Abstract
The functional reprogramming of a differentiated cell to a pluripotent state presents potential beneficial applications in disease mechanisms and regenerative medicine. Epigenetic modifications enable differentiated cells to perpetuate molecular memory to retain their identity. Therefore, the aim of this study was to investigate the reprogramming modification of yak fibroblast cells that were permeabilized and incubated in the extracts of mesenchymal stem cells derived from mice adipose tissue [adipose-derived stem cells (ADSCs)]. According to the results, the treatment of ADSC extracts promoted colony formation. Moreover, pluripotent gene expression was associated with the loss of repressive histone modifications and increased global demethylation. The genes Col1a1 and Col1a2, which are typically found in differentiated cells only, demonstrated decreased expression and increased methylation in the 5′-flanking regulatory regions. Moreover, yak fibroblast cells that were exposed to ADSC extracts resulted in significantly different eight-cell and blastocyst formation rates of cloned embryos compared with their untreated counterparts. This investigation provides the first evidence that nuclear reprogramming of yak fibroblast cells is modified after the ADSC extract treatment. This research also presents a methodology for studying the dedifferentiation of somatic cells that can potentially lead to an efficient way of reprogramming somatic cells toward a pluripotent state without genetic alteration.
Introduction
Successful reprogramming of differentiated somatic cells toward pluripotency is a promising approach for studying disease mechanisms and regenerative medicine. Several systems have accomplished dedifferentiation through cell fusion, overexpression of transcription factors, and nuclear transfer to oocytes (Cowan et al., 2005; Takahashi and Yamanaka, 2006; Wilmut et al., 1997). Each method facilitates the reacquisition of pluripotency in differentiated somatic cell nuclei. The fusion of a somatic cell with an embryonic stem cell (ESC) elicits a reprogramming of the somatic genome, which acquires ESC properties, including contribution to all germ layers in teratomas and aggregation chimeras (Cowan et al., 2005). Recently, reports have shown that ectopic expression of a defined set of transcription factors (Oct-4, Sox-2, Klf-4, Nanog, and c-Myc) can directly produce an induced pluripotent stem cell (iPSC) (Takahashi et al. 2007; Yu et al. 2007). Somatic cell nuclear transfer (SCNT) technology is another powerful strategy that is performed by transferring the somatic cell nucleus into an enucleated oocyte. In this process, the somatic cell nucleus is transformed into an undifferentiated zygote with the potential to develop into a newborn animal. Nuclear transplantation into oocytes has demonstrated that functional nuclear reprogramming is possible through the production of nuclear transfer ESCs.
Many species have been cloned successfully, but the relatively inefficient nature of SCNT is often accompanied with numerous abnormalities in clones, which has overshadowed its benefits and limited its application (Farin et al., 2006; Yang et al., 2007). The incomplete reprogramming or reprogramming errors of donor nucleus have been widely suggested as the major reason for the inefficiency of nuclear transfer. Various strategies have been employed to improve the success rate of SCNT. The use of DNA methyltransferase and histone deacetylate inhibitors, such as trichostatin A, Scriptaid, and valproic acid, to treat the donor nuclei or cloned embryo can significantly improve the efficiency of SCNT (Gómez et al., 2011; Lee et al., 2010; Sangalli et al., 2012; Wang et al., 2012; Xiong et al., 2013a). In addition, pretreatment of the somatic cell with cell-free extract derived from differentiated or undifferentiated cells results in removal of epigenetic memory in the donor nuclei, inducing the expression of pluripotency genes, downregulating the somatic cell marker genes, and facilitating the establishment of new epigenetic status after SCNT (Miyamoto et al., 2008; Taranger et al., 2005).
Notably, the oocyte extracts of Xenopus and mammals have been used for reprogramming somatic cells in previous studies. These extracts have a significant positive effect on nuclear reprogramming and cloned embryo development (Miyamoto et al., 2008; Xiong et al., 2013b). Epigenetic reprogramming, such as DNA demethylation and histone acetylation, is also modified after treatment with oocyte extract (Liu et al., 2013). The extract derived from cells that have biological functions is safer and less toxic than chemical agents.
However, oocyte sources are extremely limited, and this oocyte lacks the ability to proliferate in vivo or in vitro. Thus, pluripotent cell lines might be a good choice for preparing the extract for the analysis of molecular mechanisms associated with differentiation and nuclear reprogramming. Extracts derived from teratocarcinoma cells can dedifferentiate NIH/3T3 cells and modify the expression of the transcription factors associated with totipotency (Zhang et al., 2012). The cell fate of 293T cells has been altered after exposure to T cell extract (Håkelien et al., 2004). These observations demonstrate that exposure of a differentiated cell to factors derived from pluripotent or undifferentiated cells is sufficient to elicit partial or complete reprogramming of its nuclear function. Considering that the genome of mesenchymal stem cells derived from mice adipose tissue [adipose-derived stem cells (ADSCs)] is inherently less differentiated than other somatic cells, ADSCs have an equal potential to be differentiated into cells and tissues of mesodermal origin and potentially influence regenerative cell therapy for ischemic diseases (Schäffler and Büchler, 2007). Exposure to ADSC extracts may result in a low or undifferentiated state. Extract treatment reprogramming using ADSCs can be a promising and plausible approach toward the production of replacement cells for therapeutic purposes and the production of suitable donor cells for SCNT.
Therefore, to test the hypothesis that extracts of ADSCs can elicit dedifferentiation in somatic cells and facilitate nuclear reprogramming, yak fibroblast cells were treated with ADSC extracts to determine the effects on DNA methylation and histone acetylation status, to detect if treatment of fibroblasts would up-regulate the expression of pluripotency factor genes, and to investigate if treatment of donor nucleus with extract before nuclear transfer would improve subsequent development of cloned embryos.
Materials and Methods
All chemicals used in this study were purchased from Sigma (St. Louis, MO) unless otherwise specified. Disposable, sterile plastic wares were purchased from Nunclon (Roskilde, Denmark). All procedures in this experiment were approved by the Animal Care and Use Committee of Southwest University for Nationalities, and performed in accordance with animal welfare and ethics.
Cell culture
Yak fibroblast cell cultures were derived from the ear skin of a 6-month-old female yak, as described previously (Xiong et al., 2013b). Passages two to five were used for treatment with extract and SCNT. ADSCs were cultured in RPMI-1640 medium (Hyclone) supplemented with 10% fetal bovine serum (FBS; Gibco), 1 mM sodium pyruvate, 1 μg/mL epidermal growth factor (EGF), 2 mM l-glutamine, 100 IU/mL penicillin, and 100 IU/mL streptomycin under 5% CO2 in air at 38.5°C.
Preparation of ADSC extract
The ADSC extract was prepared as described in a previous study with minor modifications (Zhang et al., 2010). Briefly, ADSCs were washed twice in ice-cold phosphate-buffered saline (PBS) by suspension and sedimentation at 700×g for 10 min at 4°C. The extract was then resuspended in 1 mL of ice-cold cell lysis buffer containing 1 mM adenosine triphosphate, 10 mM phosphocreatine, and 25 μg/mL creatine kinase at pH 7.4 [energy regeneration system (ERS)] and centrifuged twice at 700×g for 10 min at 4°C. Approximately 2×108 ADSCs were added to 100 μL of ERS supplemented with protease inhibitor cocktail in a 0.5-mL Eppendorf tube and held on ice for 45 min. The cells were sonicated on ice at 30% amplitude, 0.4-sec pulses over 2 min until all cells and nuclei were lysed (determined by microscopy). The lysate was then centrifuged at 15,000×g for 30 min at 4°C. The supernatant was used as extract and stored at −80°C. The procedure was repeated to acquire a sufficient amount of extract.
Permeabilization and extract treatment
The yak fibroblast cells used as donor cells were permeabilized based on our previous report (Xiong et al., 2013b). The cells were washed in Ca2+- and Mg2+-free PBS, and approximately 5×106 cells were permeabilized with 200 ng/mL streptolysin O (SLO) for 20 min on ice. Permeabilization was terminated by adding an excess of PBS and centrifuging at 700×g for 5 min. The permeabilized cells were resuspended in 1.5 mL of the culture medium [Dulbecco's modified Eagle medium (DMEM) containing 10% FBS], 1 mM sodium pyruvate, 1 μg/mL EGF, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 IU/mL streptomycin, and then divided into two equal portions in a 35-mm Petri dish. The permeabilized cells were added into 0 (control group, added 100 μL of ERS) and 100 μL (treated group) of the ADSC extracts, and incubated at 38.5°C for 24 h prior to further experimentation. As a negative control, the permeabilized cells were resealed with 100 μL of DMEM instead of ERS and extract.
Immunofluorescence for histone H3K9 and DNA methylation
The cells were washed briefly in PBS, fixed with 4% paraformaldehyde in PBS for 30 min, permeabilized with 0.2% Triton X-100 in PBS for 30 min at room temperature, and then incubated overnight at 4°C with a primary antibody diluted in PBS, anti-histone H3 lysine 9 acetylation (H3K9ac) rabbit polyclonal antibody, and anti-5-methyl cytidine (5MeC) mouse monoclonal antibody (Abcam, Cambridge, MA). The cells were then washed three times in PBS for 5 min and incubated with a 1:200 dilution of fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G (IgG) or anti-mouse IgG for 2 h. The cells were washed three times for 5 min in PBS and incubated for 7 min in 10 μg/mL propidium iodide. For the negative control, immunostaining was performed without the primary antibodies. Fluorescence was detected using a laser-scanning confocal microscope.
RNA isolation, cDNA synthesis, and qRT-PCR
Approximately 72 h after extract exposure, the cells were collected and processed for RNA extraction using TRIzol (Invitrogen) in accordance with the instruction manual with minor modifications. Complementary DNA (cDNA) synthesis was performed using a cDNA synthesis kit (Takara, China) according to the manufacturer's guidelines. The primers for all genes were designed as cross-introns by Primer 5.0 software (Premier Biosoft International, Palo Alto, CA, USA), and were based on bovine and yak RNA sequences in the GenBank National Center for Biotechnology Information (NCBI) database (Table 1). qRT-PCR was performed using the CFX96 detection system (Bio-Rad, USA) with SYBR Premix ExTaq™II (TaKaRa, China). Melting curve analysis was performed to check for primer specificity. Amplification efficiency for each cDNA and growth condition was determined as described in a previous study (Ruijter et al., 2009). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was initially used as housekeeping reference gene. Thus, the relative quantification method (2−ΔΔCt) was used to calculate the gene expression level of each target gene relative to Gapdh for each sample and to determine eventually the relative amount of the target mRNA. For ease of comparison, the average expression level of each gene from the control group was set at 1.
Table 1.
Primer Sequences and PCR Conditions Used for qRT-PCR
| Gene | Primer sequences (5′-3′) | Accession no. | Tm | Product size (bp) |
|---|---|---|---|---|
| Oct-4 | F: GTGGAGGGATGGCCTACTGT | NM_174580 | 59 | 259 |
| R: TTCTGCTTTAGGAGCTTGGCA | ||||
| Sox-2 | F: TTCTTCGCCTGATTTTCCTC | NM_001105463 | 60 | 275 |
| R: GGGCTGTTCTTCTGGTTGCC | ||||
| c-Myc | F: TACAACATCCGAGCGACACC | NM_001046074 | 58 | 229 |
| R: TGCACCGAATCGTAGTCGAG | ||||
| Klf-4 | F: GGTCCCACCGCTCCATTAC | NM_001105385 | 60 | 247 |
| R: ATCTGAGCGGGCAAACTTCC | ||||
| Col1a1 | F: CCAGCCGCAAAGAGTCTACA | NM_00103403 | 59 | 296 |
| R: GGGACTTTGGCGTTAGGACA | ||||
| Col1a2 | F: CCACTGGAGAAATCGGACCC | NM_174520 | 61 | 280 |
| R: CTGCCGTCAATACCAGGGAG | ||||
| Gapdh | F: CCTGCCCGTTCGACAGATAG | NM_001034034 | 59 | 249 |
| R: CCGTTCTCTGCCTTGACTGT |
F, forward primer; R, reverse primer; Tm, annealing temperature.
Bisulfite sequencing analysis
DNA extraction and bisulfite sequencing of mock-treated and fibroblast cells were performed as described in our previous study (Xiong et al., 2013b). Genomic DNA was isolated using a DNeasy kit (Qiagen). Bisulfite genomic sequencing was performed using an EZ DNA Methylation-Direct Kit (Zymo Research, Irvine, CA, USA) in accordance with the instruction manual. Briefly, bisulfite-modified DNA was amplified using the primers designed according to the online MethPrimer software (www.urogene.org/methprimer/). Purified PCR products were subcloned into the pMD19-T vector (TaKaRa, China). Three independent amplification experiments were performed for each sample. Three to four clones from each independent set of amplification and cloning were sequenced, in which a minimum of nine clones were selected for DNA sequencing (BGI, China). Bisulfite sequencing data and C-T conversion rates were analyzed by BIQ analyzer software (Bock et al., 2005).
Interspecies somatic cell nuclear transfer
The cells from the extract treatment groups were used as donor nuclei. Ovary collection, oocyte maturation in vitro, and interspecies SCNT (iSCNT) were performed as described in our previous study (Xiong et al., 2013b). Briefly, bovine ovaries were collected from a local abattoir and transported to the laboratory at 25°C within 2 h. Cumulus oocyte complexes were matured in oocyte maturation medium at 38.5°C in 5.5% CO2 for 24 h. Metaphase II (MII) oocytes were enucleated with an approximately 20-μm (internal diameter) glass pipette by aspirating the first polar body and a small amount of the surrounding cytoplasm. The expelled cytoplasm was stained with 10 μg/mL of Hoechst 33342 to confirm the removal of the nuclear material. A single donor cell was placed in the perivitelline space of the enucleated bovine oocyte. Fusion was induced by applying two 35 V electrical pulses for 10 μsec.
The successfully reconstructed embryos were activated in 5 mM ionomycin for 5 min followed by 4 h of exposure to 2 mM 6-dimethylaminopurine in modified synthetic oviduct fluid (mSOF). Activated embryos were cultured in mSOF and then transferred into the new medium droplets on day 3 of the culture under 5.5% CO2 atmosphere at 38.5°C.
Statistical analysis
The experiment was repeated at least three times for each treatment group. The total fluorescence intensity was measured by Image-Pro Plus 6 software (Media Cybernetics, Silver Spring, MD, USA) after background subtraction. All results were analyzed via one-way analysis of variance (ANOVA) and Tukey's least significant difference (LSD) test using SPSS 13 software (SPSS Inc., IL, USA). Data were presented as mean±standard error of the mean (SEM) unless indicated otherwise, and the differences were considered significant at p<0.05.
Results
Treatment of yak fibroblast cells with extract promoted colony formation
Yak fibroblast cells were permeabilized with SLO and exposed to the extract of ADSCs. For the control, permeabilized fibroblasts were treated with DMEM instead of the extract. The first result of the extract exposure was a modification in the morphology of fibroblast cells. The somatic cells began to form around cell aggregates (Fig. 1) within 3 days after the treatment with ADSC extracts. The phenotype was not a mere consequence of the treatment with any extract, because yak fibroblast cells incubated in their own extract did not form any colonies (Fig. 1).
FIG. 1.
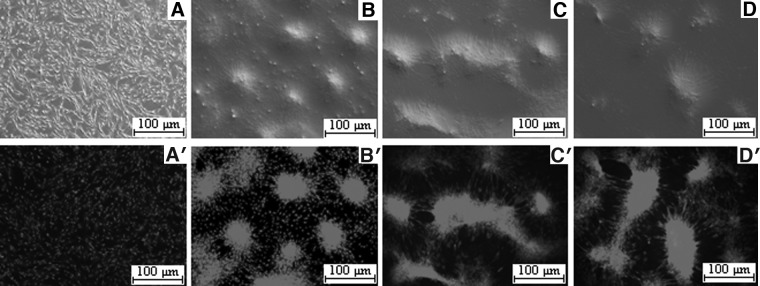
Morphological observation of yak fibroblasts after with or without ADSC extract incubated. (A, A′) Yak fibroblasts without ADSC extract incubated (control). (B, B′) Yak fibroblasts 2 days after exposure to ADSC extract. (C, C′) Yak fibroblasts 3 days after exposure to ADSC extract. (D, D′) Yak fibroblasts 5 days after exposure to ADSC extract.
Extract treatment promoted dynamic reprogramming of histone acetylation and DNA methylation
To determine whether or not the treatment of yak fibroblasts with ADSC extracts modified histone acetylation and global DNA methylation, antibodies to H3K9ac and 5MeC were used. The intensity of H3K9ac staining in the cells was significantly increased after the treatment with ADSC extracts (p<0.05) (Fig. 2). However, the intensity of 5MeC staining in the cells was significantly decreased after the treatment. In summary, the change in epigenetic modification detected on H3K9ac and DNA methylation after extract treatment are indicative of a remodeling of chromatin on yak fibroblasts to establish a new epigenetic state.
FIG. 2.
Quantitative analysis of the histone acetylation of H3K9 (A, B) and methylation status of 5MeC (C, D) in control and extract-treated cells using immunostaining. (A, a) H3K9ac in yak fibroblasts without ADSC extract incubated (control). (B, b) H3K9ac in yak fibroblasts with ADSC extract incubated. (C, c) 5MeC in yak fibroblasts without ADSC extract incubated (control). (D, d) 5MeC in yak fibroblasts with ADSC extract incubated. The histogram represents average optical intensity and an asterisk (*) indicates significant differences (p<0.05).
Extract treatment upregulated pluripotent-associated genes
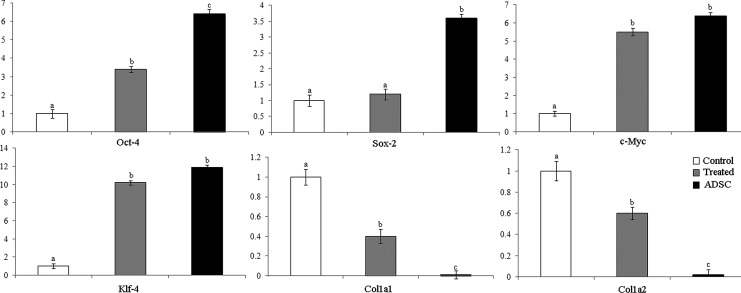
After yak fibroblasts were incubated in ADSC extracts for 3 days, the quantitative expression profiles of Oct-4, Sox-2, c-Myc, and Klf-4 were analyzed by qRT-PCR, as shown in Figure 3. No significant difference was observed in c-Myc and Klf-4 expression between the ADSCs and yak fibroblast cells exposed to ADSC extracts. Interestingly, a significant difference in Oct-4 expression between the untreated yak fibroblast cells and yak fibroblast cells exposed to ADSC extracts was observed, but it was still lower than that in ADSCs. In addition, no significant change in Sox-2 after yak fibroblast cells exposed to ADSCs was observed. However, the expression levels of fibroblast marker genes (Col1a1 and Col1a2) decreased more noticeably after the yak fibroblast cells were treated with ADSC extracts compared with the expression levels in the control samples.
FIG. 3.
Relative expression of pluipotent associated genes (Oct-4, Sox-2, c-Myc, and Klf-4) and fibroblast marker genes (Col1a1 and Col1a2) in ADSCs and yak fibroblast cells treated with extracts or untreated groups. Transcript levels in yak fibroblast cells untreated were used as the calibrator (relative expression=1.0). Values with different superscripts (a, b, and c) are significantly different (p<0.05).
Extract treatment decreased DNA methylation levels in the 5′-flanking regulatory regions
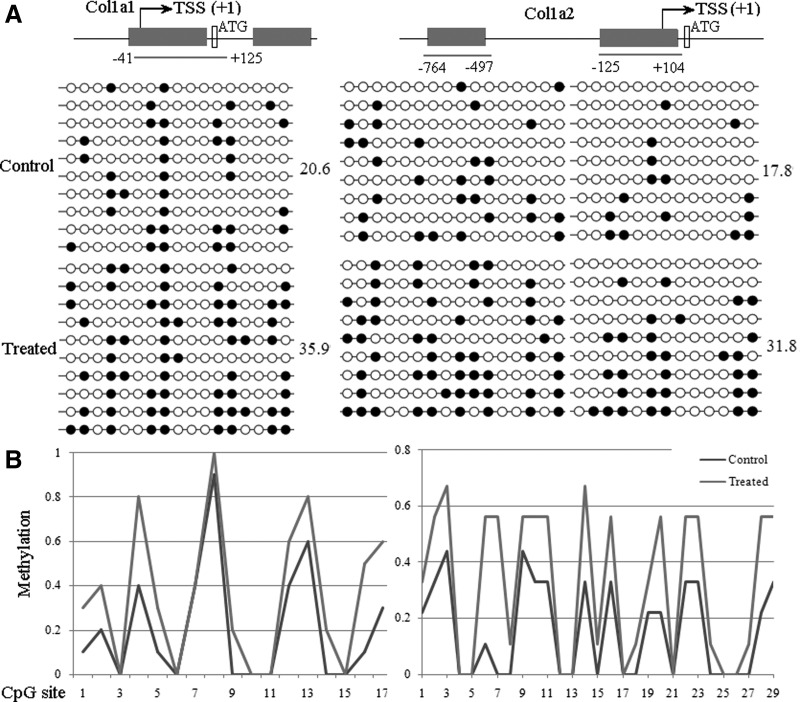
To determine the capability of ADSC extracts to elicit epigenetic modifications in yak fibroblast cells, the cytosine guanine (CpG) dinucleotides in the 5′-flanking regulatory regions of the fibroblast marker genes (Col1a1 and Col1a2) were examined. About nine to ten amplicons were analyzed, which collectively covered 17 potentially methylated CpG dinucleotides within nucleotides −41 to +251 relative to the transcription start site (TSS) (+1) of Col1a1. These amplicons also covered 29 potentially methylated CpG dinucleotides within nucleotides −764 to −497 and −125 to +104 relative to the TSS (+1) of Col1a2. Results of bisulfite sequencing analysis are shown in Figure 4, which shows that Col1a1 and Col1a2 were highly methylated in yak fibroblast cells after the extract treatment, but were largely unmethylated in their untreated counterparts. These results suggest that fibroblast marker gene 5′-flanking regulatory regions exhibited partial methylation induced by ADSC extracts compared with the control group.
FIG. 4.
Exposure of yak fibroblast cells to ADSC extracts elicits DNA demethylation of Col1a1 and Col1a2. (A) Bisulfite sequencing analysis of Col1a1 and Col1a2 methylation in control and treated groups. Global percentages of methylated cytosines (%Me) are shown. Each row of circles for a given amplicon represents the methylation status of each CpG in one bacterial clone for that region. (B) Percentages of methylated cytosines in each position in Col1a1 and Col1a2 determined from data shown in A. On the x axes, CpG No. 1 is the 5′ cytosine examined in each region. Positions of genomic regions examined are shown.
Extract treatment improved in vitro developmental competence of cloned embryos
A total of 503 reconstructed embryos were produced from five replicates and cultured in mSOF for 7 days. Results are shown in Table 2. No significant difference was observed in the percentage of cleaved embryos between the extract-treated group and control group (P>0.05). However, the percentage of cloned embryos that developed to the eight-cell and blastocyst stages in the extract-treated group increased compared with the untreated control (P<0.05). Furthermore, the total cell number in day-7 blastocyst in the extract-treated group was higher than its counterparts (P<0.05).
Table 2.
Developmental Competence of Yak iSCNT Embryos Produced with Extracts Pretreatment and Nontreated Donor Cells
| Cloned embryo development (mean %±SEM) | |||||
|---|---|---|---|---|---|
| Group | Embryos cultured | Cleaved | Eight-cell | Blastocysts | Total nuclei |
| Treated | 263 | 205 (77.9±1.1) | 105 (51.2±1.7) a | 44 (21.5±1.9) a | 82.6±2.8 a |
| Control | 240 | 188 (78.3±0.8) | 76 (40.4±1.5) b | 23 (12.2±2.1) b | 71.5±3.6 b |
a and b within a group indicate without a common superscript differed (p<0.05).
iSCNT, interspecies somatic cell nuclear transfer; SEM, standard error of the mean.
Discussion
Numerous studies have demonstrated that pluripotency can be restored in terminally differentiated cells, which proves that the epigenetic state of somatic cells is not irreversibly fixed (Boland et al., 2009; Jaenisch and Young, 2008). In the present study, we provide evidence on the changes during the reprogramming of yak fibroblasts exposed to ADSC extracts and establish a new approach to facilitate nuclear reprogramming. Considering that endogenous expression of Oct-4, Sox-2, c-Myc, and Klf-4 contribute toward reprogramming efficiency, we endeavored to increase their expression level in yak fibroblast cells through exposure to nuclear and cytoplasmic extracts of ADSC. Extract-based reprogramming approaches have shown that differentiated cells may be induced to transdifferentiate into other differentiated cell types or dedifferentiate toward pluripotency (Bru et al., 2008; Taranger et al., 2005). On the basis of our knowledge, this research is the first report of yak fibroblasts treated with ADSC extracts.
Epigenetic factors are barriers to nuclear reprogramming, such as histone modification and DNA methylation (Pasque et al., 2011). Histone tails are subjected to numerous posttranslational modifications that are important for the regulation of chromatin structure and gene expression (Bannister and Kouzarides, 2011). Moreover, histone deacetylation commonly accompanies gene repression in differentiated cells. Another hindrance to nuclear reprogramming is DNA methylation. Demethylation of repressed genes is required for gene reactivation during reprogramming, and failure of this mechanism has been correlated with inefficient SCNT (Bhutani et al., 2010; Mikkelsen et al., 2008). However, many useful methods have been used to improve the epigenetic pattern of differentiated somatic cells. Rathbone et al. (2010) showed that the global methylation pattern of ovine somatic cells is significantly decreased after pretreatment with Xenopus laevis oocyte extract, which then promotes nuclear reprogramming. Teratoma cellular extract also induces DNA demethylation of NIH/3T3 fibroblasts (Zhang et al., 2012). Moreover, acetylation of H3K9 in yak fibroblasts increased significantly when treated with ADSC extracts, and the global DNA methylation level apparently decreased. The combination of these findings indicates that ADSC extracts can provide the necessary regulatory components required to induce somatic cell nuclear reprogramming and modify the epigenetic status of fibroblast cells.
In addition, the patterns of DNA methylation and histone acetylation affect gene expression after extract treatment. Oct-4, Sox-2, c-Myc, and Klf-4 are associated with pluripotent cells and are present in yak fibroblast cells and ADSCs. Previous studies have reported that expression of Oct-4, Sox-2, c-Myc, and Klf-4 in differentiated cells is upregulated after treatment with extracts derived from ESCs (Bru et al., 2008; Freberg et al., 2007; Taranger et al., 2005; Zhang et al., 2012). In contrast to these studies, our results revealed that genes were upregulated, including c-Myc (∼5-fold) and Klf-4 (∼10-fold), and approached the level in ADSCs, whereas Oct-4 (∼4-fold) was still significantly lower than that of ADSCs. However, no significant difference was observed in the Sox-2 expression level between the untreated fibroblast cells and fibroblast cells treated with ADSC extracts. One possible explanation for this result may be due to the expression differences between species. Another explanation for this may be the use of reprogramming extracts derived from different undifferentiated cells (including the passage number). Treatment time was also inconsistent.
For further confirmation of the effects of ADSC extracts on fibroblasts, the relative expression levels of fibroblast marker genes were analyzed. Fibroblast-associated genes (Col1a1 and Col1a2) were downregulated significantly after the extract treatment. This finding suggests that yak fibroblast cells had initiated the induction toward a more pluripotent state after the exposure to ADSC extracts, but had not achieved full reprogramming to pluripotent state. Consistent with this interpretation, the methylated statuses of 5′-flanking regulatory regions of Col1a1 and Col1a2 were partially methylated after induction by the extract, as compared with the control group.
Incomplete epigenetic reprogramming is the major cause of developmental failure of cloned embryos (Farin et al., 2006; Yang et al., 2007), which can be attributed to the extensive chromatin modification characteristics of terminally differentiated somatic cells. Thus, the nucleus of a less differentiated cell may be more suitable or require less reprogramming than the nucleus of a fully differentiated somatic cell (Rideout et al., 2001). A possible reason is the increasing difficulty of resetting gene expression as the cells become more differentiated. The differentiated state becomes more firmly established as cells embark on their terminal pathways and inappropriate lineages are shut down (Gurdon and Melton, 2008). A close relationship exists with epigenetic modifications, such as DNA and histone modifications, and we propose that combinations of DNA-binding or chromosomal proteins become more tightly associated with the regulatory regions of inactive genes. Therefore, improving the differentiated state and epigenetic reprogramming of donor nuclei might be one of the key issues that should be addressed to improve SCNT efficiency.
Notably, the extracts of pluripotent cells (such as ESCs and oocytes) can significantly improve the epigenetic reprogramming of donor nucleus and efficiency of SCNT (Bru et al., 2008; Miyamoto et al., 2008; Yang et al., 2012). Moreover, blastocyst formation and quality of yak cloned embryos significantly improved after the donor nucleus was pretreated with ADSC extracts. We suggest that the pluripotent factors of ADSCs induce the somatic cells to undergo dedifferentiation and demethylation, thus facilitating nuclear reprogramming and cloned embryo development. Research is in progress to further explore the mechanisms of this phenomenon and evaluate the long-term effects of pre-exposure of the donor nucleus to ADSC extracts on in vivo developmental competence of yak cloned embryos.
In conclusion, yak fibroblast cells can upregulate the expression of pluripotency genes and decrease the expression of fibroblast marker genes after exposure to ADSC extracts. In addition, the treatment of fibroblasts with ADSC extracts can modify the patterns of histone acetylation and global DNA methylation. Furthermore, the pretreated donor nuclei with ADSC extracts can improve the efficiency of yak iSCNT.
Acknowledgments
The present study was supported by the National Science and Technology Program of China (no. 2012BAD13B06) and the Fundamental Research Funds for the Central Universities of Southwest University for Nationalities (13NZYQN24 and 2011XWD-S0905).
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Bannister A.J., and Kouzarides T. (2011). Regulation of chromatin by histone modifications. Cell Res. 21, 381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani N., Brady J.J., Damian M., Sacco A., Corbel S.Y., and Blau H.M. (2010). Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 463, 1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C., Reither S., Mikeska T., et al. (2005). BiQ Analyzer: Visualization and quality control for DNA methylation data from bisulfite sequencing. Applications Note. 21(21), 4067–4068 [DOI] [PubMed] [Google Scholar]
- Boland M.J., Hazen J.L., Nazor K.L., Rodriguez A.R., Gifford W., Martin G., Kupriyanov S., and Baldwin K.K. (2009). Adult mice generated from induced pluripotent stem cells. Nature 461, 91–94 [DOI] [PubMed] [Google Scholar]
- Bru T., Clarke C., McGrew M.J., Sang H.M., Wilmut I., and Blow J.J. (2008). Rapid induction of pluripotency genes after exposure of human somatic cells to mouse ES cell extracts. Exp. Cell Res. 314, 2634–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan C.A., Atienza J., Melton D.A., and Eggan K. (2005). Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373 [DOI] [PubMed] [Google Scholar]
- Farin P.W., Piedrahita J.A., and Farin C.E. (2006). Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology 65, 178–191 [DOI] [PubMed] [Google Scholar]
- Freberg C.T., Dahl J.A., Timoskainen S., and Collas P. (2007). Epigenetic reprogramming of Oct-4 and Nanog regulatory regions by embryonal carcinoma cell extract. Mol. Biol. Cell 18, 1543–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez M.C., Pope C.E., Biancardi M.N., Dumas C., Galiguis J., Morris A.C., Wang G., and Dresser B.L. (2011). Trichostatin A modified histone covalent pattern and enhanced expression of pluripotent genes in interspecies Black-Footed cat cloned embryos but did not improve in vitro and in vivo viability. Cell. Reprogram. 13, 315–329 [DOI] [PubMed] [Google Scholar]
- Gurdon J.B., and Melton D.A. (2008). Nuclear reprogramming in cells. Science 322, 1811–1815 [DOI] [PubMed] [Google Scholar]
- Håkelien A.M., Gaustad K.G., and Collas P. (2004). Transient alteration of cell fate using a nuclear and cytoplasmic extract of an insulinoma cell line. Biochem. Biophys. Res Commun. 316, 834–841 [DOI] [PubMed] [Google Scholar]
- Jaenisch R., and Young R. (2008). Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132, 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.S., Yu X.F., Bang J.I., Cho S.J., Deb G.K., Kim B.W., and Kong I.K. (2010). Enhanced histone acetylation in somatic cells induced by a histone deacetylases inhibitor improved inter-generic cloned leopard cat blastocysts. Theriogenology 74, 1439–1449 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ostrup O., Li R., Li J., Vajta G., Kragh P.M., Schmidt M., Purup S., Poul Hyttel P., Klærke D., and Callesen H. (2013). Long-term effect on in vitro cloning efficiency after treatment of somatic cells with Xenopus egg extract in the pig. Reprod. Fertil. Dev. Published online: 8August2013; doi. 10.1071/RD13147 [DOI] [PubMed] [Google Scholar]
- Mikkelsen T.S., Hanna J., Zhang X.L., Ku M., Wernig M., Schorderet P., Bernstein B.E., Jaenisch R., Lander E.S., and Meissner A. (2008). Dissecting direct reprogramming through integrative genomic analysis. Nature 454, 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Yamashita T., Tsukiyama T., Kitamura N., Minami N., Yamada M., and Imai H. (2008). Reversible membrane permeabilization of mammalian cells treated with digitonin and its use for inducing nuclear reprogramming by Xenopus egg extracts. Cloning Stem Cells 10, 535–542 [DOI] [PubMed] [Google Scholar]
- Pasque V., Jullien J., Miyamoto K., Halley-Stott R.P., and Gurdon J.B. (2011). Epigenetic factors influencing resistance to nuclear reprogramming. Trends Genet. 27, 516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone A.J., Fisher P.A., Lee J.H., Craigon J., and Campbell K.H. (2010). Reprogramming of ovine somatic cells with xenopus laevis oocyte extract prior to SCNT improves live birth rate. Cell. Reprogram. 12, 609–616 [DOI] [PubMed] [Google Scholar]
- Rideout W.M., Eggan K., and Jaenisch R. (2001). Nuclear cloning and epigenetic reprogramming of the genome. Science 293, 1093–1098 [DOI] [PubMed] [Google Scholar]
- Ruijter J.M., Ramakers C., Hoogaars W.M.H., Karlen Y., Bakker O., van den Hoff M.J., and Moorman A.F. (2009). Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 6, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangalli J.R., De Bem T.H.C., Perecin F., Chiaratti M.R., Oliveira L.de J, de Araújo R.R., Valim Pimentel J.R., Smith L.C., and Meirelles F.V. (2012). Treatment of nuclear-donor cells or cloned zygotes with chromatin-modifying agents increases histone acetylation but does not improve full-term development of cloned cattle. Cell. Reprogram. 14, 235–247 [DOI] [PubMed] [Google Scholar]
- Schäffler A.M.D., and Büchler C. (2007). Concise review: Adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells 25, 818–827 [DOI] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- Taranger C.K., Noer A., Sørensen A.L., Håkelien A.M., Boquest A.C., and Collas P. (2005). Induction of dedifferentiation, genome wide transcriptional programming, and epigenetic reprogramming by extracts of carcinoma and embryonic stem cells. Mol. Biol. Cell 16, 5719–5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.J., Xiong X.R., Zhang H., Li Y.Y., Li Q., Wang Y.S., Xu W.B., Hua S., and Zhang Y. (2012). Defined media optimization for in vitro culture of bovine somatic cell nuclear transfer (SCNT) embryos. Theriogenology 78, 2110–2119 [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke A.E., McWhir J., Kind A.J., and Campbell K.H. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813 [DOI] [PubMed] [Google Scholar]
- Xiong X.R., Lan D.L., Li J., Zi X., Ma L., and Wang Y. (2013a). Zebularine and scriptaid significantly improve epigenetic reprogramming of yak fibroblasts and cloning efficiency. Cell. Reprogram. 15, 293–300 [DOI] [PubMed] [Google Scholar]
- Xiong X.R., Li J., Fu M., Gao C., Wang Y., and Zhong J.C. (2013b). Oocyte extract improves epigenetic reprogramming of yak fibroblast cells and cloned embryo development. Theriogenology 79, 462–469 [DOI] [PubMed] [Google Scholar]
- Yang X.Z., Smith S.L., Tian X.C., Lewin H.A., Renard J.P., and Wakayama T. (2007). Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 39, 295–302 [DOI] [PubMed] [Google Scholar]
- Yang X.Y., Mao J.D., Walters E.M., Zhao M.T., Teson J., Lee K., and Prather R.S. (2012). Xenopus egg extract treatment reduced global DNA methylation of donor cells and enhanced somatic cell nuclear transfer embryo development in pigs. BioRes. Open Access 1, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Victor Ruotti V., Stewart R., Slukvin I.I., and Thomson J.A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 [DOI] [PubMed] [Google Scholar]
- Zhang X.M., Li Q.M., Su D.J., Wang N., Shan Z.Y., Jin L.H., and Lei L. (2010). RA induces the neural-like cells generated from epigenetic modified NIH/3T3 cells. Mol. Biol. Rep. 37, 1197–1202 [DOI] [PubMed] [Google Scholar]
- Zhang X.M., Wang N., Li D.M., Jin L.H., and Lei L. (2012). Induction of epigenetic reprogramming in fibroblast by extracts of carcinoma. Afr. J. Biotechnol. 11, 2855–2861 [Google Scholar]