene expression and epigenetic profiles, and are able to differentiate to three germ lineages in teratoma assays. However, the functional properties of ES–somatic cell hybrids remain largely unclear. The ES–somatic cell hybrids contribute minimally to the late gestation epiblast (Tada et al., 2001) and chimeras (Ying et al., 2002). This may be due to the tetraploid nature of ES–somatic cells, which may be out-competed by diploid cells in the developing embryo due to their longer cell cycle (Nagy et al., 1990). Furthermore, because there is a mixing of the DNA/genomes in ES–somatic cell hybrids, it has also remained unclear whether the somatic genome is an active partner in the hybrid cells or simply retained as silent cargo (Hochedlinger and Jaenisch, 2006).
In the present study, Rag2−/− ESCs were fused to wild-type autologous somatic cells, and the resulting cell hybrids were characterized both in vitro and in vivo. Furthermore, we investigated whether the somatic genome is actively functioning in ES–somatic cell hybrids and determine whether they have the same hematopoietic differentiation potential as ESCs.
Materials and Methods
Cell lines and cell fusion
The Rag2−/− ESCs and control C5/7B6 ESCs have been previously described elsewhere (Kyba et al., 2002; Rideout et al., 2002). Mesenchymal stem cells (MSCs) were isolated from the same strain of mice as the Rag 2−/− ESCs—6- to 8-week-old 129SvEv×C57BL/6 mice as previously described (Sumer et al., 2009). They were then transduced with pSicoR-Hygro-Tk via lentiviral transduction (Sumer et al., 2009).
ES–somatic cell hybrids were produced by cell fusion between the neomycin-resistant Rag2−/− ESCs and the hygromycin-resistant MSCs. The fusion was performed in a four-well Nunc tissue culture plate, as previously described (Sumer et al., 2010a; Sumer et al., 2010b). In brief, 0.5×106 Rag2−/− ESCs were plated on a cellular fibronectin- (Sigma) coated 1-cm Nunc well and cultured overnight. A total of 1×106 MSCs were centrifuged onto the dense monolayer in at 400×g for 10 min. The culture medium was removed and fusion was performed by adding 500 μL of 50% polyethylene glycol 1500 (PEG1500)/150 mM HEPES and incubated at room temperature for 2 min. The PEG was removed, the cells washed four times in calcium-and magnesium-free phosphate-buffered saline (PBS) and allowed to recover in ES medium in the incubator for at least 4 h before the contents of the plate were trypsinized and plated in 2-×6-cm dishes for culture overnight. Double antibiotic-resistant clones were then selected over 10 days using 200 μg/mL neomycin and 150 μg/mL hygromycin. The ES–somatic cell hybrids were picked and expanded clonally for further analyses.
Cell culture and differentiation
Rag2−/− ESCs, control C5/7B6 ESCs, and ES–somatic cell hybrids were cultured on irradiated feeders in ES medium, consisting of Dulbecco's modified Eagle medium (DMEM; Invitrogen) supplemented with 15% Hyclone™ fetal bovine serum (FBS), 1 mM L-glutamine (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma), 1000 U/mL leukemia inhibitory factor (LIF; Chemicon), 1% nonessential amino acids (NEAA; Invitrogen), and 0.5% penicillin-streptomycin (Invitrogen). MSCs were cultured in α-minimum essential medium (α-MEM) supplemented with 20% Hyclone™ FBS. Cultures were maintained in a humidified incubator at 37°C, with 5% CO2 in air.
The Rag2−/− ESCs, control C5/7B6 ESCs, and ES–somatic cell hybrids were induced to differentiate using the hanging drop method. Cells were dissociated with 0.25% trypsin-EDTA (Sigma) for 2 min, resuspended into ES medium (minus LIF), and plated as 20 μL droplets (approximately 450 cells per drop) on the lid of an inverted Petri dish for 48 h to promote the formation of embyroid bodies (EBs). EBs were then placed into suspension for a further 5 days before being plated onto 0.1% gelatin-coated six-well plates and cultured up to day 14 of differentiation. To examine teratoma formation, 1–2×106 cells were injected into the rear leg muscle of 4- to 6-week-old severe combined immunodeficient (SCID) mice. After 4 weeks, the teratomas were excised and fixed in 4% paraformaldehyde, embedded in paraffin, sectioned at 5 μM, and stained with Hematoxylin & Eosin by the MIMR Histology Laboratory core facility.
Hematopoietic differentiation and transplantation of cell lines
The Rag2−/− ESCs, control C5/7B6 ESCs, and ES–somatic cell hybrids were differentiated into hematopoietic progenitors as described (Kyba et al. 2002). Day-6 EBs were dissociated with collagenase and plated into six-well dishes of OP9 stromal cells at 105 cells/well. Retroviral infection was performed with MSCVHoxB4iGFP as described (Kyba et al. 2002). Colonies (adherent and nonadherent cells) were expanded by trypsinization onto a 15-cm dish with OP9 cells. The hematopoietic potential of the cell lines was determined by differentiation in methylcellulose medium with interleukin-3 (IL-3), IL-6, erythropoietin (EPO), and stem cell factor (SCF) (M3434, StemCell Tech.) as described (Kyba et al. 2002). Hematopoietic colony types were determined on day 10. Colony identity was confirmed by leukostain analysis of cytospin preparation of the methylcellulose colonies. Differentiated cells were used for transplantation on day 14 after retroviral infection. Two- to 3-month-old 129SvEv females (isogenic to the Rag2−/− ESCs) were given 2×500 cGy doses of γ-irradiation, separated by 4 h, and injected with 2×106 cells in 500 μL of Iscove's modified Dulbecco's medium (IMDM)/10% inactivated fetal serum (IFS) via the lateral tail vein.
Polymerase chain reaction
Genomic DNA was extracted from Rag2−/− ESCs, 129SvEv×C57BL/6 MSCs, and ES–somatic cell hybrid cells using the DNeasy Blood and Tissue kit (Qiagen) and used for amplification of neomycin, hygromycin, and Rag2 sequences by PCR. The PCR cycle parameters included an initial denaturation at 94°C for 5 min followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 58°C for 45 sec, and extension at 72°C for 75 sec, followed by final extension at 72°C for 5 min. PCR products were run on a 1% agarose gel at 100 V for 1 h. Primer sequences were: Neo F, AGACAATCGGCTGCTCTGAT, Neo R, CAATAGCAG CCAGTCCCTTC; Hygro F, CGCAAGGAATCGGTCAATAC, Hygro R, ACATTGTTGGAGCCGAAATC; Rag2-Exon3 F, GACCTATTCACAATCAAAAATGTCC, Rag2-Exon3 R, GAAATAGAATGCTTCTGACATAGCC.
Reverse transcription PCR
Total RNA was extracted from Rag2−/− ESCs, 129SvEv×C57BL/6 MSCs, and ES–somatic cell hybrid cells using the RNeasy kit (Qiagen) according to the manufacturer's instructions. To remove contaminating genomic DNA, the resulting total RNA was subjected to DNase I treatment using the DNA-free kit (Ambion). A 2-μg amount of RNA was used to synthesize cDNA using the SuperScript III reverse transcriptase kit (Invitrogen). cDNA samples were subjected to PCR amplification with the following primer pairs:
Oct4 F, GTTCAGCCAGACCACCATCT, R, CCTGGGA AAGGTGTCCTGTAG;
Rex1F, GGACTAAGAGCTGGGACACG, R, GCTGCT TCCTTCTTGAACAAT;
Nanog F, TCAAGGACAGGTTTCAGAAGCA, R, GCT GGGATACTCCACTGGTG;
Sox2 F, GAGGAGAGCGCCTGTTTTT, R, GGAGATC TGGCGGAGAATAG;
β-actin F, GGAATCCTGTGGCATCCATGAAAC, R, AAAACGCAGCTCAGTAACAGTCCG; Rag2 F, CCAGA GAACCACAGAAAAAT, R, TGATAACCACCCACAAT AACAAAT.
Histochemistry and immunohistochemistry
The Rag2−/− ESCs, control C5/7B6 ESCs, 129SvEv×C57BL/6 MSCs, and ES–somatic cell hybrids cells were cultured in four-well glass culture slides (BD Falcon) and fixed in 4% paraformaldehyde for 10 min, washed three times with phosphate-buffered saline (PBS), and incubated with blocking solution [5% goat serum, 1% bovine serum albumin (BSA) in PBS] or blocking solution with 0.1% Triton-X for stage-specific embryonic antigen-1 (SSEA-1) and Oct4 primary antibodies, respectively, before being exposed to the primary antibodies at 1:100 dilution overnight at 4°C. Following three washes with PBS, the slides were incubated at room temperature for 1 h with secondary antibodies goat anti-mouse immunoglobulin M (IgM) Alexa 594 or goat anti-mouse IgG Alexa 594 (Invitrogen, Australia), respectively, diluted 1:1000 in blocking solution. Following three washes with PBS, the slides were mounted in Vectashield+4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories) with a coverslip. For immunohistochemistry of teratoma sections, the paraffin-embedded 5μm sections were dewaxed and rehydratred. Heat-mediated antigen retrieval was performed in citrate buffer for 15 min. After blocking the endogenous peroxidase by hydrogen peroxidase (H2O2) and unmasking the antigen by 5% serum, all sections were incubated with primary antibody (all diluted 1:100) overnight at 4°C. The following antibodies were used: smooth muscle actin (SMA; Abcam, catalog no. Ab5694), mucin (Abcam, catalog no. Ab3649,), and cytokeratin 6 (Abcam, catalog no. Ab24646). After washing, sections were incubated with biotinylated secondary antibodies (diluted 1:200) for 30 min at room temperature. The Avidin-Biotin-Peroxidase System (ABC Elite Kit, Vector) was then applied for 30 min, and the peroxidase activity was detected with diaminobenzidine (DAB; Dako). Sections were counterstained with Hematoxylin for 10–15 sec and mounted with aqueous mounting medium (Dako). Alkaline phosphatase (AP) activity was detected with an AP Kit (Chemicon) according to the manufacturer's instructions.
Ploidy and karyotype analysis
Cultured cells were harvested then fixed with ethanol. The samples were washed with 1 mL of ice-cold PBS supplemented with 1% FBS and resuspended in PBS supplemented with propidium iodide at 5 μg/mL and incubated in the dark for 30 min before fluorescence-activate cell sorting (FACS) analysis. Cells recovered from teratomas were dissociated with 4 mg/mL Collagenase IV and cultured for three days in mouse embryonic fibroblast (MEF) medium before preparation for ploidy analysis. Karyotype analysis on cultures of cell hybrids were performed by Southern Cross Pathology Australia. At least 20 metaphase spreads were assessed by G-banding for each sample.
Flow cytometry analysis
Hematopoietic samples were analyzed by flow cytometer (LSI-II, Flow Facility at Children's Hospital Boston) and stained with lineage-specific antibodies (B220; RA3-6B2, CD19; 1D3, CD3; 145-2c11, CD4; GK1.5, CD8; 536.7, NK1.1; PK136, Ter119; ter119, Gr-1; RB6-8C5, Mac1; M1/70) and hematopoietic cell antibodies (CD41; MWReg30, CD45; 30-F11, cKit; 2B8, Flk1; AVaS12α1).
Animals
All animal experimentation was housed and conducted with protocols and ethics approved by the Animal Welfare Committees from the institutions, Monash University animal ethics committee permit MMCA 2006/36, and protocols 09-12-1556R, -1555R, -1553R approved by the Children1s Hospital Institutional Animal Use and Care Committee.
Results
Generation and characterization of ES–somatic cell hybrids
PEG-mediated cell fusion was performed with the Rag2−/− ESCs and MSCs isolated from autologous wild-type 129Sv/Ev×C57BL/6 mice, as outlined in Figure 1A. Ten days after fusion, a number of double antibiotic-resistant colonies were observed, picked, and expanded clonally for further analysis (Fig. 1B). The ES–somatic cell hybrids were confirmed to be tetraploid by DNA-staining profile using flow cytometry and had arisen from a fusion event from the two parental cell lines by PCR for the neomycin and hygromycin transgenes from the parental lines, as well as the presence of both the mutant and wild-type Rag2 alleles (Fig. 1C).
FIG. 1.
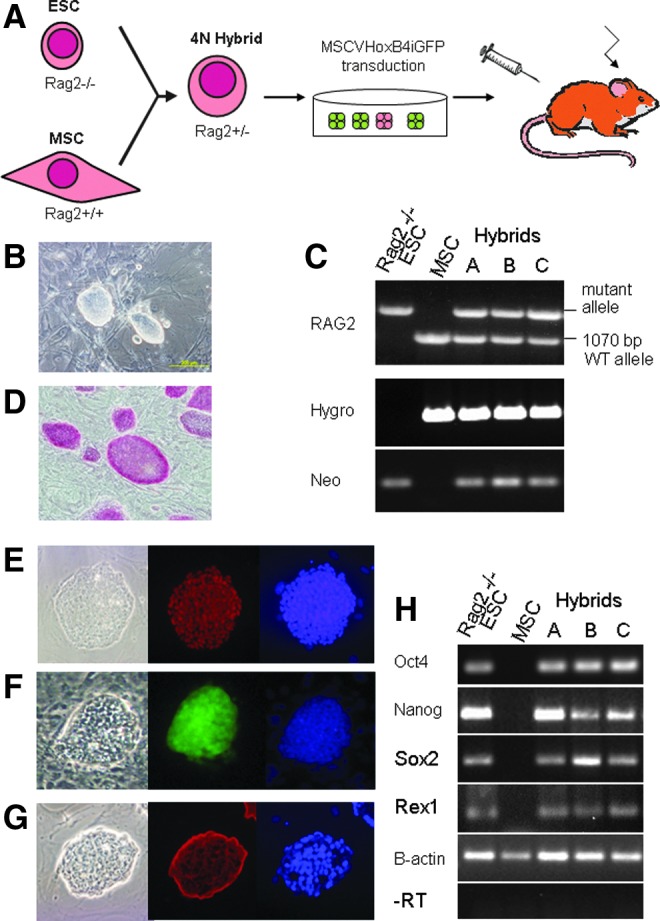
Generation of Rag2 ES–somatic cell hybrids. (A) Experimental outline of ES–somatic cell hybrid formation, hematopoietic differentiation, and transplantation. (B) Typical morphology of Rag2 ES–somatic cell hybrid. (C) PCR amplification of hygromycin (Hygro) and neomycin (Neo) transgenes as well as confirmation of the presence of both wild-type and mutant Rag2 alleles. (D) Alkaline phosphatase staining. Immunostaining of hybrids for Oct4 (E), NANOG (F), and SSEA-1 (G), showing phase contrast, antibody staining, and counterstain with DAPI. (H) Gene expression profile. Color images available online at www.liebertpub.com/cell
Furthermore, we analyzed the ES–somatic hybrids for their pluripotent properties, both in vitro and in vivo. The hybrids cells showed high levels of alkaline phosphatase activity (Fig. 1D) and the expected protein localization for the pluripotency markers Oct4 and Nanog to the nucleus (Fig. 1E, F), and SSEA-1 to the cell surface (Fig. 1G). Control immunostaining of mouse embryonic stem cells was also performed (Supplementary Fig. S1). The hybrid colonies also expressed the ESC markers Oct 4, Nanog, Sox2, and Rex1 (Fig. 1H).
Expression of Rag2 from the somatic genome and maintenance of tetraploidy following differentiation
To determine whether the somatic genome is an active transcriptional partner in ES–somatic cell hybrids, we examined the expression of the Rag2 gene following differentiation in vitro. We found that both ESCs and ES–somatic hybrids did not express Rag2 in the undifferentiated state. However, following 14 days of EB differentiation, Rag2 gene expression was observed in the hybrids but not in the differentiated Rag2−/− ESCs (Fig. 2A).
FIG. 2.
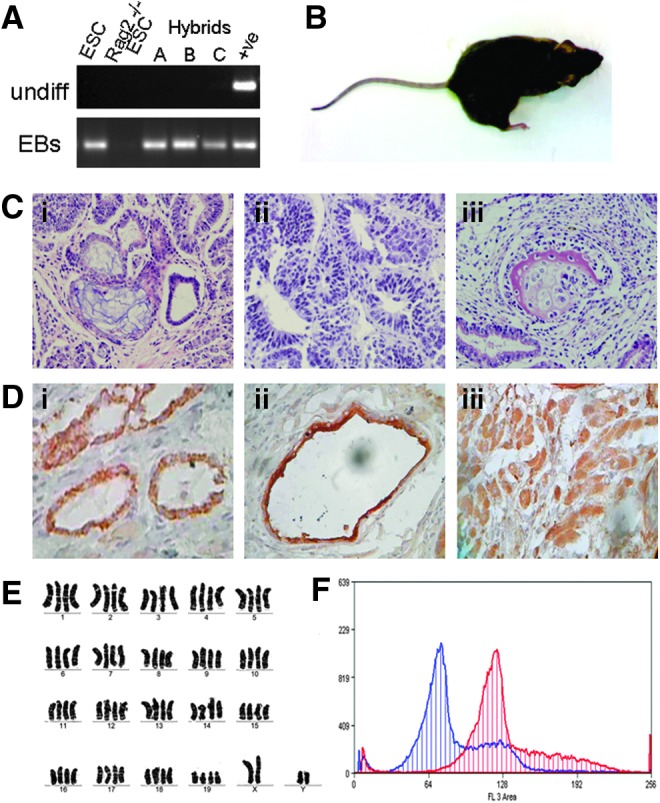
Characterization of Rag2 ES–somatic cell hybrids. (A) Rag2 gene expression in undifferentiated cells and following 14 days of EB formation and differentiation. (B) Teratoma formation of hybrid cells in 129SvEv×C57BL/6 autologous mice. (C) Histology of teratoma tissue showing (i) secretory epithelium, (ii) neural rosettes, and (iii) articular cartilage. (D) Immunohistochemical analysis of teratoma sections revealed positive staining for the lineage germ-line markers for (i) endoderm (mucin), (ii) ectoderm (cytokeratin 6, CK6), and (iii) mesoderm (SMA). (E) Karyotype analysis of Rag 2 Hyb C showing an expected tetraploid karyotype of 80 chromosomes. (F) FACS ploidy analysis of hybrid cells recovered from teratomas (blue), overlaid with diploid ESC controls (red) stained with Propidium Iodide. Color images available online at www.liebertpub.com/cell
To test whether hybrid cells could maintain their ploidy upon differentiation and whether they would engraft into autologous recipient mice, we attempted to form teratomas by hind leg injection in 129SvEv×C57BL/6 mice. We first injected undifferentiated cells subcutaneously into major histocompatibility complex (MHC)-matched immunocompetent mice and saw no engraftment/teratoma formation for both Rag2−/− ESCs (0/3) and ES–somatic cell hybrids (0/6). This phenomenon of rejection of undifferentiated mouse ESCs, possibly due to the action of natural killer (NK) cells on pluripotent stem cells lacking MHC antigens, has been previously reported (Kim et al., 2007). To circumvent this, we predifferentiated the pluripotent cells into EBs and cultured them for 14 days before subcutaneous injection into the 129SvEv×C57BL/6 MHC-matched immunocompetent mice and observed teratomas in all mice (Fig. 2B). Histological analysis revealed cell types indicative of all three germ layers (Fig. 2C), which was further confirmed by immunohistochemical analysis for the three germ lineages (Fig. 2D): Endoderm (mucin), ectoderm (cytokeratin 6, CK6), and mesoderm (α- SMA) in teratoma sections. The ploidy of the hybrids was confirmed by karyotype analysis (Fig. 2E); furthermore, the cells were also recovered from and cultured from the teratomas and tested for ploidy by FACs analysis (Fig. 2F). The hybrid cells were shown to maintain their tetraploid state, even after 4 weeks of differentiation in vivo (Fig. 2E).
Directed differentiation of ES–somatic cell hybrids into hematopoietic precursors and subsequent transplantation
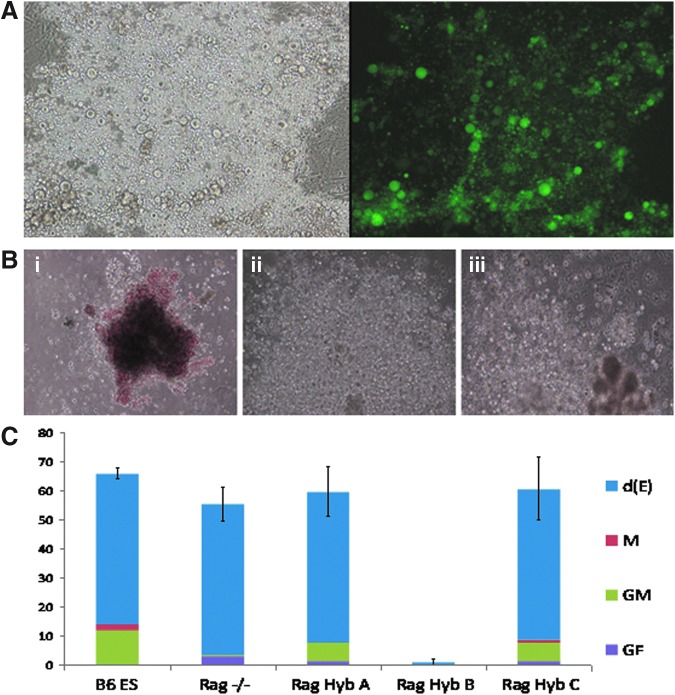
We next determined whether ES–somatic cell hybrids could undergo directed differentiation in vitro. Using the HoxB4-induced differentiation method for differentiating ESCs into embryonic hematopoietic stem cells, we transduced EBs generated from ES–somatic cell hybrids, along with controls, with the MSCVHoxB4iGFP vector. The transduced cells were then cultured for 14 days on OP9 stromal cells, which can support both myeloid and lymphoid hematopoietic development in the presence of hematopoietic cytokines, and assessed for hematopoietic potential (Fig. 3A). We tested three hybrid cell lines, designated Hyb A, Hyb B, and Hyb C, and found that Rag2 Hyb C had the best hematopoietic differentiation. Furthermore, Hyb C had a similar proportion of definitive erythroid, macrophage, granulocytic macrophage, and granulocytic erythroid colonies, when compared to ESC controls (Fig. 3B, C). Therefore, we chose to perform in vivo experiments with the Rag2 Hyb C cell line. Rag2 Hyb A produced a similar proportion of hematopoietic colonies but lacked macrophage colonies, and Rag2 Hyb B showed minimal hematopoietic differentiation.
FIG. 3.
Hematopoietic differentiation potential of Rag2 ES–somatic cell hybrids. (A) OP9 cultured hematopoietic colonies and green fluorescent protein (GFP) signal from MSCVHoxB4iGFP-transduced Rag2 Hyb C. (B) Methylcellulose analysis of Rag2 Hyb C showing differentiation into: (i) definitive erythroid, (ii) granulocyte, and (iii) granulocyte/erythroid/macrophage colonies. (C) Proportion of differentiation of various cell lines into definitive erythroid [d(E)], macrophage (M), granulocytic macrophage (GM), and granulocyte/erythroid/macrophage (GEMM) colonies. Color images available online at www.liebertpub.com/cell
To determine whether the derivatives of ES–somatic cell hybrids were functional in vivo, we tested whether the Rag2 Hyb C-derived hematopoietic progenitor cells could repopulate the hematopoietic system and rescue lethally irradiated mice (outlined in Fig. 1A). Following HoxB4-induced differentiation and culture on OP9 stromal cells, the hematopoietic cell development of the Rag2 Hyb C cells was determined by flow cytometry analysis and staining with hematopoietic lineages-specific markers (Fig. 4). The gates were set using both positive (C57B6 ESCs differentiated on OP9 cells) and negative controls, as previously described (Kyba et al., 2002; McKinney-Freeman et al., 2009). The differentiated Rag2 Hyb C cells expressed a high level of hematopoietic markers (Fig. 4A; CD41, cKit, and Flk1), and both myeloid (Fig. 4B; Gr1, Mac1, and Ter119) and lymphoid (Fig. 4C; Nk1.1, CD4, CD8, and B220) development were observed. The level of the positive population is similar to diploid ESCs cells (Kyba et al., 2002; McKinney-Freeman et al., 2009). However, we found the altered phenotype in the CD45-positive population (Fig. 4D). CD45 is known as a common hematopoietic marker, but both CD45-positive and -negative populations are observed in OP9 cultured cells and have potential to engraft in the lethally irradiated mice. A recent report showed that a CD45-negative population contributes to long-term hematopoietic development, whereas the CD45-positive cells have a more active role in short-term hematopoiesis (McKinney-Freeman et al., 2009). We transplanted OP9 cultured Rag2 HybC cells into lethally irradiated mice via tail vein injections alongside controls. We found that from a total of 23 mice representing three separate experiments none of them were able to successfully engraft upon transplantation (21 mice died within 3 weeks posttransplantation, and the two surviving mice had no detectable level of the transplanted cells), whereas all control ESCs were able to rescue lethally irradiated mice (n=5).
FIG. 4.
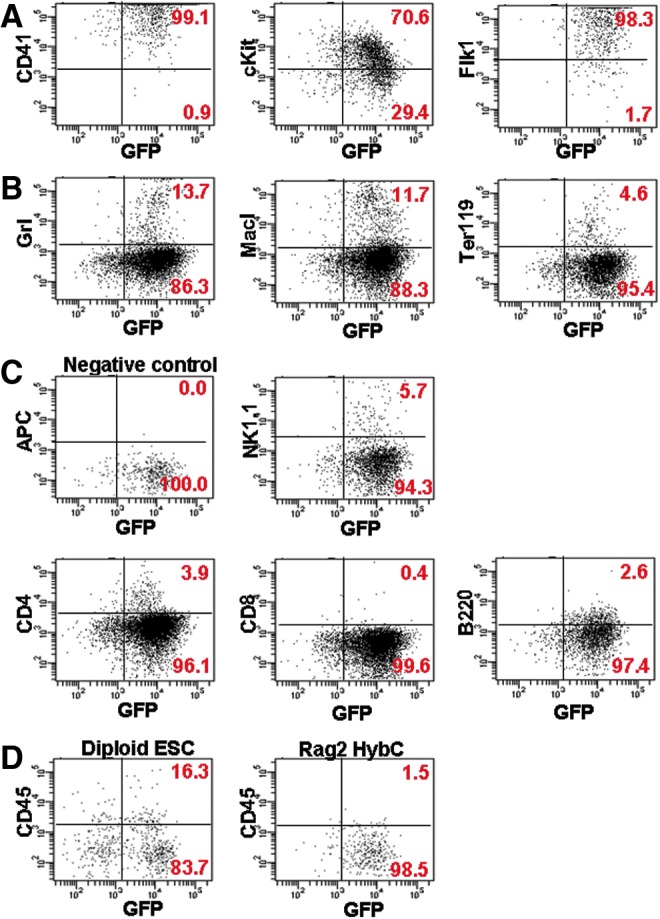
Flow cytometry analysis of the OP9 cultured Rag2 ES–somatic cell hybrid. (A) Common hematopoietic markers CD41, cKit, and Flk1. (B) Myeloid markers Gr1, Mac1, and Ter19. (C) Lymphoid markers Nk1.1, CD4, CD8, and B220. (D) CD45 marker. Color images available online at www.liebertpub.com/cell
Discussion
Following cell fusion between an ES and somatic cell, the transcriptional program of ESCs is dominant, resulting in a cell hybrid that has ESC properties (Kimura et al., 2004; Tada et al., 2003). However, the extent of reprogramming and the functional activity of the somatic genome remain unclear (Hochedlinger and Jaenisch, 2006). In this study, we show that the somatic genome is an active partner in differentiated hybrid cells. Despite the disruption of the Rag2 gene in the ESC genome, the somatic alleles were expressed in the correct temporal manner during EB formation and produced lymphoid lineages on the OP9 stroma cell culture. Furthermore, the interactions between the ESC and somatic genome appeared to be regulated and functioned appropriately because the hybrid cells were able to differentiate in vitro into hematopoietic cells, such as definitive erythroid, macrophage, granulocytic macrophage, and granulocytic erythroid cells in similar proportions to normal pluripotent ESCs. It became evident that the functional properties of ES–somatic cell hybrids were compromised only when the in vivo properties of hybrid cells were tested in the transplantation studies.
It is not clear whether the tetraploid nature of the cells or reprogramming is the reason for the lack of engraftment of the ES–somatic cell hybrids. There are a few examples of normal functioning cells with increased ploidy. Normal and healthy muscle fibers are multinucleated, and there are cells in the liver that are tetraploid. Cell fusion studies of cell hybrids between donor monocytes and diseased recipient hepatocytes resulted in heptatocytes with normal hepatocellular function and proliferative capability following transplantation into a mouse liver injury model (Willenbring et al., 2004). Furthermore, the tetraploid complementation assay, considered the most stringent test for pluripotency, involves the fusion of the cells of a two-cell-stage embryo, resulting in a tetraploid embryo to which diploid pluripotent stem cells are injected/aggregated to test the growth and development of live-born or viable mice (Nagy et al., 1990). The placenta and the yolk sac endoderm have been confirmed to be completely of tetraploid origin and are able to support the development of the embryo proper (Nagy et al., 1990). Furthermore, following blastocyst injection, tetraploid ESCs have been shown to localize to the ICM of embryos (Pralong et al., 2005a), and ES–somatic cell hybrids have been detected in the late-gestation epiblast (Tada et al., 2001) and chimeras (Ying et al., 2002). During normal hematopoietic development, polyploidization of megakaryocyte development is also involved (Zimmet and Ravid, 2000). The current result shows that tetraploid Rag2 Hyb C cells have substantial hematopoietic activities in both myeloid and lymphoid lineages. However, gene dose effects during embryo development and in vitro tissue development from ESCs cannot be ignored. For example, imprinted gene expression has been known to affect embryo development, even with haploid genomes, and a number of imprinted genes are reported to be related to blood development (DeChiara et al., 1991; Giannoukakis et al., 1996). Tetraploid cells will have an increased gene dosage by two-fold, which could alter developmental potential. In fact, Down syndrome in humans, associated with trisomy 21, alters differentiation of blood cells manifesting in blood disorders (Tigay, 2009). These altered phenotypes are defined by a triple gene dose rather than a diploid gene dose from chromosome 21. Therefore, in tetraploid pluripotent cells, gene dose effect will be saturated and could alter the blood differentiation.
The current in vitro blood differentiation patterns of the tetraploid pluripotent cells were similar to diploid pluripotent cells, as reported previously (Kyba et al., 2002; McKinney-Freeman et al., 2009). However, we found a difference in the CD45-positive population of tetraploid pluripotent cells in OP9 culture. Multiple reports indicate that OP9 culture produces distinct CD45-positive and -negative populations. However, tetraploid pluripotent cells could not generate a distinct CD45-positive population. Although CD45-positive cells have an active role in short-term hematopoiesis, the lack of a distinct CD45-positive population cannot explain the failure of the transplantations because both CD45-positive and -negative populations have the potential to engraft in lethally irradiated mice (McKinney-Freeman et al., 2009). However, it is evident that tetraploid pluripotent cells have altered blood differentiation potential, and the precise reason for the lack of engraftment of the ES–somatic cell hybrids remains unclear. This is possibly due to the inability to generate a distinct CD45-positive population and would require further alteration to overcome the technical challenges. This remains to be studied.
To determine whether the somatic genome can maintain pluripotency in the absence of the ESC genome and whether the failure of the hybrids to function adequately in vivo was due to aberrant interactions between the ESC and somatic DNA, the two genomes need to be separated from the hybrid cells after fusion. There have been two methods that have been proposed to establish this: The first is the removal of the ESC chromosomes from ES–somatic hybrids by integration of a universal chromosome elimination cassette onto ESC chromosomes (Matsumura et al., 2007). Cre-mediated sister chromatid recombination would selectively eliminate the ESC chromosomes during cell division, resulting in the presence of only the somatic genome in the reverted hybrid. Alternatively, the reprogramming of a somatic cell without hybridization of the ESC and somatic genomes in a heterokaryon (fused cells with two separate nuclei and a common cytoplasm) could be explored following the selective removal of the ESC-derived nucleus from heterokaryons (Pralong et al., 2005b; Sumer et al., 2009).
In summary, the correct temporal transcription of the Rag2 gene and production of lymphocytes from the somatic genome in ES–somatic cell hybrids during in vitro differentiation were observed. Furthermore, the ability of ES–somatic cell hybrids to differentiate into hematopoietic precursors is equivalent to diploid ESCs. However, using the established differentiation protocol (Kyba et al., 2002), derivatives of these cells are not able to rescue a lethally irradiated mouse upon transplantation.
Supplementary Material
Acknowledgments
This project was supported by funding from the Australian Stem Cell Centre. We would like to thank R. Jaenisch for the kind gift of the Rag2 ntES cells and the pSicoR-Hygro-Tk vector. H.S. was supported by an NH & MRC Biomedical Training Fellowship and an Establishment Gift from the Clive and Vera Ramaciotti Foundation. H.S. and P.J.V. acknowledge funding support from Dairy Australia, funding from the Dairy Futures CRC, and MIMR infrastructure funding supported by the Victorian Government's Operational Infrastructure Support Program. K.K. was supported by the National Institutes of Health (NIH; grant no. K99HL093212-01), Leukemia & Lymphoma Society (LLS; grant no. 3567-07), and Cooley's Anemia Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Boland M.J., Hazen J.L., Nazor K.L., Rodriguez A.R., Gifford W., Martin G., Kupriyanov S., and Baldwin K.K. (2009). Adult mice generated from induced pluripotent stem cells. Nature 461, 91–94 [DOI] [PubMed] [Google Scholar]
- Brambrink T., Foreman R., Welstead G.G., Lengner C.J., Wernig M., Suh H., and Jaenisch R. (2008). Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.A., Atienza J., Melton D.A., and Eggan K. (2005). Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 [DOI] [PubMed] [Google Scholar]
- DeChiara T.M., Robertson E.J., and Efstratiadis A. (1991). Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64, 849–859 [DOI] [PubMed] [Google Scholar]
- Giannoukakis N., Deal C., Paquette J., Kukuvitis A., and Polychronakos C. (1996). Polymorphic functional imprinting of the human IGF2 gene among individuals, in blood cells, is associated with H19 expression. Biochem. Biophys. Res. Commun. 220, 1014–1019 [DOI] [PubMed] [Google Scholar]
- Hanna J., Wernig M., Markoulaki S., Sun C.W., Meissner A., Cassady J.P., Beard C., Brambrink T., Wu L.C., Townes T.M., and Jaenisch R. (2007). Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318, 1920–1923 [DOI] [PubMed] [Google Scholar]
- Hochedlinger K., and Jaenisch R. (2006). Nuclear reprogramming and pluripotency. Nature 441, 1061–1067 [DOI] [PubMed] [Google Scholar]
- Kim K., Lerou P., Yabuuchi A., Lengerke C., Ng K., West J., Kirby A., Daly M.J., and Daley G.Q. (2007). Histocompatible embryonic stem cells by parthenogenesis. Science 315, 482–486 [DOI] [PubMed] [Google Scholar]
- Kimura H., Tada M., Nakatsuji N., and Tada T. (2004). Histone code modifications on pluripotential nuclei of reprogrammed somatic cells. Mol. Cell. Biol. 24, 5710–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M., Perlingeiro R.C., and Daley G.Q. (2002). HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109, 29–37 [DOI] [PubMed] [Google Scholar]
- Maherali N., and Hochedlinger K. (2008). Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell 3, 595–605 [DOI] [PubMed] [Google Scholar]
- Matsumura H., Tada M., Otsuji T., Yasuchika K., Nakatsuji N., Surani A., and Tada T. (2007). Targeted chromosome elimination from ES-somatic hybrid cells. Nat. Methods 4, 23–25 [DOI] [PubMed] [Google Scholar]
- McKinney-Freeman S.L., Naveiras O., Yates F., Loewer S., Philitas M., Curran M., Park P.J., and Daley G.Q. (2009). Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood 114, 268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Gocza E., Diaz E.M., Prideaux V.R., Ivanyi E., Markkula M., and Rossant J. (1990). Embryonic stem cells alone are able to support fetal development in the mouse. Development 110, 815–821 [DOI] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., and Yamanaka S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 [DOI] [PubMed] [Google Scholar]
- Pralong D., Lim M.L., Vassiliev I., Mrozik K., Wijesundara N., Rathjen P., and Verma P.J. (2005a). Tetraploid embryonic stem cells contribute to the inner cell mass of mouse blastocysts. Cloning Stem Cells 7, 272–278 [DOI] [PubMed] [Google Scholar]
- Pralong D., Mrozik K., Occhiodoro F., Wijesundara N., Sumer H., Van Boxtel A.L., Trounson A., and Verma P.J. (2005b). A novel method for somatic cell nuclear transfer to mouse embryonic stem cells. Cloning Stem Cells 7, 265–271 [DOI] [PubMed] [Google Scholar]
- Rideout W.M., 3rd, Hochedlinger K., Kyba M., Daley G.Q., and Jaenisch R. (2002). Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell 109, 17–27 [DOI] [PubMed] [Google Scholar]
- Sumer H., Jones K.L., Liu J., Rollo B.N., van Boxtel A.L., Pralong D., and Verma P.J. (2009). Transcriptional changes in somatic cells recovered from embryonic stem-somatic heterokaryons. Stem Cells Dev. 18, 1361–1368 [DOI] [PubMed] [Google Scholar]
- Sumer H., Jones K.L., Liu J., Heffernan C., Tat P.A., Upton K.R., and Verma P.J. (2010a). Reprogramming of somatic cells after fusion with induced pluripotent stem cells and nuclear transfer embryonic stem cells. Stem Cells Dev. 19, 239–246 [DOI] [PubMed] [Google Scholar]
- Sumer H., Nicholls C., Pinto A.R., Indraharan D., Liu J., Lim M.L., Liu J.P., and Verma P.J. (2010b). Chromosomal and telomeric reprogramming following ES-somatic cell fusion. Chromosoma 119, 167–176 [DOI] [PubMed] [Google Scholar]
- Tada M., Takahama Y., Abe K., Nakatsuji N., and Tada T. (2001). Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 11, 1553–1558 [DOI] [PubMed] [Google Scholar]
- Tada M., Morizane A., Kimura H., Kawasaki H., Ainscough J.F., Sasai Y., Nakatsuji N., and Tada T. (2003). Pluripotency of reprogrammed somatic genomes in embryonic stem hybrid cells. Dev. Dyn. 227, 504–510 [DOI] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- Tigay J.H. (2009). A comparison of acute lymphoblastic leukemia in Down syndrome and non-Down syndrome children: The role of trisomy 21. J. Pediatr. Oncol. Nurs. 26, 362–368 [DOI] [PubMed] [Google Scholar]
- Wernig M., Zhao J.P., Pruszak J., Hedlund E., Fu D., Soldner F., Broccoli V., Constantine-Paton M., Isacson O., and Jaenisch R. (2008). Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc. Natl. Acad. Sci. USA 105, 5856–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbring H., Bailey A.S., Foster M., Akkari Y., Dorrell C., Olson S., Finegold M., Fleming W.H., and Grompe M. (2004). Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat. Med. 10, 744–748 [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Nichols J., Evans E.P., and Smith A.G. (2002). Changing potency by spontaneous fusion. Nature 416, 545–548 [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., He P., Slukvin II, and Thomson J.A. (2006). Human embryonic stem cells reprogram myeloid precursors following cell-cell fusion. Stem Cells 24, 168–176 [DOI] [PubMed] [Google Scholar]
- Zhao X.Y., Li W., Lv Z., Liu L., Tong M., Hai T., Hao J., Guo C.L., Ma Q.W., Wang L. and others. (2009). iPS cells produce viable mice through tetraploid complementation. Nature 461, 86–90 [DOI] [PubMed] [Google Scholar]
- Zimmet J., and Ravid K. (2000). Polyploidy: Occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Exp. Hematol. 28, 3–16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.