Abstract
One of the primary challenges in translating tissue engineering to clinical applicability is adequate, functional vascularization of tissue constructs. Vascularization is necessary for the long-term viability of implanted tissue expanded and differentiated in vitro. Such tissues may be derived from various cell sources, including mesenchymal stem cells (MSCs). MSCs, able to differentiate down several lineages, have been extensively researched for their therapeutic capabilities. In addition, MSCs have a variety of roles in the vascularization of tissue, both through direct contact and indirect signaling. The studied relationships between MSCs and vascularization have been utilized to further the necessary advancement of vascularization in tissue engineering concepts. This review aims to provide a summary of relevant relationships between MSCs, vascularization, and other relevant cell types, along with an overview discussing applications and challenges related to the roles and relationships of MSCs and vascular tissues.
Introduction
Complete vascularization of engineered tissues is currently a major hurdle in the field of tissue engineering, inhibiting successful postimplantation viability. Several strategies have been investigated to overcome this problem, often involving overexpression of angiogenic and vasculogenic factors, such as vascular endothelial growth factor (VEGF),1,2 or combining bioactive scaffolds with encapsulated cells.3–5 In addition to molecular signals, several other environmental factors have been considered to play an important role in promoting vascularization in the presence of mesenchymal stem cells (MSCs). These include environmental effects and interactions with various cell populations. Still, complete vascularization of tissue-engineered constructs and subsequent host integration for clinical applications has yet to be fully realized.
To overcome these challenges, research has focused on optimizing culturing conditions in vitro in preparation for clinical applications. MSCs have become a standard cell population to be cultured with vascular cell types due to their ability to act as support cells and accelerate vascularization and angiogenesis. The availability and differentiation potential of MSCs makes them a popular choice in many developing technologies designed for clinical applications.
To further illustrate the importance of the role of MSCs in vascularization, a survey was conducted by querying leaders in the field of tissue engineering to compile and rank strategies for achieving the clinical development of tissue engineering technologies.6 The analysis of the survey results identified key strategic concepts in the future development of the field. The two most important strategies were found to be angiogenic control and stem cell science. Thus, in the spirit of these findings, we present current concepts and strategies that focus on the interactions of MSCs and vascularization. The interactions between MSCs and the process of tissue vascularization are intimately related, revealing interdependent roles in the goal of developing functional tissue constructs with MSCs. This review will evaluate current strategies used to improve vascularization of engineered constructs using MSCs and a variety of vascular cell types. The individual roles of these various cells in vascularization have been extensively characterized and reviewed, as illustrated by Table 1, and we present how environmental conditions and these cell types influence vascularization.
Table 1.
Mesenchymal Stem Cells and Other Relevant Cell Types Involved in Vascularization of Tissues
| Cell type | Function | Common markers | Relevant reviews |
|---|---|---|---|
| MSC | Nonhematopoietic stromal cells | CD34−, CD106, CD166, CD146, SH2, Stro-1 | 106–109 |
| Can differentiate into bone, cartilage, fat, or muscle lineages | |||
| Homing ability for tissue regeneration | |||
| EC | Innermost layer of blood vessels | CD31, VE-cadherin, VEGFR-1, VEGFR-2, vWF | 110–112 |
| Performs crucial regulatory roles | |||
| Enables nutrient and waste transfer | |||
| EPC | Express VEGF | Early EPCs: CD14, CD31, CD34, CD45, VEGFR-2, VE-cadherin, vWF | 94,113,114 |
| Support new vessel formation | |||
| May differentiate into ECs | Late EPCs: CD31, CD133, VEGFR-2, VE-cadherin, vWF | ||
| Pericyte | Wrap around EC layer | α-SMA, PDGFRβ, NG2-proteoglycan, annexin A5 (markers dependent on resident tissue) | 59,95,115 |
| Initiate vessel maturation | |||
| Regulate microvessel integrity, structure, and function |
EC, endothelial cell; EPCs, endothelial progenitor cells; MSC, mesenchymal stem cell; PDGFRβ, platelet-derived growth factor receptor-beta; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; α-SMA, alpha-smooth muscle actin; vWF, von Willebrand Factor.
Vascularization Interactions with MSCs
Hypoxia
The optimal culturing conditions for MSCs have long been studied, with oxygen tension being one major characteristic.7 It has been determined that developing embryos have much lower oxygen tension than most normal adult tissue, while tissues known to contain stem cells have even lower oxygen.8 This low oxygen tension has been shown to maintain the undifferentiated state of MSCs as well as prolong their lifespan and proliferation capabilities.9 However, differentiation into adipocytes and osteocytes was hindered at such low oxygen levels and required subsequent stimulation at higher oxygen concentrations. This discovery highlights the importance of oxygen levels as a critical influence on MSC growth and differentiation. Therefore, these culturing conditions have been widely investigated in order to optimize vascularization in engineered tissues.10–12
The molecular mechanism of hypoxia has been closely examined, for the purpose of neovascularization as well as angiogenesis for cancer metastasis. It has been found that hypoxia-inducing factor 1 (HIF-1) is one of the major regulators that orchestrates the cellular response to hypoxia.10 As a transcription factor, it is able to modulate vascularization through activation of endothelial growth factors and transcription factors. Under hypoxic conditions, HIF-1's alpha subunit is upregulated exponentially, triggering a series of downstream transcription cascades that result in increased expression of vascular proteins such as VEGF.12
MSCs have been found in close association with blood vessels in a wide variety of tissues.13,14 Even though MSCs are located very closely to vascular structures, they are often still found in a relatively low oxygen environment, which further supports the finding that a hypoxic surrounding may be necessary in order to maintain the cells' undifferentiated state.7 Hypoxia leads to decreased adipogenic and osteogenic differentiation,15,16 triggering the release of angiogenic factors as well as promoting the expression of vasculogenic characteristics and functions in MSCs.17,18
With physiological conditions in mind, there are proliferation benefits for cells cultured in low oxygen environments. The effects on MSCs' behavior include better survival, proliferation, and differentiation capabilities. More specifically, hypoxia can stimulate proangiogenic factors in MSCs. For example, VEGF and interleukin-6 show increased expression after hypoxic stimulation. In addition, MSCs that were cultured under physiologically relevant hypoxia (2% oxygen) in a three-dimensional (3D) environment saw longer proliferation periods as well as an increase in MSC gene expression compared to those cultured at normoxic (20%) conditions.8
The level of hypoxia, as well as the time of application, has also shown to be important for in vivo vascularization applications. Preculturing of MSCs in hypoxic conditions has demonstrated improved angiogenic function once transferred into an in vivo environment.19–21 MSCs were shown to have enhanced migration rates and a number of upregulated growth factors and corresponding receptors, such as hepatocyte growth factor, which is responsible for MSC recruitment to damaged and ischemic tissues.19 These results demonstrate the sensitivity of MSCs to their culturing conditions and the potential to fine-tune their environment to enhance vascularization in tissue constructs as well as in vivo transplantations. MSC tissue constructs cultured with 2% oxygen demonstrated a switch in metabolic pathways and exhibited increased proliferation potential compared with those cultured at normal oxygen tensions.22 Changes in total protein levels and extracellular matrix (ECM) expression suggest that hypoxia altered the MSC tissue development processes. Further, hypoxic conditions are able to better maintain the stemness of undifferentiated MSCs, preserving their multilineage differentiation ability. These findings indicate that oxygen tension may be an important culture parameter in developing in vitro tissues using MSCs.
Nevertheless, while considerable work with hypoxia has been done in the field of tissue engineering, there is no convincing evidence that a preculture of cells under hypoxic conditions alone will be sufficient to sustain tissue-engineered constructs larger than a few millimeters after implantation long term. Therefore, a combination of vascularization techniques may be required to complete vascularization in vivo.
Physical blood flow
While paracrine and endocrine signals play a large role in controlling MSC behavior, mechanical forces and stimuli may also have an important impact on vascularization. Specifically, shear stress, a mechanical force generated by fluid flow, has been shown to induce MSC differentiation and activation of vasculogenic pathways.23–25 In the body, shear stress is generated by blood flow through the endothelium, which applies physical tension to cells. To mimic this type of mechanical stress in vitro, a flow chamber can supply steady fluid shear stress ranging from 5 to 30 dyn/cm2. Results after dynamic culturing have indicated an increase in genetic vascular markers and a decrease in MSC characteristics, demonstrating endothelial differentiation of MSCs for potential use in tissue engineering applications.26
More specifically, molecular blood vessel formation pathways can be triggered by shear stress. It was observed that in some cells, levels of transforming growth factor (TGFβ) and monocyte chemoattractant protein-1 (MCP-1) greatly increased in response to shear stress, while VEGF expression remained unaffected. Similarly, studies have identified shear stress receptors on the surface of MSCs that may be involved with molecular events related to vascularization. For example, CD31 receptors activated by shear stress have been shown to increase the recruitment of neutrophils and expression of tumor necrosis factor-α (TNF-α), both indicators of early vascularization.27 Several other studies have shown that ion channels, specifically Ca2+ channels, are sensitive to shear stress and can induce angiogenesis via VEGF receptor-2 (VEGFR-2) activation and resulting in phosphorylation of p38 and increased expression of VEGF.28 The applied force of fluid flow has also led to remodeling of the actin cytoskeleton, which regulates important intracellular processes and protein expression, indicating the important role that mechanotransduction induced by shear stress plays in MSCs' vascularization pathways.29
Different flow patterns also have an effect on MSC differentiation. Laminar flow has shown increased VEGF production by MSCs but no change in cell morphology,30 while dynamic rotational seeding resulted in vascular tube formation of MSCs.31 The use of shear flow has also become especially popular in tissue-engineered vascular grafts. Using pulsatile flow on a 3D graft, MSCs were successfully differentiated into endothelial cells (ECs) as seen through an increase in endothelial markers, such as platelet-endothelial cell adhesion molecule-1 (PECAM-1) and VE-cadherin. In order to withstand a range of shear stress to induce endothelial differentiation of MSCs in vivo, tissue-engineered scaffolds have been modified with a variety of different bioactive molecules to improve cell adhesion and ensure immobilization in flow environments.32
Several different factors have been combined in order to promote endothelial differentiation for the purpose of vascularization. For example, cultured MSCs under shear flow conditions as well as hypoxia have shown increased production of angiogenic factors and formation of microvasculature.25 Such applications validate the complex in vivo culturing environment experienced by MSCs. Therefore, the use of flow stimulation may be a crucial step in advancing the field of vascularization in tissue engineering because it is able to imitate the dynamic in vivo environment most closely.
Interactions with ECs
MSCs are known to adopt a supporting role when mixed with cells derived from tissues, such as muscle, skin, endothelial, and renal epithelial layers.33 It has been demonstrated that MSCs can promote tumor growth by increasing the secretion of proangiogenic factors, which enhance blood vessel formation in the surrounding areas.34 With a higher blood and oxygen supply, tumor cells are able to proliferate much faster resulting in increased tumor size.
The interaction between MSCs and ECs has shown to be highly regulated and requires precise spatial and temporal control. For example, formation of microvasculature is most successful after a delayed addition of MSCs to ECs encapsulated in collagen scaffolds and cocultured in an in vitro environment.35 This setup emulates the in vivo environment most accurately because MSCs are recruited to the site of vascularization after the ECs have begun the initial formation of nascent microstructures.35,36
The interaction between ECs and MSCs has been most pronounced in the application of wound healing. MSCs near the location of the wound secrete paracrine factors, such as VEGF, to recruit macrophages and ECs, accelerating the wound healing process. This process requires a complex series of molecular events, including cell migration, ECM deposition, angiogenesis, and remodeling.37 At the same time, damaged ECs are able to recruit MSCs for the same purpose of tissue repair via chemokine receptors found on the surface of MSCs. These MSCs were then able to aid in the wound healing process through growth factor release as well as differentiation into ECs.38
Direct cell-to-cell contact between ECs and MSCs has been investigated to understand their signaling pathway and complex interactions. Utilizing a parallel-plate flow chamber to mimic blood flow conditions, MSCs and human umbilical vein ECs were cocultured with the objective to study the initial steps of contact.39 Results showed rapid extension of the podia, followed by rolling and firm adhesion of MSCs to ECs. These results were enhanced when TNF-α was added to the culture, or suppressed when treated with anti-P-selectin or anti-vascular cell adhesion protein 1 (VCAM-1), indicating that binding is both selectin and integrin dependent. Additionally, combining any of those parameters would vary the degree of adhesion of MSCs to endothelial progenitor cells (EPCs). These collaborative pathways indicate that MSCs and ECs are capable of coordinating their rolling and adhesion behaviors.38
While ECs and MSCs may interact closely in vivo, their coculture has been less successful in vitro, with many microvessels turning out to be leaky and unstable once implanted.40 An improved coculturing system with a higher ratio of supporting MSCs, for example, may accelerate the maturation of blood vessels. Additionally, a mixed population of vascular cell types will also closely represent the native populations necessary for vascularization.
Interactions with EPCs
EPCs circulate the bloodstream and promote neovascularization in places of injury, ischemia, hypoxia, and tumorigenesis.41,42 Beneficial interactions between MSCs and EPCs promote the development of tubular structures and vascular networks.43,44 Such vascularization and vascular structure formation has been observed in vitro and in vivo, lending insight into the mechanisms underlying this process.44–47 MSCs interact with EPCs both directly through gap junctions and indirectly through paracrine signaling, with major pathways highlighted in Figure 1.47 Direct contact between MSCs and EPCs may lead to induced endothelial phenotypes, without the addition of exogenous growth factors, in both cell types.47,48 These cell–cell interactions elicit dynamic, temporal changes in cocultured EPCs and MSCs. Initially, adhesion protein, growth factors, and signaling cytokines are upregulated.47 Proteins, such as CDh-5 and PECAM-1, present in vascular cell junctions and regulators of vessel permeability are upregulated.47,49 VEGF, IGF1, and angiopoietin-1 (ANG-1), responsible for vessel formation, pericyte recruitment, and EC differentiation, also experience early, increased expression.47,50,51 In addition to these changes in RNA expression, MSCs have been observed to participate with EPCs in forming tube-like structures,47 further supporting the synergistic relationship between MSCs and EPCs in neovascularization.
FIG. 1.
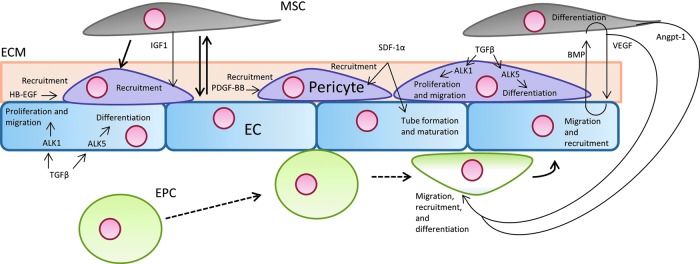
Several cell types are involved in angiogenesis and vascularization. Signaling pathways, indicated by thin arrows, between these cell types direct vascularization and differentiation. Differentiation is shown through bold, solid arrows. Dashed lines show endothelial progenitor cells' (EPCs') method of rolling and attachment to endothelial cells (ECs).
In EPC and MSC interactions, bone morphogenetic protein-2 (BMP-2) appears to have an important influence on EPCs through chemotactic effects.52 Such effects are bolstered by the elution of angiogenic growth factors from MSCs. Secretion of these growth factors from MSCs was found to be dependent on MSC exposure to BMP-2.52 These secreted factors include placental growth factor, which is a cytokine associated with the recruitment of EPCs.53 Importantly, paracrine signaling between MSCs and EPCs is strongly dependent on the interplay between VEGF and BMP-2. BMP-2 plays an integral role in the osteogenic differentiation and function of MSCs. However, BMPs have also been shown to stimulate VEGF production. As VEGF stimulation drives angiogenesis, BMP-stimulated VEGF promotes angiogenesis.54 Particularly, BMP-2 has been shown to stimulate angiogenesis in fracture-healing models.55,56
These pathways have been explored in functional assessments of EPC and MSC interactions. EPC and MSC synergistic interactions have been studied in critical bone defect repair in rats.44 The bone defects were repaired with one of the following treatments: autologous bone, β-tricalcium phosphate (β-TCP) as a scaffold, MSCs seeded on β-TCP, EPCs seeded on β-TCP, and a coculture of MSCs and EPCs seeded on β-TCP. The coculture of MSCs and EPCs produced the highest amount of vascularization, demonstrating the combined effects of MSCs and EPCs in bone repair. Cotransplantation of EPCs and MSCs shows good bone regeneration and vascularization potential.45 In addition to the enhanced vascularization demonstrated with the coimplantation of EPCs and MSCs, it is thought that coimplantation leads to MSCs acting as perivascular mural cells.57 When cocultured with EPCs, MSCs have also shown a committed differentiation toward smooth muscle cell and pericyte phenotypes.58 Differentiation appeared to occur due to direct cell-to-cell contact and extracellular signal-regulated kinase signaling, demonstrating the various pathways influencing the reciprocal interactions between EPCs and MSCs.
Pericytes, MSCs, and vascularization
Pericytes are embedded within capillaries, wrapping around ECs within the basement membrane.59,60 While it has been shown that some pericytes, also known as mural cells, represent a subpopulation of MSCs, pericytic behavior is not characteristic of all cells classified as MSCs.61 Still, this particular subpopulation of MSCs appears to interact with ECs much like bone-marrow-derived MSCs, utilizing paracrine and direct-contact signaling, and has been extensively reviewed.62 Pericytes utilize a variety of signaling mechanisms that act on ECs, influencing vascularization and vessel maturation. Like ECs, pericytes are capable of TGFβ signaling. TGFβ has been directly implicated in pericyte proliferation and differentiation, as well as in the regulation of EC differentiation and proliferation. In pericytes, TGFβ acts on two receptors: Alk-1 and Alk-5. Alk-1 appears to induce cell proliferation and migration, while Alk-5 leads to vessel maturation through differentiation and ECM formation.59 Similar to TGFβ signaling, angiopoietin secreted by mural cells act on Tie receptors expressed by ECs.62 Angiopoietins, acting on Tie receptors, are secreted by pericytes and are crucial to vascular development and remodeling.50,62 These signaling loops have been implicated in vascular remodeling, vascular development, and the adhesion of the ECs, MSCs, and ECM.63,64
Several pathways appear to influence perivascular cell recruitment to ECs, as illustrated by Figure 1. On the surface of pericytes, platelet-derived growth factor receptor-beta (PDGFRβ) is expressed, which binds soluble PDGF-BB produced by ECs. This particular signaling pathway plays a significant role in pericyte recruitment to ECs and has been regarded as the major pathway of pericyte recruitment in physiological angiogenesis. In undifferentiated mesenchymal cells, PDGF-BB induces mural cell fate.65,66 By regulating PDGFRβ expression, Notch signaling has been suggested to possess a role in mural cell recruitment.67,68 Notch signaling is also crucial for angiogenic sprouting and plays a role in endothelial–pericyte interactions.67,69,70
Demonstrating the possibility of other pathways associated with pericyte recruitment, stromal-derived factor 1-a (SDF-1α) has been recently implicated in pericyte recruitment, along with its role in endothelial tube formation and maturation.60,71 Still, pericyte recruitment associated with SDF-1α may be due to crosstalk between SDF-1α and PDGF-BB pathways.71 Another pathway involved in pericyte recruitment is through heparin-binding epidermal growth factor (HB-EGF).72 HB-EGF, implicated in cardiovascular development, has also been found to have protective effect on pericytes by supporting pericyte proliferation and minimizing the effects anoxia-induced apoptosis.73 Several studies have demonstrated that HB-EGF pathways may experience crosstalk with PDGF pathways.60,74 Because of the varied pathways affecting pericyte recruitment and function, opportunities to affect change in MSC function as pericytes are numerous.
Direct endothelial-like differentiation
While much of the MSC's influence on vascularization is primarily through paracrine and endocrine effects on other cells, MSCs may also have a direct role in vascularization through direct endothelial differentiation. Through endothelial differentiation, MSCs have been used in a variety of models, in vitro and in vivo to enhance vascularization.75–78 In vitro, MSCs exhibited endothelial-specific markers, such as VEGFR-1, VEGFR-2, and von Willebrand Factor (vWF), after incubation with 2% fetal calf serum and 50 ng/mL VEGF.79 While VEGF may be crucial to inducing arterial fate, VEGF alone has also been shown to be ineffective at enhancing or accelerating the endothelial differentiation of MSCs.80 Still, further angiogenesis tests demonstrated the functional behavior of conduits formed by the MSCs.75 Conversely, ECs have been found to differentiate into MSCs.81,82 EC-derived MSCs have displayed the capability to differentiate into adipocytes, chondrocytes, and osteoblasts.82 Such differentiation could be induced by TGFβ2 or BMP-4. The relationship between EC and MSC differentiation pathways provides alternative approaches to study and solve issues of vascularization in tissue-engineered constructs.
In addition to utilizing natural growth factors, researchers are investigating synthetic chemicals and drugs to induce endothelial differentiation of stem cells. Chemical small molecules have been used to induce mouse embryonic stem cell (ESC) differentiation into ECs. For example, the compound R-ABO effectively induced ESC differentiation into ECs via upregulation of a molecule acting upstream of fibroblast growth factor 2 (FGF-2).83 Another study used a DNA methyltransferase inhibitor to induce endothelial differentiation of ESCs via epigenetic activation more efficiently than traditional VEGF treatment strategies.84 Extending these strategies to MSCs, one study treated adipose-derived MSCs with an epigenetic drug, BIX-01294.85 Treatment resulted in significantly increased expression of several markers and factors associated with ECs and blood vessel formation, including VCAM-1, PECAM-1, vWF, VEGFR-2, PDGF, and ANG-1. Continued research into synthetic-chemical-induced EC differentiation could improve the efficiency of differentiation and thereby reduce the costs of therapeutic MSC differentiation strategies.
In addition to soluble natural and synthetic chemicals, microenvironmental effects are critical to MSC behavior and fate. Substrate topology and mechanical properties are crucial determinants of cell function and fate.86–88 For example, MSCs differentiation along neural, myogenic, and osteogenic lineages was dependent on the modulus of two-dimensional substrate gels on which MSCs were cultured.89 MSCs cultured using either vasculogenic or nonvasculogenic media and seeded on 3D tubular collagen scaffolds experienced EC and smooth muscle cells (SMC) differentiation.90 Thus, it is apparent that the 3D microenvironment can effectively induce differentiation even without the influence of soluble growth factors. MSCs have also been seeded on 3D nanofiber matrices with elastic moduli tuned to ranges that corresponded with the intima and media layers of blood vessels.91 The tuned nanofiber matrix moduli enabled control of MSC differentiation into ECs or SMCs. Controlling MSC differentiation into ECs has also been tuned by modification of fibrinogen with various polyethylene glycol (PEG) derivatives to achieve a range of mechanical and physical properties.92 Adjusting the properties of these substrates resulted in drastic alterations in cell morphology, gene expression, and overall transdifferentiation of MSCs to an EC-like phenotype. Thus, when inducing differentiation of MSCs to an endothelial phenotype, it is crucial to consider both chemical and physical microenvironmental conditions for therapeutic applications.
In an example of therapeutic benefit, researchers utilized MSCs' ability to differentiate into ECs in an in vivo canine chronic ischemia model.78 Injected MSCs were found to have differentiated into SMCs and transdifferentiated into ECs, as suggested by the luminal location of the MSCs and their expressed factor VIII.78 Such transdifferentiation may have led to the higher capillary density observed in the MSC-treated canines. Canine MSCs have also been seeded on decellularized arterial matrices and cultured in pulsatile flow bioreactors.93 These MSCs cultured on the matrices expressed vWF and oriented themselves in the flow direction. Endothelial differentiation of MSCs can be promoted by growth supplements and shear force.75 While growth supplement administration and shear force exposure were not sufficient alone to differentiate MSCs, the cells produced an endothelial gene expression profile, including CD31, KDR, and vWF, and exhibited morphology consistent with ECs, when seeded in Matrigel. In addition, these MSCs, after growth supplement and shear force priming, were capable of forming a functional capillary network in 3D culture environments, both in vitro and in vivo. These results demonstrate the complex environment that leads to MSC to EC differentiation, which may be difficult to replicate in an in vitro experiment. Still, these studies validate the potential capability of MSCs to be used as a cell source for not only supporting vascular cells, but also directly vascularizing tissue constructs.
Applications
As with most developing tissue engineering obstacles, several challenges remain in translating the fundamental relationship between MSCs and vascularization into clinical applications. There is a need to define and develop optimized culture and 3D microenvironmental conditions for MSCs and any vascular cells that may be included in order to promote healthy tissue development and vascularization. Utilizing these cells in a clinical environment must involve a careful understanding of the safety issues involved with the biomaterials chosen, source of the cells, and any modification (genetic or otherwise) to the cells. While much work has been performed in applying MSCs and vascularization in in vivo experiments, a complete understanding of the signaling pathways and cell types involved has yet to be elucidated. Clarifying these more basic fundamental questions will provide greater insight into the results and advancements made in therapeutic in vivo applications. For example, considerable debate still remains regarding the precise identities and subtypes of EPCs and pericytes, and, as such, their functional relationship with MSCs must be more closely examined.94,95 To complement the growing understanding of these relationships, a variety of strategies have been utilized to take advantage of both MSCs' influence on vascularization and vascular cells' influence on MSCs. Many of these concepts directly incorporate one or more signaling pathways, various vascular cell types, and deliberate cellular microenvironment design to reach these ends.
MSC 3D microenvironment
MSCs may be seeded onto scaffolds, injected, or implanted on their own to improve tissue vascularization. To examine the perivascular therapeutic potential of MSCs, MSCs were embedded in alginate beads and the beads were implanted in ischemic mice.96 Beads were implanted in the perivascular space around the femoral artery. The implantation of beads containing MSCs activated proangiogenic signaling pathways leading to the activation of VEGF-A. Through these mechanisms, the perivascular MSCs appeared to support neovascularization, significantly improved blood flow, increased tissue oxygenation, and reduced toe necrosis. In another application, bone-marrow-derived MSCs were seeded into pullulan-collagen hydrogels and implanted in a murine model simulating an excisional wound.97 MSCs seeded on the hydrogels best secreted angiogenic factors compared with those grown in standard culture conditions. Once implanted, the seeded MSCs were discovered to have differentiated into fibroblasts, ECs, and pericytes, while also demonstrating significantly increased angiogenesis in wounds treated with the MSC-seeded hydrogels.
To bolster cell–cell communication, the 3D microenvironment must be considered. Such an environment may directly affect cell–cell signaling, survival, proliferation, and differentiation. For example, MSCs aggregated into 3D spheroids produce higher amounts of VEGF and FGF-2.98 Because of these effects, 3D MSC spheroids were seeded onto porous polyurethane scaffolds.99 Compared with nonseeded scaffolds and scaffolds seeded with individual MSCs, MSC-spheroid-seeded scaffolds demonstrated improved scaffold vascularization and higher microvessel functionality. In the absence of biomaterial scaffolds, simply encouraging 3D organization of MSCs also enhances MSC survival and, subsequently, vascularization. For example, in both in vitro and in vivo environments, MSCs transplanted in spheroids produced by a hanging-drop method improved the viability of the MSCs.100 MSC spheroid transplantation, compared with MSCs from monolayer, resulted in increased microvessel formation and reduced limb loss and necrosis in ischemic mice. While the 3D environment may increase paracrine signaling and function in the MSCs, the spheroids may also have allowed for longer MSC residency in the tissues compared with MSCs transplanted from monolayer.
Culturing and implanting MSCs in a 3D microenvironment appears to improve MSCs' promotion of angiogenesis and neovascularization. These strategies appear to work well on small implant sizes and when used in a more supportive role, like perivascular delivery to ischemic tissues, instead of a direct role, such as osteogenesis in a critical-sized bone defect. For larger structures, culturing unmodified MSCs alone has not been sufficient for promoting tissue vascularization.
Genetic modification of MSCs
To bolster the natural influence of MSCs on vascularization, MSCs have been genetically modified to expedite and improve vascularization and tissue formation. Bmp2-gene-modified MSCs and EPCs were delivered in injectable calcium sulfate/alginate scaffolds, providing drastic increases in osteoblast differentiation and endothelial differentiation, resulting in increased bone and vascular formation.101 Another genetic modification strategy involved modifying MSCs to express VEGF.102 The increased expression of VEGF led to a threefold increase of vascular density compared with control MSC-seeded grafts. However, the increased VEGF levels resulted in decreased quantities of bone formation and increased osteoclast populations. Such information demonstrates the importance of carefully weighing the benefits of methods to improve vascularization, while ensuring minimal deleterious effects of MSC contributions to bone formation and homeostasis. Relying on genetic modifications of MSCs introduces additional safety concerns, increasing the barriers to clinical applicability.
Combinatorial cell seeding and scaffold incorporation
Besides utilizing MSCs alone, a variety of strategies have incorporated the implantation of ECs, EPCs, and pericytes to better improve engineered vascularization. One group seeded MSCs and EPCs in macroporous polycaprolactone-TCP scaffolds that were subsequently cultured within a biaxial bioreactor.103 Interestingly, scaffolds cultured within the bioreactor did not demonstrate vessel formation as shown in static controls, despite greater mineralization. Despite these in vitro results, dynamically cultured scaffolds displayed both earlier vasculogenesis and increased bone formation in vivo compared with statically cultured constructs. Dynamically cultured scaffolds yielded 1.2- and 2.3-fold more capillary formation than static and acellular controls, respectively. Prevascularization of tissue-engineered bone constructs through the insertion of a vascular bundle has been found to augment both new bone formation and vessel formation once implanted in β-TCP scaffolds seeded with MSCs.104 In scaffolds with MSCs and inserted vascular bundles, VEGF levels were markedly increased over control constructs. Combinatorial cell seeding appears to be a good potential strategy for ensuring adequate vascularization in implanted tissue constructs. Prevascularization of tissue constructs, though, could take considerable time and effort before implanting the scaffold in a clinical application, in addition to the safety concerns associated with autologous cell seeding.
Combining strategies
Scaffold design and modification, genetic modification, combinatorial cell seeding, and other vascularization techniques are often not uniquely applied. For example, the close relationship between MSCs and pericytes was used to design a scaffold-free construct of MSCs, ECs, and perivascular-like cells.105 The perivascular-like cells were differentiated from MSCs and seeded onto an MSC monolayer, along with ECs. ECs and perivascular-like cells self-assembled into colonies in vitro and vascularized the osteogenic tissue sheets when implanted in vivo when seeded by themselves. Using MSCs for perivascular-like and osteogenic functions proved to stabilize the vascular network formed in vivo, demonstrating the importance of crosstalk between all these cells during the vascularization process. Likely, successful tissue-engineered constructs to support vascularization will necessarily incorporate multiple strategies discussed here.
Conclusions
While tremendous strides have been made in understanding the complex interactions between MSCs and vascularization, the need for vascularizing MSC-derived tissue constructs still demands the continued development of current and future strategies to enhance the clinical potential of MSC-based therapies. Such strategies will not only entail incorporating vascular cells to support MSCs, successful strategies will likely utilize MSCs' beneficial paracrine and autocrine effects on vascular cells to further improve and expedite vascularization. Vasculogenic MSC pathways can be bolstered through methods such as careful scaffold design, growth factor immobilization, or genetic modification. The optimal construct will incorporate the synergistic effects of MSCs and vascularization.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR061460 and a seed grant from Children's National Sheikh Zayed Institute for Pediatric Surgical Innovation and the A. James Clark School of Engineering at the University of Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was additionally supported by National Science Foundation Graduate Research Fellowships (to AJM and BNBN), as well as National Science Foundation CBET 1264517.
Disclosure Statement
No competing financial interests exist.
References
- 1.Koyama S., Sato E., Tsukadaira A., Haniuda M., Numanami H., Kurai M., et al. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J 20,1449, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Villars F., Bordenave L., Bareille R., and Amédée J.Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J Cell Biochem 79,672, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Wernike E., Montjovent M.O., and Liu Y.VEGF Incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cells Mater 19,30, 2010 [DOI] [PubMed] [Google Scholar]
- 4.De la Riva B., Nowak C., Sánchez E., Hernández A., Schulz-Siegmund M., Pec M.K., et al. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur J Pharm Biopharm 73,50, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Ribatti D.The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. Br J Haematol 128,303, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Johnson P.C., Mikos A.G., Fisher J.P., and Jansen J.A.Strategic directions in tissue engineering. Tissue Eng 13,2827, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Mohyeldin A., Garzón-Muvdi T., and Quiñones-Hinojosa A.Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7,150, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Grayson W.L., Zhao F., Izadpanah R., Bunnell B., and Ma T.Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol 207,331, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Fehrer C., Brunauer R., Laschober G., Unterluggauer H., Reitinger S., Kloss F., et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell 6,745, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hirota K., and Semenza G.L.Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol 59,15, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Das R., Jahr H., van Osch G.J.V.M., and Farrell E.The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 16,159, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P., Dor Y., Herbert J.M., Fukumura D., Brusselmans K., Dewerchin M., et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394,485, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Shi S., and Gronthos S.Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18,696, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Crisan M., Yap S., Casteilla L., Chen C.-W., Corselli M., Park T.S., et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3,301, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Potier E., Ferreira E., Andriamanalijaona R., Pujol J.-P., Oudina K., Logeart-Avramoglou D., et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 40,1078, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Wagegg M., Gaber T., Lohanatha F.L., Hahne M., Strehl C., Fangradt M., et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. Covas D.T., editor. PLoS One 7,e46483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwezdaryk K.J., Coffelt S.B., Figueroa Y.G., Liu J., Phinney D.G., LaMarca H.L., et al. Erythropoietin, a hypoxia-regulated factor, elicits a pro-angiogenic program in human mesenchymal stem cells. Exp Hematol 35,640, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Rochefort G.Y., Delorme B., Lopez A., Hérault O., Bonnet P., Charbord P., et al. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 24,2202, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Rosová I., Dao M., Capoccia B., Link D., and Nolta J.A.Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26,2173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliasson P., and Jönsson J.-I.The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol 222,17, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Valorani M.G., Montelatici E., Germani A., Biddle A., D'Alessandro D., Strollo R., et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif 45,225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T., Grayson W.L., Fröhlich M., and Vunjak-Novakovic G.Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog 25,32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto K., Takahashi T., Asahara T., Ohura N., Sokabe T., Kamiya A., et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol 95,2081, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto K., Sokabe T., Watabe T., Miyazono K., Yamashita J.K., Obi S., et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol 288,H1915, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Zhang P., Baxter J., Vinod K., Tulenko T.N., and Di Muzio P.J.Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev 18,1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang N.F., and Li S.Mesenchymal stem cells for vascular regeneration. Regen Med 3,877, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glen K., Luu N.T., Ross E., Buckley C.D., Rainger G.E., Egginton S., et al. Modulation of functional responses of endothelial cells linked to angiogenesis and inflammation by shear stress: differential effects of the mechanotransducer CD31. J Cell Physiol 227,2710, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Gee E., Milkiewicz M., and Haas T.L.p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J Cell Physiol 222,120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Titushkin I., and Cho M.Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys J 93,3693, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng L., Xiao Q., Margariti A., Zhang Z., Zampetaki A., Patel S., et al. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol 174,1059, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasseri B.A., Pomerantseva I., Kaazempur-Mofrad M.R., Sutherland F.W.H., Perry T., Ochoa E., et al. Dynamic rotational seeding and cell culture system for vascular tube formation. Tissue Eng 9,291, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Bidarra S.J., Barrias C.C., Barbosa M.A., Soares R., and Granja P.L.Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules 11,1956, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Pittenger M.F., Mosca J.D., and McIntosh K.R.Human mesenchymal stem cells: progenitor cells for cartilage, bone, fat and stroma. Curr Top Microbiol Immunol 251,3, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Zhang T., Lee Y.W., Rui Y.F., Cheng T.Y., Jiang X.H., and Li G.Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther 4,70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFadden T.M., Duffy G.P., Allen A.B., Stevens H.Y., Schwarzmaier S.M., Plesnila N., et al. The delayed addition of human MSCs to pre-formed endothelial cell networks results in functional vascularisation of a collagen-GAG scaffold in vivo. Acta Biomater 9,9303, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Au P., Tam J., Fukumura D., and Jain R.K.Bone marrow–derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111,4551, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L., Tredget E.E., Wu P.Y.G., and Wu Y.Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3,e1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., and Shimizu H.Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180,2581, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Rüster B., Göttig S., Ludwig R.J., Bistrian R., Müller S., Seifried E., et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108,3938, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tsigkou O., Pomerantseva I., Spencer J.A., Redondo P.A., Hart A.R., O'Doherty E., et al. Engineered vascularized bone grafts. Proc Natl Acad Sci U S A 107,3311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill J.M., Zalos G., Halcox J.P.J., Schenke W.H., Waclawiw M.A., Quyyumi A.A., et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348,593, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Balbarini A., Barsotti M.C., Di Stefano R., Leone A., and Santoni T.Circulating endothelial progenitor cells characterization, function and relationship with cardiovascular risk factors. Curr Pharm Des 13,1699, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Kolbe M., Xiang Z., Dohle E., Sc M., Tonak M., Kirkpatrick C.J., et al. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Part A 17,2199, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Seebach C., Henrich D., Wilhelm K., Barker J.H., and Marzi I.Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transplant 21,1667, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Zigdon-Giladi H., Bick T., Lewinson D., and Machtei E.E.Co-transplantation of endothelial progenitor cells and mesenchymal stem cells promote neovascularization and bone regeneration. Clin Implant Dent Relat Res 2013[Epub ahead of print]; DOI: 10.1111/cid.12104 [DOI] [PubMed] [Google Scholar]
- 46.Amini A.R., Laurencin C.T., and Nukavarapu S.P.Differential analysis of peripheral blood- and bone marrow-derived endothelial progenitor cells for enhanced vascularization in bone tissue engineering. J Orthop Res 30,1507, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Aguirre A., Planell J.A., and Engel E.Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun 400,284, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Rahbarghazi R., Nassiri S.M., Khazraiinia P., Kajbafzadeh A.-M., Ahmadi S.H., Mohammadi E., et al. Juxtacrine and paracrine interactions of rat marrow-derived mesenchymal stem cells, muscle-derived satellite cells, and neonatal cardiomyocytes with endothelial cells in angiogenesis dynamics. Stem Cells Dev 22,855, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dejana E., Tournier-Lasserve E., and Weinstein B.M.The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16,209, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Thomas M., and Augustin H.G.The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 12,125, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Cao Y.Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal 2,re1, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Raida M., Heymann A.C., Günther C., and Niederwieser D.Role of bone morphogenetic protein 2 in the crosstalk between endothelial progenitor cells and mesenchymal stem cells. Int J Mol Med 18,735, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Iwasaki H., Kawamoto A., Tjwa M., Horii M., Hayashi S., Oyamada A., et al. PlGF repairs myocardial ischemia through mechanisms of angiogenesis, cardioprotection and recruitment of myo-angiogenic competent marrow progenitors. PLoS One 6,e24872, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deckers M.M.L., Van Bezooijen R.L., Van Der Horst G., Hoogendam J., Van Der Bent C., Papapoulos S.E., et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 143,1545, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Date T., Doiguchi Y., Nobuta M., and Shindo H.Bone morphogenetic protein-2 induces differentiation of multipotent C3H10T1/2 cells into osteoblasts, chondrocytes, and adipocytes in vivo and in vitro. J Orthop Sci 9,503, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Carano R.A.D., and Filvaroff E.H.Angiogenesis and bone repair. Drug Discov Today 8,980, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Koob S., Torio-Padron N., Stark G.B., Hannig C., Stankovic Z., and Finkenzeller G.Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A 17,311, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Goerke S., Plaha J., Hager S., Strassburg S., Torio-Padron N., Stark B., et al. Human endothelial progenitor cells induce ERK-dependent differentiation of mesenchymal stem cells into smooth-muscle cells upon cocultivation. Tissue Eng Part A 18,2395, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Armulik A., Genové G., and Betsholtz C.Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21,193, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Stratman A.N., Schwindt A.E., Malotte K.M., and Davis G.E.Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 116,4720, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blocki A., Wang Y., Koch M., Peh P., Beyer S., Law P., et al. Not all MSCs can act as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev 22,2347, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaengel K., Genové G., Armulik A., and Betsholtz C.Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29,630, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Maisonpierre P.C., Suri C., Jones P.F., Bartunkova S., Wiegand S.J., Radziejewski C., et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277,55, 1997 [DOI] [PubMed] [Google Scholar]
- 64.Suri C., Jones P.F., Patan S., Bartunkova S., Maisonpierre P.C., Davis S., et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87,1171, 1996 [DOI] [PubMed] [Google Scholar]
- 65.Abramsson A., Lindblom P., and Betsholtz C.Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest 112,1142, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellström M., Gerhardt H., Kalén M., Li X., Eriksson U., Wolburg H., et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153,543, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu H., Zhang W., Kennard S., Caldwell R.B., and Lilly B.Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res 107,860, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin S., Hansson E.M., Tikka S., Lanner F., Sahlgren C., Farnebo F., et al. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res 102,1483, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Domenga V., Fardoux P., Lacombe P., Monet M., Maciazek J., Krebs L.T., et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18,2730, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu H., Kennard S., and Lilly B.NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res 104,466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song N., Huang Y., Shi H., Yuan S., Ding Y., Song X., et al. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res 69,6057, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Krampera M., Pasini A., Rigo A., Scupoli M.T., Tecchio C., Malpeli G., et al. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 106,59, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Yu X., Radulescu A., Chen C.-L., James I.O., and Besner G.E.Heparin-binding EGF-like growth factor protects pericytes from injury. J Surg Res 12,1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahin U., Weskamp G., Kelly K., Zhou H.-M., Higashiyama S., Peschon J., et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164,769, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janeczek Portalska K., Leferink A., Groen N., Fernandes H., Moroni L., van Blitterswijk C., et al. Endothelial differentiation of mesenchymal stromal cells. PLoS One 7,e46842, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X., Nan Y., Wang H., Chen J., Wang N., Xie J., et al. Model microgravity enhances endothelium differentiation of mesenchymal stem cells. Naturwissenschaften 100,125, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Warrier S., Haridas N., and Bhonde R.Inherent propensity of amnion-derived mesenchymal stem cells towards endothelial lineage: vascularization from an avascular tissue. Placenta 33,850, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Silva G.V., Litovsky S., Assad J.A., Sousa A.L., Martin B.J., Vela D., et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 111,150, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Oswald J., Boxberger S., Jørgensen B., Feldmann S., Ehninger G., Bornhäuser M., et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22,377, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Galas R.J., and Liu J.C.Vascular endothelial growth factor does not accelerate endothelial differentiation of human mesenchymal stem cells. J Cell Physiol 229,90, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Medici D., and Kalluri R.Endothelial–mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin Cancer Biol 22,379, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medici D., Shore E.M., Lounev V.Y., Kaplan F.S., Kalluri R., and Olsen B.R.Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med 17,1400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han L., Shao J., Su L., Gao J., Wang S., Zhang Y., et al. A chemical small molecule induces mouse embryonic stem cell differentiation into functional vascular endothelial cells via Hmbox1. Stem Cells Dev 21,2762, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Banerjee S., and Bacanamwo M.DNA methyltransferase inhibition induces mouse embryonic stem cell differentiation into endothelial cells. Exp Cell Res 316,172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi Z., Neoh K.G., Kang E.T., Poh C.K., and Wang W.Enhanced endothelial differentiation of adipose-derived stem cells by substrate nanotopography. J Tissue Eng Regen Med 8,50, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Kulangara K., and Leong K.W.Substrate topography shapes cell function. Soft Matter 5,4072, 2009 [Google Scholar]
- 87.Yim E.K.F., Darling E.M., Kulangara K., Guilak F., and Leong K.W.Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 31,1299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watt F.M., and Huck W.T.S.Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol 14,467, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Engler A.J., Sen S., Sweeney H.L., and Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Valarmathi M.T., Davis J.M., Yost M.J., Goodwin R.L., and Potts J.D.A three-dimensional model of vasculogenesis. Biomaterials 30,1098, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Wingate K., Bonani W., Tan Y., Bryant S.J., and Tan W.Compressive elasticity of three-dimensional nanofiber matrix directs mesenchymal stem cell differentiation to vascular cells with endothelial or smooth muscle cell markers. Acta Biomater 8,1440, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang G., Drinnan C.T., Geuss L.R., and Suggs L.J.Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater 6,3395, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Dong J.-D., Huang J.-H., Gao F., Zhu Z.-H., and Zhang J.Mesenchymal stem cell-based tissue engineering of small-diameter blood vessels. Vascular 19,206, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Pearson J.D.Endothelial progenitor cells—hype or hope? J Thromb Haemost 7,255, 2009 [DOI] [PubMed] [Google Scholar]
- 95.Dar A., and Itskovitz-Eldor J.Therapeutic potential of perivascular cells from human pluripotent stem cells. J Tissue Eng Regen Med 2013[Epub ahead of print]; DOI: 10.1002/term.1698 [DOI] [PubMed] [Google Scholar]
- 96.Katare R., Riu F., Rowlinson J., Lewis A., Holden R., Meloni M., et al. Perivascular delivery of encapsulated mesenchymal stem cells improves postischemic angiogenesis via paracrine activation of VEGF-A. Arterioscler Thromb Vasc Biol 33,1872, 2013 [DOI] [PubMed] [Google Scholar]
- 97.Rustad K.C., Wong V.W., Sorkin M., Glotzbach J.P., Major M.R., Rajadas J., et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 33,80, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhang S.H., Cho S.-W., La W.-G., Lee T.-J., Yang H.S., Sun A.-Y., et al. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32,2734, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Laschke M.W., Schank T.E., Scheuer C., Kleer S., Schuler S., Metzger W., et al. Three-dimensional spheroids of adipose-derived mesenchymal stem cells are potent initiators of blood vessel formation in porous polyurethane scaffolds. Acta Biomater 9,6876, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Bhang S.H., Lee S., Shin J., Lee T., and Kim B.Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A 18,2138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He X., Dziak R., Yuan X., Mao K., Genco R., Swihart M., et al. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS One 8,e60473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Helmrich U., Di Maggio N., Güven S., Groppa E., Melly L., Largo R.D., et al. Osteogenic graft vascularization and bone resorption by VEGF-expressing human mesenchymal progenitors. Biomaterials 34,5025, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Yeow C.Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng 19,893, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Wang L., Fan H., Zhang Z.-Y., Lou A.-J., Pei G.-X., Jiang S., et al. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 31,9452, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Mendes L.F., Pirraco R.P., Szymczyk W., Frias A.M., Santos T.C., Reis R.L., et al. Perivascular-like cells contribute to the stability of the vascular network of osteogenic tissue formed from cell sheet-based constructs. PLoS One 7,e41051, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ding D.-C., Shyu W.-C., and Lin S.-Z.Mesenchymal stem cells. Cell Transplant 20,5, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Bianco P., Riminucci M., Gronthos S., and Robey P.G.Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19,180, 2001 [DOI] [PubMed] [Google Scholar]
- 108.Caplan A.I.Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213,341, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Chamberlain G., Fox J., Ashton B., and Middleton J.Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25,2739, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91,3527, 1998 [PubMed] [Google Scholar]
- 111.Sumpio B.E., Riley J.T., and Dardik A.Cells in focus: endothelial cell. Int J Biochem Cell Biol 34,1508, 2002 [DOI] [PubMed] [Google Scholar]
- 112.Lamalice L., Le Boeuf F., and Huot J.Endothelial cell migration during angiogenesis. Circ Res 100,782, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Hirschi K.K., Ingram D.A., and Yoder M.C.Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 28,1584, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yoder M.C.Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2,a006692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ribatti D., Nico B., and Crivellato E.The role of pericytes in angiogenesis. Int J Dev Biol 55,261, 2011 [DOI] [PubMed] [Google Scholar]


