Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is a technologically complicated procedure that represents the only cure for many hematologic malignancies. However, HSCT is often complicated by life-threatening toxicities related to the chemo-radiation conditioning regimen, poor engraftment of donor HSCs, the hyperinflammatory syndrome of graft-versus-host disease (GVHD), infection risks from immunosuppression, and end-organ damage. Bone marrow stromal cells (MSCs), also known as “mesenchymal stromal cells,” not only play a nurturing role in the hematopoietic microenvironment but also can differentiate into other cell types of mesenchymal origin. MSCs are poorly immunogenic, and they can modulate immunological responses through interactions with a wide range of innate and adaptive immune cells to reduce inflammation. They are easily expanded ex vivo and after infusion, home to sites of injury and inflammation to promote tissue repair. Despite promising early trial results in HSCT with significant responses that have translated into survival benefits, there have been significant barriers to successful commercialization as an off-the-shelf therapy. Current efforts with MSCs in the HSCT setting are geared toward determining the factors determining potency, understanding the precise mechanisms of action in human HSCT, knowing their kinetics and fate, optimizing dose and schedule, incorporating biomarkers as response surrogates, addressing concerns about safety, optimizing clinical trial design, and negotiating the uncharted regulatory landscape for licensable cellular therapy.
Allogeneic Hematopoietic Stem Cell Transplantation and Its Complications
Allogeneic hematopoietic stem cell transplantation (HSCT) is a high-risk medical procedure representing the only curative option for many malignant and nonmalignant hematologic disorders. A preparative conditioning regimen (of myeloablative or reduced intensity) is administered prior to donor stem cell infusion to optimally cytoreduce the underlying malignancy and to make immunologic space so that the host does not reject the graft. Donor HSCs derived from a variety of potential sources (marrow, peripheral blood progenitors, or umbilical cord blood) are then infused to replace recipient hematopoiesis and donor lymphoid cells reconstitute the immune system. The donor immune system is capable of detecting major or minor histocompatibility differences with the recipient and exerting a powerful graft-versus-malignancy (GVM) effect, however, this may overlap with potentially lethal acute or chronic graft-versus-host disease (GVHD) directed against normal tissues. Eradication of malignant conditions therefore relies upon two factors: the intensity of the preparative regimen and a GVM/GVHD effect. Despite decades of progress, HSC transplantation remains a high-risk procedure with significant nonrelapse morbidity and mortality related to the conditioning regimen-related toxicity, graft failure, infectious complications, and GVHD. Lethal organ injury can result from the combination of uncontrolled inflammation, drug side effects, and infections. While mortality from these complications has been reduced in recent years, there is still much room for improvement. With the advent of improvements in HSCT, the numbers of human leucocyte antigen (HLA)-mismatched HSCT are poised to exceed HLA-identical transplants with the expectation of even greater transplant-related complications. Steroid refractory GVHD has been reported to have a survival rate of only 17% at 2 years.1 There is critical need for nontoxic treatments that will reduce inflammation and permit tissue and organ regeneration. Marrow stromal cells (MSCs) could provide novel options for reducing the morbidity and mortality of HSC transplantation. This could potentially expand the use of HSC transplantation for treatment of a wider variety of disorders. Also, growing experience with using MSCs in HSCT informs the treatment of a wide variety of other disorders.
Definitions
MSCs are multipotent bone marrow (BM) cells able to differentiate in vitro and in vivo into tissues of mesenchymal origin and are capable of suppressing immune responses and promoting repair of tissue injury (Fig. 1). MSCs were originally reported by Friedenstein as an adherent, fibroblast-like population derived from rodent marrow and capable of regenerating rudimentary bone in vivo and supporting hematopoiesis.2 MSCs comprise a small fraction (<0.1%) of adult BM cells and either directly, or through their osteoblast progeny, support growth, and differentiation of HSCs and progenitor cells in vitro and in in vivo models.3–5 MSCs are capable of differentiating into other cells of mesenchymal lineage including bone, cartilage, and fat.6 MSCs from BM are most commonly isolated by plastic adherence of plated aspirate mononuclear cells, followed by serial passage.
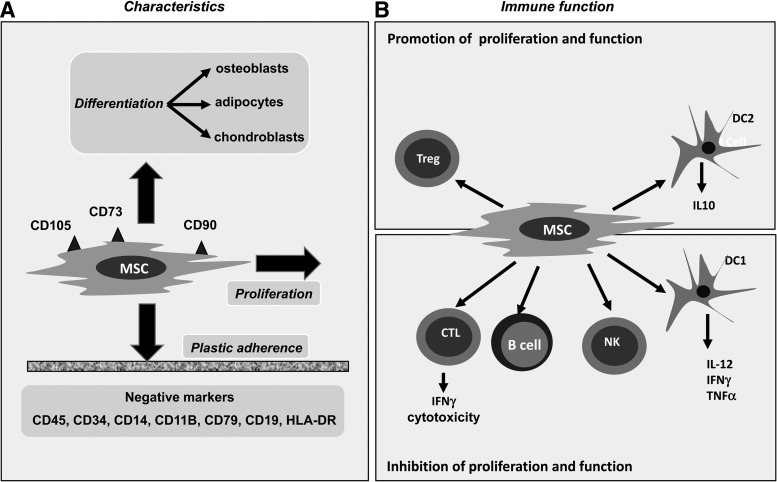
FIG. 1.
(A) Marrow stromal cells (MSCs) are characterized by surface expression of CD105, CD73, and CD90 while lacking CD45, CD34, CD14, CD11B, CD79, CD19, and human leucocyte antigen (HLA)-DR. They adhere to plastic and are capable of massive in vitro expansion in culture. Under appropriate conditions, they may differentiate into other cells of mesenchymal origin. (B) MSCs inhibit the activation and/or proliferation of conventional T cells, B cells, natural killer cells, and inhibit type 1 dendritic cells (DC1). On the other hand, they promote the activity and/or proliferation of DC2 cells and regulatory T cells (Tregs), and the net balance is to overwhelmingly suppress immune responses.
MSCs in Animal Models
Distribution
The fate of recipient and donor MSCs is of great interest after allogeneic HSCT. MSCs are part of the BM stromal microenvironment and low doses of donor MSCs are copassengers in the infusion of an allogeneic BM graft. Despite the potential for donor MSC to engraft, it is recipient MSCs that surprisingly survive the conditioning regimens and remain the predominant marrow MSC population after BM transplantation.7–9 MSC biodistribution has been extensively studied in animal models. Such studies have been helpful in showing that MSCs rapidly home to the lungs but can migrate to tissues injured by GVHD but with limited survival.10,11 Thus, although donor MSCs are coinfused in marrow grafts they do not replace recipient MSCs despite complete engraftment of a new hematopoietic system. Nevertheless, this lack of donor MSC engraftment may not obviate potential clinical utility of infused MSCs derived from ex vivo culture expanded donor marrow.
Graft-versus-host disease
Because of their immunosuppressive and tissue repair properties many investigators have sought to use MSCs to treat animal models of GVHD, identify their mechanism of action, and define dose and treatment schedules for clinical use (reviewed in Kebriaei and Robinson12). Using mouse MSCs in a MHC mismatched spleen cell transplant of H-2Kb female mice into H-2Kd male recipients, Polchert failed to demonstrate a therapeutic effect of MSCs in established GVHD but MSCs did confer protection when administered between 2 to 20 days after transplant.13 Murine MSCs differ in important respects from human MSCs and results can vary with the timing of MSC administration. To approximate more closely to the clinical situation, other investigators have therefore used human MSCs to treat murine GVHD or GVHD induced by human T cells xenografts in immune-deficient mice. Using placental-derived human MSCs Jang showed that doses of 106 MSCs inhibit secretion of interferon-γ (IFNγ), TNFα, and IL12 from alloactivated T cells and suppress murine GVHD.14 In a human xenograft GVHD model Gregoire-Gauthier et al. found that a single injection of cord-blood-derived MSCs reduced morbidity and mortality from GVHD. Importantly, they identified both an immunomodulating effect of MSCs on T cells and a repairative effect on irradiated tissues.15 Tisato and colleagues established a human xeno-GVHD in a NOD/SCID animal model transplanted with human mononuclear cells. Umbilical cord blood MSCs were effective prophylactically if given in repeated doses suggesting the need for repeated MSC treatments given before or early after GVHD onset.16 However, not all studies have yielded positive results with reports of failure of MSCs to prevent GVHD in pure murine17 and rat18 models. Also, in mouse/human xenograft GVHD experiments Bruck et al. reported that MSC doses as high as 3×106 cells/mouse failed to prevent GVHD.19
Properties of MSCs Relevant to HSCT
MSCs have several unique and exciting properties justifying their clinical exploitation in allogeneic HSCT. These include their capacity to differentiate into a variety of cell types, their ability to support and stimulate proliferation and survival of hematopoietic progenitor cells, their tropism for migration into sites of injury or inflammation after intravenous infusion, and their therapeutic tendency to promote recovery of damaged tissues through secretion of a variety of cytokines and chemokines. Properties of MSCs relevant to their use after HSCT are listed below:
(1) MSCs support the growth and differentiation of HSCs.3,20
(2) Infused MSCs home to sites of tissue injury in mice and non-human primates.21,22
(3) MSCs are immunomodulatory and anti-inflammatory, which is critical to the therapy of refractory GVHD.23–25
(4) MSCs are tolerogenic and avoid immune destruction. They do not induce lymphocyte proliferation in vitro, and escape being targeted by cytotoxic T cells or natural killer cells.26–29 Their ability to evade immune destruction makes it possible to use HLA-mismatched third party donor MSC infusions to treat patients after HSCT. MSCs do not induce proliferation or IFNγ production, or upregulation of activation markers on allogeneic lymphocytes.30 In a baboon skin-graft model, the infusion of ex vivo expanded MSCs prolonged the time to rejection of histoincompatible skin grafts. Suppression of skin graft rejection was effective whether MSCs came from the same donor as the stimulator lymphocytes, the same donor as the responder lymphocytes, or from an unrelated (third-party) donor.21 Further, MSCs also block established lymphocyte responses. Addition of MSCs 4 days after the initial mixing and stimulation of lymphocytes led to a suppression of proliferation comparable to that seen when MSCs were present from the onset of the mixed lymphocyte reaction culture.31 Suppression of T-cell responses requires cell–cell contact but is in part mediated by incompletely defined soluble factors and may also suppress T-cell reactivity by inducing T-regulatory cells.32 Immediately after infusion (hours to days), MSCs induce rapid reduction in inflammatory cytokines (Battiwalla and Barrett, unpublished data) and over durations exceeding 6 months are associated with increases in Tregs.33 Properties of immunological stealth enable MSCs to be transplanted over major histocompatibility complex barriers in humans.31,34 Thus, there is specific tolerance to MSC infusions, which allows MSCs from any source to be given to recipients without being rapidly rejected. HLA alloimmunization does not occur (Battiwalla and Barrett, unpublished data).
(5) MSCs promote tissue repair: Infused MSCs improve the outcome of acute renal, neural, and lung injury, possibly by promoting a shift from production of proinflammatory cytokines to anti-inflammatory cytokines at the site of injury. MSCs promote healing after radiation injury in experimental animals.35–37
(6) Human MSCs can be isolated from BM, cultured ex vivo, and expanded many fold.4 Culture-expanded MSCs represent a homogeneous population by flow-cytometric measures of cell-surface markers whose minimal criteria have been defined as positive for CD146, CD90, HLA class I, and negative for hematopoietic cell markers.38 Gene expression profiling shows that MSCs produce abundant extracellular matrix proteins that may contribute to their clinical immune modulatory and anti-inflammatory effects.39
Capitalizing on these unique characteristics, many HSCT studies have used “off-the-shelf” ex vivo culture expanded BM-derived-MSCs from HLA-mismatched “third party” donors, and to a more limited extent, MSCs derived from other tissues such as fat.
Early Passage MSCs Have Greater Potency
Ex vivo expansion exploits the massive proliferative potential of MSCs to generate large numbers of cells for therapeutic administration, with exponential cell yields generated by late passage. Risks of extensive passage including senescence and chromosomal abnormalities have been well described.40,41 Even with less extensive passage, biological evidence shows that the properties of MSCs may change.42,43 Clinical trial evidence also shows that immunosuppressive potency is reduced with late passage. In EU trials for GVHD, 1-year survival was 75% in patients who received early-passage MSCs (from passages 1–2) in contrast to 21% using later passage MSCs (from passages 3–4) (p<0.01).44 It is also speculated that the failure of the large phase III US trial with extensively passaged MSCs from a universal donor (Prochymal; Osiris Therapeutics, Inc., Columbia, MD) to meet its primary clinical endpoints could have been attributed to biological differences related to a single donor undergoing a relatively higher passage number (five passages).
Safety Aspects of MSCs in HSCT
The broad areas of concern about safety of MSCs in HSCT, the risk of tumorigenesis, ex vivo cell culture issues, and heterogeneity of the cell sources and conditions being treated have been extensively reviewed.45 Most troublesome is the risk of promoting malignancy demonstrated in animal models. Culture conditions (media and passage length), donor (autologous vs. allogeneic), and source (marrow, cord, placental, or adipose tissue) are additional variables that may potentially influence the safety profile.
A theoretical risk of promoting malignancy recurrence in allogeneic HSCT was reported by Ning et al. In a small randomized controlled trial of MSCs cotransplantation in HLA-identical sibling transplantation for hematologic malignancies, a higher relapse rate (60% vs. 20%) was seen in the patients who received MSCs. Although the incidence of GVHD was reduced, disease-free survival at 3 years was only 30% in those patients receiving MSCs versus 66.7% in those who did not receive MSCs.46 The conclusions have been criticized for paucity of information regarding relapse likelihood in the two groups and the variable nature of the graft source47 and increased relapse not been observed in the EU trials.44 Nevertheless, monitoring relapse rates will be critical in any future phase III trial with MSCs.
Results from Early Clinical Trials in GVHD
MSC infusions have shown great promise in the treatment of HSCT complications.48 First, shown to successfully abrogate steroid refractory acute GVHD, investigators subsequently demonstrated that responders to MSC infusions had improved overall survival.49,50 Favorable factors predictive for response include younger age and GVHD occurring in the gut and liver. Based on their capability to promote differentiation and tissue regeneration from damaged tissue progenitors, MSCs have also been used to treat hemorrhagic cystitis and pneumoperitoneum after HSCT.51,52
While there is a consensus that MSCs have some therapeutic effect in complications following HSCT, there are unanswered questions whether therapeutic responses of acute GVHD to MSCs are due to immunosuppression or due to the promotion of repair in tissues damaged by the alloimmune attack, the mechanisms underlying immune modulation and tissue repair, the optimum dose and schedule of infusions, and the impact of the method of manufacture on MSC function. MSCs are typically given in repeated infusions of about 2×106 cells per kilogram a week apart. Whether the dose and schedule is optimal and whether MSCs from different donors have distinct therapeutic characteristics are not known. The culture conditions and degree of expansion used for MSC treatments vary widely and may influence the clinical outcomes obtained. For example, the utilization of late passage MSCs in the industry led phase III clinical trial (NCT00366145) of a proprietary MSC product (Prochymal; Osiris Therapeutics, Inc.) for steroid–refractory acute GVHD is widely attributed to have been the cause for failure to meet primary endpoints but did show an improvement in secondary endpoints of complete response in liver GVHD and intestinal GVHD. Although peer-reviewed publication of the trial results are not in the public domain, an extensive failure analysis has been presented to compare the apparent discrepancy between the European experience versus Prochymal.53 Issues identified are donor heterogeneity (IFNγ responsiveness is not uniform among human subjects and that MSCs derived from low indoleamine 2,3-dioxygenase inducers may be substantially less potent than cells derived from high inducers), loss of potency with extensive passage (10,000 or more doses of Prochymal were generated from a single “universal” donor), different immunogenicity (reaction to fetal calf serum or development of alloimmunization to MSCs), and the loss of potency or longevity after freeze-thaw.
Challenges with Further Clinical Development as a Licensed Cellular Therapeutic
The exciting properties of MSCs have resulted in clinical trials long before some critical properties were known: longevity, fate, factors determining potency (including choice of donor, passage, and senescent cell content) and the precise mechanisms of action in GVHD. Exuberance in rushing to clinical trials without taking into consideration all these factors risks an impasse in further development.
There are several challenges to commercialization of MSCs including funding, intellectual property issues, lack of equivalence between different sources of MSCs, possible batch to batch variability, absence of a universally acceptable potency assay, and the lack of successful precedents in cellular therapy in terms of regulatory guidance. Funding from government sources, such as the National Institutes of Health (NIH), typically dries up beyond early development (phase I and II). The chasm between phase II trials and a definitive FDA registrational trial is termed the “Valley of Death” and, in the U.S. model, is dependant on Industry for further development. From the standpoint of a commercial sponsor, proceeding with a registrational trial depends on having clear intellectual property or licensing, a consistent method of manufacture starting from phase I and a method to address batch-to-batch variability. The failure of the Prochymal study is important because ambitious endpoints in the clinical trial design may have resulted in premature abandonment of an effective therapy, and potentially locked up intellectual property in a failed product.
NIH Approach to MSCs in Transplant Conditions
At the NIH Clinical Center, we developed a clinical grade MSC cell bank from third party donors.54 Our Institute has approached the development of MSCs by selecting the validated EU manufacturing approach with early passage (three passages) and a comprehensive approach to biological correlative studies. The infusion schedule of doses of 2×106 MSC/kg weekly followed previously used schedules effective for controlling GVHD. We chose to evaluate MSC infusions in patients with post-transplant complications after HSCT (including steroid-refractory GVHD) that are associated with a high mortality. We conducted a phase I trial using third party, early passage, MSCs for patients with steroid-refractory liver or gastrointestinal GVHD, tissue injury or marrow failure following HSCT to investigate safety and clinical responses following MSC infusion. The study schema is depicted in Figure 2. MSCs were prepared from marrow aspirates from healthy volunteers with the expansion of 3 passages. Subjects, all allogeneic stem cell transplant recipients, provided written informed consent for the phase I study (clinicaltrials.gov identifier No. NCT01633229) approved by the Institutional Review Board (IRB) at the National Heart, Lung and Blood Institute, NIH (protocol No. 12-H-0010). Ten subjects were infused a fixed dose of 2×106 MSCs/kg intravenously weekly for three doses. There was no treatment related toxicity (primary endpoint). In addition to clinical endpoints of demonstration of safety and exploration of efficacy, the major objective was to understand the biology of MSCs in post-transplant complications. The MSCs infused were fully characterized in terms of viability, freeze-thaw characteristics, and gene expression profiles with the goal of identifying markers for potency. We examined the impact of MSCs in terms of their immunogenicity by HLA-alloimmunization, their homing, and fate (by chimerism analysis). Advances in new diagnostic tools using GVHD-relevant biomarkers and markers of tissue injury provided the opportunity to more clearly define responses to MSCs.55 To identify mechanisms of MSC immunomodulation and tissue repair, patients were monitored for validated plasma GVHD biomarkers, cytokines, growth factors, and lymphocyte phenotype before and after MSC infusion.
FIG. 2.
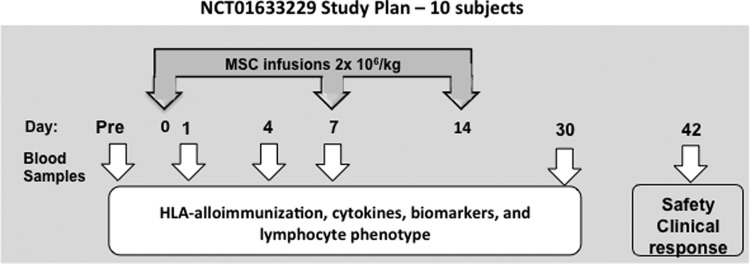
Study schema of NCT 01633229, a phase I trial of bone MSCs for transplant complications.
NIH Phase I Clinical Trial Results
Eight subjects were evaluable for response assessment at 4 weeks after the last infusion. Five of the seven patients with steroid-refractory acute GVHD achieved complete remission, two of two patients with tissue injury (pneumomediastinum/pneumothorax) achieved resolution but there was no response in two subjects with delayed marrow failure. Rapid reductions in inflammatory cytokines occurred after the first MSC infusion. Clinical responses correlated with a fall in biomarkers (Reg 3α, CK18, and Elafin) relevant for the site of GVHD, or CK18 for tissue injury. The GVHD complete responders survived significantly longer (>300 days vs. a median of 33 days), had higher baseline absolute lymphocyte and central memory CD4 and CD8 counts but there was no clear difference in natural or induced Tregs. Cytokine changes also segregated with survival.
In summary our results confirm that MSCs induce rapid clinical responses and biomarker normalization in patients with steroid-refractory GVHD and tissue injury. MSCs appeared ineffective in patients with more aggressive GVHD with lower lymphocyte counts. A relatively intact immune system with higher absolute lymphocyte counts and favorable cytokine and T-cell phenotype patterns may be required for effective GVHD control by MSCs. Early detection and MSC treatment appear important in patients with refractory GVHD. Our study is limited by its small sample size; both the clinical findings and biomarker changes need to be confirmed in larger sample studies. These results are submitted for publication.
Conclusions
Because of their immunomodulatory and reparative properties, MSCs show great promise to improve the outcome of HSCT by preventing or treating a variety of common post-transplant complications. Our interest in the application of MSCs to treat HSCT stems from a number of encouraging clinical observations published from centers in Europe and the United States. However, a sound mechanistic understanding of MSC therapeutic effects has been lacking. For this reason we focused in our clinical trial on evaluating biological markers alongside clinical outcomes. The strong temporal relationship between falls in cytokines and markers of tissue damage support a therapeutic role for MSCs notably in treating GVHD. However, much work remains to be done before it is clear how MSCs are distributed in the recipient, and whether their therapeutic effect is mediated through tissue repair immunosuppression or both. Future studies plan to expand on these preliminary observations.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
Disclosure Statement
The authors have no conflicts of interest.
References
- 1.Westin J.R., Saliba R.M., De Lima M., Alousi A., Hosing C., Qazilbash M.H., et al. Steroid-refractory acute GVHD: predictors and outcomes. Adv Hematol 2011,601953, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., and Frolova G.P.Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6,230, 1968 [PubMed] [Google Scholar]
- 3.Bensidhoum M., Chapel A., Francois S., Demarquay C., Mazurier C., Fouillard L., et al. Homing of in vitro expanded Stro-1- or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood 103,3313, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Wu J.Y., Scadden D.T., and Kronenberg H.M.Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res 24,759, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan A.I.Mesenchymal stem cells. J Orthop Res 9,641, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Koc O.N., Peters C., Aubourg P., Raghavan S., Dyhouse S., DeGasperi R., et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol 27,1675, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Rieger K., Marinets O., Fietz T., Korper S., Sommer D., Mucke C., et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol 33,605, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Awaya N., Rupert K., Bryant E., and Torok-Storb B.Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol 30,937, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Joo S.Y., Cho K.A., Jung Y.J., Kim H.S., Park S.Y., Choi Y.B., et al. Bioimaging for the monitoring of the in vivo distribution of infused mesenchymal stem cells in a mouse model of the graft-versus-host reaction. Cell Biol Int 35,417, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Gao J., Dennis J.E., Muzic R.F., Lundberg M., and Caplan A.I.The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169,12, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Kebriaei P., and Robinson S.Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy 13,262, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Polchert D., Sobinsky J., Douglas G., Kidd M., Moadsiri A., Reina E., et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol 38,1745, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang M.J., Kim H.S., Lee H.G., Kim G.J., Jeon H.G., Shin H.S., et al. Placenta-derived mesenchymal stem cells have an immunomodulatory effect that can control acute graft-versus-host disease in mice. Acta Haematol 129,197, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Gregoire-Gauthier J., Selleri S., Fontaine F., Dieng M.M., Patey N., Despars G., et al. Therapeutic efficacy of cord blood-derived mesenchymal stromal cells for the prevention of acute graft-versus-host disease in a xenogenic mouse model. Stem Cells Dev 21,1616, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Tisato V., Naresh K., Girdlestone J., Navarrete C., and Dazzi F.Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia 21,1992, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Sudres M., Norol F., Trenado A., Gregoire S., Charlotte F., Levacher B., et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol 176,7761, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Zinocker S., Wang M.Y., Rolstad B., and Vaage J.T.Mesenchymal stromal cells fail to alleviate experimental graft-versus-host disease in rats transplanted with major histocompatibility complex-mismatched bone marrow. Scand J Immunol 76,464, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Bruck F., Belle L., Lechanteur C., de Leval L., Hannon M., Dubois S., et al. Impact of bone marrow-derived mesenchymal stromal cells on experimental xenogeneic graft-versus-host disease. Cytotherapy 15,267, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Noort W.A., Kruisselbrink A.B., in't Anker P.S., Kruger M., van Bezooijen R.L., de Paus R.A., et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol 30,870, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Devine S.M., Bartholomew A.M., Mahmud N., Nelson M., Patil S., Hardy W., et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol 29,244, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Mouiseddine M., Francois S., Semont A., Sache A., Allenet B., Mathieu N., et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol 80, Spec No 1:S49, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Uccelli A., Pistoia V., and Moretta L.Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 28,219, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K., and Ringden O.Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 11,321, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Prockop D.J., and Olson S.D.Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood 109,3147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tse W.T., Pendleton J.D., Beyer W.M., Egalka M.C., and Guinan E.C.Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75,389, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., and Ringden O.HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31,890, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Klyushnenkova E., Mosca J.D., Zernetkina V., Majumdar M.K., Beggs K.J., Simonetti D.W., et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 12,47, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Rasmusson I., Ringden O., Sundberg B., and Le Blanc K.Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76,1208, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nauta A.J., and Fibbe W.E.Immunomodulatory properties of mesenchymal stromal cells. Blood 110,3499, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Sundin M., Barrett A.J., Ringden O., Uzunel M., Lonnies H., Dackland A.L., et al. HSCT recipients have specific tolerance to MSC but not to the MSC donor. J Immunother 32,755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel G., Schafer R., and Dazzi F.The immunosuppressive properties of mesenchymal stem cells. Transplantation 87,S45, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Jitschin R., Mougiakakos D., Von Bahr L., Volkl S., Moll G., Ringden O., et al. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem-cell transplantation. Stem Cells 31,1715, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Le Blanc K., Gotherstrom C., Ringden O., Hassan M., McMahon R., Horwitz E., et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation 79,1607, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Keating A.Mesenchymal stromal cells. Curr Opin Hematol 13,419, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herdrich B.J., Lind R.C., and Liechty K.W.Multipotent adult progenitor cells: their role in wound healing and the treatment of dermal wounds. Cytotherapy 10,543, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Semont A., Francois S., Mouiseddine M., Francois A., Sache A., Frick J., et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol 585,19, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8,315, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Ren J., Jin P., Sabatino M., Balakumaran A., Feng J., Kuznetsov S.A., et al. Global transcriptome analysis of human bone marrow stromal cells (BMSC) reveals proliferative, mobile and interactive cells that produce abundant extracellular matrix proteins, some of which may affect BMSC potency. Cytotherapy 13,661, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izadpanah R., Kaushal D., Kriedt C., Tsien F., Patel B., Dufour J., et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res 68,4229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolar J., Nauta A.J., Osborn M.J., Panoskaltsis Mortari A., McElmurry R.T., Bell S., et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 25,371, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Larson B.L., Ylostalo J., Lee R.H., Gregory C., and Prockop D.J.Sox11 is expressed in early progenitor human multipotent stromal cells and decreases with extensive expansion of the cells. Tissue Eng Part A 16,3385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larson B.L., Ylostalo J., and Prockop D.J.Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells 26,193, 2008 [DOI] [PubMed] [Google Scholar]
- 44.von Bahr L., Sundberg B., Lonnies L., Sander B., Karbach H., Hagglund H., et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant 18,557, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Battiwalla M., and Barrett A.J.Safety Issues in MSC Therapy. In: Hematti P., Keating A., eds. Mesenchymal Stromal Cell. New York: Springer, 2013, pp. 377–387 [Google Scholar]
- 46.Ning H., Yang F., Jiang M., Hu L., Feng K., Zhang J., et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 22,593, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Behre G., Theurich S., Weber T., and Christopeit M.Reply to ‘The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rates in hematologic malignancy patients: outcome of a pilot clinical study’ by Ning et al. Leukemia 23,178; author reply 9, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Battiwalla M., and Hematti P.Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy 11,503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371,1579, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Le Blanc K., Rasmusson I., Sundberg B., Gotherstrom C., Hassan M., Uzunel M., et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363,1439, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Prasad V.K., Lucas K.G., Kleiner G.I., Talano J.A., Jacobsohn D., Broadwater G., et al. Efficacy and safety of ex-vivo cultured adult human mesenchymal stem cells (Prochymal(TM)) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 17,534, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Ringden O., Uzunel M., Sundberg B., Lonnies L., Nava S., Gustafsson J., et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia 21,2271, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Galipeau J.The mesenchymal stromal cells dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15,2, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Sabatino M., Ren J., David-Ocampo V., England L., McGann M., Tran M., et al. The establishment of a bank of stored clinical bone marrow stromal cell products. J Transl Med 10,23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paczesny S.Discovery and validation of graft-versus-host disease biomarkers. Blood 121,585, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]