Abstract
Background
Retinal microvascular abnormalities have been associated with cognitive impairment, possibly serving as a marker of cerebral small vessel disease. This relationship has not been evaluated among persons with chronic kidney disease (CKD), a condition associated with increased risk of both retinal pathology and cognitive impairment.
Study Design
Cross-sectional study
Setting & Participants
588 participants ≥ 52 years old with CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study
Predictor
Retinopathy graded using the Early Treatment Diabetic Retinopathy Study severity scale and diameters of retinal vessels.
Outcomes
Neuropsychological battery of six cognitive tests
Measurements
Logistic regression models were used to evaluate the association of retinopathy, individual retinopathy features, and retinal vessel diameters with cognitive impairment (≤1 SD from the mean), and linear regression models were used to compare cognitive test scores across levels of retinopathy adjusting for age, race, sex, education, and medical comorbidities.
Results
The mean age of the cohort was 65.3 +/− 5.6 (SD) years; 51.9% were non-White, and 52.6% were male. The prevalence of retinopathy was 30.1% and 14.3% for cognitive impairment. Compared to those without retinopathy, participants with retinopathy had increased likelihood of cognitive impairment on executive function (35.1% vs. 11.5%; OR, 3.4; 95% CI, 2.0-6.0), attention (26.7% vs. 7.3%; OR, 3.0; 95% CI, 1.8-4.9), and naming (26.0% vs. 10.0%; OR, 2.1; 95% CI, 1.2-3.4) after multivariable adjustment. Increased level of retinopathy was also associated with lower cognitive performance on executive function and attention. Microaneurysms were associated with cognitive impairment on some domains, but there were no significant associations with other retinal measures after multivariable adjustment.
Limitations
Unknown temporal relationship between retinopathy and impairment.
Conclusions
In adults with CKD, retinopathy is associated with poor performance on several cognitive domains including executive function and attention. Evaluation of retinal microvascular abnormalities may be a promising tool for identifying patients with CKD who are at increased risk of cognitive impairment.
Cerebrovascular disease (CVD) is associated with an increased risk of cognitive impairment, but traditional methods of assessing CVD are both expensive and invasive. Alternatively, evaluation of fundus photography to identify retinal microvascular abnormalities such as retinopathy could be used to evaluate markers of CVD and target those at risk for cognitive impairment.1 Several cross-sectional studies have reported that participants with retinopathy performed worse on cognitive tests2-5 and had an increased risk of cognitive decline.6 This relationship between retinal microvascular abnormalities and cognitive impairment is especially significant for populations at high risk for cognitive impairment.7-9 For example, among participants with type I or type II diabetes, retinopathy has been reported to be associated with cognitive impairment,8, 10 and among participants with hypertension, it has been shown that those with retinopathy may be two times more likely to have dementia or cognitive impairment.2, 4
Individuals with chronic kidney disease (CKD) are also at high risk for cognitive impairment,11-13 and, despite reports that retinopathy is also associated with a greater risk of decreased kidneyfunction,14, 15 to our knowledge no studies have examined the association between retinopathy and cognitive impairment in this high risk population. A recent study found that diabetic participants with cognitive decline were more likely to have both retinopathy and nephropathy,16 indicating that cognitive impairment, decreased kidneyfunction, and retinopathy could share similar metabolic and inflammatory pathways.17, 18 The aim of our study is to investigate the association between retinopathy and cognitive function in a cohort of patients with chronic kidney disease.
Methods
Study Population
Between May 2003 and March 2007, participants were enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study, which included 3,612 adults with chronic kidney disease (CKD) and was designed to investigate the epidemiology of CKD and its relationship to cardiovascular disease. The study design and methods as well as the baseline cohort characteristics have been described elsewhere.19, 20 Two of the ancillary studies of the CRIC study are RCRIC (Retinopathy in Chronic Renal Insufficiency Cohort)21 and CRIC COG (Cognitive Function in Chronic Renal Insufficiency Cohort).22 In RCRIC, digital photos of the disc and macula in both eyes of 1936 of the 2605 (74%) participants in the CRIC Study from 6 of the 7 recruitment sites (Ann Arbor, Michigan; Baltimore, Maryland; Chicago, Illinois; Cleveland, Ohio; Philadelphia, Pennsylvania; and Oakland, California) were collected and evaluated. In CRIC COG, cognitive function measures from 825 participants (83.9 % of those asked participated,; eligibility criteria, age ≥ 52 years) from 4 of the study sites (Chicago, Cleveland, Philadelphia, and Oakland) were annually collected. Of the 638 participants enrolled in both ancillary studies, whether retinopathy was present could be determined in 588, and central retinal artery and vein diameters were measured in 512. We were able to assess individual features of retinopathy in at least 538 participants (microaneurysms, 538; hemorrhages, 546; soft exudates, 546; and hard exudates, 551). Compared to participants enrolled in both ancillary studies, other CRIC participants had slightly lower education levels, higher BMI, lower eGFR, and lower 3MS score (p<0.05).
Fundus Pathology and Retinopathy
Between June 2006 and May 2008, fundus photography was completed for both eyes by trained staff, and photographs were evaluated for fundus pathology and vascular abnormalities by blinded trained graders at a centralized site, the Fundus Photograph Reading Center at the University of Pennsylvania.23 The primary retinal measure used in these analyses was the overall retinopathy score, which was graded according to the Early Treatment Diabetic Retinopathy Study (ETDRS) severity scale, a grading protocol previously used in nondiabetic populations.24, 25 Presence of retinopathy was defined as an ETDRS score of 14, 15, 20, 35, 43, 47, 53, 60, 65, 70, or 80. We also grouped the retinopathy scores into four categories: no retinopathy (ETDRS score: 10 or 12), mild non-proliferative retinopathy (ETDRS score: 14, 15, or 20), non-proliferative retinopathy (ETDRS score: 35, 43, 47, or 53), and proliferative retinopathy (ETDRS score: 60, 65, 70, or 80). Presence/absence of each of the individual components of the ETDRS score, microaneurysms, hemorrhages, and hard and soft exudates, was also evaluated. Each of these measures was done for both eyes of each participant when possible. For CRIC participants with eye examinations, both eyes were measured 90.0% of the time for retinopathy scores, 80.2% for central retinal artery and vein diameters, 72.2% for microaneurysms, 73.3% for retinal hemorrhages, 74.1% for hard exudates, and 73.5% for soft exudates. For retinopathy features, when measures were made on both eyes, we chose the higher of the two scores. Retinal artery and vein diameters were also measured using the ARIC protocol and IVAN software (University of Wisconsin;the 6 largest retinal arteries and 6 largest retinal veins were measured to determine the mean diameters for arteries and for veins),26, 27 and we used the mean of the two eyes. For all of these measures, if only one eye was evaluated, the score for that eye was used. Further details on methods of retinal photography can be found elsewhere.21, 23
Cognitive Measures
The CRIC COG study included a battery of 6 interviewer-administered cognitive function tests: Modified Mini-Mental State Examination (3MS) to assess global cognitive function;28 Trail-Making Test, Forms A and B (Trails A and B), which are tests of attention and executive function;29 Category (verbal) Fluency, which measures verbal production, semantic memory, and language; Buschke Selective Reminding Test, which assesses delayed memory;30 and the Boston Naming Test, which measures language function based on naming pictures of objects.31 For each participant, we used the CRIC COG information collected nearest to the date of the RCRIC photographs (between July 2006 and October 2008). These measures were collected on the same day for 81% of participants and within 6 months for 96% of participants, and all were within 14 months of each other (median, 0 [range, 0-409] days). For all measures except the Trails A and B, a higher score indicated better cognitive function. Cognitive impairment was defined as a test score which was more than 1 standard deviation (SD) smaller than the mean for 3MS, Category Fluency, Boston Naming, and Buschke, and a score larger than 1 SD greater than the mean for Trails A and B.
Covariates
Sociodemographic risk factors such as age, sex, race, education, smoking, and self-reported medical history (including diagnosed hypertension and diabetes and self-reported coronary artery disease and stroke) were evaluated as part of the CRIC study visit, which coincided with the CRIC-COG cognitive evaluation. Body mass index (BMI) was calculated from measured weight and height, and estimated glomerular filtration rate (eGFR) was determined using the IDMS-traceable 4-variable Modification of Diet in Renal Disease (MDRD) Study equation.32,32a Blood pressure was measured using a standardized protocol.33 High sensitivity C-reactive protein was assayed using a BN II nephelometer (Siemens), urine albumin was measured with spot urine samples using an Immulite system (Siemens), and proteinuria was measured by 24 hour urine collection. Each covariate had <5% missing data, except for proteinuria which had 12% missing data.
Statistical analysis
Demographic and clinical measures were compared between those with and without retinopathy, and those with and without cognitive impairment, using two-sample t-tests for normally distributed continuous variables (using the Satterthwaite adjustment when variances are not equal), Wilcoxon rank sum tests for non-Normally distributed continuous variables, and chi-squared tests for categorical variables. To study the relationship between the cognitive measures and level of retinopathy, we first computed the mean and SD of each cognitive measure for each level of retinopathy. The means of the cognitive measures were compared across level of the retinal pathology using either an analysis of variance (ANOVA) for the 4-level retinopathy category (no, mild non-proliferative, non-proliferative, and proliferative retinopathy), or two-sample t-tests for dichotomous retinopathy designation (yes, no). For the ANOVA models using the 4-level retinopathy variable, we tested for trend using orthogonal polynomial contrasts. Finally, to adjust for possible confounders, we fitted linear regression models with cognitive test score as the dependent variable, and the following independent variables: presence or absence of retinal pathology, age, race, and education. We used logistic regression to calculate the odds of cognitive impairment associated with retinal pathology, adjusting for age, race, and education. In additional models, we adjusted for medical comorbidities including coronary artery disease, hypertension, diabetes, and stroke. SAS v9.1 (SAS Institute Inc) was used for all analyses. Statistical testing was two-tailed with significance level set at 0.05.
Results
The mean age of participants whose retinopathy status could be determined was 65.3 +/− 5.6 (SD) years; 51.9% of the cohort were non-White, and 52.6% were male. Thirty percent of participants showed evidence of retinopathy: 7.7% with mild non-proliferative, 12.9% with non-proliferative, and 9.5% with proliferative retinopathy. Participants with retinopathy were younger; less likely to have completed high school; less likely to be White; and more likely to have higher systolic blood pressure, diabetes, urinary protein coronary artery disease, and a history of stroke (p<0.05 for all). In addition, mean eGFR was lower for those with retinopathy (p<0.001) (Table 1). Compared to those without cognitive impairment, participants with cognitive impairment were older; less likely to be White; less likely to have completed high school; more likely to have a history of stroke; and more likely to have higher systolic blood pressure, higher urinary protein, lower BMI, and lower eGFR (p<0.05) (Table 2).
Table 1.
Baseline characteristics of CRIC Study participants by retinopathy status
| Baseline characteristics | Total N=588 |
No Retinopathy n=411 |
Retinopathy n=177 |
p- valuea |
|---|---|---|---|---|
|
| ||||
| Age (y) | 65.3 (5.6) | 65.6 (5.4) | 64.5 (6.0) | 0.03 |
|
| ||||
| Male sex | 309 (52.6) | 209 (50.9) | 100 (56.5) | 0.2 |
|
| ||||
| White | 283 (48.1) | 235 (57.2) | 48 (27.1) | <0.001 |
|
| ||||
| ≥High School education | 500 (85.0) | 366 (89.1) | 134 (75.7) | <0.001 |
|
| ||||
| Systolic BP (mmHg) | 128.8 (22.1) | 125.7 (20.2) | 136.0 (24.6) | <0.001 |
|
| ||||
| Diastolic BP( mmHg) | 68.0 (12.1) | 67.6 (11.2) | 69.0 (14.1) | 0.2 |
|
| ||||
| Diabetes | 275 (46.8) | 133 (32.4) | 142 (80.2) | <0.001 |
|
| ||||
| Coronary Artery Disease | 168 (28.6) | 105 (25.6) | 63 (35.6) | 0.01 |
|
| ||||
| Stroke | 76 (12.9) | 43 (10.5) | 33 (18.6) | 0.007 |
|
| ||||
| BMI (kg/m2) | 31.2 (6.7) | 31.0 (6.7) | 31.9 (6.6) | 0.1 |
|
| ||||
| Ever Smoker | 347 (59.0) | 246 (59.9) | 101 (57.1) | 0.5 |
|
| ||||
| eGFR categoryb | <0.001 | |||
|
| ||||
| 0-29 mL/min/1.73m2 | 120 (21.0) | 59 (14.6) | 61 (36.1) | |
| 30-44 mL/min/1.73m2 | 202 (35.3) | 139 (34.5) | 63 (37.3) | |
| 45-59 mL/min/1.73m2 | 200 (35.0) | 161 (40.0) | 39 (23.1) | |
| ≥60 mL/min/1.73m2 | 50 (8.7) | 44 (10.9) | 6 (3.6) | |
|
| ||||
| Albumin (g/dL) | 3.98 (0.40) | 4.04 (0.39) | 3.84 (0.40) | <0.001 |
|
| ||||
| Proteinuria (g/24 h) | 0.13 (0.05, 0.42) |
0.10 (0.05, 0.24) |
0.32 (0.09, 1.17) |
<0.001 |
|
| ||||
| C-reactive protein (mg/dL) | 2.17 (0.97, 5.83) |
2.15 (0.97, 5.69) |
2.22 (0.95, 6.25) |
0.6 |
|
| ||||
| Cognitive Impairmentc | ||||
|
| ||||
| 3MS | 84 (14.3) | 50 (12.2) | 34 (19.2) | 0.03 |
|
| ||||
| Trails A | 77 (13.1) | 30 (7.3) | 47 (26.7) | <0.001 |
|
| ||||
| Trails B | 108 (18.5) | 47 (11.5) | 61 (35.1) | <0.001 |
|
| ||||
| Category Fluency | 111 (18.9) | 63 (15.4) | 48 (27.3) | <0.001 |
|
| ||||
| Buschke Selective Reminding | 91 (15.7) | 58 (14.3) | 33 (18.8) | 0.2 |
|
| ||||
| Boston Naming | 87 (14.8) | 41 (10.0) | 46 (26.0) | <0.001 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables are given as mean +/− SD or median [interquartile range].
CRIC, Chronic Renal Insufficiency Cohort; BP, blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate;3MS, Modified Mini-Mental State Examination; SD, standard deviation.
p-value for comparison of no retinopathy and retinopathy groups
n=572
Cognitive impairment defined by the listed neuropsychological tests, as values more than 1 SD above the mean for Trails A and B, and more than 1 SD below the mean for the other tests
Table 2.
Baseline characteristics of CRIC Study participants by cognitive impairment status
| Baseline characteristics | Total N=588 |
No Cognitive Impairment n=504 |
Cognitive Impairment n=84 |
p- valueb |
|---|---|---|---|---|
|
| ||||
| Age (y) | 65.3 (5.6) | 65.0 (5.6) | 66.8 (5.7) | 0.005 |
|
| ||||
| Male sex | 309 (52.6) | 267 (53.0) | 42 (50.0) | 0.6 |
|
| ||||
| White | 283 (48.1) | 268 (53.2) | 15 (17.9) | <0.001 |
|
| ||||
| ≥High School education | 500 (85.0) | 460 (91.3) | 40 (47.6) | <0.001 |
|
| ||||
| Systolic BP (mmHg) | 128.8 (22.1) | 126.8 (20.6) | 140.5 (26.9) | <0.001 |
|
| ||||
| Diastolic BP (mmHg) | 68.0 (12.1) | 67.7 (11.8) | 69.9 (13.9) | 0.2 |
|
| ||||
| Diabetes | 275 (46.8) | 229 (45.4) | 46 (54.8) | 0.1 |
|
| ||||
| Coronary Artery Disease | 168 (28.6) | 143 (28.4) | 25 (29.8) | 0.8 |
|
| ||||
| Stroke | 76 (12.9) | 52 (10.3) | 24 (28.6) | <0.001 |
|
| ||||
| BMI(kg/m2) | 31.2 (6.7) | 31.5 (6.9) | 29.8 (4.8) | 0.008 |
|
| ||||
| Ever Smoker | 347 (59.0) | 296 (58.7) | 51 (60.7) | 0.7 |
|
| ||||
| eGFRc category | 0.002 | |||
|
| ||||
| 0-29 mL/min /1.73m2 | 120 (21.0) | 93 (18.9) | 27 (33.8) | |
| 30-44 mL/min /1.73m2 | 202 (35.3) | 170 (34.6) | 32 (40.0) | |
| 45-59 mL/min /1.73m2 | 200 (35.0) | 181 (36.8) | 19 (23.8) | |
| ≥60 mL/min/1.73m2 | 50 (8.7) | 48 (9.8) | 2 (2.5) | |
|
| ||||
| Albumin(g/dL) | 3.98 (0.40) | 3.98 (0.40) | 3.99 (0.44) | 0.8 |
|
| ||||
| C-reactive protein (mg/dL) | 2.17 (0.97, 5.83) |
2.03 (0.92, 5.71) |
2.76 (1.25, 6.25) |
0.09 |
|
| ||||
| Proteinuria (g/24 h) | 0.13 (0.05, 0.42) |
0.12 (0.05, 0.38) |
0.22 (0.08, 0.63) |
0.04 |
Note: Cognitive impairment defined as values more than 1 SD below the mean on the Modified Mini-Mental State Examination. Values for categorical variables are given as number (percentage); values for continuous variables are given as mean +/− SD or median [interquartile range].
CRIC, Chronic Renal Insufficiency Cohort; BP, blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate;SD, standard deviation.
p-value for comparison of no cognitive impairment and cognitive impairment groups
n=572
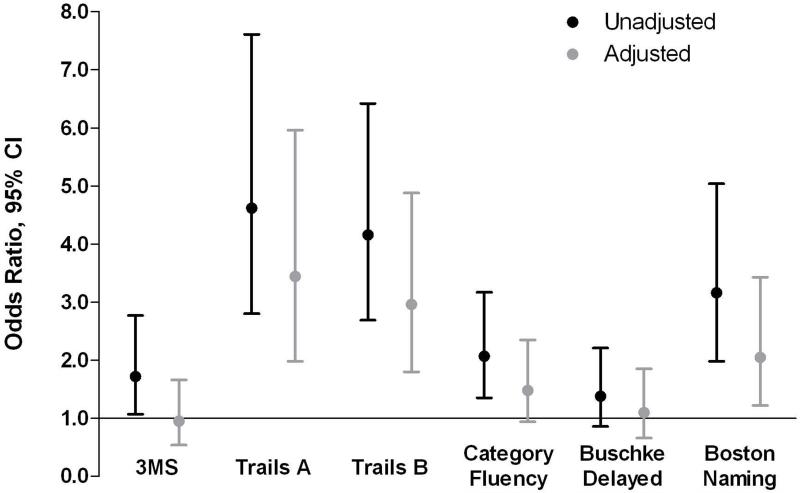
Participants with retinopathy were more likely to have cognitive impairment than those without retinopathy. In unadjusted logistic regression models of cognitive impairment, retinopathy was significantly associated with impairment on all cognitive measures except for Buschke Selective Reminding (3MS: OR, 1.7; 95% CI, 1.1-2.8; Trails A: OR, 4.6; 95% CI, 2.8-7.6; Trails B: OR, 4.2; 95% CI, 2.7-6.4; Category Fluency: OR, 2.1; 95% CI, 1.3-3.2; Boston Naming Test: OR, 3.2; 95% CI, 2.0-5.0; Buschke Selective Reminding: OR, 1.4; 95% CI, 0.9-2.2) (Figure 1). After multivariable adjustment for age, race, sex, and education, retinopathy remained associated with risk of cognitive impairment on Trails A (OR,3.4; 95% CI, 2.0-6.0), Trails B (OR,3.0; 95% CI, 1.8-4.9), and the Boston Naming Test (OR, 2.1; 95% CI, 1.2-3.4).
Figure 1.
Unadjusted and adjusted (for age, race, sex, and education) odds of cognitive impairment for those with versus without retinopathy
Performance on cognitive tests was worse as severity of retinopathy increased. For example, on Trails A, there was a trend towards worse performance with increasing severity of retinopathy (no retinopathy, mean of 45.2 +/− 1.5 [SE]; mild non-proliferative retinopathy, 52.9 +/− 4.5; non-proliferative retinopathy, 64.7 +/− 3.4; and proliferative retinopathy, 82.7 +/− 4.0; p for trend <0.001) (Table 3). Unadjusted scores on the 3MS, Trails B, Category Fluency, Buschke Selective Reminding, and Boston Naming tests also had significant trends across levels of retinopathy (Table 3). After multivariable adjustment for age, race, sex, and education, the relationship between increased severity of retinopathy and worse performance on Trails A and Trails B remained statistically significant.
Table 3.
Cognitive test scores by level of retinopathy
| None (n=411) |
Mild Non- Proliferative (n=45) |
Non- Proliferative (n=76) |
Proliferative (n=56) |
p-valuea | |
|---|---|---|---|---|---|
| 3MS | |||||
| Unadjusted | 94.1 (0.4) | 92.5 (1.1) | 91.9 (0.8) | 90.8 (1.0) | 0.002 |
| Adjustedb | 91.0 (0.4) | 91.1 (1.0) | 91.1 (0.8) | 90.8 (0.9) | 0.9 |
| Trails A | |||||
| Unadjusted | 45.2 (1.5) | 52.9 (4.5) | 64.7 (3.4) | 82.7 (4.0) | <0.001 |
| Adjustedb | 53.0 (2.0) | 56.7 (4.4) | 65.5 (3.5) | 84.1 (4.1) | <0.001 |
| Trails B | |||||
| Unadjusted | 122.1 (3.7) | 152.8 (11.0) | 171.5 (8.6) | 192.7 (10.0) | <0.001 |
| Adjustedb | 150.0 (4.5) | 168.9 (10.0) | 174.8 (8.1) | 195.8 (9.3) | 0.01 |
| Category Fluency | |||||
| Unadjusted | 18.8 (0.3) | 16.6 (0.8) | 17.5 (0.6) | 15.9 (0.7) | <0.001 |
| Adjustedb | 17.4 (0.3) | 15.8 (0.7) | 17.6 (0.6) | 16.0 (0.7) | 0.8 |
| Buschke Selective Reminding | |||||
| Unadjusted | 7.8 (0.1) | 7.2 (0.4) | 7.5 (0.3) | 6.6 (0.4) | 0.03 |
| Adjustedb | 7.2 (0.2) | 6.9 (0.4) | 7.3 (0.4) | 6.5 (0.4) | 0.5 |
| Boston Naming | |||||
| Unadjusted | 14.1 (0.1) | 13.4 (0.2) | 13.5 (0.2) | 12.9 (0.2) | <0.001 |
| Adjustedb | 13.6 (0.1) | 13.1 (0.2) | 13.4 (0.2) | 13.0 (0.2) | 0.6 |
Note: N=588. Values presented as mean (standard error).
3MS, Modofied Mini-Mental State Examination.
for trend
Adjusted for age, race, sex, and education
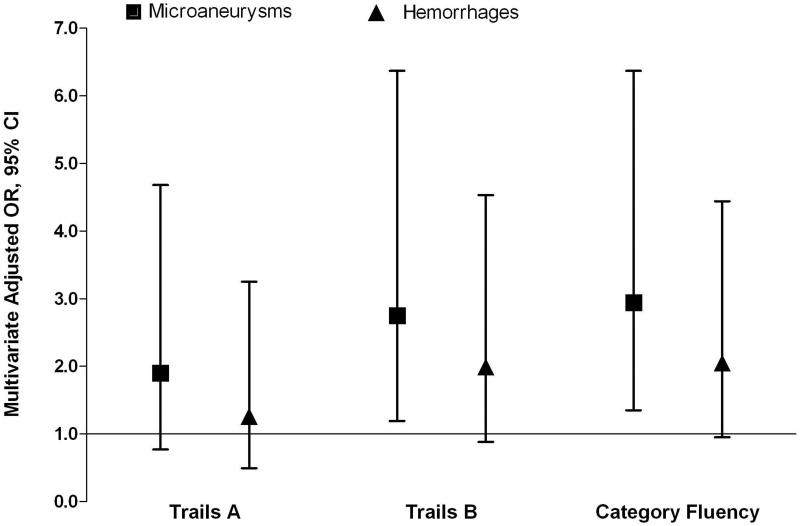
We next determined whether individual retinal microvascular abnormalities were associated with cognitive function among patients with CKD. In models of cognitive impairment adjusted for age, race, sex, and education (Figure 2), microaneurysms were significantly associated with impairment on Trails B and Category Fluency (ORs of 2.8 [95% CI, 1.2-6.4] and 2.9 [95% CI, 1.4-6.4], respectively). Mean cognitive scores on Trails A and B were also significantly worse for those with microaneurysms compared to those without (Table 4). None of the cognitive impairment measures were significant for hemorrhages or hard exudates, and there were too few participants with soft exudates (n=14) to determine their association with cognitive impairment.
Figure 2.
Odds of cognitive impairment in those with versus without microaneurysms or with versus without hemorrhages, adjusted for age, race, sex and education
Table 4.
Cognitive test scores by presence or absence of microaneurysms, hemorrhages, and hard and soft exudates
| Microaneurysms and Hemorrhages |
Microaneurysms | p -value |
Hemorrhages | p- value |
||
|---|---|---|---|---|---|---|
| Absent n=461 |
Present n=77 |
Absent n=446 |
Present n=100 |
|||
| 3MS | 91.1 (0.4) | 89.5 (1.2) | 0.2 | 91.1 (0.4) | 90.1 (1.1) | 0.4 |
| Trails A | 59.5 (1.6) | 69.2 (4.7) | 0.04 | 59.8 (1.7) | 62.5 (4.4) | 0.6 |
| Trails B | 161.2 (4.2) | 190.8 (12.3) | 0.02 | 161.8 (4.3) | 178.0 (11.5) | 0.2 |
| Category Fluency | 17.3 (0.3) | 15.6 (0.9) | 0.06 | 17.3 (0.3) | 16.0 (0.8) | 0.1 |
| Buschke Selective Reminding |
7.2 (0.2) | 6.4 (0.5) | 0.1 | 7.2 (0.2) | 7.4 (0.5) | 0.6 |
| Boston Naming | 13.4 (0.1) | 13.3 (0.3) | 0.7 | 13.4 (0.1) | 13.0 (0.3) | 0.1 |
| Exudates | Hard Exudates | p- value |
Soft Exudates | p- value |
||
|---|---|---|---|---|---|---|
| Absent n=513 |
Present n=38 |
Absent n=532 |
Present n=14 |
|||
| 3MS | 91.0 (0.4) | 92.6 (1.8) | 0.3 | 91.0 (0.4) | 90.5 (2.2) | 0.8 |
| Trails A | 60.0 (1.6) | 60.6 (7.0) | 0.9 | 60.0 (1.6) | 60.2 (8.7) | 0.9 |
| Trails B | 162.9 (4.2) | 171.6 (18.2) | 0.6 | 163.4 (4.2) | 151.2 (23.9) | 0.6 |
| Category Fluency | 17.1 (0.3) | 17.1 (1.3) | 0.9 | 17.2 (0.3) | 14.6 (1.6) | 0.1 |
| Buschke Selective Reminding |
7.1 (0.2) | 8.1 (0.8) | 0.2 | 7.2 (0.2) | 6.2 (1.0) | 0.3 |
| Boston Naming | 13.4 (0.1) | 13.9 (0.4) | 0.2 | 13.4 (0.1) | 13.2 (0.5) | 0.7 |
Note: Data adjusted for age, race, sex, and education. Values are presented as mean (standard error).
3MS, Modofied Mini-Mental State Examination.
In order to determine whether the association between retinopathy and cognitive impairment was mediated by cardiovascular disease, diabetes, or albumin (as a proxy for small vessel disease), we also adjusted for additional variables including systolic blood pressure, coronary artery disease, stroke, BMI, diabetes, or albumin. The addition of each of the variables did not attenuate the association between cognitive impairment and retinopathy. To determine the effects of kidney function, we stratified models by eGFR level. The pattern of the association between retinopathy and cognitive performance was similar but tended to be more robust for eGFR>30 mL/min/1.73 m2 on Trails A, Trails B, and Boston Naming. In models stratified for diabetes status, retinopathy was more consistently associated with cognitive performance for those without diabetes.
Analyses testing for trend in unadjusted mean test scores across quartiles of central artery and central vein diameter were not significant for most cognitive measures. Unadjusted scores on Trails A had significant trends across quartiles of central vein diameter, but after adjusting for age, race, sex, and education, the association was no longer significant. Central vein diameter was associated with impairment on the 3MS and Boston Naming Test in unadjusted models, but was not significant for any of the adjusted models.
Discussion
In this cohort of persons with chronic kidney disease, retinopathy was significantly associated with cognitive impairment. In particular, the odds of cognitive impairment on executive function, attention, and naming were two to three times higher in those with retinopathy compared to those without retinopathy, but there was no association for global cognition, category fluency, or delayed recall. Significant associations between retinopathy and cognitive impairment on executive function, attention, and naming persisted after adjusting for diabetes and cardiovascular disease. Similar relationships were observed for specific retinal microvascular abnormalities including microaneurysms, while central vein and central artery diameter, hemorrhages, and soft and hard exudates were not associated with cognitive function.
Impairment on executive function, as linked to retinopathy in this study, is associated with the frontal subcortical region of the brain, an area which is particularly vulnerable to damage from cerebral small vessel disease.34 Our findings are supported by previous reports on the relationship between retinopathy and cognitive impairment in both general populations as well as among patients with hypertension and diabetes. In the Cardiovascular Health Study, retinopathy was associated with impairment on the Digit Symbol Substitution Test, a test of executive function, but not on the Modified Mini-Mental State Examination.2 In the Atherosclerosis Risk in the Community (ARIC) cohort, the presence of retinopathy increased the risk of cognitive decline on tests of executive function and psychomotor speed, and the likelihood of cognitive impairment was almost three times higher for those with microaneurysms or hemorrhages.5, 6 Measures of central retinal artery and central retinal venular diameter were not significant in our study. While both venular dilation and arteriolar narrowing have been associated with risk of cognitive impairment and dementia in previous studies,4, 35 the evidence of a relationship between these measures of retinal microvascular abnormalities and cognitive impairment is not as consistent as with retinopathy. Similarly, retinopathy has also been associated with decreased kidneyfunction,14, 15, 36 but associations of CKD with central vein and central artery diameter have been mixed.36-38 It is possible that in retinopathy, microaneurysms may reflect more severe microvascular damage than arteriolar narrowing or venular dilation.39
Our findings support the hypothesis that CKD, retinopathy, and cognitive impairment share common vascular pathways. These mechanisms could include endothelial dysfunction, inflammation, oxidative stress, and vascular calcification,18, 40 suggesting that a systemic approach to small vessel disease which links outcomes in multiple organs may be appropriate.17 This perspective would be especially critical for patients with chronic kidney disease, a population at increased risk for cognitive impairment.13, 22, 41 Moreover, in dialysis patients, cognitive impairment and dementia have also been linked to increased risk of other adverse health outcomes including dialysis withdrawal and death.42-44 Although several studies have demonstrated a link between CKD and cognitive impairment, little is known about effective strategies to prevent cognitive decline in this population. As such, our study, which demonstrates a relationship between retinopathy and cognitive impairment independent of coronary artery disease, hypertension, diabetes, and stroke, has possible implications for monitoring patients with CKD and suggests that fundus pathology would be a useful noninvasive and inexpensive marker for identifying those at risk of cognitive impairment. This is supported by a recent study which demonstrated an association between retinopathy and markers of cerebral small vessel disease, including cerebral and lacunar infarcts identified by magnetic resonance imaging 10 years later.45
To our knowledge, this is the first study to examine the relationship between retinopathy and cognitive impairment in patients with CKD. Unlike previous investigations of retinal microvascular abnormalities and cognition, we administered an extensive battery of six cognitive tests in the CRIC Study to assess impairment on multiple cognitive domains. However, because we report on cross sectional findings, the temporal relationship between retinopathy and cognitive impairment is unclear. In addition, although protocols for retinal photography and grading were standardized, there may be some variation between graders.
In our cohort of adults with CKD, retinopathy was significantly associated with cognitive impairment on several domains including executive function, attention and naming. Our initial findings indicate that cerebral small vessel disease may play a role in the association between CKD and development of cognitive impairment. The prevalence of CKD is expected to rise in the U.S. as the population ages and the incidence of cardiovascular risk factors such as obesity also increases.46 Thus, approaches are needed to target persons with CKD who are at increased risk for cognitive decline and dementia if the public health burden of these diseases is to be reduced. Identification of retinal pathology by fundus photography as a proxy for cerebral small vessel disease is a promising approach, but longitudinal studies are needed to establish the temporal relationship between retinopathy and changes in cognitive function in the setting of CKD.
Acknowledgements
Support: The CRIC study is funded by National Institute of Health Grants U01 DK060980, U01 DK060902, U01 DK060963, U01 DK060984, U01 DK060990, U01 DK061021, U01 DK061022, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants UL1 RR-024134, UL1 RR-025005, MO1 RR-16500, UL1 RR-024989, MO1 RR-000042, UL1 RR-024986, UL1 RR-029879, UL1 RR-024131. The ancillary studies were also supported by NIDDK grant R01 DK069406, which was administered by the Northern California Institute for Research and Education and with resources of the Veterans Affairs Medical Center, San Francisco, California, and by NIH R01 DK74151, Vivian S. Lasko Research Fund, Nina C. Mackall Trust, and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Patton N, Aslam T, MacGillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. Journal of Anatomy. 2005;206(4):319. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker ML, Marino Larsen EK, Kuller LH, et al. Retinal microvascular signs, cognitive function, and dementia in older persons: the Cardiovascular Health Study. Stroke. 2007;38(7):2041–2047. doi: 10.1161/STROKEAHA.107.483586. [DOI] [PubMed] [Google Scholar]
- 3.Ding J, Patton N, Deary IJ, et al. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. British Journal of Ophthalmology. 2008;92(8):1017–1025. doi: 10.1136/bjo.2008.141994. [DOI] [PubMed] [Google Scholar]
- 4.Liew G, Mitchell P, Wong TY, et al. Retinal Microvascular Signs and Cognitive Impairment. Journal of the American Geriatrics Society. 2009;57(10):1892–1896. doi: 10.1111/j.1532-5415.2009.02459.x. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the Atherosclerosis Risk in Communities Study. Stroke. 2002;33(6):1487–1492. doi: 10.1161/01.str.0000016789.56668.43. [DOI] [PubMed] [Google Scholar]
- 6.Lesage SR, Mosley TH, Wong TY, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology. 2009;73(11):862–868. doi: 10.1212/WNL.0b013e3181b78436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Strachan MWJ, Reynolds RM, et al. Diabetic Retinopathy and Cognitive Decline in Older People With Type 2 Diabetes. Diabetes. 2010;59(11):2883–2889. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia. 2003;46(7):940–948. doi: 10.1007/s00125-003-1128-2. [DOI] [PubMed] [Google Scholar]
- 9.Tekin O, Çukur S, Urald C, et al. Relationship between retinopathy and cognitive impairment among hypertensive subjects. European neurology. 2004;52(3):156–161. doi: 10.1159/000081855. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson SC, Blane A, Perros P, et al. Cognitive Ability and Brain Structure in Type 1 Diabetes. Diabetes. 2003 Jan;52(1):149–156. doi: 10.2337/diabetes.52.1.149. 2003. [DOI] [PubMed] [Google Scholar]
- 11.Kurella M, Chertow GM, Fried LF, et al. Chronic Kidney Disease and Cognitive Impairment in the Elderly: The Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2005 Jul 1;16(7):2127–2133. doi: 10.1681/ASN.2005010005. 2005. [DOI] [PubMed] [Google Scholar]
- 12.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate Renal Impairment and Risk of Dementia among Older Adults: The Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004 Jul 1;15(7):1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. 2004. [DOI] [PubMed] [Google Scholar]
- 13.Slinin Y, Paudel ML, Ishani A, et al. Kidney Function and Cognitive Performance and Decline in Older Men. Journal of the American Geriatrics Society. 2008;56:2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. American journal of kidney diseases. 2005;46(2):214–224. doi: 10.1053/j.ajkd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. Journal of the American Society of Nephrology. 2004;15(9):2469–2476. doi: 10.1097/01.ASN.0000136133.28194.E4. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson A, Ryan C, Cleary P, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia. 2011;54(2):245–255. doi: 10.1007/s00125-010-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson CS, Hakim AM. Living Beyond Our Physiological Means: Small Vessel Disease of the Brain Is an Expression of a Systemic Failure in Arteriolar Function: A Unifying Hypothesis. Stroke. 2009 May 1;40(5):e322–330. doi: 10.1161/STROKEAHA.108.542266. 2009. [DOI] [PubMed] [Google Scholar]
- 18.Cheung N, Wong TY. Diabetic Retinopathy and Systemic Complications. In: Duh E, editor. Diabetic Retinopathy. Humana Press; Totowa, NJ: 2008. [Google Scholar]
- 19.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003 Jul;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 20.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clinical Journal of the American Society of Nephrology. 2009 Aug 1;4(8):1302–1311. doi: 10.2215/CJN.00070109. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunwald JE, Alexander J, Maguire M, et al. Prevalence of Ocular Fundus Pathology in Patients with Chronic Kidney Disease. Clinical Journal of the American Society of Nephrology. 2010;5(5):867–873. doi: 10.2215/CJN.08271109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaffe K, Ackerson L, Tamura MK, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. Journal of the American Geriatrics Society. 2010;58(2):338–345. doi: 10.1111/j.1532-5415.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunwald JE, Ying G-S, Maguire M, et al. Association Between Retinopathy and Cardiovascular Disease in Patients With Chronic Kidney Disease (from the Chronic Renal Insufficiency Cohort [CRIC] Study) American Journal of Cardiology. 2012;110(2):246–253. doi: 10.1016/j.amjcard.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. Ophthalmology. 1991 May;98(5 Suppl):786–806. ETDRS report number 10. [PubMed] [Google Scholar]
- 25.Chao JR, Lai MY, Azen SP, Klein R, Varma R, the Los Angeles Latino Eye Study G Retinopathy in Persons without Diabetes: The Los Angeles Latino Eye Study. Investigative ophthalmology & visual science. 2007 Sep 1;48(9):4019–4025. doi: 10.1167/iovs.07-0206. 2007. [DOI] [PubMed] [Google Scholar]
- 26.Wong TY, Knudtson MD, Klein R, Klein BEK, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 27.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BEK. Revised formulas for summarizing retinal vessel diameters. Current Eye Research. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 28.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 29.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Battery: Theory and Clinical Interpretation. Neuropsychology Press; Tuscon, AZ: 1985. [Google Scholar]
- 30.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 31.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 32.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. American Journal of Kidney Diseases. 2002 May;39(5):920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 32a.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study Equation for estimating glomerular filtration rate. Annals Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5):2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 34.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005 Sep;128(9):2034–2041. doi: 10.1093/brain/awh553. 2005. [DOI] [PubMed] [Google Scholar]
- 35.de Jong FJ, Schrijvers EMC, Ikram MK, et al. Retinal vascular caliber and risk of dementia. Neurology. 2011;76(9):816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabanayagam C, Shankar A, Koh D, et al. Retinal Microvascular Caliber and Chronic Kidney Disease in an Asian Population. American Journal of Epidemiology. 2009 Mar 1;169(5):625–632. doi: 10.1093/aje/kwn367. 2009. [DOI] [PubMed] [Google Scholar]
- 37.Sabanayagam C, Shankar A, Klein BEK, et al. Bidirectional Association of Retinal Vessel Diameters and Estimated GFR Decline: The Beaver Dam CKD Study. American Journal of Kidney Diseases. 2011;57(5):682–691. doi: 10.1053/j.ajkd.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yau JWY, Xie J, Kawasaki R, et al. Retinal Arteriolar Narrowing and Subsequent Development of CKD Stage 3: The Multi-Ethnic Study of Atherosclerosis (MESA) American Journal of Kidney Diseases. 2011;58(1):39–46. doi: 10.1053/j.ajkd.2011.02.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong TY, Klein R, Klein BEK, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Survey of Ophthalmology. 2001;46(1):59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 40.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MMB. Kidney Function Is Related to Cerebral Small Vessel Disease. Stroke. 2008 Jan 1;39(1):55–61. doi: 10.1161/STROKEAHA.107.493494. 2008. [DOI] [PubMed] [Google Scholar]
- 41.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009 Sep 22;73(12):920–927. doi: 10.1212/WNL.0b013e3181b72629. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive Impairment and 7-Year Mortality in Dialysis Patients. American Journal of Kidney Diseases. 2010;56(4):693–703. doi: 10.1053/j.ajkd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrology Dialysis Transplantation. 2006 Sep;21(9):2543–2548. doi: 10.1093/ndt/gfl275. 2006. [DOI] [PubMed] [Google Scholar]
- 44.Cohen LM, Ruthazer R, Moss AH, Germain MJ. Predicting Six-Month Mortality for Patients Who Are on Maintenance Hemodialysis. Clinical Journal of the American Society of Nephrology. 2009 Dec 3;5(1):72–79. doi: 10.2215/CJN.03860609. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung N, Mosley T, Islam A, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010 Jul 1;133(7):1987–1993. doi: 10.1093/brain/awq127. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens LA, Viswanathan G, Weiner DE. Chronic Kidney Disease and End-Stage Renal Disease in the Elderly Population: Current Prevalence, Future Projections, and Clinical Significance. Advances in Chronic Kidney Disease. 2010;17(4):293–301. doi: 10.1053/j.ackd.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]